Abstract
The durability of adhesive bonds to enamel and dentin and the mechanisms of degradation caused by cyclic loading are important to the survival of composite restorations. In this study a novel method of evaluation was used to determine the strength of resin-enamel bonded interfaces under both static and cyclic loading, and to identify the mechanisms of failure. Specimens with twin interfaces of enamel bonded to commercial resin composite were loaded in monotonic and cyclic 4-point flexure to failure within a hydrated environment. Results for the resin-enamel interface were compared with those for the resin composite (control) and values reported for resin-dentin adhesive bonds. Under both modes of loading the strength of the resin-enamel interface was significantly (p≤0.0001) lower than that of the resin composite and the resin-dentin bonded interface. Fatigue failure of the interface occurred predominately by fracture of enamel, adjacent to the interface, and not due to adhesive failures. In the absence of water aging or acid production of biofilms, the durability of adhesive bonds to enamel is lower than that achieved in dentin bonding.
Keywords: Bonding, Durability, Enamel, Fatigue, Hybrid Layer, Resin Composite
INTRODUCTION
In the field of restorative dentistry, bond strength testing has been used to characterize the adhesion of restorative materials to dentin and enamel since the invention of acid etching [Buonocore, 1955]. Micro-tensile and micro-shear tests are the primary approaches for investigating the potential performance of new dental materials and/or the bonding procedures [Pashley et al., 1999; Spencer et al., 2010; De Munck et al., 2012]. There are several variations of these two methods, and a number of reviews have been presented to discuss their qualities and the advantages of particular configurations [e.g. Armstrong et al., 2010; Braga et al., 2010; Scherrer et al., 2010]. The bond strength obtained using these techniques has been adopted as a metric of performance, and high strength is considered to be indicative of longevity in the oral environment. But there are concerns regarding the applicability of these methods, and their relevance towards understanding the true causes of clinical failures [Van Meerbeek et al., 2003; Kelly et al., 2012; Roulet, 2012; Söderholm, 2012]. Perhaps the prevailing concern is that the results of in vitro experiments do not reflect the reality of failures in vivo, and there is little correlation to clinical behavior [Ferracane, 2012].
A load-bearing restoration bonded to dentin and enamel must resist damage over many years of function. Cyclic stresses transmitted across the resin adhesive and hybrid layers may cause degradation of the interface by fatigue [Spencer et al., 2010; Pashley et al., 2011]. Yet, in comparison to quasi-static loading, the influence of cyclic loading to the adhesive interface has received limited attention. Ruse et al. [1995] reported one of the earliest studies to evaluate the strength of resin-enamel bonds under in response to cyclic loading. Since then, surprisingly few studies have experimentally examined fatigue of the resin-enamel interface [De Munck et al., 2005; Erickson et al., 2006; 2008; 2009a; Barkmeier et al., 2009]. Reported studies on fatigue properties of the resin-dentin interface are equally scant [Drummond et al., 1996; Frankeberger et al., 1999; 2003; 2005; De Munck et al., 2005; Soappman et al., 2007; Staninec et al., 2008a and 2008b, Belli et al., 2010]. In fact, a quick survey of the literature shows that more manuscripts are published on micro-tensile testing in one month than the total number of studies reported on fatigue degradation of the bonded interface overall!
Two qualities summarize the findings of prior studies on the fatigue strength of adhesive bonds to dentin and enamel. Firstly, the fatigue strength of the interface is lower than that found in monotonic testing, with ratios of fatigue to static strength ranging from roughly 0.35 to 0.6. Secondly, adhesive bonds to dentin appear to be more durable than those to enamel [De Munck et al., 2005]. One potential limitation of the reported studies on enamel bonding is that cyclic loading has been limited to 1×105 cycles or lower. Considering that there are several thousands cycles of mastication per day [Anusavice, 1996], the aforementioned duration of previous fatigue evaluations is much less than one year of oral function. In addition, previous studies on enamel bonding have been conducted primarily using a cyclic shear configuration, whereas the bonded interface appears least durable in cyclic tension [Staninec et al., 2008a].
