Abstract
OBJECTIVES
Publications in the surgical literature are very consistent in their conclusions that blood is dangerous with regard to in-hospital mortality, morbidity and long-term survival. Blood is frequently used as a volume expander while simultaneously increasing the haematocrit. We investigated the effects of a single-unit blood transfusion on long-term survival post-cardiac surgery in isolated coronary artery bypass grafting patients.
METHODS
A prospective single-institution cardiac surgery database was analysed involving 4615 patients. Univariate, multivariate stepwise Cox regression analysis and propensity matching were performed to identify whether a single-unit blood transfusion was detrimental to long-term survival.
RESULTS
Univariate analysis revealed that blood was significantly associated with a reduced long-term survival even with a single-unit transfused, P = 0.0001. Cox multivariate regression analysis identified age, ejection fraction, preoperative dialysis, logistic EuroSCORE, postoperative CKMB, blood transfusion, urgent operative status and atrial fibrillation as significant factors determining long-term survival. When the Cox regression was repeated with patients who received no blood or only one unit of blood, transfusion was not a risk factor for long-term survival. An interaction analysis revealed that blood transfusion was significantly interacting with preoperative haemoglobin levels, P = 0.02. Propensity analysis demonstrated that a single-unit transfusion is not associated with a detrimental long-term survival, P = 0.3.
CONCLUSIONS
Cox regression and propensity matching both indicate that a single-unit transfusion is not a significant cause of reduced long-term survival. Preoperative anaemia is a significant confounding factor. Despite demonstrating the negligible risks of a single-unit blood transfusion, we are not advocating liberal transfusion and would recommend changing from a double-unit to a single-unit transfusion policy. We speculate that blood is not bad, but that the underlying reason that it is given might be.
Keywords: Coronary, Survival, Blood transfusion
INTRODUCTION
Publications in the surgical literature are very consistent in their conclusions that blood is dangerous with regard to in-hospital mortality, morbidity and long-term survival [1–5]. The life-saving properties of blood in massive haemorrhage are undeniable; however, cardiac surgery blood transfusion is frequently not given for massive haemorrhage and merely acts as a volume expander while simultaneously increasing the haematocrit.
We investigated the effects of a single-unit blood transfusion on long-term survival post-cardiac surgery in isolated coronary artery bypass grafting (CABG) patients.
MATERIALS AND METHODS
Local institutional review board approval was granted for this study.
Database
Consecutive patients were included from a prospective single-institutional cardiac surgery database from February 2003 to June 2009, n = 4615, which was 100% validated by the hospital data analysis department and accredited by the Society of Cardiothoracic Surgeons of Great Britain and Ireland (SCTS), and was utilized in conjunction with the National Strategic Tracing Service for long-term follow-up that exists in the UK, as has been described previously [6–10]. Long-term survival was assessed as the time interval between operation date and October 2010, when the National Strategic Tracing Service was utlized to assess who was alive or dead. Our unit performs about 1800 cardiac cases per year. Transfusion was at the discretion of the operating surgeon and intensivist. No cut-off criteria for transfusion were in place.
Analysis
Univariate and multivariate stepwise Cox proportional hazards regression analyses were utilized to identify the potential significant determining factors with regard to long-term survival. Entry and removal criteria were P < 0.05 and P > 0.1, respectively. The results of the Cox regression were plotted at the mean of the covariates.
Propensity analysis
A propensity analysis was performed as patients who receive blood have a different risk profile compared with those who do not. Two propensity analyses were performed, patients who received a blood transfusion vs those who did not receive a blood transfusion, and patients who received only one unit of blood vs those who received no blood transfusion.
Logistic regression for group membership of who received a blood transfusion or a single-unit blood transfusion was used to calculate the propensity score for 1:1 matching for the two analyses. Nearest-neighbour matching without replacement with a caliper of 0.2 was utilized.
Variables used in the propensity match included: logistic EuroSCORE, preoperative haemoglobin, body mass index (BMI), age, cardiopulmonary bypass time, ejection fraction, preoperative atrial fibrillation, priority of surgery, female sex, diabetes, preoperative intra-aortic balloon pump, left internal mammary artery usage, blood loss and postoperative creatinine kinase muscle-brain isoenzyme. A dotplot of standardized mean differences (Cohen's d) for all covariates before and after matching was produced for patients who only received a single-unit transfusion.
A Kaplan–Meier survival post-matching was performed for each propensity match.
Statistical software
All statistical analysis other than the propensity matching was performed with MedCalc for Windows, (version 12.1.4, MedCalc Software, Mariakerke, Belgium). The propensity matching was performed with SPSS (version 20.0 for Windows, SPSS, Inc., Chicago, IL, USA), SPSS Statistics Integration Plug-In for R, and R 2.12.2.
RESULTS
A 100% long-term follow-up via the National Strategic Tracing Service was achieved. Benchmarking of our institutional mortality rates compared with the UK did not reveal any differences (part of the continuous UK cardiac surgery quality assessment programme by the society of cardiothoracic surgeons). Two thousand five hundred and thirty-seven (55%) patients received no transfusion post-isolated CABG, and 590 (13%) received only one unit. 28% of patients with haemoglobin <12 g/dl received a single-unit blood transfusion post-cardiac surgery, compared with 18% with no preoperative anaemia. In-hospital mortality was 2.1%, and the cohort mortality over the study period was 10.3%. Less than 1% of CABG patients had bilateral internal mammary arteries utilized in our institute. The patient characteristics are presented in Table 1.
