Abstract
Despite technological advances in a newer generation of ventricular assist devices (VAD), complications, such as pump thromboses, remain a significant cause of morbidity and indeed mortality in these patients. We present the case of a 34-year old patient who underwent HeartAssist 5 (HA5) implantation as a bridge to cardiac transplant. After an initial uneventful recovery, he developed a pump thrombosis that was refractory to medical treatment. We present the surgical technique used to exchange the HA5 with a HeartWare (HVAD), leaving the old inflow-sewing ring in situ.
Keywords: Circulatory assist devices
CASE REPORT
A 34-year old gentleman suffering from congenital aortic valve disease (quadricuspid aortic valve) causing critical aortic stenosis and resulting in end-stage left ventricle dysfunction (ejection fraction of 10%) underwent elective aortic valve replacement (AVR) at our institution. Following AVR, he developed a post-cardiotomy shock necessitating a Levitronix® short-term biventricular support device (Levitronix LLC, Waltham, MA, USA) as a bridge to expected recovery. Unfortunately, after 28 days of support, the patient showed no significant improvement in the left ventricular function.
The first option was to list him for an urgent heart transplant, but the estimated waiting time was too long. Thus, it was decided to upgrade the support to a permanent ventricular assist device (VAD) as a bridge to transplantation for which a HeartAssist 5 (HA5) was chosen. This is an axial flow device, which has a curved and slightly funnelled inflow non-flexible cannula with an attached Teflon suture ring. For insertion, a second placement/fixation ring with a purse string mechanism is sutured to the ventricular apex, an insertion hole is cored and the device inserted. The purse string is tightened to achieve haemostasis, and the Teflon ring is sutured to the placement ring.
The device was implanted in a semi-elective procedure, at which the right ventricular support was weaned. The patient had an uneventful postoperative period and was discharged on warfarin and aspirin, as per institutional protocol. Five months thereafter, an abnormal VAD sound was detected, accompanied by a significant decrease in flow to 3.5 l/m from a stable baseline of 4.5 l/m and an increase in power consumption to 9 Watts (baseline 8 Watts). His plasma haemoglobin was 0.8 mg/dl and lactate dehydrogenase >3000 mg/dl. He was started on intravenous heparin, which was escalated to tirofiban for 7 days, after which the haemolysis markers and the sound profile returned to normal. At this point, he was restarted on warfarin and subsequently discharged. Unfortunately, he had to be readmitted a month later, because he experienced a similar episode. Additionally, echocardiography revealed indirect signs of thrombosis within the device itself. In addition, he was initially treated with heparin and tirofiban and this time, the therapy was increased to intraventricular tissue plasminogen activator (TPA) administration. As the medical therapy did not resolve the signs of VAD thrombosis, it was decided to replace the HA5.
It is our policy to use a different device if we experience problems with the device initially implanted. For this purpose, an HVAD was chosen, as the outflow grafts have the same diameter and the placement/fixation ring of the HVAD could easily be attached to its HA5 equivalent, which would allow for easy implantation.
The surgery was performed via a redo sternotoma. The device and outflow graft were mobilized carefully and the patient was put on cardiopulmonary bypass through cannulation of the right femoral vessels. The HA5 device was stopped, the outflow graft protector was removed and the graft was clamped and divided about 3 cm from its anastomosis with the ascending aorta. The pump was disconnected from the inflow-sewing ring by cutting the four sutures between them (Fig. 1). The HA5 driveline and the device were explanted. Keeping the sewing ring in place, the sewing ring hat lining the transmyocardial tunnel was incised vertically and removed in order to allow placement of the HVAD inflow cannula, which is of slightly larger diameter (Fig. 2). The HA5 sewing ring was left attached to the ventricle, and the sewing ring of the HVAD was secured to it by using interrupted pledgetted 2-0 Ethibond (Ethicon, Somerville, NJ, USA) sutures and by sealing them with BioGlue (CryoLife, Kennesaw, GA, USA).
Figure 1:
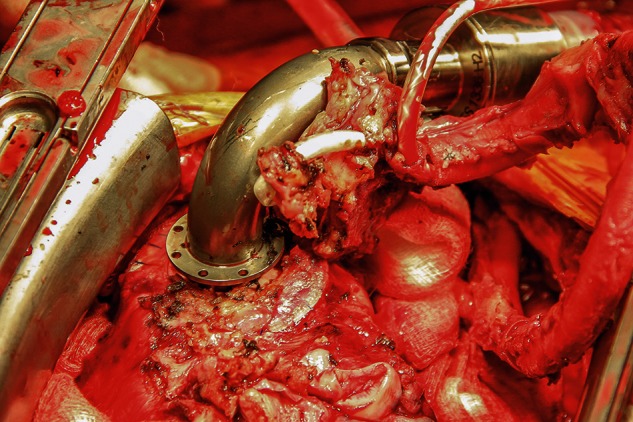
HA5 pump disconnected from the inflow-sewing ring.
