Abstract
Posterior coronary looping remains a risk factor when performing an arterial switch operation for transposition of the great arteries. In such a situation, to avoid the risk of overstretching, we use an ‘inverse’ flap technique, resulting in a tension-free relocation.
Keywords: Congenital, Great vessels, Arterial switch operation, Transposition of great arteries
INTRODUCTION
Posterior coronary looping, e.g. a single right coronary artery with a posterior left coronary artery (Yacoub Type B) and circumflex artery from a right aortic sinus (Yacoub Type D), has been identified by many as an independent risk factor for mortality in patients undergoing an arterial switch operation (ASO) for transposition of great arteries [1, 2]. These anatomical settings are at high risk for kinking and/or overstretching of single reimplanted coronary artery with the risk of ischaemic injury. Despite technical advances, these arrangements still represent a notable surgical challenge, particularly in the cases with two separate ostia opening in the right coronary sinus. Different approaches have been described to circumvent the likelihood of postoperative myocardial ischaemia after coronary transfer: large mobilization and reimplantation of the coronary button into a previously anastomosed neoaorta [3], the use of trap-door technique associated with pericardial or pulmonary artery hoods [4], in situ coronary artery relocation and tube reconstruction of the coronary artery [5]. Our recent approach to reimplantation of a posterior looping coronary artery aims at keeping the geometry of the vessel as close as possible to the native course. In essence, the coronary button is anastomosed to a J-shaped door consisting in a medially based rectangular flap created in the right antero-lateral aspect of the distal aortic stump at the end of the neoascending aorta reconstruction.
MATERIALS AND METHODS
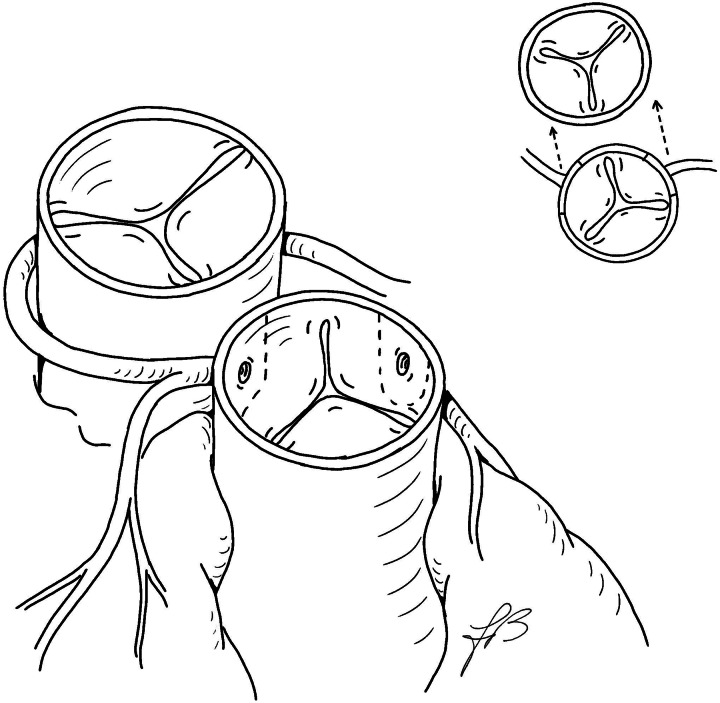
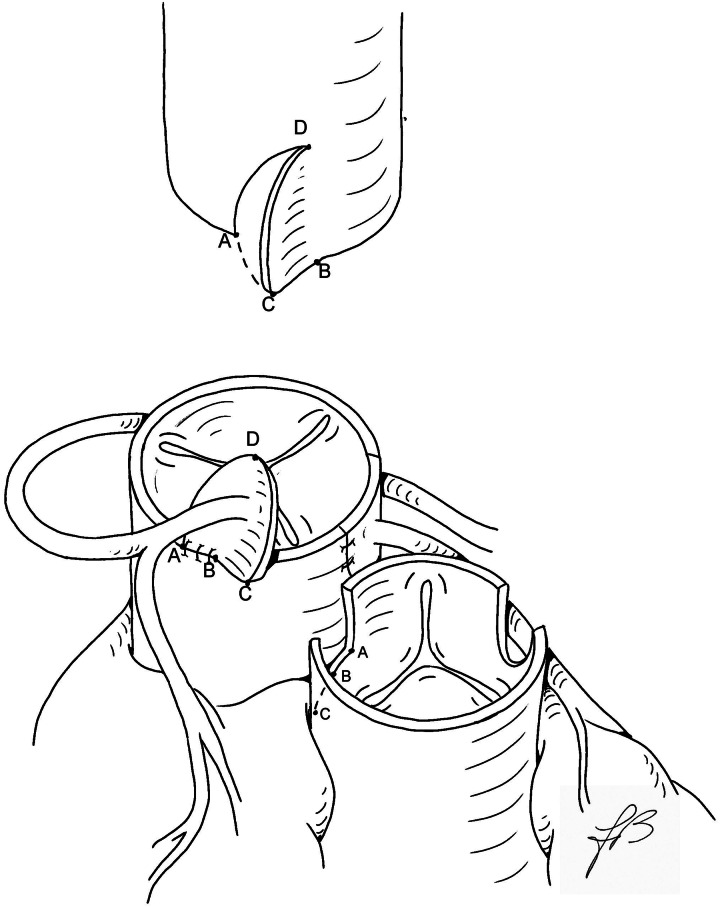
The great arteries are dissected free and extensively mobilized. Standard cardiopulmonary bypass is established with cooling down to 28°C. After dividing the patent ductus arteriosus, the pulmonary arteries are also widely mobilized. Myocardial protection is achieved by antegrade cold-blood cardioplegia. The aorta is transected 2–3 mm above the sinotubular junction. The pulmonary trunk is divided just proximal to its bifurcation, followed by the Lecompte manoeuvre. The coronary ostia are detached in the usual fashion, and the coronary arteries are generously mobilized (Fig. 1). The left coronary button is anastomosed first either a vertical or a trap-door incision on the facing aspect of the neoaortic root, just above the valve annulus. At this point, the right coronary button is transferred without any sort of rotation. The right-sided half of the lower rim of the right coronary button is anchored to the right lateral aspect of the free edge of the neoaortic root, from the posterior corner to the midpoint of the button, just underneath the single ostium or between two separate ostia (Points A to B of the right coronary button, Fig. 2). The ascending aorta is then reconstructed end-to-end, matching Points A and B of the distal aortic stump with Points A and B of the attached right coronary button, respectively, leaving a redundant segment of the free edge (B–C) with a length closely matching that of the still-unattached portion of the lower rim of the right coronary button (also B–C). At this point, starting from the lateral corner (Point A), a reverse J incision is made in the distal aorta and the remaining low free edges (B–C segments) of the composite right coronary button and the ‘inverse’ J flap are sutured together (Fig. 2). The coronary reimplantation is completed by anastomosing together the lateral free edges of the button and the aortic flap. The subsequent cardioplegic injection into the neoaortic root demonstrates the morphological adequacy of the new coronary arrangement. The pulmonary trunk is repaired with a large U-shaped patch of autologous pericardium, with or without a short split in the lower posterior edge to accommodate the native intercoronary commissure. The remainder of the operation is conducted in the usual manner.