Table 1:
Pre-, peri- and postoperative characteristics of patients in the study group
| Data (n = 4615) | |
|---|---|
| Preoperative | |
| Age (years) | 65.6 [58.9–71.6] |
| Female (%) | 905 (19.6) |
| Body mass index (kg/m2) | 27.9 [25.4–30.9] |
| Diabetes (%) | 1694 (36.7) |
| Preoperative dialysis (%) | 19 (0.4) |
| Previous myocardial infarction (%) | 2257 (48.9) |
| Peripheral vascular disease (%) | 628 (13.6) |
| Hypertension (%) | 2774 (60.1) |
| Preoperative intra-aortic balloon pump (%) | 60 (1.3) |
| Ejection fraction | |
| Good (%) | 2755 (59.7) |
| Moderate (%) | 1449 (31.4) |
| Poor (%) | 411 (8.9) |
| Previous percutaneous coronary intervention (%) | 295 (6.4) |
| Status | |
| Elective (%) | 3715 (80.5) |
| Urgent (%) | 840 (18.2) |
| Emergency (%) | 60 (1.3) |
| Logistic EuroSCORE | 2.6 [1.5–5.1] |
| Preop haemoglobin | 13.9 [12.9–14.8] |
| Operative | |
| Left internal mammary artery (%) | 4232 (91.9) |
| No. of grafts | 3.3 (1.5–5.2) |
| Cardiopulmonary bypass time (min) | 109 [46–173] |
| Cross-clamp time | 65 [50–79] |
| Postoperative | |
| Intensive care length of stay (days) | 1 [1–2] |
| Hospital length of stay (days) | 7 [6–9] |
| Blood loss (ml) | 730 [500–1010] |
| Creatinine kinase muscle-brain isoenzyme (IU) | 15 [4–34] |
| Transfused (units) (%) | |
| 0 | 2537 (55) |
| 1 | 590 (12.8) |
| 2 | 708 (15.3) |
| 3 | 241 (5.2) |
| 4 | 190 (4.1) |
| ≥5 | 349 (7.6) |
| In-hospital mortality (%) | 97 (2.1) |
| Median survival (years) [range] | 5.3 [0–8.7] |
| Study period group mortality (%) | 475 (10.3) |
Continuous variables that are normally distributed are shown as mean with 95% confidence intervals shown in brackets. Continuous variables that are skewed in distributed are shown as median [25th–75th centiles]. Categorical variables are shown as numbers (percentage).
Ejection fraction was defined as good (EF ≥50%), moderate (EF ≥30–50%) and poor (EF <30%). Diabetes was defined as oral, medication or insulin controlled.
Univariate analysis
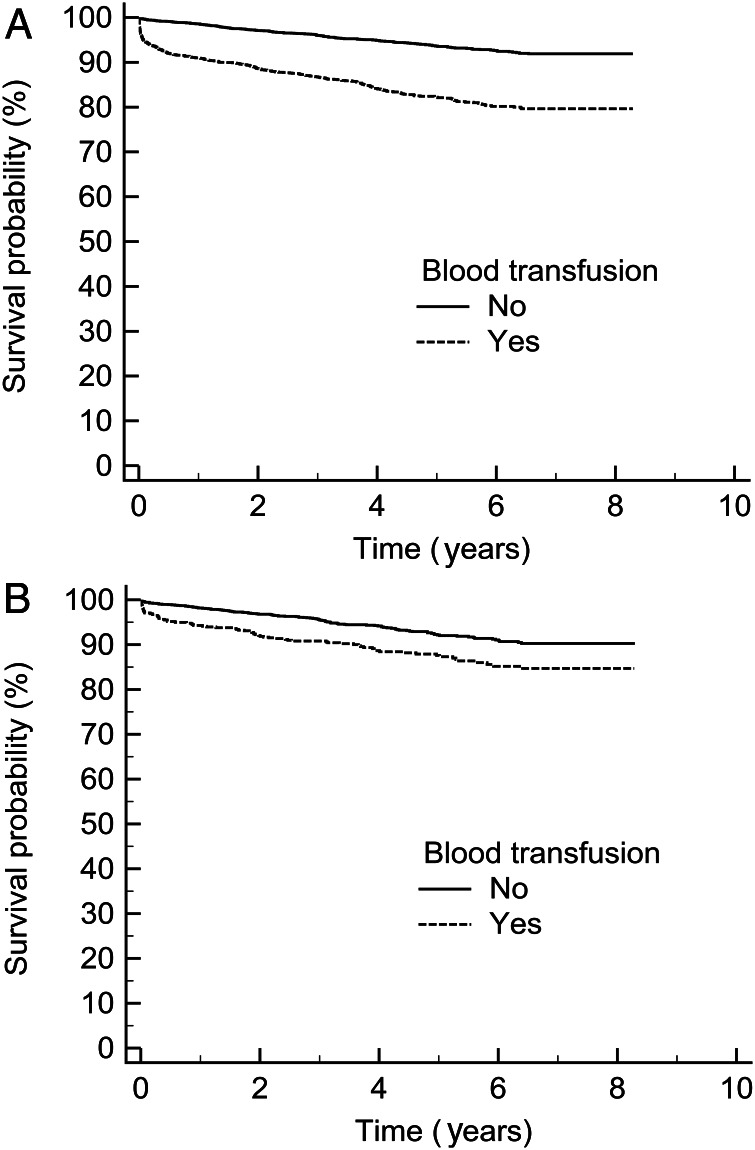
Kaplan–Meier analysis revealed that blood was significantly associated with a reduced long-term survival even with a single unit transfused, P = 0.0001 (Fig. 1).
Figure 1:
Kaplan–Meier survival curves comparing patients who did not receive a blood transfusion, n = 2537 vs (A) patients who received a blood transfusion regardless of amount, n = 2078, P < 0.0001, (B) patients who only received one unit transfusions, n = 590, P = 0.0001.
