Abstract
OBJECTIVES
Although pulmonary valve-sparing repair is preferable for patients with tetralogy of Fallot, the repair of very small pulmonary valves is challenging. The present study evaluates our modification for preserving severely hypoplastic pulmonary valves in patients with tetralogy of Fallot.
METHODS
Sixty-eight consecutive patients who underwent complete repair of a tetralogy of Fallot between 2005 and 2011 were retrospectively reviewed. Patients with pulmonary atresia, absence of a pulmonary valve, atrioventricular septal defect and/or subarterial ventricular septal defect were excluded. There were 19 (28%) patients with a severely hypoplastic pulmonary annulus determined by preoperative echocardiography (z-score <−4). For these patients, we collected echocardiographic data and information about their postoperative course.
RESULTS
Valve preserving was successful in 11 of 19 (58%) of the z < −4 group, compared with 48 of 49 (98%) of the z > −4 group. In the z < −4 valve-sparing subgroup (n = 11), the preoperative pulmonary valve diameter z-score was −4.9 (range −6.3 to −4.3), and an approach involving ventriculotomy with no transannular patch was employed at a mean age of 6.9 (range 2.2–16.1) months. In this subgroup, residual right ventricular outflow tract velocity was 2.4 ± 0.6 m/s at discharge from the hospital. During a mean follow-up of 2.6 ± 2.4 years, no reintervention was necessary. Late right ventricular outflow tract velocity was 2.2 ± 0.6 m/s, and there was no severe pulmonary regurgitation. The pulmonary valve annulus grew in relation to the patient's body surface area (z = −0.51, range −4.2–0.24) without any aneurysmal changes in the right ventricular outflow tract.
CONCLUSIONS
Although our modification of valve-sparing repair for severely hypoplastic pulmonary valves in patients with tetralogy of Fallot could not be applied in all patients, this strategy enabled acceptable growth of the valve annulus, with only mild stenosis during the early to mid-term follow-up. This modification seems to be an option, even for a very small pulmonary valve.
Keywords: Tetralogy of Fallot, Pulmonary stenosis, Valve-sparing repair, Right ventricular outflow tract obstruction
INTRODUCTION
Surgical repair has progressed over the past 50 years since the initial successful repair of tetralogy of Fallot (TOF) [1]. Early repairs closed ventricular septal defects via a large right ventriculotomy, and right ventricular outflow tract (RVOT) obstruction was relieved using a transannular patch (TAP) [2, 3]. Although this strategy resulted in low short-term mortality and morbidity, the principal issue for patients with repaired TOF has shifted to long-term outcomes. Repair using a TAP provides reasonable or complete relief of obstruction, but such relief is associated with the potential for severe pulmonary regurgitation. Several studies have identified pulmonary regurgitation as a risk factor for late right ventricular dilatation [4], arrhythmia [5] and sudden death [6]. Over the past 20 years, preservation of the native pulmonary valve function has been considered desirable, even at the cost of some level of residual stenosis [7]. Although aggressive preservation of the pulmonary valve, which requires right ventriculotomy when the pulmonary valve is hypoplastic, is preferable [8–10], some institutions try to avoid right ventriculotomy to preserve the valve, because their focus is on the right ventricular function itself and on arrhythmogenesis [11, 12]. Therefore, valve-preserving repair for a severely hypoplastic pulmonary valve has been controversial.
We have directed our TOF strategy towards preserving the native pulmonary valve function by aggressively avoiding a TAP. The aim was to alleviate late complications of the right ventricular function caused by pulmonary regurgitation and to avoid frequent late intervention for an impaired pulmonary valve. The application of this strategy has been gradually extended to preserving even severely hypoplastic pulmonary valves. The present study evaluates our modification of valve-sparing repair for severely hypoplastic pulmonary valves in patients with TOF.
MATERIALS AND METHODS
A retrospective chart review was undertaken to identify all patients undergoing repair of TOF. Between March 2005 and September 2011, 68 consecutive patients with TOF underwent complete repair at the Mt. Fuji Shizuoka Children's Hospital, Japan. TOF with pulmonary atresia, an absent pulmonary valve, an atrioventricular septal defect and subarterial ventricular septal defects were excluded.
The surgical approach to valve-sparing repair was as follows: a no-ventriculotomy approach was used in 44% of cases (30 of 68); a ventriculotomy with the no-TAP approach was used in 43% (29 of 68) and a ventriculotomy with the TAP approach was used in 13% (9 of 68).
The pulmonary valve annulus diameter (PVD) was measured between the hinge points of the pulmonary valve in the longitudinal view during the systolic phase using transthoracic echocardiography immediately before surgery. All patients had a detailed clinical and echocardiographic assessment before discharge from the hospital, and 6, 12 and 36 months later.
