Abstract
OBJECTIVES
To explore the rule of subcarinal lymph node metastasis in thoracic oesophageal cancer and its clinical significance in the radical resection of oesophageal cancer.
METHODS
We retrospectively analysed 2223 patients with oesophageal cancer who were admitted to Henan Cancer Hospital during 2004–2011 and underwent surgery as the first treatment option. Routine subcarinal lymph node dissections were performed, and the sections from the resected lymph nodes were embedded in paraffin for routine pathological examination.
RESULTS
Subcarinal lymph node metastasis was observed in 200 patients (9%). Logistic regression analysis identified the following risk factors (P < 0.05): tumour location, depth of invasion into the oesophageal wall, tissue type, number of lymph node metastases, paraoesophageal lymph node metastasis (level 8 lymph nodes), left gastric cardiac lymph node metastasis. Unpaired t-test and χ2-test showed that more lymph node metastases, longer tumour length, deeper tumour invasion, middle oesophageal cancer, squamous-cell carcinoma, lower degree of differentiation, paraoesophageal lymph node metastasis and left gastric cardiac lymph node metastasis were associated with a higher frequency of subcarinal lymph node metastases (P < 0.05). Using the Kaplan–Meier method, recurrence and metastasis were shown to be more likely with solitary subcarinal lymph node metastasis than with solitary paraoesophageal lymph node metastasis (P = 0.001).
CONCLUSIONS
Tumour location, depth of invasion, pathological type, degree of differentiation and other factors are closely associated with subcarinal lymph node metastasis. Recurrence and metastasis after oesophageal dissection are more likely with subcarinal lymph node metastasis.
Keywords: Oesophageal cancer, Oesophageal surgery, Subcarinal lymph node dissection
INTRODUCTION
Oesophageal cancer is 1 of the top 10 most-common cancers, and its morbidity is ranked eighth among all cancers globally [1]. An estimated 16 470 new oesophageal cancer patients and 14 280 deaths were recorded in the USA in 2008 [2]. China has one of the highest oesophageal cancer rates in the world. At present, surgical resection is still the main approach in the treatment of oesophageal cancer [3]. Nodal-stage classification is considered the most useful predictor of survival and is an indicator of the risk of disease recurrence [4]. However, the extent of lymph node dissection is associated with increased trauma and complications [5–7]; thus, the appropriate extent of lymph node dissection may not only guarantee the effect of operation and the postoperative quality of life, but may also reduce the incidence of postoperative complications. Therefore, it is of great clinical significance to explore the characteristics of lymph node metastasis in oesophageal cancer and to define the appropriate extent of lymph node dissection accompanying oesophagectomy.
Subcarinal lymph nodes have traditionally been considered to occur in the region of oesophageal cancer [8]; thus, subcarinal lymph node dissection has become a routine procedure during oesophagectomy. However, few studies have reported the details of subcarinal lymph node metastasis in oesophageal cancer. Therefore, further relevant studies are necessary.
PATIENTS AND METHODS
The medical history data of 2223 patients undergoing radical oesophagectomy in Henan Cancer Hospital during 2004–2011 were collected. None of the patients underwent radiotherapy and chemotherapy prior to oesophagectomy. The selected patients did not have tumours located in the cervical oesophagus or below the gastro-oesophageal junction. Subcarinal lymph node dissection was performed in all cases, and from each lymph node, paraffin-embedded sections were made for pathological examination. In total, 22 936 lymph nodes were dissected, and of these, the number of lymph node metastases was 2165. Postoperative pathological staging followed the oesophageal cancer tumour-node metastasis (TNM) staging system of the American Joint Committee on Cancer (AJCC2009, 7th edition) (Table 1) [9].