Multivariate analysis
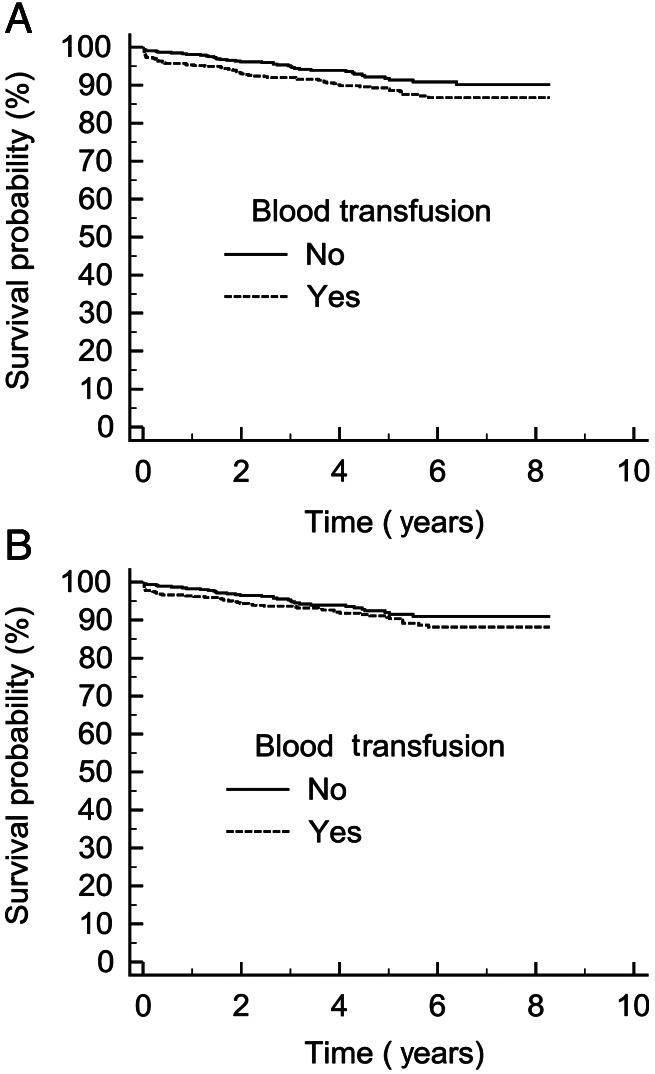
Cox regression analysis identified age, ejection fraction, preoperative dialysis, logistic EuroSCORE, postoperative CKMB, blood transfusion, urgent operative status and atrial fibrillation as significant factors determining the long-term survival (Table 2a). The following variables were excluded by the stepwise analysis: sex, BMI, hypertension, diabetes, peripheral vascular disease, blood loss and cardiac pacemaker. When the Cox regression was repeated with patients who received no blood or only one unit of blood, transfusion was not a risk factor for long-term survival (Table 2b). The effect of blood transfusion plotted at the mean of the covariates is shown in Fig. 2.
Table 2.
Cox regression analysis of long-term survival for (a) all patients, n = 4615 and (b) patients who received no blood transfusion, n = 2537, or a single-unit transfusion only, n = 590.
| Covariate | Relative risk (RR) | 95% CI of RR | P-value |
|---|---|---|---|
| (a) All patients | |||
| Age | 1.04 | 1.03–1.06 | <0.0001 |
| EF moderate | 1.53 | 1.24–1.89 | 0.0001 |
| Poor | 3.13 | 2.38–4.11 | <0.0001 |
| Preoperative dialysis | 5.43 | 3.03–9.72 | <0.0001 |
| Logistic EuroSCORE | 1.02 | 1.01–1.03 | 0.0004 |
| CKMB | 1.00 | 1.00–1.01 | 0.0003 |
| Blood transfusion | 2.02 | 1.66–2.45 | <0.0001 |
| Urgent | 1.41 | 1.14–1.74 | 0.001 |
| Atrial fibrillation | 2.02 | 1.21–3.36 | 0.0071 |
| (b) No blood transfusion or single-unit transfusion only | |||
| Age | 1.03 | 1.01–1.05 | 0.004 |
| EF moderate | 1.83 | 1.33–2.52 | 0.0002 |
| Poor | 2.95 | 1.87–4.65 | <0.0001 |
| Preoperative dialysis | 7.15 | 2.27–22.49 | 0.001 |
| LIMA not used | 1.73 | 1.06–2.81 | 0.03 |
| Logistic EuroSCORE | 1.02 | 1.00–1.04 | 0.06 |
| CKMB | 1.00 | 1.00–1.01 | 0.03 |
| Preoperative haemoglobin | 0.84 | 0.77–0.93 | 0.001 |
| Interaction blood transfusion and preoperative Hb | 1.03 | 1.00–1.05 | 0.02 |
| Atrial fibrillation | 2.48 | 1.09–5.62 | 0.03 |
EF: ejection fraction; LIMA: left internal mammary artery; CKMB: creatinine kinase muscle-brain isoenzyme; Hb: haemoglobin.
Figure 2:
Cox survival plotted at the mean of the covariates (A) all patients, n = 4615, (B) patients who only received one unit, n = 590, with preoperative anaemia as an interacting factor.
Interaction analysis
An interaction analysis revealed that blood transfusion was significantly interacting with preoperative haemoglobin levels, P = 0.02, implying that it is not the transfusion itself that is the sole determinant with regard to long-term survival, as anaemia is a known risk factor. The effect of only one-unit transfusion is shown in Fig. 2B. It can be seen that a transfusion of one unit does not affect long-term survival. An interaction analysis with mediastinal blood loss and blood transfusion was not significant, P = 0.67 (data not shown).