Of the total of 68 patients, there were 19 (28%) with a severely hypoplastic pulmonary annulus, defined as a preoperative echocardiographic z-score <−4. For these patients, we collected echocardiographic data, including RVOT velocity, PVD and pulmonary regurgitation, and information about their postoperative course. Pulmonary regurgitation was graded on a scale of 0–3: 0 = none; 0.5 = trivial; 1 = mild; 2 = moderate and 3 = severe.
Surgical technique
The standard technique of cardiopulmonary bypass with bicaval cannulation, full flow and moderate hypothermia up to 28°C was applied. Myocardial protection was provided by crystalloid antegrade cardioplegia. The ventricular septal defect was initially closed using a 0.4-mm expanded polytetrafluoroethylene (ePTFE) patch and continuous 6-0 or 7-0 polypropylene sutures through a right atriotomy. RVOT muscle bands were resected through the tricuspid valve as far as possible. A longitudinal pulmonary arteriotomy was performed. Basically, TOF has a bicuspid pulmonary valve, and incising two sinuses can completely relieve supravalvular stenosis. Unless, the main pulmonary artery was well developed, a longitudinal arteriotomy was made in the main pulmonary artery into both sides of the sinuses with an antero-posterior oriented commissure. When bicuspid valve commissures were arranged horizontally, namely without an anterior commissure, a longitudinal incision was made from a main pulmonary artery into the anterior sinus. This type of pulmonary valve has a narrowed sinotubular junction of the posterior sinus. The posterior ridge at the sinotubular junction was incised longitudinally for the full thickness of the pulmonary artery wall and resutured horizontally using interrupted 8-0 polypropylene sutures (Z-plasty of the posterior ridge). We applied the commissurotomy with a small release of tethering to preserve the leaflet structure, while ensuring that the leaflets remained attached to the pulmonary artery wall. We then sliced the sub-leaflet muscle, including fibrous tissue, through this enlarged orifice of the pulmonary valve. Patients with a very small pulmonary valve annulus have leaflets that are buried in the RVOT muscles. This slicing of sub-leaflet tissues allowed excavation and elongation of the leaflets and provided sufficient motion (freeing of leaflets). When such slicing was inadequate, more aggressive and vertical slicing proceeded through an additional small longitudinal infundibular incision (about 10 mm in length) placed as close to the annulus as possible (Fig. 1). Hegar's dilators were inserted to aim for the normal pulmonary annular size predicted by the modified Rowlatt chart [13]. If 100% of the normal size was not exceeded after all these procedures, we applied an additional aggressive commissurotomy up to the normal size of a pulmonary valve (Fig. 2). Although we did not apply a transannular incision, this additional subcommissural vertical incision may divide valve ring tissue. Some cases have poor extensibility of the pulmonary valve ring, even if the ring tissue is cut. When pulmonary valve leaflets were not injured and a normally sized dilator could be passed after all these procedures, we adopted valve-sparing repair for these patients with a severely hypoplastic pulmonary valve. The pulmonary arteriotomy was augmented using an autologous pericardial patch. When a Y-shaped incision was performed, we augmented an arteriotomy with a Y-shaped pericardial patch. The infundibular incision was closed with an ePTFE patch augmentation. When we could not achieve valve-sparing repair, a transannular incision was made and the pulmonary valve was reconstructed using a transannular ePTFE monocusp patch. We consider that it is acceptable for the RVOT velocity in operation room to be <3.0 m/s using transoesophageal echocardiography. If a cardiologist detects supravalvular stenosis, it must be repaired, but if the gradient is centred on the valve or is subvalvular, we consider that it is acceptable.
Figure 1:
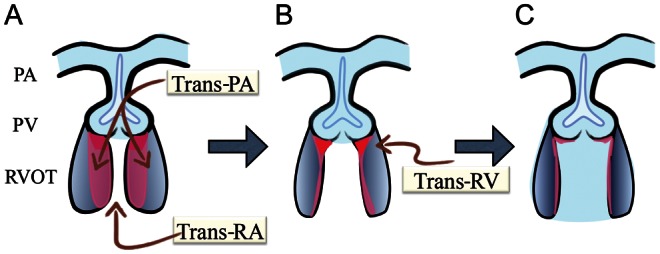
Freeing of leaflets. (A) A trans-right atrium/trans-pulmonary artery approach allows cutting muscle band and excision of subvalvular fibrous/muscular tissue. (B) Tissues remain just beneath the leaflets (red-coloured zone). An additional trans-right ventriculotomy (about 10 mm longitudinal incision placed as close to the annulus as possible) is prepared if necessary. (C) Slicing fibrous/muscular tissue just beneath the leaflets vertically using scissors to obtain sufficient leaflet motion. PA: pulmonary artery; PV: pulmonary valve; RA: right atrium; RV: right ventricle; RVOT: right ventricular outflow tract.