Table 1:
General clinical data of patients [n (%)]
| Variables | X1 | X2 | P |
|---|---|---|---|
| Age | 58.26 ± 7.61 | 59.06 ± 7.84 | 0.17 |
| Sex | |||
| Male | 135 (67.5) | 1349 (66.7) | 0.82 |
| Female | 65 (32.5) | 674 (33.3) | |
| Tumour location | |||
| Upper | 6 (3.0) | 177 (8.7) | 0.001 |
| Middle | 176 (88.0) | 1521 (75.2) | |
| Lower | 18 (9.0) | 325 (16.1) | |
| Number of lymph node metastasis | 3.96 ± 2.8 | 0.6 ± 1.2 | 0.000 |
| Number of lymph node dissected | 11.31 ± 4.5 | 10.95 ± 4.4 | 0.000 |
| Tissue types | |||
| Squamous | 182 (91.0) | 1890 (93.4) | 0.32 |
| Adenocarcinoma | 7 (3.5) | 63 (3.1) | |
| Other | 11 (5.5) | 70 (3.5) | |
| Level 8 lymph nodes | |||
| Metastasis | 100 (50.0) | 321 (15.9) | 0.001 |
| Not | 100 (50.0) | 1702 (84.1) | |
| Left gastric cardia lymph node | |||
| Metastasis | 97 (48.5) | 355 (17.5) | 0.001 |
| Not | 103 (51.5) | 1668 (82.5) | |
| Depth of invasion | |||
| Tis | 0 (0.0) | 32 (1.6) | 0.001 |
| T1 | 4 (2.0) | 255 (12.6) | |
| T2 | 41 (20.5) | 596 (29.5) | |
| T3 | 144 (72.0) | 1094 (54.1) | |
| T4 | 11 (5.5) | 46 (2.3) | |
| Length of cancer | 5.72 ± 2.1 | 4.47 ± 1.9 | 0.000 |
| Degree of differentiation | |||
| G1 | 34 (17.0) | 523 (25.9) | 0.041 |
| G2 | 68 (34.0) | 659 (32.6) | |
| G3 | 51 (25.5) | 426 (21.1) | |
| Gx | 47 (23.5) | 415 (20.4) | |
X1: subcarinal lymph node metastasis; X2: no subcarinal lymph node metastasis.
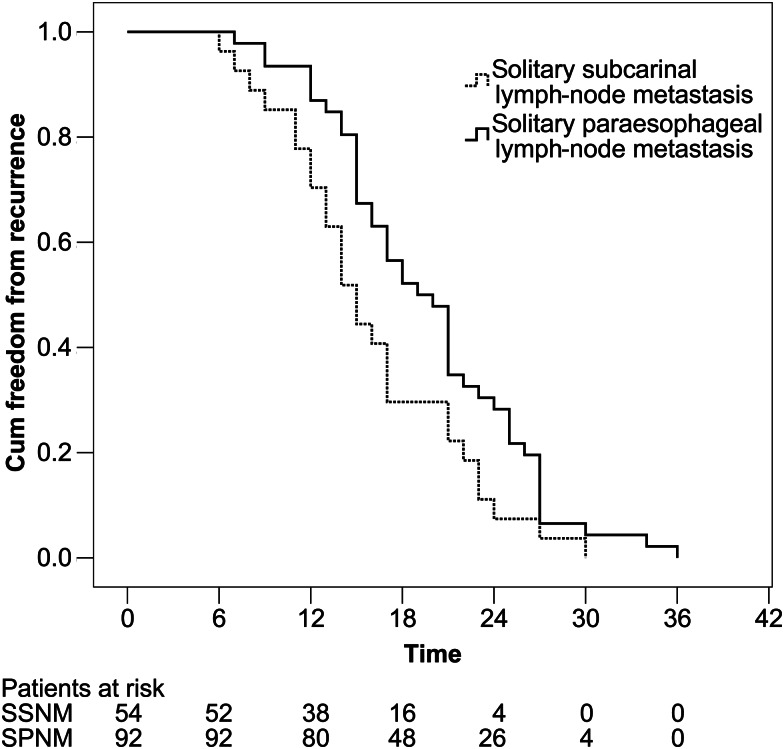
Patients with solitary subcarinal (SSNM) or paraoesophageal lymph node metastasis (SPNM) for whom we had intact clinical data and medical history were included. In accordance with the standard requirement for radical resection for treatment of oesophageal carcinoma in China, patients with lymph node metastasis identified by postoperative lymph node biopsy were given adjuvant chemoradiotherapy. All of the patients underwent 4–6 courses of chemotherapy after oesophagectomy. The time of recurrence was considered as the period from the end of chemotherapy to the occurrence of haematogenous, lymphatic, or other metastasis (Fig. 1).
