Abstract
OBJECTIVES
To investigate the combined influence of blood flow and haemodilution with either a miniaturized (Mini-CPB) or a conventional cardiopulmonary bypass (C-CPB) circuit on average oxygen delivery during bypass. The influence of this on clinical outcome, particularly renal dysfunction after routine coronary artery bypass surgery (CABG), was measured.
METHODS
Retrospective analysis in two groups of 160 patients based on the surgeon's preference for bypass circuit. We compared consecutive patients undergoing isolated CABG surgery by two surgeons using Mini-CPB with a matched cohort of patients, from the same period, undergoing isolated CABG surgery by four other surgeons using a C-CPB. No trial-related intervention occurred. Data on bypass circuit parameters and clinical outcomes were acquired from routinely collected data sources.
RESULTS
Average cardiopulmonary bypass pump flow was significantly lower with Mini-CPB compared with C-CPB. Mini-CPB resulted in significantly less haemodilution. The resultant calculated average oxygen delivery provided by the two systems was the same. Percentage change in plasma creatinine was significantly and inversely related to the oxygen delivery during CPB. There was no difference in percentage change in plasma creatinine between groups. The risk of having Acute Kidney Injury Network (AKIN) score ≥1 increased 1% for every 1 ml min−1 m−2 decrease in oxygen delivery (P = 0.0001, OR 0.990, 95% CI 0.984–0.995).
CONCLUSIONS
Despite aiming for the same target pump flow, periodic limitations of venous return to the pump resulted in a significant reduction in average flow delivered to the patient by Mini-CPB. Less haemodilution compensated for this reduction, so that the average oxygen delivery was the same. The association between oxygen delivery and postoperative change in plasma creatinine was evident in both groups. Further work to understand whether there is a particular cohort of patients who benefit (or are put at risk) by one method of CPB vs the other is warranted.
Keywords: Oxygen delivery, Extracorporeal circulation, Miniaturized cardiopulmonary bypass, Cardiac surgery
INTRODUCTION
The factors related to the process of cardiopulmonary bypass (CPB) that contribute most significantly to ‘optimal perfusion’ remain an important topic of debate. Randomized data on best practice in managing haemodilution, perfusion pressure, haematocrit and pump flow are extremely limited, but in an extensive review of the literature [1], the authors conclude that oxygen delivery remains ‘one of the most important determinants of optimal perfusion’.
Organ dysfunction after cardiac surgery has been linked to a decrease in oxygen delivery during CPB [2, 3]. Conventional CPB (C-CPB) requires an initial crystalloid prime of 1500–2000 ml, resulting in dilutional anaemia at the onset of bypass. Autologous priming of the circuit after cannulation reduces the prime, but is incomplete, and haemodilution still occurs. Acute haemodilution during cardiac surgery is associated with an increased risks of renal failure [4–6], stroke [7] and mortality [8–10].
A recent development, miniaturized cardiopulmonary bypass (Mini-CPB), has appeared to offer theoretical advantages. These include the absence of a venous reservoir, considerably lowering the priming volume to 200–500 ml after the circuit is retrogradedly primed, resulting in minimal haemodilution. This should increase the oxygen delivery (DO2) during bypass, but there are suggestions that the pump flow achieved with Mini-CPB is lower than with C-CPB [11], and that periods of ‘low flow’ are more frequent and last longer [12].
We evaluated the combined influence of blood flow during bypass together with any change in haematocrit concentration on average oxygen delivery. Because of the frequently reported association between oxygen delivery and subsequent renal dysfunction [2, 13], we used this as the main marker of differences in clinical outcome.
MATERIALS AND METHODS
Over a 1-year period, a total of 160 patients underwent coronary artery bypass grafting (CABG) with the Mini-CPB circuit (two surgeons). After approval from the Cornwall and Plymouth Research Ethics Committee, we compared these patients with the first 160 who had undergone CABG with C-CPB during the same period (four other surgeons). We used data from the cardiac surgery and perfusion database.
Conventional and miniaturized cardiopulmonary bypass
The C-CPB circuit was a standard open circuit with a centrifugal arterial pump, membrane oxygenator and hard-shell reservoir (Quadrox-I, Maquet Cardiopulmonary). Venous return was gravity-dependent with ½ in. tubing. The circuit was primed with 1000 ml Hartmann's solution, 700 ml gelofusine, 100 ml 20% mannitol and 5000 IU heparin. No retrograde autologous priming was used. Cardiotomy suction was used and aspirated blood returned into the reservoir. A cell-saver device was used depending on surgeon preference.