Figure 2:
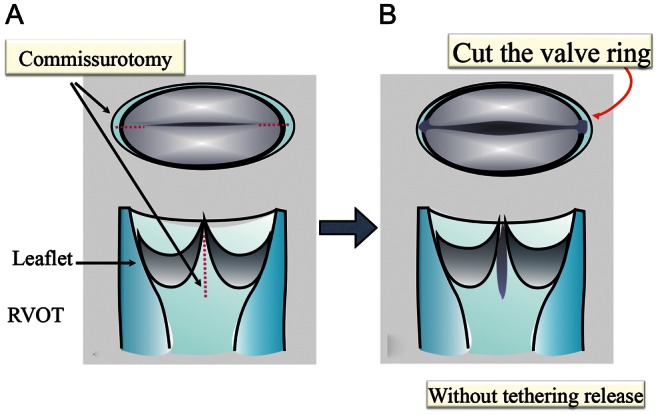
Aggressive additional commissurotomy. (A) Complete longitudinal commissurotomy (dotted line) with small tethering release to maintain leaflet structure. (B) Preserving tethering, this incision is continued to the valve ring tissue and sometimes over the valve ring tissue if needed. RVOT: right ventricular outflow tract.
Statistical analysis
All data are presented as counts and ratios (%), median values with ranges or means ± standard deviation. All tests were two-sided and P-values <0.05 were considered significant. Specific parameters over time were compared using analysis of variance, continuous variables were compared using an unpaired t-test and categorical data were compared using Fisher's exact test. GraphPad Prism 5.0b for Macintosh (GraphPad Software, San Diego, CA, USA) was used for the data analysis.
RESULTS
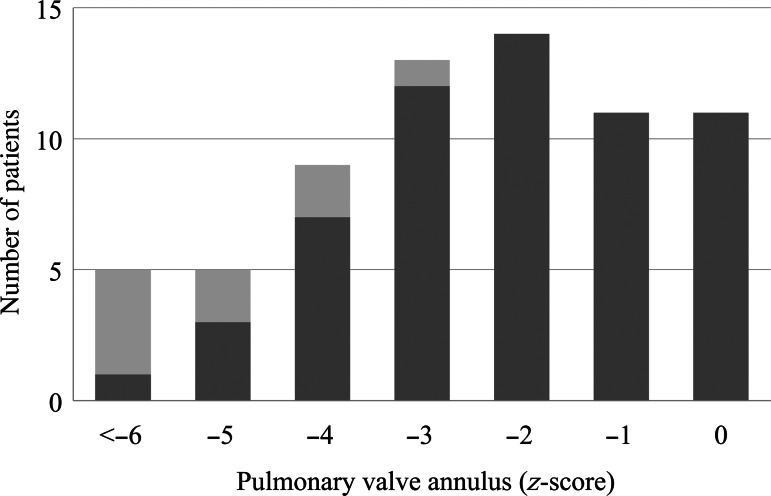
Overall, pulmonary valve-sparing was achieved in 59 of the 68(87%) patients and in 48 of the 49 (98%) patients with z-scores >−4. Figure 3 shows the distribution of the patients with the valve-sparing repair, based on the preoperative PVD z-score. One TAP repair patient had a small pulmonary annulus (z = −3.9) with a severely dysplastic pulmonary valve.
Figure 3:
Distribution of valve-sparing (black bars) and transannular patch repairs (grey bars) based on the pulmonary valve annulus z-score.
Patients with hypoplastic pulmonary valves
Nineteen (28%) patients had preoperative z-scores <−4. A preparative shunt procedure was associated with more TAP repair in these patients (P = 0.018).