Figure 1.
Time of recurrence and metastasis of oesophageal cancer patients with solitary subcarinal lymph node metastasis and solitary paraoesophageal lymph node metastasis.
Surgical procedures
Surgical procedures included primary tumour resection and lymph node dissection. The most commonly used surgical approaches included the left transthoracic procedure, the Ivor-Lewis approach, and the cervical-thoracoabdominal procedure. The left transthoracic procedure and Ivor-Lewis procedure with anastomosis of the upper chest were performed for all tumours of the lower thoracic oesophagus and some tumours of the middle thoracic oesophagus. The cervical-thoracoabdominal procedure was used for all tumours of the upper thoracic oesophagus and some tumours of the middle thoracic oesophagus. In this cohort of patients, en bloc lymph node dissection was performed, including the subcarinal, paraoesophageal, pulmonary ligament, diaphragmatic and paracardial lymph nodes, as well as those located along the lesser gastric curvature, the origin of the left gastric artery. In addition, the lymph nodes, such as aortopulmonary, anterior mediastinal and left tracheobronchial nodes were removed when the left transthoracic procedure was used, and the right superior mediastinal lymph nodes and right recurrent laryngeal nerve nodes were removed when the Ivor-Lewis procedure or cervical-thoracoabdominal procedure was used. Cervical lymphadenectomy was not systematically undertaken. For patients with cervical anastomosis, the lymph nodes exposed by the cervical incision were also dissected. The alimentary tract was reconstructed using the gastric pull-up technique; if the stomach was unavailable, a jejunal loop or the transverse colon was used. The surgeons identified the sites of the nodes during the operation. All resected specimens were submitted for pathological examination.
Statistical analysis
Data were analysed using SPSS version 17.0 (SPSS, IBM, Chicago, IL, USA). Continuous data were expressed as the mean ± SD and were analysed using the unpaired t-test. Categorical variables were presented as frequency (%) and were analysed using the χ2 test. Multiple logistic regression analysis of factors correlated to subcarinal lymph node metastasis. The Kaplan–Meier method was used to test the associated risk factors for the time of recurrence and metastasis. Comparison between groups was calculated by log-rank test. P < 0.05 was considered significant.
RESULTS
According to our pathological examinations, subcarinal lymph node metastasis was observed in 200 cases, with a rate of 9% (200/2223). The t-test and χ2-test showed the following factors to be associated with a higher frequency of subcarinal lymph node metastasis (P < 0.05, Table 1): more lymph node metastases, longer tumour length, deeper invasion, middle oesophageal cancer, squamous-cell carcinoma, lower degree of differentiation, paraoesophageal lymph node metastasis and left gastric cardiac lymph node metastasis.
The logistic regression analysis showed that the following factors were associated with a risk of subcarinal lymph node metastasis (P < 0.05, Table 2): tumour location, depth of invasion into the oesophageal wall, tissue type, number of lymph node metastases, number of lymph nodes dissected, paraoesophageal lymph node metastasis, left gastric cardiac lymph node metastasis and cervical lymph node metastasis.
Table 2:
Logistic regression analysis of factors correlated to subcarinal lymph node metastasis
| Variables | SE | Sig. | OR (95% CI) |
|---|---|---|---|
| Tumour location | 0.3029 | 0.0318 | 0.522 (0.360–0.693) |
| Number of lymph node metastasis | 0.0918 | 0.0001 | 2.498 (1.939–3.155) |
| Number of lymph node dissection | 0.0171 | 0.0001 | 0.839 (0.808–0.873) |
| Level 8 lymph nodes | 0.2472 | 0.0067 | 0.512 (0.410–0.626) |
| Left gastric cardia lymph node metastasis | 0.2620 | 0.0001 | 0.360 (0.243–0.501) |
| Depth of invasion | 0.2419 | 0.0413 | 1.638 (1.233–2.183) |
OR: odds ration; SE: standard error.
