Abstract
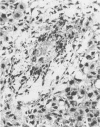
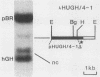
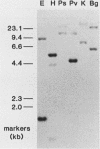
Insertional mutagenesis was investigated in a transgenic mouse strain (HUGH/4) derived from a fertilized egg injected with plasmid DNA containing the human growth hormone gene. Lethality occurred in homozygous embryos and was traced to the egg cylinder stage on days 4 to 5 of gestation, shortly after implantation. The mutation is on chromosome 12 and is distinct in location and integration pattern from another mutation also leading to lethality of homozygotes in the egg cylinder stage. Based on this and other evidence, relatively many genes may be recruited to activity near the time of implantation and may therefore present a large target of vulnerability to mutagenesis. The single insert in HUGH/4, consisting of approximately three tandem copies of plasmid sequences, is flanked by mouse cellular sequences that have undergone rearrangements, including a probable deletion. The data suggest the hypothesis that DNA rearrangements, which appear to be commonplace in transgenic mice, may arise because the initial insertional complex is unstable; stepwise changes may then be generated until a more stable conformation is achieved.
Full text
PDF
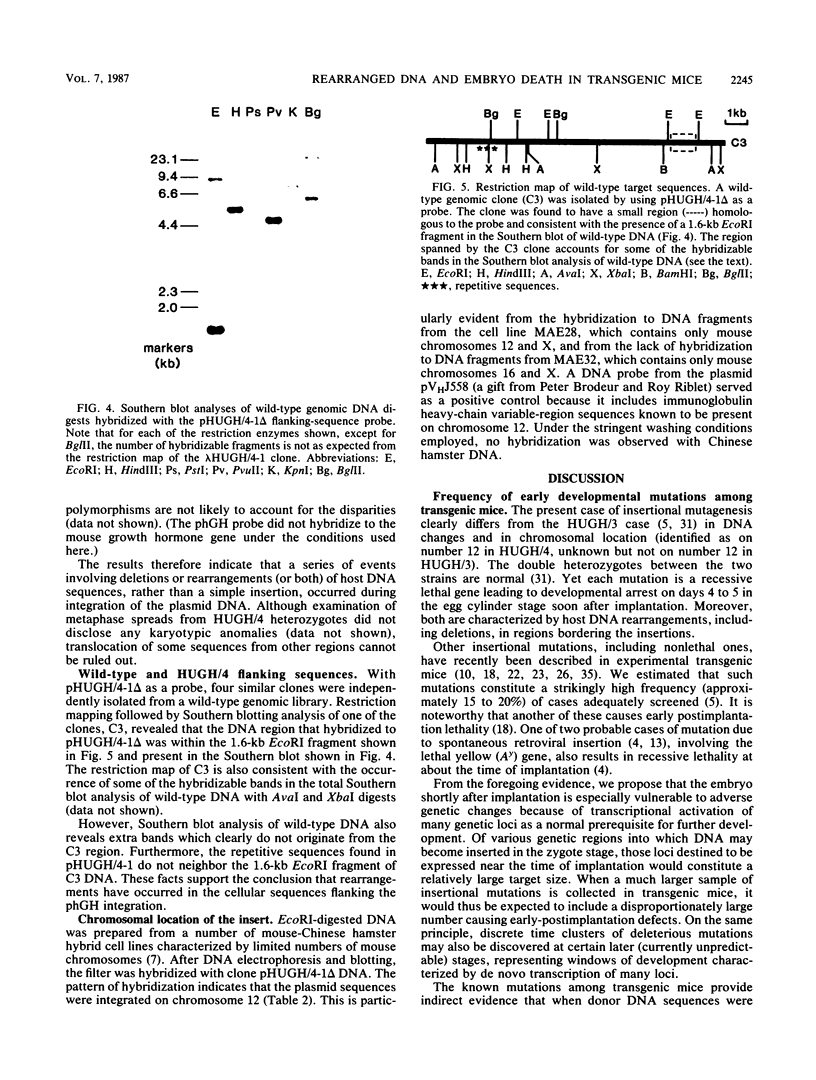
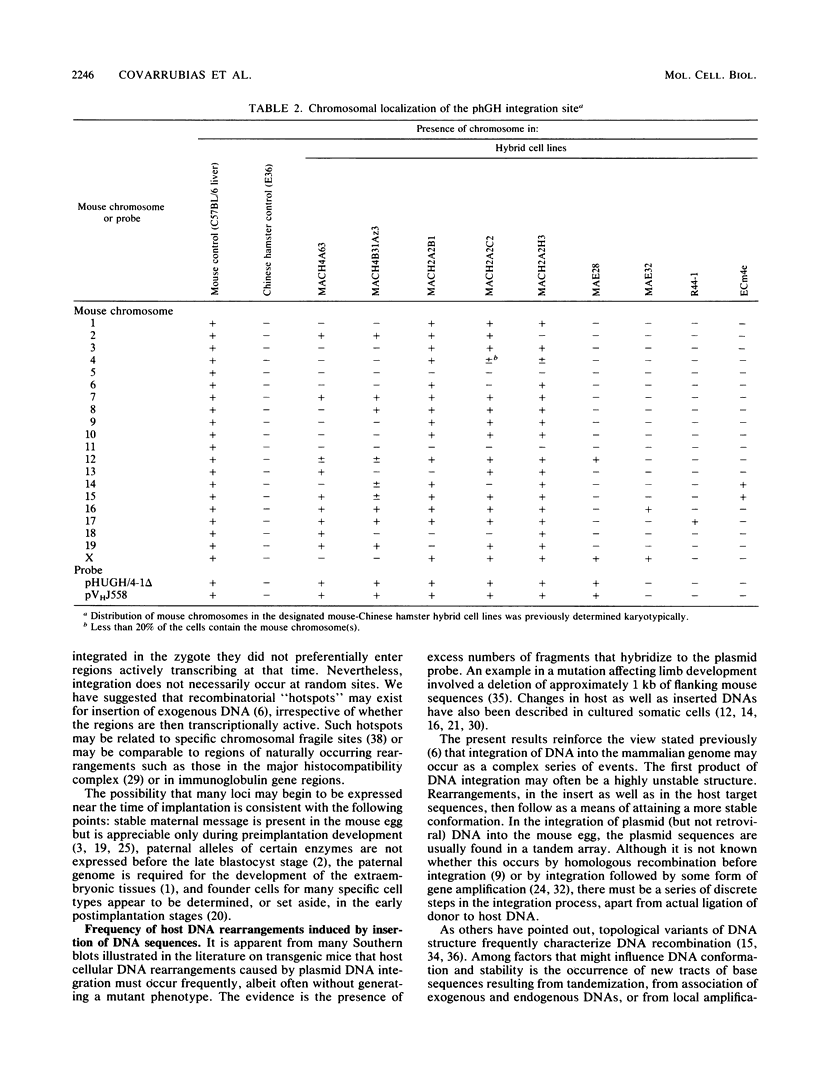

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton S. C., Surani M. A., Norris M. L. Role of paternal and maternal genomes in mouse development. 1984 Sep 27-Oct 3Nature. 311(5984):374–376. doi: 10.1038/311374a0. [DOI] [PubMed] [Google Scholar]
- Chapman V. M., Whitten W. K., Ruddle F. H. Expression of paternal glucose phosphate isomerase-1 (Gpi-1) in preimplantation stages of mouse embryos. Dev Biol. 1971 Sep;26(1):153–158. doi: 10.1016/0012-1606(71)90114-x. [DOI] [PubMed] [Google Scholar]
- Clegg K. B., Pikó L. Poly(A) length, cytoplasmic adenylation and synthesis of poly(A)+ RNA in early mouse embryos. Dev Biol. 1983 Feb;95(2):331–341. doi: 10.1016/0012-1606(83)90034-9. [DOI] [PubMed] [Google Scholar]
- Copeland N. G., Jenkins N. A., Lee B. K. Association of the lethal yellow (Ay) coat color mutation with an ecotropic murine leukemia virus genome. Proc Natl Acad Sci U S A. 1983 Jan;80(1):247–249. doi: 10.1073/pnas.80.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covarrubias L., Nishida Y., Mintz B. Early developmental mutations due to DNA rearrangements in transgenic mouse embryos. Cold Spring Harb Symp Quant Biol. 1985;50:447–452. doi: 10.1101/sqb.1985.050.01.056. [DOI] [PubMed] [Google Scholar]