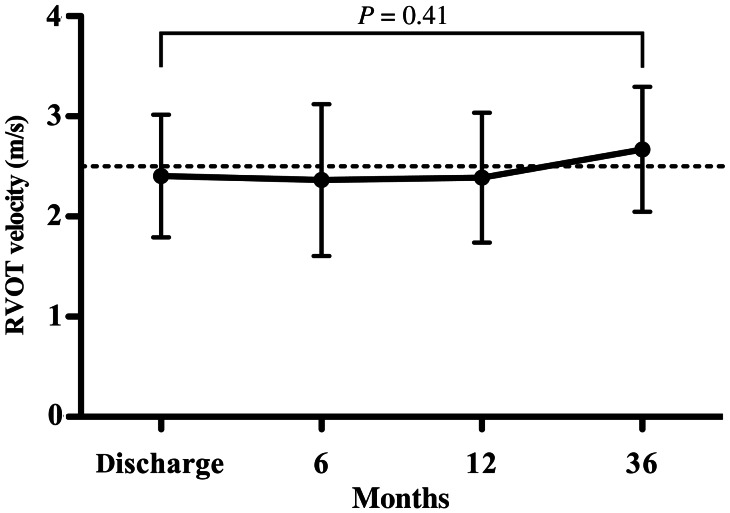
Valve-sparing repair was achieved in 11 of the 19 patients, using the surgical approach of ventriculotomy with no TAP. We compared the outcomes, including growth of the PVD, RVOT velocity and pulmonary regurgitation, in the valve-sparing z < −4 group (n = 11) and the z < −4 TAP group (n = 8). Tables 1 and 2 summarize the data for these two subgroups. The mean follow-up for the entire group was 2.6 ± 2.4 years and there was no mortality. No reoperation was required in the z < −4 valve-sparing group, whereas one intervention was needed in the TAP group for RVOT obstruction. No patient developed cardiac arrhythmia or required pacemaker implantation. The postoperative RVOT velocity at discharge was greater in the valve-sparing z < −4 group than in the TAP repair group (2.4 ± 0.6 vs 1.6 ± 0.8 m/s, P = 0.044). However, the RVOT velocity did not change in the valve-sparing group during the follow-up (Fig. 4). Postoperative PVD at discharge was smaller in the z < −4 valve-sparing group (z = −3.1; range −4.8–0.24) than in the TAP group (z = −0.53; range −2.7–0.75; P = 0.019).
Table 1:
Preoperative data for patients with a small pulmonary valve (echocardiographic z < −4) who underwent either valve-sparing or transannular patch (TAP) surgery
| Valve sparing (n = 11) | TAP (n = 8) | P-value | |
|---|---|---|---|
| Age (months) | 6.9 (2.2 to 16.1) | 8.7 (1.0 to 13) | 0.65 |
| Body weight (kg) | 6.9 (4.6 to 9.2) | 6.4 (3.6 to 8.9) | 0.68 |
| PVD (mm) | 5.7 (4.9 to 6.7) | 5.1 (3.3 to 6.8) | 0.19 |
| PVD (z-score) | −4.9 (−6.3 to −4.3) | −6.1 (−7.9 to −4.3) | 0.04 |
| Bicuspid pulmonary valve | 10 (90%) | 9 (100%) | 0.55 |
| Prior shunt | 0 | 4 | 0.02 |
PVD: pulmonary valve annulus diameter.
Table 2:
Postoperative results from patients with a small pulmonary valve (echocardiographic z < −4) who underwent either valve-sparing or transannular patch (TAP) surgery.
| Valve sparing (n = 11) | TAP (n = 8) | P-value | |
|---|---|---|---|
| Postoperative RVOT velocity (m/s) | 2.4 ± 0.6 | 1.7 ± 0.8 | 0.09 |
| Postoperative PR (0–3) | 1.3 ± 0.6 | 1.6 ± 0.4 | 0.25 |
| Postoperative PVD (z-score) | −3.1 (−4.8 to 0.24) | −0.73 (−2.65 to 0.75) | 0.03 |
| Late right ventricular outflow tract velocity (m/s) | 2.2 ± 0.6 | 2.3 ± 0.7 | 0.74 |
| Late PR (0–3) | 1.6 ± 0.5 | 1.8 ± 0.6 | 0.51 |
| Late PVD (z-score) | −0.32 (−2.7 to 0.24) | −2.5 (−5.7 to 1.58) | 0.08 |
| Aneurysm of right ventricular outflow tract | 0 | 0 | NA |
| Re-right ventricular outflow tract repair | 0 | 1 | NA |
PR: pulmonary valve regurgitation (0, none; 0.5, trivial; 1, mild; 2, moderate; 3, severe); PVD: pulmonary valve annulus diameter.
Figure 4:
Right ventricular outflow tract (RVOT) velocity in children with pulmonary valve preservation, whose preoperative pulmonary valve annulus was below z = −4 (n = 11). The RVOT velocity remained unchanged during the follow-up.