Figure 2:
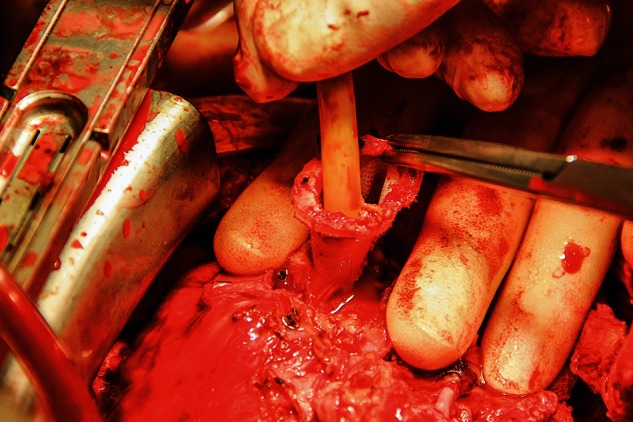
HA5 sewing ring hat removed.
The HVAD was inserted into the sewing ring and locked into place. The driveline was tunnelled subcutaneously to the left lower abdominal quadrant avoiding the previous driveline tunnel on the right side.
Finally, the outflow graft of the HVAD was anastomosed to the remaining stump of the old HA5 graft using a 4-0 polypropylene running suture. After complete de-airing and satisfactory transoesophageal echocardiography examination, CPB was terminated, and the HVAD was set at full flow.
Due to borderline right ventricular function and significant coagulopathy, the chest was initially stented and the mediastinum packed. On the second postoperative day (POD), the chest was closed electively after which the patient had an uneventful recovery. He was extubated on POD 3 and discharged to the ward after 1 week. He was discharged home on POD 32.
DISCUSSION
VADs have become clinical routine and represent an effective therapy for patients with advanced heart failure. They have been demonstrated to increase both survival and quality of life. Over the last 10 years, a new generation of small-size pumps, such as the Thoratec HeartMate II, HeartWare HVAD and MicroMed DeBakey HeartAssist 5, have been developed [1]. The HeartAssist 5™ represents a further development in this series being the only VAD equipped with the in-built flow probe attached to the outflow tract, which enables direct flow measurements [2].
Despite technological advances, there remains, among others, a risk of pump thrombosis that may lead to pump failure. Pump thromboses are difficult to treat medically and often require a device replacement, which may be technically challenging and carries significant perioperative mortality. The accurate diagnosis of this entity remains challenging and it is often established by indirect evidence. It usually presents with haemolysis and increased pump power consumption. The echocardiography may show indirect signs of pump failure. In addition, the sound profile of the device usually changes [3]. In our case, there were periods of paradoxical flows vs power demand data, abnormal sound profiles and increased haemolytic parameters, all consistent with the suspected pump thrombosis. An echocardiogram also suggested obstruction to the VAD flow within the device itself.
Despite maximum medical treatment, the suspected pump thrombosis could not be resolved. The intraventricular infusion of TPA for VAD thrombosis has been reported as a successful approach that can be considered as an acceptable alternative to pump exchange in select cases [4, 5]. In our patient, intraventricular TPA was used, but without any success.
A simple device explantation was not a possibility as the patient had not shown any signs of recovery and his underlying ventricular function was not good enough to support the circulation. As an alternative to a pump exchange, it was considered to list him for an urgent heart transplant, but he had developed antihuman leucocyte antigen (HLA) antibodies, directed against HLA antigens present in approximately 58% of the UK donor population. As the expected waiting time was too long with the permanent risk of an imminent total pump failure, we decided to replace the device.
To our knowledge, this is the first report of a successful HA5 pump exchange. It was performed without removing the HA5 inflow-sewing ring to avoid the dissection around it and the subsequent damage to the left ventricle. The removal of the sewing ring hat allowed creating enough space inside the inlet for the insertion of the new pump.
In summary, the exchange of a HA5 pump with a HeartWare can be accomplished with relatively low morbidity, despite the need for reoperation and the use of a different device. The technique can be simplified by keeping the previous inflow-sewing ring in situ.
Conflict of interest: none declared.
REFERENCES
- 1.Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–51. doi: 10.1056/NEJMoa0909938. doi:10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 2.Noon GP, Loebe M. Current status of the MicroMed DeBakey Noon Ventricular Assist Device. Tex Heart Inst J. 2010;37:652–3. [PMC free article] [PubMed] [Google Scholar]
- 3.Aissaoui N, Börgermann J, Gummert J, Morshuis M. HeartWare continuous-flow ventricular assist device thrombosis: the Bad Oeynhausen experience. J Thorac Cardiovasc Surg. 2012;143:e37–9. doi: 10.1016/j.jtcvs.2011.12.035. doi:10.1016/j.jtcvs.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 4.Rothenburger M, Wilhelm MJ, Hammel D, Schmidt C, Tjan TD, Böcker D, et al. Treatment of thrombus formation associated with the MicroMed DeBakey VAD using recombinant tissue plasminogen activator. Circulation. 2002;106(12 Suppl 1):I189–92. [PubMed] [Google Scholar]
- 5.Kamouh A, John R, Eckman P. Successful treatment of early thrombosis of heartware left ventricular assist device with intraventricular thrombolytics. Ann Thorac Surg. 2012;94:281–83. doi: 10.1016/j.athoracsur.2011.12.024. doi:10.1016/j.athoracsur.2011.12.024. [DOI] [PubMed] [Google Scholar]