As given in Table 3, the difference (in location) of recurrence and metastasis of oesophageal cancer patients with SSNM and SPNM were statistically significant (P = 0.025). The Kaplan–Meier method was used to test the relevant factors for the time of recurrence and metastasis. The median time of solitary subcarinal lymph node metastasis was 15 months, and that of solitary paraoesophageal metastasis was 19 months. The difference in both groups was statistically significant (P = 0.001, Table 4).
Table 3:
Difference (in location) of recurrence and metastasis of oesophageal cancer patients with solitary subcarinal lymph node metastasis (SSNM) and solitary paraoesophageal lymph node metastasis (SPNN) [n (%)]
| Variables | SSNM | SPNM | P |
|---|---|---|---|
| Anastomotic stoma | 10 (18.5) | 15 (16.3) | 0.025 |
| Superior mediastinum lymph nodes | 21 (38.9) | 20 (21.7) | |
| Supraclavicular lymph nodes | 13 (24.1) | 15 (16.3) | |
| Abdominal lymph nodes | 3 (5.6) | 19 (20.7) | |
| Bone metastasis | 3 (5.6) | 6 (6.5) | |
| Liver | 4 (7.4) | 17 (18.5) |
Table 4:
Difference of median times of oesophageal cancer patients with solitary subcarinal lymph node metastasis (SSNM) and solitary paraoesophageal lymph node metastasis (SPNN)
| 1 year |
2 years |
3 years |
||||
|---|---|---|---|---|---|---|
| Median (95% CI) | P | Median (95% CI) | P | Median (95% CI) | P | |
| SSNM | 9 (7.040–10.960) | 0.23 | 14 (12.615–15.385) | 0.40 | 15 (13.569–16.431) | 0.001 |
| SPNM | 9 (7.303–10.697) | 16 (14.915–17.085) | 19 (17.120–20.880) | |||
DISCUSSION
Compared with the Western world, oesophageal squamous-cell cancer is more common in Asian countries. The prevalence of the squamous-cell subtype of oesophageal cancer in some regions of China and Japan is reported to be >90% [10, 11]. The 5-year survival rate after oesophageal cancer surgery increased from 15–18% in the 1980s [12] to 32–37% by 2000 [13, 14]. At present, oesophagectomy is still the first line of treatment for oesophageal cancer, but there is not yet a standard for the extent of lymph node dissection. Currently, the extent of lymph node dissection in oesophageal cancer surgery around the world can be classified into four types: standard, extended, total and three-field. Because the rates of metastasis from upper, middle and lower thoracic oesophageal cancer to the cervical nodes account for 39–47%, 26–41% and 19∼27%, respectively, many treatment centres in Japan have listed three-field lymph node dissection as a routine surgical procedure [15]. For the patients who were diagnosed with N0 by traditional pathological methods, micrometastatic carcinoma could still be found in their negative lymph nodes, indicating that it is nearly impossible to dissect all of the lymph nodes at risk of cancer metastasis using surgery only. Therefore, some European and American scholars have held the opinion that mediastinal lymph node dissection is not required at all, suggesting that oesophageal cancer should be considered a systemic disease and that excess lymph node dissection may not improve outcomes. For example, Orringer et al. [16] conducted oesophagectomy through the oesophageal hiatus without opening the chest. They supplemented the surgery with radiotherapy and chemotherapy before and after the operation. The 2-year survival rate reached 60%, and the curative effect was similar to that of extensive resection. In addition, Japanese scholars reported that three-field lymph node dissection was beneficial for the survival of patients, but the duration of the operation was long, with trauma and high operative mortality as well as more postoperative complications [5, 6]. The results of a randomized controlled trial reported by Western scholars showed that three-field lymph node dissection did not significantly improve the 5-year survival rate of oesophageal cancer but did increase some complications, such as recurrent laryngeal nerve injury and pulmonary infection, in addition to the surgical risks [17]. Therefore, the decrease in the postoperative quality of life and the forward advantages of three-field lymph node dissection cancelled each other. Li et al. [18] reported that depth of invasion was an important risk factor for lymph node metastasis. Siewert et al. [19] reported that a higher degree of differentiation in oesophageal cancer indicated a lower risk of lymphatic metastasis, and that the degree of differentiation was an independent risk factor for lymphatic metastasis in oesophageal cancer. The retrospective data of this project showed that the following factors were associated with a lower risk of lymph node metastasis: upper oesophageal cancer, high degree of differentiation, squamous-cell carcinoma, shorter tumour length, superficial invasion and some other factors. Therefore, for the patients with relatively early, local and superficial oesophageal cancer, further study should be made to determine whether routine subcarinal lymph node dissection is required.