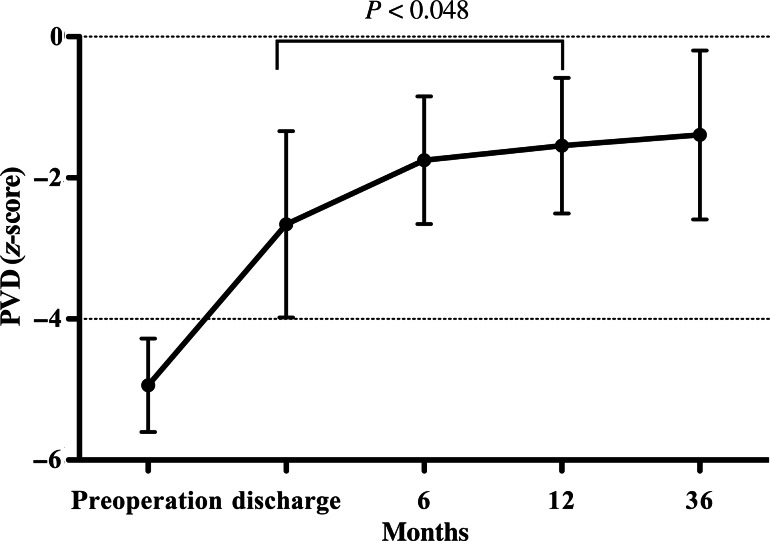
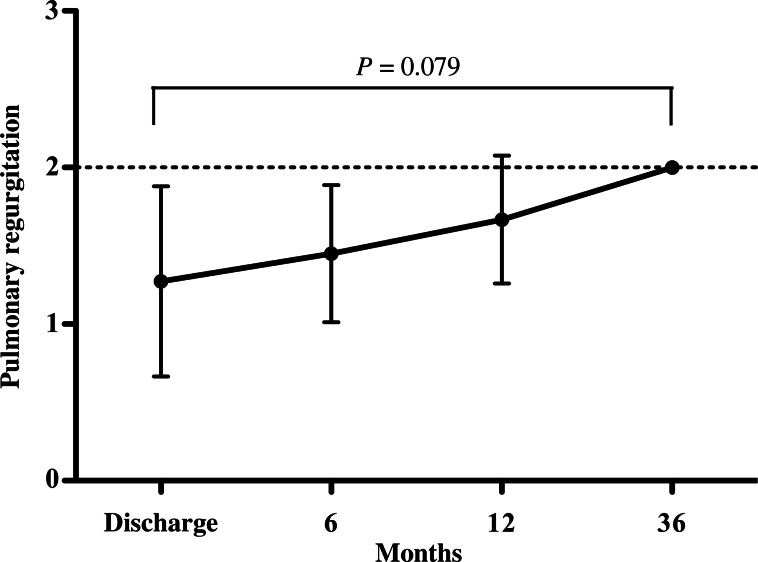
The small PVD in the z < −4 valve-sparing group increased significantly during the follow-up in relation to the patients' body surface area (Fig. 5), without any aneurysmal changes being noted in the RVOT. Postoperative pulmonary regurgitation at discharge and later did not differ significantly between the two subgroups. Although severe pulmonary regurgitation was observed in 3 patients with TAP repair, none was evident in patients with preserved pulmonary valves (P = 0.073). The grade of pulmonary regurgitation in the z < −4 valve-sparing group gradually increased during the follow-up, but not to a statistically significant degree (Fig. 6).
Figure 5:
Children with a preserved pulmonary valve, whose pulmonary valve annulus diameter (PVD) z-score before surgery was below z = −4 (n = 11). During the follow-up, PVD grew in relation to the patient's body surface area without aneurysms developing in the RVOT.
Figure 6:
Grade of pulmonary regurgitation (PR) in children with the preserved pulmonary valve, whose pulmonary valve annulus before surgery was below z = −4 (n = 11). The grade of PR increased gradually during the follow-up, but no severe PR was related to the divided pulmonary valve annulus and no right ventricular dilatation occurred.
DISCUSSION
Pulmonary valve insufficiency has been recognized as a risk factor for functional deterioration of both right ventricular and left ventricular performance after the repair of TOF. TAP repair can relieve RVOT obstruction sufficiently, resulting in good early mortality and morbidity and limiting the need for reoperation. However, the disrupted and enlarged annulus leaves the pulmonary valve prone to severe regurgitation. Several studies have shown that residual pulmonary regurgitation is closely associated with TAP, and that the regurgitation results in significant right ventricular dilatation and dysfunction [4, 14]. While the avoidance of TAP repair is preferable in patients with TOF, pulmonary valve-preserving repair in patients with severely hypoplastic pulmonary valves has been challenging and controversial.
We endeavour to avoid TAP repair, even in patients with severely hypoplastic pulmonary valves (z < −4). This study shows that our strategy was able to preserve the valves in 11 of the 19 such patients. We have extended the application of the pulmonary valve-preserving technique to z = −6 using these modifications. Rao et al. [9] reported that they were able to preserve the pulmonary valve down to z = −6, and that the pulmonary valve-preserving ratio reached 90% of all patients with TOF. Although they had no reoperation for RVOT in the early phase, 5 children (5 of 27, 18%) with preserved pulmonary valves showed severe regurgitation within a year. This suggests that valve preserving for severely hypoplastic pulmonary valves may not be preferable to TAP repair. However, our study indicated that the early to mid-term outcomes in the valve-sparing z < −4 group and the TAP repair group were comparable. Furthermore, though there was mild pulmonary stenosis in the valve-sparing z < −4 group with the growth of the pulmonary valve annulus, no severe pulmonary regurgitation was observed. Although several prior studies have found that the early reoperation rate for RVOT obstruction tends to be higher after valve sparing than after TAP repair [10, 15, 16], no reoperation for RVOT was needed in this group in our study.