Propensity analysis
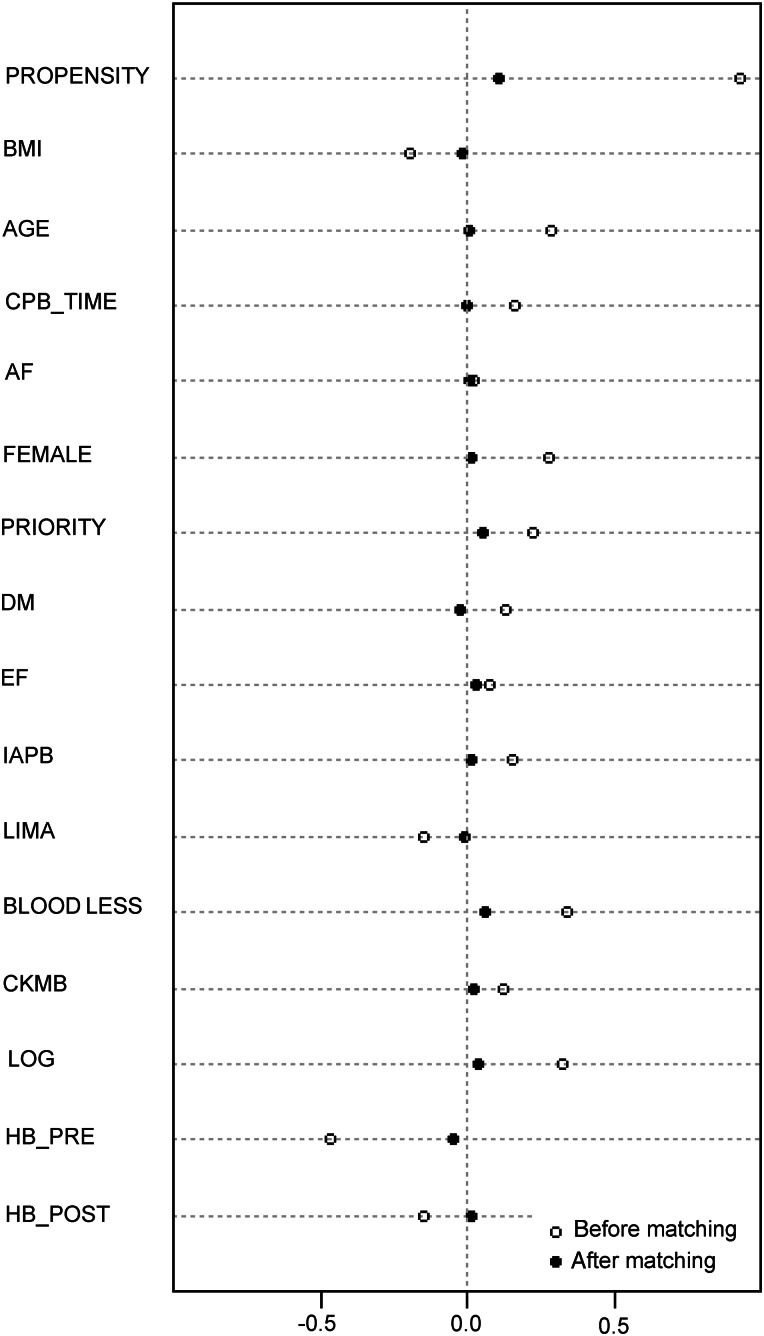
A dotplot of standardized mean differences (Cohen's d) for all covariates before and after matching for patients who received only one unit of blood is shown in Fig. 3. The median EuroSCORE post-propensity matching was 3.2 (95% confidence interval [CI] 3.2–3.6), which was significantly higher than prematching 2.6 (95% CI 2.6–2.7), P < 0.0001 (Mann–Whitney U-test independent samples).
Figure 3:
Dotplot of standardized mean differences (Cohen's d—x axis) for all covariates before and after matching for a single-unit blood transfusion. Overall χ2 balance test was not significant, χ2(15) = 12.2, P = 0.7. BMI: body mass index; CPB_TIME: cardiopulmonary bypass time; AF: atrial fibrillation; DM: diabetes; EF: ejection fraction; IABP: intra-aortic balloon pump preoperatively; LIMA: left internal mammary artery usage; BLOOD LOSS: mediastinal blood loss post-surgery; CKMB: creatinine kinase muscle-brain isoenzyme; LOG: logistic EuroSCORE; HB_PRE: preoperative haemoglobin and HB_POST: postoperative haemoglobin.