The Mini-CPB circuit (Maquet Cardiopulmonary) was a preconnected closed-loop circuit incorporating a RotaFlow centrifugal pump, a Quadrox-I diffusion membrane oxygenator and a Quart arterial blood filter. There was both an arterial and a venous bubble detector and a venous bubble trap (Maquet Cardiopulmonary). Pericardial blood was aspirated into a cell-saver device (Electa, Sorin). If sufficient volume was aspirated, it had been processed and re-transfused at the end of the operation. In cases where large volumes returned to the cell saver, the processed blood was returned to the circuit via the bubble trap. The circuit was heparin coated (Bioline Coating, Maquet Cardiopulmonary) and primed with 800 ml Gelofusine that was reduced to between 200 and 500 ml by retrograde autologous priming following aortic cannulation.
Anaesthesia and surgical management
All patients were anaesthetized with a low-dose opioid technique, using 10 µg kg−1 of fentanyl. Maintenance of anaesthesia was done with a combination of isoflurane and propofol. Surgical technique did not differ between groups. Arterial access was the same in all patients and was achieved through an ascending aorta cannulation [24-fr angled (72 524, Medtronic)], and venous access was done through a two-stage venous cannula inserted through the right atrial appendage (91251C, Medtronic).
Perfusion management
Moderate hypothermia to 34°C, alpha-stat pH management and target flow rates of 2.5 l min−1 m−2 were used for both groups. Mean arterial pressure target was 50–60 mmHg. Anticoagulation to achieve an activated clotting time >400 s was achieved with heparin 300 IU kg−1. Final cardioplegia concentrations were the same (K+ 20 mmol l−1 induction and 10 mmol l−1 maintenance). In the Mini-CPB group, retrograde autologous priming of the circuit was undertaken over ≤1 min prior to the commencement of bypass. Target haematocrit (Hct) during bypass was maintained at 21% or higher, with red blood cell transfusion given as necessary.
Intensive care unit
A unit-based transfusion policy was used for both packed red blood cell transfusion, to maintain the haematocrit >24%, and for the transfusion of platelets and fresh-frozen plasma depending on the results obtained with thrombo-elastography. Standard protocols were used for weaning from the ventilator, discharge from the Intensive Care Unit (ICU) and High-Dependency Unit (HDU).
Study end-points
Average pump flow over the whole period of bypass, arterial PaO2, and arterial oxygen saturation (SaO2) (Sorin, Datamaster) were automatically collected by the JocapXL data acquisition system on the bypass machine. The JocabXL has a sample frequency every 15 s. Arterial blood gas measurements were taken every 30 min during bypass, measured with the ABL815 blood gas analyser (Radiometer) and entered onto the electronic database management system. An average haemoglobin (Hb) concentration was calculated from these measurements, together with the peak lactate concentration. Average indexed oxygen delivery (DO2I) was calculated:
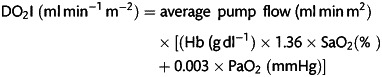 |
Routine blood counts, biochemistry and number and type of blood transfusion given were recorded by the hospital pathology system. Time of discharge from the critical care and hospital was recorded on the hospital patient manager system. Postoperative information, including requirement for renal replacement therapy or reoperation, was recorded on the cardiac surgery database.
Data acquisition and statistical analysis
Continuous variables were compared using a Student t-test to test for a difference in means between the two patient groups. Two-sample tests of proportions were used to test for differences in proportions of patients with given characteristics or outcomes in the two groups. Comparisons of dichotomous data were made using Fisher's exact test. A Bonferroni adjustment was applied to allow for multiple testing.
For all the tests, a P-value of ≤0.05 was deemed statistically significant. Statistical analysis was performed using the software package R (R Development Core Team, Vienna, Austria).
Summary statistics for patient demographics, comorbidities, intraoperative and ICU variables and clinical outcomes are reported as Bonferroni adjusted mean ± standard deviation or n (%), [95% confidence interval (Mini-CPB – C-CPB)].
RESULTS
Patients’ demographics, comorbidities and procedural characteristics are presented in Table 1, and show a well-matched patient population, but patients in the Mini-CPB group started at a slightly higher Hb and had a marginally lower left-ventricular ejection fraction. One patient in the C-CPB group had an incomplete dataset and was excluded from the final analysis.