A recent study on the durability of resin-dentin adhesive interfaces adopted the use of a novel twin bonded interface specimen subjected to cyclic flexure loading [Mutluay et al., 2012a]. Fatigue failures occurred by cyclic tension and the ratio of the apparent endurance limit to the ultimate strength of this interface was 0.26. That estimate is essentially half that reported for cyclic shear loading of enamel. If cyclic tensile stresses are indeed most detrimental [Staninec et al., 2008a], then the durability of enamel bonds should be assessed under that mode of loading. Therefore, the present study adopted a novel approach to evaluate the strength of resin-enamel adhesive bonds under both monotonic and cyclic flexure loading. The objectives were to characterize the interface strength under the two modes of loading, distinguish the mechanisms of degradation and failure, and compare the results with those achieved for dentin bonding.
MATERIALS AND METHODS
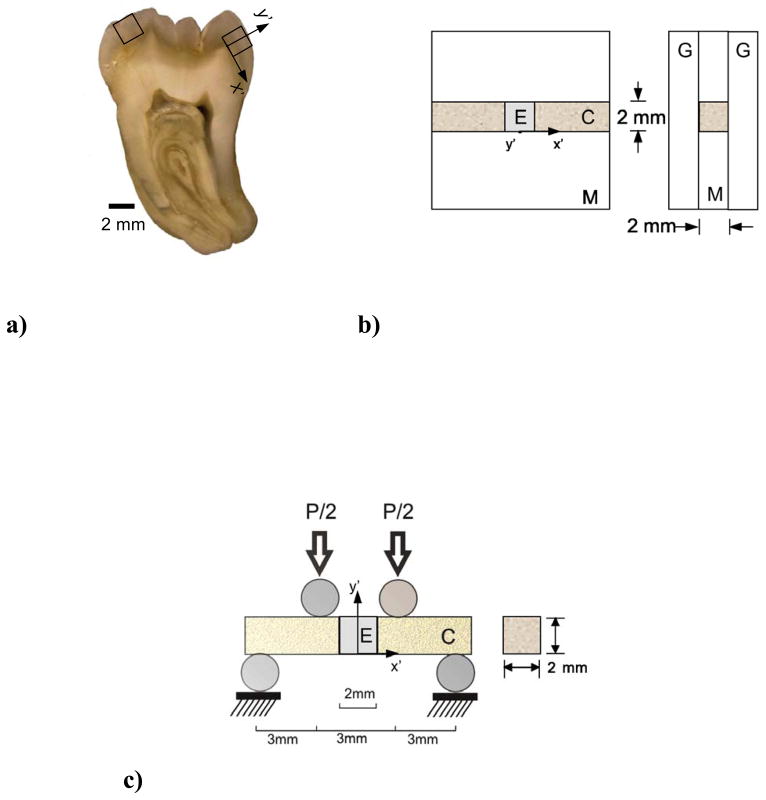
Bonded interface specimens were prepared from the enamel of caries-free human third molars. All extracted teeth were obtained with record of patient age (18≤age≤30) and gender from participating clinics in Maryland according to a protocol approved by the University of Maryland Baltimore County (#Y04DA23151). The teeth were stored in Hank’s Balanced Salt Solution (HBSS) for less than 1 month, and then sectioned using a computer-controlled grinder (Chevalier Smart-H818II, Chevalier Machinery, Santa Fe Springs, CA, USA) with diamond abrasive slicing wheels (#320 mesh abrasives) and water spray coolant. Sections of cuspal enamel with cross-section of approximately 2×2×2 mm3 were obtained (one from each tooth) as indicated in Fig. 1(a)).
Figure 1.