- Covarrubias L., Nishida Y., Mintz B. Early postimplantation embryo lethality due to DNA rearrangements in a transgenic mouse strain. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6020–6024. doi: 10.1073/pnas.83.16.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Eustachio P., Bothwell A. L., Takaro T. K., Baltimore D., Ruddle F. H. Chromosomal location of structural genes encoding murine immunoglobulin lambda light chains. Genetics of murine lambda light chains. J Exp Med. 1981 Apr 1;153(4):793–800. doi: 10.1084/jem.153.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiddes J. C., Seeburg P. H., DeNoto F. M., Hallewell R. A., Baxter J. D., Goodman H. M. Structure of genes for human growth hormone and chorionic somatomammotropin. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4294–4298. doi: 10.1073/pnas.76.9.4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folger K. R., Wong E. A., Wahl G., Capecchi M. R. Patterns of integration of DNA microinjected into cultured mammalian cells: evidence for homologous recombination between injected plasmid DNA molecules. Mol Cell Biol. 1982 Nov;2(11):1372–1387. doi: 10.1128/mcb.2.11.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. W. A foreign dihydrofolate reductase gene in transgenic mice acts as a dominant mutation. Mol Cell Biol. 1986 Jun;6(6):2158–2167. doi: 10.1128/mcb.6.6.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosschedl R., Weaver D., Baltimore D., Costantini F. Introduction of a mu immunoglobulin gene into the mouse germ line: specific expression in lymphoid cells and synthesis of functional antibody. Cell. 1984 Oct;38(3):647–658. doi: 10.1016/0092-8674(84)90259-9. [DOI] [PubMed] [Google Scholar]
- Hawley R. G., Shulman M. J., Murialdo H., Gibson D. M., Hozumi N. Mutant immunoglobulin genes have repetitive DNA elements inserted into their intervening sequences. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7425–7429. doi: 10.1073/pnas.79.23.7425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins N. A., Copeland N. G., Taylor B. A., Lee B. K. Dilute (d) coat colour mutation of DBA/2J mice is associated with the site of integration of an ecotropic MuLV genome. Nature. 1981 Oct 1;293(5831):370–374. doi: 10.1038/293370a0. [DOI] [PubMed] [Google Scholar]