Table 1:
Patient demographics
| Variables | Mini-CPB (n = 160) | C-CPB (n = 159) | Statistics P [95% CI (Mini-CPB to C-CPB)] |
|---|---|---|---|
| Age (years) | 67.1 ± 10 | 66.3 ± 10 | NS (−1.40 to 3.00) |
| Height (cm) | 171.9 ± 8 | 172.1 ± 8 | NS (−1.96 to 1.56) |
| Weight (kg) | 84.1 ± 16 | 84.7 ± 14 | NS (−3.91 to 2.71) |
| Body surface area (m2) | 2.0 ± 0.2 | 2.0 ± 0.2 | NS (−0.04 to 0.04) |
| Logistic EuroSCORE | 4.4 ± 6.0 | 3.7 ± 5.0 | NS (−0.52 to 1.92) |
| ‘Urgent’ case (n) | 45 | 37 | NS (P = 0.37) |
| Ejection fraction (%) | 55.1 ± 10.5 | 60.7 ± 14.2 | P < 0.0005 (−8.35 to −2.85) |
| Diabetes (n) | 33 | 26 | NS (P = 0.39) |
| NYHA grade 3/4 (n) | 32 | 40 | NS (P = 0.29) |
| Hypertension (n) | 97 | 106 | NS (P = 0.30) |
| Preop Hb (g dl−1) | 14.0 ± 1.5 | 13.6 ± 1.3 | P < 0.011 (0.091 to 0.71) |
| Preop Cr (µmol l−1) | 95 ± 25 | 99 ± 43 | NS (−11.7 to 3.74) |
| CPB time (min) | 68 ± 20 | 68 ± 21 | NS (−4.52 to 4.52) |
| Aortic cross-clamp (min) | 42 ± 14 | 40 ± 14 | NS (−1.08 to 5.08) |
Differences in preoperative demographic variables, co-morbidities and bypass times between patients before either Mini-CPB or C-CPB. Each variable is shown as either mean ± SD, or absolute number.
Intraoperative variables
Table 2 shows the intraoperative variable between the two groups. Initiation of bypass diluted patients on Mini-CPB 30.5% compared with 39.3% with C-CPB (P < 0.0005). This resulted in a greater percentage of C-CPB patients requiring a perioperative packed red blood cell (PRBC) transfusion to maintain Hb above target, compared with Mini-CPB. Patients in the C-CPB group were also more likely to require a platelet transfusion, while the fresh-frozen plasma (FFP) requirement, based on coagulation parameters, was the same (Table 2). Despite the higher transfusion rate on the day of surgery, patients in the C-CPB had a significantly lower Hb and percentage fall in Hb from their preoperative level by Day 1.
Table 2:
Perfusion characteristics
| Variables | Mini-CPB (n = 160) | C-CPB (n = 159) | Statistics P [95% CI (Mini-CPB to C-CPB)] |
|---|---|---|---|
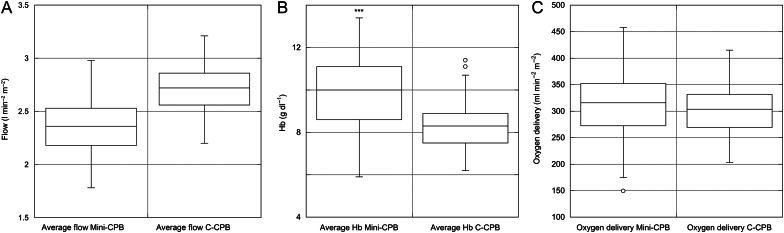
| Average Hb bypass (g dl−1) | 9.7 ± 1.6 | 8.3 ± 1.1 | P < 0.0005 (1.10 to 1.70) |
| Average CPB pump flow index (l min−2 m−2) | 2.37 ± 0.25 | 2.71 ± 0.21 | P < 0.0005 (−0.39 to −0.29) |
| Patients transfused PRBC Day 0 (n) | 37 | 54 | P = 0.035 |
| Patients transfused FFP (n) | 25 | 31 | NS (P = 0.38) |
| Patients transfused platelets (n) | 25 | 41 | P = 0.027 |
| Average oxygen delivery (ml min−2 m−2) | 311.3 ± 61.2 | 303.1 ± 43.2 | NS (P = 0.168) (−3.48 to 19.88) |
| Peak lactate (mmol l−1) | 1.35 ± 0.5 | 1.88 ± 0.7 | P < 0.0005 (−0.66 to −0.40) |
Perioperative perfusion variables between patients having either Mini-CPB or C-CPB. Each variable is shown as either mean ± SD or absolute number.