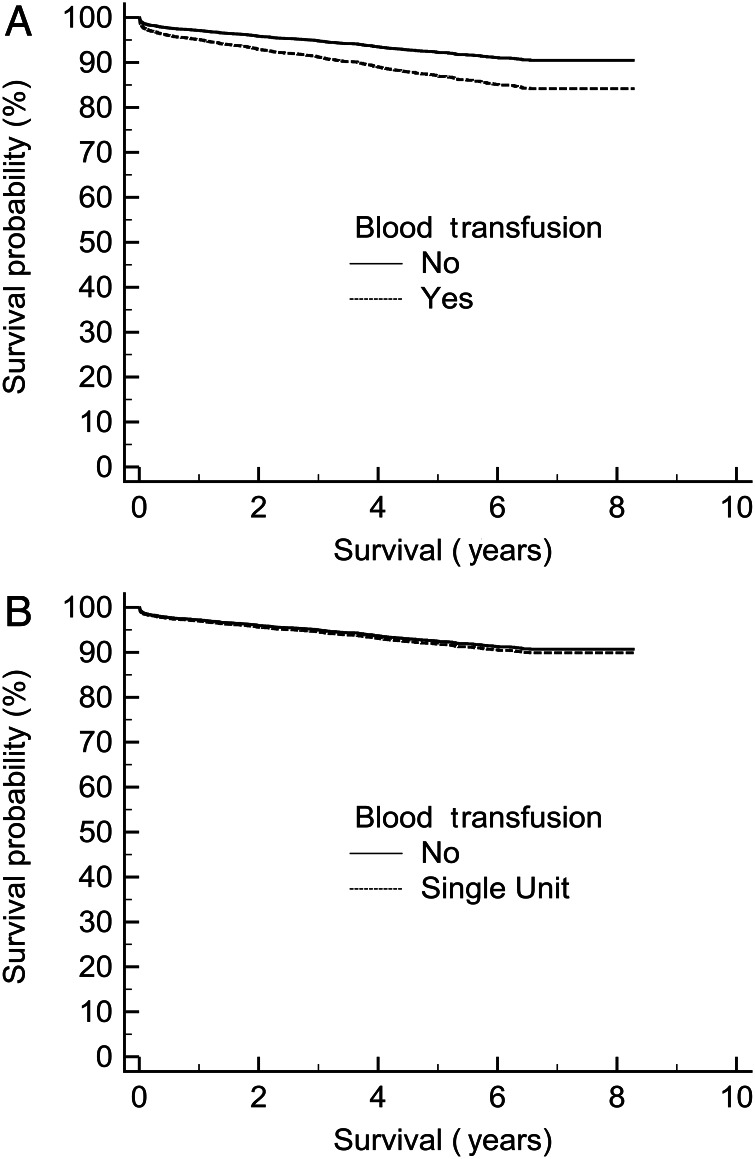
With respect to the propensity match of patients who received a single-unit transfusion, n = 514, (overall χ2 balance test was not significant, χ2(15) = 12.2, P = 0.7), a Kaplan–Meier plot of survival demonstrated that, in patients with haemoglobin >10.5 g/dl preoperatively, blood transfusion was not a significant risk factor, P = 0.06 (Fig. 4A). If a preoperative haemoglobin of 12 g/dl was utilized as a cut-off, n = 444, the difference became even less significant, P = 0.3 (Fig. 4B).
Figure 4:
Kaplan–Meier plot of survival in patients who received a single-unit transfusion, n = 514, (A) with a preoperative haemoglobin >10.5 g/dl, P = 0.06, and (B) with a preoperative haemoglobin >12 g/dl, P = 0.3.
With respect to the propensity match of patients who received a blood transfusion, n = 831 per group, (overall χ2 balance test was not significant, χ2(15) = 6.1, P = 0.98), the Kaplan–Meier survival demonstrated that as little as a two-unit transfusion was associated with a significantly reduced long-term survival, P = 0.0001; however, a Kaplan–Meier analysis demonstrated that a single-unit transfusion was not associated with a detrimental long-term survival, P = 0.2 (data not shown).
DISCUSSION
A single-unit blood transfusion is not associated with reduced long-term survival post-CABG after multivariate analysis and propensity matching have been performed. The finding that a single unit is not associated with an excess risk of death implies that patients who receive more than one unit may have detrimental outcomes secondary to the reason for transfusion, as opposed to the transfusion itself.
We have a large number of patients who only received one-unit transfusions. Changing from a double-unit to a single-unit transfusion policy may reduce the total number of units transfused. This finding has been demonstrated in other areas of medicine previously [11].
Blood is rarely administered in the operating theatres or on the ward after CABG in our institution (<2%). The vast majority is used to treat a low haemoglobin post-surgery in the intensive care unit. The exact cut-off for transfusion varied between operating surgeons and intensivists. Blood is frequently prescribed a unit at a time due to the reduction in overall transfusion in which this results [11, 12]. We feel that the variation in transfusion trigger points and prescribing only a unit at a time are actually strengths of this study. Few units are likely to have patients who only receive one-unit transfusion, due to the medical dogma, if you are going to give one, give two due to the risks of transfusion [13, 14]. In addition, if blood is being administered inappropriately due to an inappropriately liberal transfusion policy, a reduced long-term survival would be expected; however, this is not the case.
Patients who receive blood are inherently a higher-risk group. Cox analysis of skewed data may potentially result in errors. Logistic regression demonstrates that sex, diabetes, age, ejection fraction, preoperative haemoglobin, blood loss, operative priority, cardiac rhythm and logistic EuroSCORE are significant factors determining the need for blood transfusion (data not shown). Propensity matching, however, demonstrated that a single-unit transfusion is not associated with reduced long-term survival.
The shapes of the Kaplan–Meier survival curves, univariate analysis and after propensity matching demonstrate that the risk of dying after receiving a blood transfusion is highest in the first 3 months post-surgery. The rate of attrition after this period is similar regardless of the transfusion status. The above is demonstrated by removing all in-hospital deaths, as then no significant difference exists between those who receive blood and those who do not. A similar finding has been demonstrated, but not commented on previously [3]. This implies an association between blood and death in the short term. We speculate that this is secondary to the indication for transfusion and not the blood itself.
Risks of blood transfusion range from acute transfusion reactions—anaphylaxis, transfusion-related lung injury (TRALI), to more subtle organ damage, and are all associated with an increased mortality post-cardiac surgery [2, 4]. Acute catastrophic, frequently fatal reactions to blood are rare, implying a more subtle mechanism of action that is only active during the patients’ stay in hospital. We hypothesize that the reason that blood is detrimental to survival in hospital only is due to the reason for transfusion, and not the blood itself.