Our strategy for valve-sparing repair consists of the complete resection of the RVOT muscle band via the right atrium and pulmonary valve orifice; the complete release of supravalvular stenosis (including Z-plasty of the posterior ridge); freeing of the leaflets to gain better leaflet motion by slicing the sub-leaflet muscle, including fibrous tissues in the pulmonary artery; a complete commissurotomy without extreme release of tethering to maintain leaflet structure and more aggressive vertical slicing of muscles and fibrous tissues just beneath the leaflets through an additional small infundibular longitudinal incision just beneath the annulus, if necessary. Finally, the RVOT is closed with an ePTFE patch and the main pulmonary artery is augmented using an autologous Y-shaped pericardial patch when a Y-incision is performed. The complete relief of supravalvular, subvalvular and valvular stenosis could render early reoperation for RVOT obstruction unnecessary. When a pulmonary valve annulus is smaller than z < −4, we might divide the fibrous continuity of the pulmonary annulus at the time of aggressive additional commissurotomy. d'Udekem et al. [17] indicated that both RVOT patches and TAP, which might bring about aneurysmal dilatation in the RVOT, are responsible for a similar degree of long-term pulmonary insufficiency and right ventricular dilatation. However, we did not notice any aneurysmal changes in the RVOT with mild pulmonary stenosis, nor was any severe pulmonary regurgitation due to the ruptured fibrous continuity of the annulus and the RVOT patch observed during the follow-up.
Patients with an extremely small pulmonary valve annulus may also have a dysplastic pulmonary valve that is thick and short. This study indicated the possibility that these dysplastic pulmonary leaflets themselves, when preserved, tend to grow along with the annular growth, resulting in mild pulmonary stenosis but no severe regurgitation on regular echocardiographic examinations. However, the number of patients in this study is small and the follow-up period was relatively short, while no detailed observations of these leaflets have yet been made. If these spared leaflets could indeed grow, and regurgitation were caused only by valvular dilatation, then this type of pulmonary valve repair might avoid the need for pulmonary valve replacement, as well as reducing the need for repeated lifelong interventions for RVOT.
Aggressive preservation of the pulmonary valve leaflets requires right ventriculotomy when the pulmonary valve annulus is hypoplastic. Some institutions try to avoid right ventriculotomy to preserve the valve, because one of their focuses is arrhythmogenesis in the long term [12]. We performed a small longitudinal ventriculotomy (about 10 mm in length), which is augmented using an ePTFE patch. This infundibular longitudinal incision was placed as close to the annulus as possible, allowing us to slice sub-leaflet tissue to achieve better leaflet motion. When the commissure was on the anterior side of the annulus, we extended the infundibular incision as far as the fibrous triangle between the hinge points of the leaflets. This adjacent incision involved not only slicing the muscle and fibrous tissue just beneath the leaflets, but also leaving as small a width of isthmus as possible. By connecting the incisions close to the annulus, incisional re-entrant tachycardia may be prevented [18].
Palliative shunt
In terms of staged repair, we perform a palliative procedure for symptomatic neonates, early infants or patients who have a small left ventricular volume calculated from catheterization. In our cohort, a shunt was significantly more likely (P = 0.018) to lead to TAP repair in the z < −4 group. Several authors have reported pulmonary valve growth after a shunt procedure; however, precisely how shunting results in pulmonary valve growth remains unknown [10, 19]. When the pulmonary valve is embedded in the muscle, the echo annulus z-score can be underestimated, especially when there is no volume overload. This might indicate that the volume overload associated with the shunt procedure apparently increases the size of the pulmonary valve annulus in some patients. These patients who have ‘growth potential’ after a shunt might be able to preserve their pulmonary valves. On the other hand, a few patients could have truly severe hypoplastic pulmonary valve annulus with poor extensibility. Notably, patients who still have an extremely small annulus (z < −4) after the shunt procedure might not preserve the native pulmonary valve.
Limitations
In this study, 11 patients with a pulmonary valve annulus z-score <4 were evaluated in order to assess the safety and feasibility of our new technical modification. The limitations are that these are short- to mid-term results from a very small group. Furthermore, a comparison between the valve-sparing group and the TAP group is statistically not meaningful, since the surgical decision regarding the procedure introduces a heavy bias. The long-term benefits of these approaches need to be elucidated by the study of a larger number of patients.