Average CPB pump flow index with Mini-CPB was significantly lower. When this figure was combined with average Hb during bypass, average PaO2 and arterial saturation, this resulted in the same DO2I with Mini-CPB compared with C-CPB (Fig. 1A–C). The peak lactate measured during bypass was higher with C-CPB compared with Mini-CPB.
Figures 1:
(A) Average flow (indexed to body surface area), (B) Average haemoglobin concentration and (C) Calculated oxygen delivery (indexed to body surface area) in patients undergoing cardiopulmonary bypass with either Mini-CPB or C-CPB. Box-and-Whisker plots with mean, interquartile range and range is shown.
Postoperative management and morbidity
Table 3 shows estimated differences in ICU variables between the two groups, in terms of proportions of patients with a given characteristic. By the time of discharge, similar numbers of patients in each group had received PRBC transfusion.
Table 3:
Postoperative management, morbidity and mortality
| Variables | Mini-CPB (n = 160) | C-CPB (n = 159) | Statistics P [95% CI (Mini-CPB – C-CPB)] |
|---|---|---|---|
| Average Hb Day 1 (g dl−1) | 9.7 ± 1.2 | 8.9 ± 0.9 | P < 0.0005 (0.57 to 1.03) |
| Percentage change Hb (g dl−1) from preoperative to Day 1 (%) | 30.3 ± 8.5 | 34.3 ± 7.8 | P < 0.0005 (−5.80 to −2.20) |
| Patients transfused PRBC by hospital discharge (n) | 56 | 70 | NS (P = 0.11) |
| Peak rise [Cr] (µmol l−1) | 117 ± 61 | 116 ± 72 | NS (P = 0.89) |
| Percentage change [Cr] (%) | 43 ± 22 | 40 ± 16 | NS (P = 0.17) |
| Critical care LOS (days) | 2.6 ± 3.4 | 2.8 ± 4.0 | NS (P = 0.63) |
| Hospital LOS (days) | 8.5 ± 5.8 | 8.4 ± 5.3 | NS (P = 0.87) |
| Hospital mortality (n) | 3 | 4 | NS (P = 0.72) |
Postoperative transfusion, morbidity and mortality between patients having either Mini-CPB or C-CPB. Each variable is shown as either mean ± SD or absolute number.
Daily measurement of serum creatinine demonstrated the same peak rise and maximum percentage rise in plasma creatinine irrespective of group. We classified individual patients by the criteria described by the Acute Kidney Injury Network (AKIN) [14]. Similar numbers of patients in each group developed AKIN scores 1–3. Ten patients (Mini-CPB) and 9 patients (C-CPB) developed AKIN 2 or 3, with 6 patients in the Mini-CPB group and 7 in the C-CPB group requiring postoperative renal replacement therapy. Using a Fisher's exact test, there was no evidence of an association between group and AKIN scores (P = 0.21).
Both groups had an association between average oxygen delivery and postoperative percentage change in plasma creatinine (ΔCr). The difference in this effect between groups was not significant. For every 1 ml min−1 m−2 increase in oxygen delivery, ΔCr decreased by 0.0012 (P = 0.006).
A logistic regression model revealed a strong risk of developing AKIN 1 or more, compared with a score of 0, with a decreasing oxygen delivery. There was no evidence of a similar effect with flow index. The risk of having AKIN ≥1 increased by ∼1% for every 1 ml min−1 m−2 decrease in oxygen delivery (P = 0.0001, OR 0.990, 95% CI 0.984–0.995).
Length of stay
Length of stay in critical care and in hospital was the same between groups. We used a multiple regression model fitted to the log ICU stay, incorporating the covariates flow during bypass, oxygen delivery and AKIN score 2 or 3. Backward elimination, to include only the AKIN score, demonstrated that compared with those with an AKIN score of 0, patients with AKIN 1 are estimated to stay in ICU for approximately twice as long (95% CI 1.69–2.53), while those with a score of 2 or 3 are estimated to stay in ICU for approximately three times as long (95% CI 2.31–4.30).
Major morbidity and mortality
Four patients in the C-CPB group and 3 in the Mini-CPB group died before discharge from hospital.
DISCUSSION
We have shown that despite the same target flow indexed flow is limited at some point, and for long enough, to significantly reduce the average flow during perfusion with the Mini-CPB compared with C-CPB. We have also demonstrated that, despite this reduction in flow, the effects of minimal haemodilution with Mini-CPB results in the preservation of average oxygen delivery.