Utilizing the Nadler method of calculating blood volume [15], based on sex, age, weight and height, in two common scenarios indicates that blood per se is not the primary issue (data not shown). An 80-kg male who is 180 cm tall with a preoperative haemoglobin of 13.5 g/dl needs to lose 2160 ml of blood to drop his haemoglobin to 8 g/dl and receive a blood transfusion. A 60-kg female who is 150 cm tall with a preoperative haemoglobin of 11 g/dl needs to lose 930 ml of blood to drop her haemoglobin to 8 g/dl and receive a blood transfusion. These volumes of blood loss are large, particularly in the male patient. The male has had to lose 40% of his blood volume, and the female has had to lose 27% of her blood volume to receive a transfusion. Even to drop their haemoglobin to 10 g/dl, the males need to lose 25% of their blood volume, and the females 10%. It should not be forgotten that blood loss via the mediastinal chest drains is only the revealed blood loss and frequently, blood remains within the mediastium, as demonstrated by a widened mediastinum on plain radiography of the chest, suggesting concealed blood loss. This may explain our finding that mediastinal blood loss is not a significant factor determining long-term survival, but blood transfusion is.
With regard to long-term survival, the inclusion and subsequent demonstration of the significance of left internal mammary artery usage, postoperative myocardial creatinine kinase, preoperative haemoglobin and atrial fibrillation confirm previous work [16–18], but also highlight deficiencies in the blood- transfusion literature to date [4], which have not included these known prognostic factors post-cardiac surgery in their analyses [3, 19].
Though we have identified preoperative anaemia as a risk factor for transfusion, we speculate that it is also a surrogate marker for poor tissue quality and poor long-term survival. Recently, a separate group has independently identified, in octogenarians undergoing cardiac surgery, that a single-unit transfusion does not adversely affect long-term survival [20].
LIMITATIONS
Unfortunately, we do not have the haemoglobin trigger level for transfusion recorded, the timing of transfusion and the clinical situation at the time of transfusion. Our particular unit has an ethos of only transfusing in the operating theatres for major catastrophic bleeding, and although we do not have the exact rate recorded, it is <1% for isolated CABG patients. We do not have causes of death after hospital discharge.
CONCLUSION
Despite demonstrating the negligible risks of a single-unit blood transfusion, we are not advocating liberal transfusion. We speculate that blood is not bad, but that the underlying reason that it is given might be.
Conflict of interest: none declared.
APPENDIX. CONFERENCE DISCUSSION
Dr P. Matt (Basel, Switzerland): I think that since many patients having coronary artery bypass surgery nowadays, are under potent platelet inhibitors such as prasugrel or clopidogrel, the topic you study here is very important. On the other hand - and it was mentioned already during this session - there is no randomized controlled trial available at the moment on the topic. So the best way that you could address this question, whether blood is dangerous or not for long or early survival, is to do a propensity match scoring analysis as you have done.
I have two questions. The first is you showed that one unit of blood is not dangerous: is that true for all patients or do you see any differences between patients with a low EuroSCORE, high EuroSCORE, older patients, younger patients?
Dr Poullis: Well, we matched partially based on the EuroSCORE. We do not have enough patients, though, to break it down into people with a EuroSCORE less than 6, who are under 60 years, who are non-diabetic. We just do not have those numbers even though we are a big institution. I quite appreciate that. The answer is always in the middle. There will be certain groups that we know will be bad. That is why the aim of this paper is not to promote the use of blood. We are just saying maybe there is something else going on as to why people who have blood die.
Dr Matt: And the second question is, do you have any data on the other blood products, platelets, FFP, and how they influence the survival?
Dr Poullis: We do have that data, but I have not included it in this manuscript. I appreciate that could be a significant confounding factor.
Dr U. Myhre (Kuala Lumpur, Malaysia): I think it was a nice presentation, and I also think that massive transfusions and a too-liberal transfusion policy, based on all the publications, most certainly is quite bad for you. But at the same time, I think we should not also forget the benefits of transfusion, which I think you have in your presentation demonstrated to some extent. And in going for a goal of lower and lower transfusion limits, I think we also have to remember the benefits of transfusions. I think a lot of the studies are lacking in that they have not been presenting the length of stay of patients, the exercise capacity postoperatively, and so forth, because I think we have all seen patients the third or fourth day after surgery who do significantly better after having had a transfusion in terms of getting up, mobilizing, and being ready to go home rather than to a rehabilitation institution.
Dr C. Alhan (Istanbul, Turkey): I just want to ask a couple of questions. What is your transfusion trigger in terms of which patients you transfuse and which patients you do not? And did you look at the patients with low preoperative haemoglobin values as a group analysis, either transfused or not, and the outcome? And the last one, is it the patient or the physician that cannot tolerate anaemia?
Dr Poullis: Each surgeon has different opinions in my unit as to what is done, and that is potentially the strength of the study because the patients were transfused at different levels. And so it has been a random occurrence of what has happened postoperatively, so we are actually covering the full spread. We are not investigating a trigger level here for this study; we are just investigating if patients had the blood or did not have the blood.
With regard to the preoperative haemoglobin values and who did and did not have transfusion, I cannot tell you off the top of my head the exact percentages and ratios. But just looking at the raw figures, the more anaemic you are before, the more blood you get afterwards. And we think that preoperative anaemia is a major risk factor for getting blood. The third thing, of course, you are right, is it the patient or is it the physician? Quite often you are treating the physician not the patient in the ICU.
Dr T. Schwann (Toledo, OH, USA): I was wondering, did you adjust this analysis for the size of the patient? Because I would respectfully submit to you ….
Dr Poullis: Yes, we did. The hypothesis that we fought was that small people would get more blood, and so we adjusted for body mass index, and we still finally have these findings.
Dr Schwann: So a similar blood unit transfusion in a small-sized individual carried the same prognosis as a single unit transfusion in a relatively big individual?