- King W., Patel M. D., Lobel L. I., Goff S. P., Nguyen-Huu M. C. Insertion mutagenesis of embryonal carcinoma cells by retroviruses. Science. 1985 May 3;228(4699):554–558. doi: 10.1126/science.3838595. [DOI] [PubMed] [Google Scholar]
- Koo H. S., Wu H. M., Crothers D. M. DNA bending at adenine . thymine tracts. Nature. 1986 Apr 10;320(6062):501–506. doi: 10.1038/320501a0. [DOI] [PubMed] [Google Scholar]
- Lebkowski J. S., DuBridge R. B., Antell E. A., Greisen K. S., Calos M. P. Transfected DNA is mutated in monkey, mouse, and human cells. Mol Cell Biol. 1984 Oct;4(10):1951–1960. doi: 10.1128/mcb.4.10.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MINTZ B. SYNTHETIC PROCESSES AND EARLY DEVELOPMENT IN THE MAMMALIAN EGG. J Exp Zool. 1964 Oct;157:85–100. doi: 10.1002/jez.1401570114. [DOI] [PubMed] [Google Scholar]
- Mark W. H., Signorelli K., Lacy E. An insertional mutation in a transgenic mouse line results in developmental arrest at day 5 of gestation. Cold Spring Harb Symp Quant Biol. 1985;50:453–463. doi: 10.1101/sqb.1985.050.01.057. [DOI] [PubMed] [Google Scholar]
- Mintz B. Clonal basis of mammalian differentiation. Symp Soc Exp Biol. 1971;25:345–370. [PubMed] [Google Scholar]
- Mounts P., Kelly T. J., Jr Rearrangements of host and viral DNA in mouse cells transformed by simian virus 40. J Mol Biol. 1984 Aug 15;177(3):431–460. doi: 10.1016/0022-2836(84)90294-8. [DOI] [PubMed] [Google Scholar]
- Overbeek P. A., Lai S. P., Van Quill K. R., Westphal H. Tissue-specific expression in transgenic mice of a fused gene containing RSV terminal sequences. Science. 1986 Mar 28;231(4745):1574–1577. doi: 10.1126/science.3006249. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Wilkie T. M., Chen H. Y., Brinster R. L. Transmission distortion and mosaicism in an unusual transgenic mouse pedigree. Cell. 1984 Apr;36(4):869–877. doi: 10.1016/0092-8674(84)90036-9. [DOI] [PubMed] [Google Scholar]
- Roberts J. M., Buck L. B., Axel R. A structure for amplified DNA. Cell. 1983 May;33(1):53–63. doi: 10.1016/0092-8674(83)90334-3. [DOI] [PubMed] [Google Scholar]
- Sawicki J. A., Magnuson T., Epstein C. J. Evidence for expression of the paternal genome in the two-cell mouse embryo. Nature. 1981 Dec 3;294(5840):450–451. doi: 10.1038/294450a0. [DOI] [PubMed] [Google Scholar]