This study concurs with previous studies, and with the conclusion of a best-evidence review in this journal [15], by demonstrating the benefits of the Mini-CPB circuit, together with a full-circuit retrograde autologous prime, in reducing haemodilution [8, 10, 16] and in turn the requirement for red blood cell transfusion during the immediate operative period [16–19]. We are unable to explain why this early effect does not appear to persist, with patients in the Mini-CPB group being transfused later, with no difference in transfusion rate by the time of discharge from hospital.
Achieving the same oxygen delivery while reducing the requirement for intraoperative red cell transfusion should in theory confer advantages in avoiding transfusion-related postoperative organ dysfunction [20]. The magnitude of this effect may be small compared with other factors that may affect the outcome. One is that simply measuring the average flow missed the periods of profoundly harmful low flow, but that the indexed flow is adequate for the majority of the time ‘on bypass’. Alternatively, the patient may be under-filled for the duration of bypass, with a compromised venous return, and hence arterial inflow. Reduction in flow was quantified in a retrospective case–control study [12] of 120 patients comparing Mini-CPB with C-CPB. The Mini-CPB patients had a significantly lower nadir of low flow lasting >2 min (1.75 ± 0.23 vs 2.20 ± 0.22 l min−1 m−2), a longer duration of low flow (defined as <2 l min−1 m−2) during the bypass of 64 ± 41 vs 1 ± 4 min and a lower mean cardiac index during low flow (1.78 ± 0.15 vs 1.98 ± 0.06 l min−1 m−2). A prospective study using continuous measurement of all of the variables that contribute to oxygen delivery is therefore needed.
Limitations
This was a retrospective analysis in two groups of patients who were very similar in demographics and comorbidities. We accept that this retrospective analysis did not control for the heparin-bonded Mini-CPB circuit. Nor can we claim that this compared anything other that full retrograde priming of one circuit vs no retrograde priming of another. Postoperative care is not different based on the method of bypass used, and intensive care management, particularly transfusion and coagulation management, is based on a clear protocol. However, with two of six surgeons using the Mini-CPB almost exclusively for CABG, we cannot rule out the influence of their management style on the course of their patients’ recovery.
CONCLUSIONS
Miniaturized cardiopulmonary bypass is associated with lower average indexed flows compared with conventional circuits despite aiming for the same target flows. The reduction in haemodilution with Mini-CPB results in the preservation of oxygen delivery despite lower net flows. In both groups, a postoperative rise in plasma creatinine and risk of developing ≥AKIN 1 are strongly associated with any fall in oxygen delivery.
Conflict of interest: none declared
REFERENCES
- 1.Murphy GS, Hessel EA, II, Groom RC. Optimal perfusion during cardiopulmonary bypass: an evidence-based approach. Anesth Analg. 2009;108:1394–417. doi: 10.1213/ane.0b013e3181875e2e. [DOI] [PubMed] [Google Scholar]
- 2.de Somer F, Mulholland JW, Bryan MR, Aloisio T, Van Nooten GJ, Ranucci M. O2 delivery and CO2 production during cardiopulmonary bypass as determinants of acute kidney injury: time for a goal-directed perfusion management? Crit Care. 2011;15:R192. doi: 10.1186/cc10349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ranucci M, De Toffol B, Isgro G, Romitti F, Conti D, Vicentini M. Hyperlactatemia during cardiopulmonary bypass: determinants and impact on postoperative outcome. Crit Care. 2006;10:R167. doi: 10.1186/cc5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karkouti K, Beattie WS, Wijeysundera DN, Rao V, Chan C, Dattilo KM, et al. Hemodilution during cardiopulmonary bypass is an independent risk factor for acute renal failure in adult cardiac surgery. J Thorac Cardiovasc Surg. 2005;129:391–400. doi: 10.1016/j.jtcvs.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 5.Ranucci M, Romitti F, Isgro G, Cotza M, Brozzi S, Boncilli A, et al. Oxygen delivery during cardiopulmonary bypass and acute renal failure after coronary operations. Ann Thorac Surg. 2005;80:2213–20. doi: 10.1016/j.athoracsur.2005.05.069. [DOI] [PubMed] [Google Scholar]