Dr Poullis: I cannot tell you that, but that is a slightly separate question. But we did adjust for the body mass index in the Cox and in the propensity matching because there is going to be a risk factor. If your haemoglobin is 9 and you weigh 150 kilos, you will probably get away with it. But if you weigh 55 kilos, you're 100% are going to get blood.
Dr Schwann: So size does matter?
Dr Poullis: It does matter, but I cannot tell you the breakdown for the odds ratio for it.
Dr G. Whitman (Baltimore, MD, USA): Dr Poullis, I thought the presentation was great, but I think your conclusion suggests a bias which I do not understand, which is that giving a unit of blood is not bad. Giving the unit of blood is at a cost to society at least, if not to the patient. From what you present, it is not clear why you conclude that giving a unit of blood is not bad, as opposed to concluding that giving a unit of blood is an unnecessary use of resources or makes no difference whatsoever?
Dr Poullis: Okay. I will be slightly controversial. I do not care about the resources. That is not my interest. It is what is best for the patient. My philosophy is if you have to give blood, they have a surgical hole until proved otherwise. Most people do not need blood unless they have got a surgical hole. So having a high re-exploration rate I do not think is necessarily bad. Having a high transfusion rate I think is. And my theory is that when people are given multiple units of blood, it is because they have got a surgical hole that needs sorting out. And so I do not think the blood is the bad bit. It is the hole that is the bad bit. That is the angle we came from on this paper.
Dr Whitman: I thought the paper evaluated the benefit of transfusing one unit of blood?
Dr Poullis: It was because very few people will have a significant hole and only need one unit of blood to sort it out. And that is why we have the one unit. And that is why as soon as you get above one unit, blood is bad. Because if you are having two or three units of blood, why does the average 70 kilo guy coming in for elective CABG need blood? It is because he is bleeding from something. You have to lose over a litre of blood to drop your haemoglobin below 10 to require blood. That is a lot of blood loss. And people get drawn up on what is coming out of the mediastinal drains. It is the concealed blood inside that you are not measuring that is the problem.
So that is why I am not advocating blood transfusion at all. I am actually advocating no surgical bleeding. I think blood is an innocent bystander but realize that is controversial.
Dr M. Akay (Istanbul, Turkey): Did you have a chance to look at age of the blood that you are giving? Does it matter?
Dr Poullis: You are right, there is a growing basis of evidence that the older the blood you give for transfusion, the worse for patients it is. We actually do not have the dates of that. I realize that is a limitation of our study.
Dr V. Zamvar (Edinburgh, UK): I must say that your study makes a lot of sense. All the evidence that we see in the literature from observational studies suggests blood is bad. Maybe in view of what you have presented, all the other authors now should go back and look at their data. They should exclude patients who have had three, four, or five units of blood and then see if blood is bad or not.
Dr R. Habib (Beirut, Lebanon): I enjoyed the talk a lot, but I have maybe a word of caution. You found that preoperative anaemia is a predictor of worse outcomes, worse late outcomes, but not one unit of blood. There is no way you could separate these two because the predictor of that one unit is most likely that preoperative anaemia. So to be honest, at least a secondary analysis has to be done so that you do not have to include the preoperative anaemia as a covariate and match for that in comparison groups. And then if your data holds, you find the same effect, then you can make that conclusion.
Dr Poullis: Okay. If you take out the anaemic patients beforehand, preoperatively, one unit of blood does not make a difference, but that will be presenting you a subset of our database which I think will be misleading. But if you use a preop haemoglobin in the Cox regression as an interacting factor, the preoperative anaemia is a major risk. And that is why we did the propensity matching because clearly the groups are matched, and that is why I think Cox regression in this case is potentially flawed even though the literature is full of Cox regression on it.
Dr Habib: Yes, but this is exactly my point. You used propensity matching.
Dr Poullis: Yes.
Dr Habib: And as a result of the propensity matching, you found that preoperative anaemia is a predictor of worse outcomes, not one unit of transfusion.
Dr Poullis: Yes.
Dr Habib: These two entities are correlated.
Dr Poullis: Sure.
Dr Habib: So you cannot make that conclusion.
Dr Poullis: Not everyone with preoperative anaemia has blood. The trouble is the risk of dying with preoperative anaemia is a lot higher than the risk of dying from a unit of blood. That is why potentially the unit of blood does not come out.
Dr Habib: Okay. We can continue this.
Dr Poullis: Yes. It is a bit of a circle, isn't it?