The lymphatic drainage of the oesophagus runs in the longitudinal direction, resulting in regional, up–down bidirectional metastases and skips the metastasis of oesophageal cancer [20, 21]. Because the oesophagus lacks serosa, the local extension of the tumour is also easy to diffuse. Currently, the regional lymphatic drainage area of oesophageal cancer is not yet entirely clear. The domestic and foreign institutes usually define the area according to the site of lymph node metastasis in oesophageal patients, so there is no uniform standard for the definition of regional lymph node dissection in oesophageal cancer. The Japanese Society for Esophageal Diseases (ISDE) and Japan Society for Eating Disorders (JSED) defined the regional lymph nodes of oesophageal cancer in 1992 and 1999, respectively, covering the extent of three fields of cervical nodes, thorax and abdomen. As for the definition of subcarinal lymph nodes and paraoesophageal lymph nodes, neither the ISDE nor the JSED have given a specific boundary. According to the definition of thoracic lymph nodes proposed by the International Association for the Study of Lung Cancer (IASLC) in 2009, the upper bound of subcarinal lymph nodes (Group 7) is the carina of the trachea, and the lower bound is the connection between the upper opening of bronchus lobar (inferior sinister) and the lower opening of the right intermediate bronchus [22]. The anterior and posterior bounds of middle thoracic paraoesophageal lymph nodes and subcarinal lymph nodes have not yet been clearly defined. From the traditional view, subcarinal lymph nodes are the regional lymph nodes in oesophageal cancer [6]; thus, at oesophagectomy, subcarinal lymph node dissection has become a routine procedure. However, few studies have reported the details of subcarinal lymph node metastasis in oesophageal cancer.
The results of this study indicate that solitary subcarinal lymph node recurrence and metastasis mainly occur in the superior mediastinum and the supraclavicular lymph nodes, and when solitary paraoesophageal lymph node metastases occur, they are mainly located in the superior mediastinum, abdominal lymph nodes and liver. In this study, the difference between the recurrence and metastasis of solitary subcarinal lymph nodes and solitary paraoesophageal lymph nodes was statistically significant, and when there was subcarinal metastasis, the risk of recurrence and metastasis was higher than when there was solitary paraoesophageal lymph node metastasis. Therefore, subcarinal lymph nodes might not be considered regional lymph nodes of oesophageal lymphatic drainage.
At present, even when three-field lymph node dissection is used, the mean number of metastatic lymph nodes is as many as 59.5–82 [23], which is still far from the mean number of 118–234 lymph nodes in oesophageal cancer patients found by autopsy [20]. Positron emission tomography (PET), mediastinoscopy, endoscopic ultrasound (EUS) and endobronchial ultrasound (EBUS) have been used in the clinic, and they can provide relatively accurate information about the absence or presence of lymph node metastases [24, 25]. Therefore, for patients with oesophageal cancer, we advocate individualized and appropriate lymph node dissection, and the extent of dissection should be determined by preoperative examination, intraoperative probing and the results of frozen sections. When the surgery is relatively radical, extensive lymph node dissection should be avoided, because it can reduce local tumour defense function and immune function in patients. In this way, patients can recover from surgical trauma as soon as possible and can receive earlier multidisciplinary comprehensive treatment, which benefits their treatment and rehabilitation.