In conclusion, although our strategy was not successful in all patients with TOF and a severely hypoplastic pulmonary valve, it enabled acceptable growth with mild pulmonary stenosis during the early to mid-term follow-up. This modification seems to be an option, even for patients with a very small pulmonary valve.
Conflict of interest: none declared.
APPENDIX. CONFERENCE DISCUSSION
Dr M. Siepe (Freiburg, Germany): May I ask you, what gradients do you accept after your repair in the operation room?
Dr Ito: Firstly, in the OR, we do not check the direct right ventricular pressure at all. We check the transoesophageal echocardiography in all the patients if possible, and we estimate the RVOT velocity, including tricuspid valve regurgitation velocity if possible. If the velocity is less than 3 metres per second, we consider it is acceptable in this condition. If supravalvular stenosis is detected, we must repair that. But if the gradient is located at valvular or subvalvular level, we consider that acceptable.
Dr Siepe: Okay.
Dr Ito: During follow-up, below 3 metres per second is also acceptable I think.
Dr G. Stellin (Padova, Italy): We have applied similar concepts in our institution for three years and, similarly to you, we reconstruct the pulmonary valve. However, to get rid of the gradient for the annular hypoplasia, we do apply balloon dilatation to the pulmonary valve. And we have pushed our new treatment to a Z-score as low as minus 5. We are very happy with our results of pulmonary valve dilatation, and further reconstruction, obviously, of the valve is similar to what you are doing. We also found that the gradient across the valve is similar to the gradient found in patients whom I have operated upon in the same era with a transannular patch. The pulmonary valve incompetence is minimal when compared to the transannular patch group. I wonder whether some of your patients maybe could benefit from a balloon dilatation?
Dr Ito: Balloon dilatation or cutting the valve ring tissue?
Dr Stellin: You have four to five patients in whom you have cut through the annulus.
Dr Ito: Yes, we cut the valve.
Dr Stellin: So what kind of Z-score did those patients have? I wonder whether those patients would have benefit from a dilatation of the pulmonary annulus rather than cutting through the annulus itself.
Dr Ito: We can preserve the pulmonary valve leaflet without a rupture. Using the balloon dilatation makes a rupture of the pulmonary valve leaflet. But we precisely cut the ring, and we have no experience of ruptured pulmonary valve leaflet in our series.
Dr K. Sakamoto (Shizuoka City, Japan): I am a coauthor of this article. You are right. Balloon dilatation may be acceptable; however, in this technique, we completely cut the pulmonary annulus ring. And after that, it is easy to avoid fracture of the leaflet in dilating the valve by balloon or other methods. So I think our technique is a leaflet-sparing approach without fracture of the leaflet. We intentionally cut into the subvalvular fibrous ring. That is our philosophy.
Dr Stellin: Similar to you, we first perform a valve commissurotomy. At the time of the balloon dilatation, the annulus will fracture at that level. This is followed by what we call a ‘delamination’ of the leaflets from the pulmonary artery wall to reconstruct the commissure. Indeed the radial force of balloon dilatation is very much different from a diagonal force that you would apply with a Hegar dilator.
REFERENCES
- 1.Lillehei CW, Cohen M, Warden HE, Read RC, Aust JB, Dewall RA, et al. Direct vision intracardiac surgical correction of the tetralogy of Fallot, pentalogy of Fallot, and pulmonary atresia defects; report of first ten cases. Ann Surg. 1955;142:418–42. doi: 10.1097/00000658-195509000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gott VL. C. Walton Lillehei and total correction of tetralogy of Fallot. Ann Thorac Surg. 1990;49:328–32. doi: 10.1016/0003-4975(90)90167-5. [DOI] [PubMed] [Google Scholar]
- 3.Kirklin JW, Blackstone EH, Pacifico AD, Kirklin JK, Bargeron LM., Jr Risk factors for early and late failure after repair of tetralogy of Fallot, and their neutralization. Thorac Cardiovasc Surg. 1984;32:208–14. doi: 10.1055/s-2007-1023386. [DOI] [PubMed] [Google Scholar]