REFERENCES
- 1.Engoren MC, Habib RH, Zacharias A, Schwann TA, Riordan CJ, Durham SJ. Effect of blood transfusion on long-term survival after cardiac operation. Ann Thorac Surg. 2002;74:1180–6. doi: 10.1016/s0003-4975(02)03766-9. doi:10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Koch CG, Li L, Duncan AI, Mihaljevic T, Cosgrove DM, Loop FD, et al. Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006;34:1608–16. doi: 10.1097/01.CCM.0000217920.48559.D8. [DOI] [PubMed] [Google Scholar]
- 3.Murphy GJ, Reeves BC, Rogers CA, Rizvi SI, Culliford L, Angelini GD. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116:2544–52. doi: 10.1161/CIRCULATIONAHA.107.698977. [DOI] [PubMed] [Google Scholar]
- 4.Reeves BC, Murphy GJ. Increased mortality, morbidity, and cost associated with red blood cell transfusion after cardiac surgery. Curr Opin Cardiol. 2008;23:607–12. doi: 10.1097/HCO.0b013e328310fc95. doi:10.1007/s11605-007-0264-2. [DOI] [PubMed] [Google Scholar]
- 5.Kuduvalli M, Oo AY, Newall N, Grayson AD, Jackson M, Desmond MJ, et al. Effect of peri-operative red blood cell transfusion on 30-day and 1-year mortality following coronary artery bypass surgery. Eur J Cardiothorac Surg. 2005;27:592–8. doi: 10.1016/j.ejcts.2005.01.030. doi:10.1016/j.ejso.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Fontaine E, McShane J, Page R, Shackcloth M, Mediratta N, Carr M, et al. Aspirin and non-small cell lung cancer resections: effect on long-term survival. Eur J Cardiothorac Surg. 2010;38:21–6. doi: 10.1016/j.ejcts.2010.01.015. doi:10.1016/j.ejso.2006.03.048. [DOI] [PubMed] [Google Scholar]
- 7.Fontaine E, McShane J, Carr M, Shachcloth M, Mediratta N, Page R, et al. Should we operate on microscopic N2 non small cell lung cancer? Interact CardioVasc Thorac Surg. 2011;12:956–61. doi: 10.1510/icvts.2010.255323. [DOI] [PubMed] [Google Scholar]
- 8.Fontaine E, McShane J, Carr M, Shackcloth M, Mediratta N, Page R, et al. Does positron emission tomography scanning improve survival in patients undergoing potentially curative lung resections for non-small-cell lung cancer? Eur J Cardiothorac Surg. 2011;40:642–6. doi: 10.1016/j.ejcts.2010.12.053. doi:10.1097/SLA.0b013e3181b2f6ee. [DOI] [PubMed] [Google Scholar]
- 9.Attaran S, Shaw M, Bond L, Pullan MD, Fabri BM. Does off-pump coronary artery revascularization improve the long-term survival in patients with ventricular dysfunction? Interact CardioVasc Thorac Surg. 2010;11:442–6. doi: 10.1510/icvts.2010.237040. doi:10.1046/j.1365-2168.2001.01746.x. [DOI] [PubMed] [Google Scholar]
- 10.Attaran S, Saleh HZ, Shaw M, Bond L, Pullan MD, Fabri BM. Comparing the outcome of on-pump versus off-pump coronary artery bypass grafting in patients with preoperative atrial fibrillation. Interact CardioVasc Thorac Surg. 2011;13:288–92. doi: 10.1510/icvts.2011.270249. doi:10.1097/SLA.0b013e31817bbe59. [DOI] [PubMed] [Google Scholar]
- 11.Berger MD, Gerber B, Arn K, Senn O, Schanz U, Stussi G. Significant reduction of red blood cell transfusion requirements by changing from a double-unit to a single-unit transfusion policy in patients receiving intensive chemotherapy or stem cell transplantation. Haematologica. 2012;97:116–22. doi: 10.3324/haematol.2011.047035. doi:10.1097/SLA.0b013e31814697f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callaghan FM, Luechinger R, Kurtcuoglu V, Sarikaya H, Poulikakos D, Baumgartner RW. Wall stress of the cervical carotid artery in patients with carotid dissection: a case-control study. Am J Physiol Heart Circ Physiol. 2011;300:H1451–H8. doi: 10.1152/ajpheart.00871.2010. doi:10.1097/SLA.0b013e31815c4037. [DOI] [PubMed] [Google Scholar]
- 13.Chohan SS, McArdle F, McClelland DB, Mackenzie SJ, Walsh TS. Red cell transfusion practice following the transfusion requirements in critical care (TRICC) study: prospective observational cohort study in a large UK intensive care unit. Vox Sang. 2003;84:211–8. doi: 10.1046/j.1423-0410.2003.00284.x. doi:10.1002/cncr.23309. [DOI] [PubMed] [Google Scholar]
- 14.Hebert PC, Wells G, Martin C, Tweeddale M, Marshall J, Blajchman M, et al. Variation in red cell transfusion practice in the intensive care unit: a multicentre cohort study. Crit Care. 1999;3:57–63. doi: 10.1186/cc310. doi:10.1007/s00268-009-9926-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keszthelyi B, Lakatos-Novotny S, Toth A. Measurement of blood loss by total-body counting on the basis of calculated and predicted blood volumes. Acta Med Acad Sci Hung. 1979;36:71–8. doi:10.1245/s10434-010-0948-9. [PubMed] [Google Scholar]
- 16.Domanski MJ, Mahaffey K, Hasselblad V, Brener SJ, Smith PK, Hillis G, et al. Association of myocardial enzyme elevation and survival following coronary artery bypass graft surgery. JAMA. 2011;305:585–91. doi: 10.1001/jama.2011.99. [DOI] [PubMed] [Google Scholar]
- 17.Loop FD, Lytle BW, Cosgrove DM, Stewart RW, Goormastic M, Williams GW, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. 1986;314:1–6. doi: 10.1056/NEJM198601023140101. [DOI] [PubMed] [Google Scholar]
- 18.El Chami MF, Kilgo P, Thourani V, Lattouf OM, Delurgio DB, Guyton RA, et al. New-onset atrial fibrillation predicts long-term mortality after coronary artery bypass graft. J Am Coll Cardiol. 2010;55:1370–6. doi: 10.1016/j.jacc.2009.10.058. [DOI] [PubMed] [Google Scholar]
- 19.Spiess BD. Transfusion of blood products affects outcome in cardiac surgery. Semin Cardiothorac Vasc Anesth. 2004;8:267–81. doi: 10.1177/108925320400800402. [DOI] [PubMed] [Google Scholar]
- 20.Yun JJ, Helm RE, Kramer RS, Leavitt BJ, Surgenor SD, Discipio AW, et al. Limited blood transfusion does not impact survival in octogenarians undergoing cardiac operations. Ann Thorac Surg. 2012;94:2038–45. doi: 10.1016/j.athoracsur.2012.06.059. [DOI] [PubMed] [Google Scholar]