Conflict of interest: none declared.
Acknowledgements
The authors thank all of the patients included in this research.
REFERENCES
- 1.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–50. doi: 10.1200/JCO.2005.05.2308. doi:10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008.CA. Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. doi:10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Peyre CG, Hagen JA, DeMeester SR, Altorki NK, Ancona E, Griffin SM, et al. The number of lymph nodes removed predicts survival in esophageal cancer: an international study on the impact of extent of surgical resection. Ann Surg. 2008;248:549–56. doi: 10.1097/SLA.0b013e318188c474. [DOI] [PubMed] [Google Scholar]
- 4.Koenig AM, Prenzel KL, Bogoevski D, Yekebas EF, Bubenheim M, Faithova L, et al. Strong impact of micrometastatic tumor cell load in patients with esophageal carcinoma. Ann Surg Oncol. 2009;16:454–62. doi: 10.1245/s10434-008-0169-7. doi:10.1245/s10434-008-0169-7. [DOI] [PubMed] [Google Scholar]
- 5.Fujita H, Sueyoshi S, Tanaka T, Fujii T, Toh U, Mine T, et al. Optimal lymphadenectomy for squamous cell carcinoma in the thoracic esophagus: comparing the short- and long-term outcome among the four types of lymphadenectomy. World J Surg. 2003;27:571–9. doi: 10.1007/s00268-003-6913-z. doi:10.1007/s00268-003-6913-z. [DOI] [PubMed] [Google Scholar]
- 6.Tachibana M, Kinugasa S, Yoshimura H, Dhar DK, Nagasue N. Extended esophagectomy with 3-field lymph node dissection for esophageal cancer. Arch Surg. 2003;138:1383–9. doi: 10.1001/archsurg.138.12.1383. doi:10.1001/archsurg.138.12.1383. [DOI] [PubMed] [Google Scholar]
- 7.Zingg U, Smithers BM, Gotley DC, Smith G, Aly A, Clough A, et al. Factors associated with postoperative pulmonary morbidity after esophagectomy for cancer. Ann Surg Oncol. 2011;18:1460–8. doi: 10.1245/s10434-010-1474-5. doi:10.1245/s10434-010-1474-5. [DOI] [PubMed] [Google Scholar]
- 8.Japanese Society for Esophageal Diseases. Guidelines for the Clinical and Pathologic Studies on Carcinoma of the Esophagus. 9th edn. Tokyo: Kanehara Public Co; 1999. pp. 1–34. [Google Scholar]
- 9.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th edition. New York: Springer; 2009. pp. 103–15. [Google Scholar]
- 10.Isono K, Sato H, Nakayama K. Results of a nationwide study on the three-field lymph node dissection of esophageal cancer. Oncology. 1991;48:411–20. doi: 10.1159/000226971. doi:10.1159/000226971. [DOI] [PubMed] [Google Scholar]
- 11.He Z, Zhao Y, Guo C, Liu Y, Sun M, Liu F, et al. Prevalence and risk factors for esophageal squamous cell cancer and precursor lesions in Anyang, China: a population-based endoscopic survey. Br J Cancer. 2010;103:1085–8. doi: 10.1038/sj.bjc.6605843. doi:10.1038/sj.bjc.6605843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King RM, Pairolero PC, Trastek VF, Payne WS, Bernatz PE. Ivor Lewis esophagogastrectomy for carcinoma of the esophagus: early and late functional results. Ann Thorac Surg. 1987;44:119–22. doi: 10.1016/s0003-4975(10)62019-x. doi:10.1016/S0003-4975(10)62019-X. [DOI] [PubMed] [Google Scholar]
- 13.Rao YG, Pal S, Pande GK, Sahni P, Chattopadhyay TK. Transhiatal esophagectomy for benign and malignant conditions. Am J Surg. 2002;184:136–42. doi: 10.1016/s0002-9610(02)00906-6. doi:10.1016/S0002-9610(02)00906-6. [DOI] [PubMed] [Google Scholar]