Figure 1:
Anatomical pattern with coronary buttons cutting lines. Planned coronary translocation is shown in the upper insert.
Figure 2:
Technique of operation. The left button is anastomosed in the usual manner, and the right button is implanted in the upper border of the neoaorta (see text). The distal aorta with an inverse J-shaped incision is drawn on the top.
From November 2009, 5 babies admitted for a prenatally diagnosed transposition of great arteries with intact ventricular septum were found to have a posterior coronary looping. A balloon atrial septostomy was performed soon after birth in all cases. The mean age was 15 (12–21 days) and mean body weight was 3.1 (2.8–3.5 kg). In 2 patients, a single coronary ostium arose from the right sinus, and in the other 3 cases, the pattern was that of a typical circumflex artery arising from the right sinus. All 5 patients successfully underwent an ASO by the described technique.
RESULTS
The postoperative course was uneventful in all, without any evidence of coronary artery distortion or myocardial ischaemia. All patients were discharged from the hospital. During the follow-up, all patients continued to do well, except one that needed a reoperation for severe dilatation of the pulmonary trunk, secondary to right pulmonary artery stenosis.
DISCUSSION
Posterior coronary looping may still, though occasionally, represent a technical challenge during an ASO for transposition of the great arteries. This holds particularly true in the cases with double-barrelled coronary ostia.
Compared with other techniques, the described ‘inverse trap-door’ approach to reimplantation of the right coronary button is the most respectful of the original courses of the involved bifurcating coronary branches, i.e. the right coronary artery and either the entire left or an isolated circumflex coronary artery. The principles of this coronary transfer include: (i) extensive mobilization of the coronary arteries to minimize the risk of kinking or stretching; (ii) high implantation of the composite coronary button above the neoaortic root; (iii) minimal shift of the ostium/ostia from the original position; (iv) nearly perfect alignment of the coronary branches that essentially keep their native position.
In addition, the avoidance of prosthetic material to create the aortic flap preserves the growth potential of the arterial tissue.
In conclusion, the inverse trap-door technique can be a valid option to achieve successful repair for patients with transposition and posterior looping coronary artery anatomy, despite the fact that long-term outcomes still remain to be assessed.
Conflict of interest: none declared.
REFERENCES
- 1.Serraf A, Lacour-Gayet F, Bruniaux J, Touchot A, Losay J, Comas J, et al. Anatomic correction of transposition of the great arteries in neonates. J Am Coll Cardiol. 1993;22:193–200. doi: 10.1016/0735-1097(93)90834-n. doi:10.1016/0735-1097(93)90834-N. [DOI] [PubMed] [Google Scholar]
- 2.Daebritz SH, Nollert G, Sachweh JS, Engelhardt W, von BG, Messmer BJ. Anatomical risk factors for mortality and cardiac morbidity after arterial switch operation. Ann Thorac Surg. 2000;69:1880–6. doi: 10.1016/s0003-4975(00)01241-8. doi:10.1016/S0003-4975(00)01241-8. [DOI] [PubMed] [Google Scholar]
- 3.Qamar ZA, Goldberg CS, Devaney EJ, Bove EL, Ohye RG. Current risk factors and outcomes for the arterial switch operation. Ann Thorac Surg. 2007;84:871–8. doi: 10.1016/j.athoracsur.2007.04.102. doi:10.1016/j.athoracsur.2007.04.102. [DOI] [PubMed] [Google Scholar]
- 4.Parry AJ, Thurm M, Hanley FL. The use of 'pericardial hoods' for maintaining exact coronary artery geometry in the arterial switch operation with complex coronary anatomy. Eur J Cardiothorac Surg. 1999;15:159–64. doi: 10.1016/s1010-7940(98)00314-5. doi:10.1016/S1010-7940(98)00314-5. [DOI] [PubMed] [Google Scholar]
- 5.Scheule AM, Zurakowski D, Blume ED, Stamm C, del Nido PJ, Mayer JE, Jr, et al. Arterial switch operation with a single coronary artery. J Thorac Cardiovasc Surg. 2002;123:1164–72. doi: 10.1067/mtc.2002.118047. doi:10.1067/mtc.2002.118047. [DOI] [PubMed] [Google Scholar]