The resin-enamel interface specimens were developed using a molding process (Fig. 1(b)). Clearfil primer (Clearfil SE Bond, Lot 062127, Kuraray America, Houston, TX, USA) was applied to the lingual and buccal aspects of the enamel cubes according to the manufacturers instructions outside of the mold. The prisms are nominally aligned and in-plane with these two surfaces. Then adhesive (Clearfil SE Bond, Lot 062127, Kuraray America, Houston, TX, USA) was applied to the primed surfaces, and cured for 10 sec using a quartz-tungsten-halogen light-curing unit (Demetron VCL 401, Demetron, CA, USA) with output intensity of 600 mW/cm2 and tip diameter wider than 10 mm. The prepared cubes were then placed within the mold (Fig. 1(b)). Restorative resin composite (Clearfil AP-X, A2 color, Lot 1136AA; Kuraray America) was applied incrementally from the two bonding surfaces as necessary to fill the two sides of the mold cavities. After sandwiching the mold between two glass plates, the composite was performed on both sides for 30 seconds each. The sections of bonded resin composite and enamel were released from the mold and polished to obtain smooth surfaces with nominal cross section of 2×2 mm2 and length of approximately 12 mm. The specimens were stored in HBSS at room temperature (22° C) for at least 24 hours prior to further evaluation. Control specimens of resin composite with equivalent dimensions were prepared using the same procedures (without the inset enamel and bonded interfaces).
Quasi-static and cyclic four-point flexure was performed according to the loading configuration in Fig. 1(c) using a universal testing system (EnduraTEC Model ELF 3200, Minnetonka, MN, USA) with load capacity and sensitivity of 225 N and ±0.01 N, respectively. Both modes of loading were performed with the specimens maintained within a hydration bath of HBSS at room temperature. Quasi-static loading was applied using displacement control feedback at a crosshead rate of 0.06 mm/min. The flexural strength of the beams was estimated using conventional beam theory [Popov, 1978] in terms of the maximum measured load (P) and beam geometry (width b, thickness h) according to 3Pl/bh2, where l is the distance from interior and exterior supports (l=3 mm). Ten specimens of the enamel interface (N=10) and the resin composite control (N=10) were evaluated using this approach. The results of both groups were compared to those obtained for the resin-dentin interface, which was prepared according to the manufacturers recommendations using the exact same bonding materials. Details for the interested reader have been reported elsewhere [Mutluay et al., 2012a; 2012b]. The flexure strengths of the three groups were compared using a one-way ANOVA and Tukey’s HSD post-hoc analysis with the critical value (alpha) set at 0.05.
Cyclic loading of the bonded interface and control specimens was conducted using the aforementioned flexure configuration (Fig. 1(c)) under load control with frequency of 4 Hz and stress ratio (R = ratio of minimum to maximum cyclic load) of 0.1. Fatigue testing was initiated using a maximum cyclic stress of approximately 90% of the flexural strength identified from the quasi-static experiments. For successive specimens, the maximum cyclic load was decreased in increments of 5 MPa or less according to the staircase method of evaluation [Collins, 1981]. The process continued until reaching a flexure stress amplitude at which the specimens did not fail within 1.2×106 cycles. The stress-life (S–N) fatigue distribution was evaluated by plotting the cyclic stress amplitude in terms of the number of cycles to failure. The fatigue life distribution of the specimens in each group that underwent fatigue failure was fit using non-linear regression with a Basquin-type model [Stephens et al., 2001]
| (1) |
where A and B are the fatigue-life coefficient and exponent, respectively. These constants were obtained from a power law regression of the fatigue responses. The apparent endurance limit was estimated from the models for a fatigue limit defined at 1×107 cycles, consistent with that used in previous studies on dentin [Arola and Reprogel, 2005; Arola and Reprogel, 2006]. Both the fatigue life distributions and estimated endurance limits were compared with those obtained for the resin-dentin bonded interface that was prepared and tested using the same approach [Mutluay et al, 2012a; 2012b]. The fatigue life distributions were compared using the Wilcoxon Sum Rank Test with p ≤ 0.05 considered significant. A total of 45 resin-enamel bonded interface specimens were evaluated. The results obtained for Nf ≥ 1 kcycles were compared with results for the resin composite (N=20) and the resin-dentin bonded interface specimens (N=20).