- 14.Altorki N, Kent M, Ferrara C, Port J. Three-field lymph node dissection for squamous cell and adenocarcinoma of the esophagus. Ann Surg. 2002;236:177–83. doi: 10.1097/00000658-200208000-00005. doi:10.1097/00000658-200208000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ando N, Ozawa S, Kitagawa Y, Shinozawa Y, Kitajima M. Improvement in the results of surgical treatment of advanced squamous esophageal carcinoma during 15 consecutive years. Ann Surg. 2000;232:225–32. doi: 10.1097/00000658-200008000-00013. doi:10.1097/00000658-200008000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orringer MB, Forastiere AA, Perez-Tamayo C, Urba S, Takasugi BJ, Bromberg J. Chemotherapy and radiation therapy before transhiatal esophagectomy for esophageal carcinoma. Ann Thorac Surg. 1990;49:348–54. doi: 10.1016/0003-4975(90)90237-z. doi:10.1016/0003-4975(90)90237-Z. [DOI] [PubMed] [Google Scholar]
- 17.Boyle MJ, Franceschi D, Livingstone AS. Transhiatal versus transthoracic esophagectomy: complication and survival rates. Am Surg. 1999;65:1137–41. [PubMed] [Google Scholar]
- 18.Li H, Zhang Y, Cai H, Xiang J. Pattern of lymph node metastases in patients with squamous cell carcinoma of the thoracic esophagus who underwent three-field lymphadenectomy. Eur Surg Res. 2007;39:1–6. doi: 10.1159/000096925. doi:10.1159/000096925. [DOI] [PubMed] [Google Scholar]
- 19.Siewert JR, Stein HJ, Feith M, Bruecher BL, Bartels H, Fink U. Histologic tumor type is an independent prognostic parameter in esophageal cancer: lessons from more than 1,000 consecutive resections at a single center in the Western world. Ann Surg. 2001;234:360–7. doi: 10.1097/00000658-200109000-00010. doi:10.1097/00000658-200109000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma D, Thakur A, Toppo S, Chandrakar SK. Lymph node counts in Indians in relation to lymphadenectomy for carcinoma of the oesophagus and stomach. Asian J Surg. 2005;28:116–20. doi: 10.1016/S1015-9584(09)60274-8. doi:10.1016/S1015-9584(09)60274-8. [DOI] [PubMed] [Google Scholar]
- 21.Herbella FA, Del Grande JC, Colleoni R. Anatomical analysis of the mediastinal lymph nodes of normal Brazilian subjects according to the classification of the Japanese Society for Diseases of the Esophagus. Surg Today. 2003;33:249–53. doi: 10.1007/s005950300056. doi:10.1007/s005950300056. [DOI] [PubMed] [Google Scholar]
- 22.Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4:568–77. doi: 10.1097/JTO.0b013e3181a0d82e. doi:10.1097/JTO.0b013e3181a0d82e. [DOI] [PubMed] [Google Scholar]
- 23.Nishihira T, Hirayama K, Mori S. A prospective randomized trial of extended cervical and superior mediastinal lymphadenectomy for carcinoma of the thoracic esophagus. Am J Surg. 1998;175:47–51. doi: 10.1016/s0002-9610(97)00227-4. doi:10.1016/S0002-9610(97)00227-4. [DOI] [PubMed] [Google Scholar]
- 24.Maziak DE, Darling GE, Inculet RI, Gulenchyn KY, Driedger AA, Ung YC, et al. Positron emission tomography in staging early lung cancer: a randomized trial. Ann Intern Med. 2009;151:221–8. doi: 10.7326/0003-4819-151-4-200908180-00132. [DOI] [PubMed] [Google Scholar]
- 25.Hwangbo B, Kim SK, Lee HS, Lee HS, Kim MS, Lee JM, et al. Application of endobronchial ultrasound-guided transbronchial needle aspiration following integrated PET/CT in mediastinal staging of potentially operable non-small cell lung cancer. Chest. 2009;135:1280–7. doi: 10.1378/chest.08-2019. doi:10.1378/chest.08-2019. [DOI] [PubMed] [Google Scholar]