- 4.Frigiola A, Redington AN, Cullen S, Vogel M. Pulmonary regurgitation is an important determinant of right ventricular contractile dysfunction in patients with surgically repaired tetralogy of Fallot. Circulation. 2004;110:II153–7. doi: 10.1161/01.CIR.0000138397.60956.c2. [DOI] [PubMed] [Google Scholar]
- 5.Harrison DA, Siu SC, Hussain F, MacLoghlin CJ, Webb GD, Harris L. Sustained atrial arrhythmias in adults late after repair of tetralogy of Fallot. Am J Cardiol. 2001;87:584–8. doi: 10.1016/s0002-9149(00)01435-1. [DOI] [PubMed] [Google Scholar]
- 6.Gatzoulis MA, Balaji S, Webber SA, Siu SC, Hokanson JS, Poile C, et al. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet. 2000;356:975–81. doi: 10.1016/S0140-6736(00)02714-8. [DOI] [PubMed] [Google Scholar]
- 7.Apitz C, Webb GD, Redington AN. Tetralogy of Fallot. Lancet. 2009;374:1462–71. doi: 10.1016/S0140-6736(09)60657-7. [DOI] [PubMed] [Google Scholar]
- 8.Yasui H, Nakamura Y, Kado H, Yonenaga K, Aso T, Sunagawa H, et al. Preservation of the pulmonary valve during intracardiac repair of tetralogy of Fallot. J Cardiovasc Surg (Torino) 1992;33:545–53. [PubMed] [Google Scholar]
- 9.Rao V, Kadletz M, Hornberger LK, Freedom RM, Black MD. Preservation of the pulmonary valve complex in tetralogy of Fallot: how small is too small? Ann Thorac Surg. 2000;69:176–9. doi: 10.1016/s0003-4975(99)01152-2. discussion 179–80. [DOI] [PubMed] [Google Scholar]
- 10.Stewart RD, Backer CL, Young L, Mavroudis C. Tetralogy of Fallot: results of a pulmonary valve-sparing strategy. Ann Thorac Surg. 2005;80:1431–8. doi: 10.1016/j.athoracsur.2005.04.016. discussion 1438–9. [DOI] [PubMed] [Google Scholar]
- 11.Karl TR, Sano S, Pornviliwan S, Mee RB. Tetralogy of Fallot: favorable outcome of nonneonatal transatrial, transpulmonary repair. Ann Thorac Surg. 1992;54:903–7. doi: 10.1016/0003-4975(92)90646-l. [DOI] [PubMed] [Google Scholar]
- 12.Morales DL, Zafar F, Heinle JS, Ocampo EC, Kim JJ, Relyea K, et al. Right ventricular infundibulum sparing (RVIS) tetralogy of Fallot repair: a review of over 300 patients. Ann Surg. 2009;250:611–7. doi: 10.1097/SLA.0b013e3181b79958. [DOI] [PubMed] [Google Scholar]
- 13.Rowlatt UF, Rimoldi HJA, Lev M. The quantitative anatomy of the normal child's heart. Pediatr Clin North Am. 1963;10:499–588. [Google Scholar]
- 14.Roest AA, Helbing WA, Kunz P, van den Aardweg JG, Lamb HJ, Vliegen HW, et al. Exercise MR imaging in the assessment of pulmonary regurgitation and biventricular function in patients after tetralogy of Fallot repair. Radiology. 2002;223:204–11. doi: 10.1148/radiol.2231010924. [DOI] [PubMed] [Google Scholar]
- 15.Robinson JD, Rathod RH, Brown DW, Del Nido PJ, Lock JE, McElhinney DB, et al. The evolving role of intraoperative balloon pulmonary valvuloplasty in valve-sparing repair of tetralogy of Fallot. J Thorac Cardiovasc Surg. 2011;142:1367–73. doi: 10.1016/j.jtcvs.2011.02.047. [DOI] [PubMed] [Google Scholar]
- 16.Alexiou C, Chen Q, Galogavrou M, Gnanapragasam J, Salmon AP, Keeton BR, et al. Repair of tetralogy of Fallot in infancy with a transventricular or a transatrial approach. Eur J Cardiothorac Surg. 2002;22:174–83. doi: 10.1016/s1010-7940(02)00295-6. [DOI] [PubMed] [Google Scholar]
- 17.d'Udekem Y, Ovaert C, Grandjean F, Gerin V, Cailteux M, Shango-Lody P, et al. Tetralogy of Fallot: transannular and right ventricular patching equally affect late functional status. Circulation. 2000;102:III116–22. doi: 10.1161/01.cir.102.suppl_3.iii-116. [DOI] [PubMed] [Google Scholar]
- 18.Ishii Y, Nitta T, Sakamoto S, Tanaka S, Asano G. Incisional atrial reentrant tachycardia: experimental study on the conduction property through the isthmus. J Thorac Cardiovasc Surg. 2003;126:254–62. doi: 10.1016/s0022-5223(02)73603-9. [DOI] [PubMed] [Google Scholar]
- 19.Sousa Uva M, Lacour-Gayet F, Komiya T, Serraf A, Bruniaux J, Touchot A, et al. Surgery for tetralogy of Fallot at less than six months of age. J Thorac Cardiovasc Surg. 1994;107:1291–300. [PubMed] [Google Scholar]