- 6.Swaminathan M, Phillips-Bute BG, Conlon PJ, Smith PK, Newman MF, Stafford-Smith M. The association of lowest hematocrit during cardiopulmonary bypass with acute renal injury after coronary artery bypass surgery. Ann Thorac Surg. 2003;76:784–91. doi: 10.1016/s0003-4975(03)00558-7. discussion 92. [DOI] [PubMed] [Google Scholar]
- 7.Karkouti K, Djaiani G, Borger MA, Beattie WS, Fedorko L, Wijeysundera D, et al. Low hematocrit during cardiopulmonary bypass is associated with increased risk of perioperative stroke in cardiac surgery. Ann Thorac Surg. 2005;80:1381–7. doi: 10.1016/j.athoracsur.2005.03.137. [DOI] [PubMed] [Google Scholar]
- 8.DeFoe GR, Ross CS, Olmstead EM, Surgenor SD, Fillinger MP, Groom RC, et al. Lowest hematocrit on bypass and adverse outcomes associated with coronary artery bypass grafting. Northern New England Cardiovascular Disease Study Group. Ann Thorac Surg. 2001;71:769–76. doi: 10.1016/s0003-4975(00)02393-6. [DOI] [PubMed] [Google Scholar]
- 9.Fang WC, Helm RE, Krieger KH, Rosengart TK, DuBois WJ, Sason C, et al. Impact of minimum hematocrit during cardiopulmonary bypass on mortality in patients undergoing coronary artery surgery. Circulation. 1997;96:II-194–9. [PubMed] [Google Scholar]
- 10.Habib RH, Zacharias A, Schwann TA, Riordan CJ, Durham SJ, Shah A. Adverse effects of low hematocrit during cardiopulmonary bypass in the adult: should current practice be changed? J Thorac Cardiovasc Surg. 2003;125:1438–50. doi: 10.1016/s0022-5223(02)73291-1. [DOI] [PubMed] [Google Scholar]
- 11.Dobele T, Schwirtz G, Gahl B, Eckstein F. Mini ECC vs. conventional ECC: an examination of venous oxygen saturation, haemoglobin, haematocrit, flow, cardiac index and oxygen delivery. Perfusion. 2010;25:125–31. doi: 10.1177/0267659110369852. [DOI] [PubMed] [Google Scholar]
- 12.Ti LK, Goh BL, Wong PS, Ong P, Goh SG, Lee CN. Comparison of mini-cardiopulmonary bypass system with air-purge device to conventional bypass system. Ann Thorac Surg. 2008;85:994–1000. doi: 10.1016/j.athoracsur.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Ranucci M, Pavesi M, Mazza E, Bertucci C, Frigiola A, Menicanti L, et al. Risk factors for renal dysfunction after coronary surgery: the role of cardiopulmonary bypass technique. Perfusion. 1994;9:319–26. doi: 10.1177/026765919400900503. [DOI] [PubMed] [Google Scholar]
- 14.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alevizou A, Dunning J, Park JD. Can a mini-bypass circuit improve perfusion in cardiac surgery compared to conventional cardiopulmonary bypass? Interact CardioVasc Thorac Surg. 2009;8:457–66. doi: 10.1510/icvts.2008.200857. [DOI] [PubMed] [Google Scholar]
- 16.El-Essawi A, Hajek T, Skorpil J, Boning A, Sabol F, Hausmann H, et al. A prospective randomised multicentre clinical comparison of a minimised perfusion circuit versus conventional cardiopulmonary bypass. Eur J Cardiothorac Surg. 2010;38:91–7. doi: 10.1016/j.ejcts.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 17.Castiglioni A, Verzini A, Colangelo N, Nascimbene S, Laino G, Alfieri O. Comparison of minimally invasive closed circuit versus standard extracorporeal circulation for aortic valve replacement: a randomized study. Interact CardioVasc Thorac Surg. 2009;9:37–41. doi: 10.1510/icvts.2008.192559. discussion 41. [DOI] [PubMed] [Google Scholar]
- 18.Huybregts RA, Morariu AM, Rakhorst G, Spiegelenberg SR, Romijn HW, de Vroege R, et al. Attenuated renal and intestinal injury after use of a mini-cardiopulmonary bypass system. Ann Thorac Surg. 2007;83:1760–6. doi: 10.1016/j.athoracsur.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 19.Remadi JP, Rakotoarivelo Z, Marticho P, Benamar A. Prospective randomized study comparing coronary artery bypass grafting with the new mini-extracorporeal circulation Jostra System or with a standard cardiopulmonary bypass. Am Heart J. 2006;151:198. doi: 10.1016/j.ahj.2005.03.067. [DOI] [PubMed] [Google Scholar]
- 20.Koch CG, Li L, Duncan AI, Mihaljevic T, Cosgrove DM, Loop FD, et al. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006;34:1608–16. doi: 10.1097/01.CCM.0000217920.48559.D8. [DOI] [PubMed] [Google Scholar]