- Schnieke A., Harbers K., Jaenisch R. Embryonic lethal mutation in mice induced by retrovirus insertion into the alpha 1(I) collagen gene. 1983 Jul 28-Aug 3Nature. 304(5924):315–320. doi: 10.1038/304315a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Höchtl J., Schnell H., Gebhard W., Zachau H. G. Cloning of V region fragments from mouse liver DNA and localization of repetitive DNA sequences in the vicinity of immunoglobulin gene segments. Nucleic Acids Res. 1980 Apr 25;8(8):1721–1729. doi: 10.1093/nar/8.8.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz M., Stephan D., Fischer Lindahl K. Gene organization and recombinational hotspots in the murine major histocompatibility complex. Cell. 1986 Mar 28;44(6):895–904. doi: 10.1016/0092-8674(86)90012-7. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Quintrell N., Ortiz S. Retroviruses as mutagens: insertion and excision of a nontransforming provirus alter expression of a resident transforming provirus. Cell. 1981 Jul;25(1):23–36. doi: 10.1016/0092-8674(81)90228-2. [DOI] [PubMed] [Google Scholar]
- Wagner E. F., Covarrubias L., Stewart T. A., Mintz B. Prenatal lethalities in mice homozygous for human growth hormone gene sequences integrated in the germ line. Cell. 1983 Dec;35(3 Pt 2):647–655. doi: 10.1016/0092-8674(83)90097-1. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Robert de Saint Vincent B., DeRose M. L. Effect of chromosomal position on amplification of transfected genes in animal cells. Nature. 1984 Feb 9;307(5951):516–520. doi: 10.1038/307516a0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman S. A., Cozzarelli N. R. Biochemical topology: applications to DNA recombination and replication. Science. 1986 May 23;232(4753):951–960. doi: 10.1126/science.3010458. [DOI] [PubMed] [Google Scholar]
- Woychik R. P., Stewart T. A., Davis L. G., D'Eustachio P., Leder P. An inherited limb deformity created by insertional mutagenesis in a transgenic mouse. Nature. 1985 Nov 7;318(6041):36–40. doi: 10.1038/318036a0. [DOI] [PubMed] [Google Scholar]
- Wu H. M., Crothers D. M. The locus of sequence-directed and protein-induced DNA bending. Nature. 1984 Apr 5;308(5959):509–513. doi: 10.1038/308509a0. [DOI] [PubMed] [Google Scholar]
- Yunis J. J., Soreng A. L. Constitutive fragile sites and cancer. Science. 1984 Dec 7;226(4679):1199–1204. doi: 10.1126/science.6239375. [DOI] [PubMed] [Google Scholar]