Selected specimens were evaluated using Scanning Electron Microscopy (SEM) and nanoscopic Dynamic Mechanical Analysis (nanoDMA). Electron microscopy was conducted using a JEOL Model JSM-5600 (Peabody MA, USA) unit in secondary electron imaging (SEI) mode and back scattered electron (BEC) imaging mode. Prior to this analysis the specimens were dehydrated in an ascending ethanol series (70–100%), fixed in Hexamethyldisilazane, polished lightly in series using #800, #2400 and #4000 emory cloth and then sputtered with gold/palladium to enhance conductance of the enamel and resin adhesive. The fracture surfaces were inspected to identify the origins of failure and potential mechanisms of degradation.
To perform nanoDMA of the bonded interfaces, the portion of specimen containing the surviving resin-enamel interface (the bonded interface that did not break) was cold-mounted in Epofix epoxy resin (Struers, Cleveland, OH, USA). The side of the specimen was exposed, thereby revealing the tension and compression areas of the specimen. Polishing was performed with diamond particle suspensions (Buehler) of sizes 9, 3, and 0.04 μm to produce a highly polished surface with a roughness of less than 50 nm RMS. NanoDMA was performed with a Triboindenter (Model TI 900, Hysitron, Minneapolis, MN, USA) and a Berkovich diamond indenter with a 100 nm tip radius. Scanning mode dynamic loading was conducted over scan areas of 50 μm × 50 μm with 4 μN contact load, 2 μN dynamic loading amplitude and dynamic loading frequency of 100 Hz. The contact load and displacement signals were used to calculate the phase angle and to generate maps of the complex (E*) modulus distribution for the enamel, resin adhesive and both the matrix and filler of the resin composite. Scanning was performed with hydration using a layer of ethylene glycol over the specimen surface to prevent water evaporation [Ryou et al., 2011]. An unloaded interface specimen (control) was also evaluated using these techniques and after immersion in HBSS for 24 hours. Additional details regarding application of nanoDMA and evaluating adhesive interfaces using this approach are described in [Ryou et al., 2011; 2012].
Numerical Model and Analysis
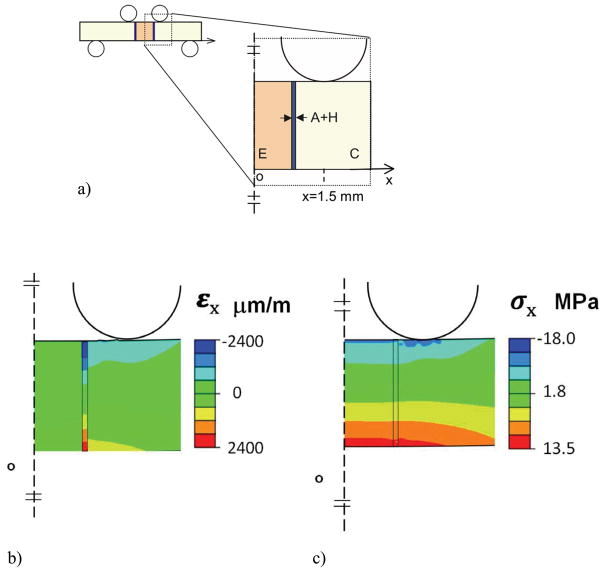
The flexure specimen geometry adopted for evaluating the resin-enamel bond strength was designed with two identical interfaces after the double-notched beam commonly used for evaluating engineering and natural materials [e.g. Nalla et al., 2003]. This configuration subjects both interfaces to a constant bending moment and corresponding normal stress. One of the twin interfaces undergoes failure and the second interface effectively “freezes” the status of the microstructure at that moment, thereby providing an interface for evaluation that has undergone cycle loading, but has not fractured. Owing to the presence of two interfaces it was necessary to evaluate the stress distribution and to examine the applicability of beam theory for estimating the stress in the resin-enamel specimens. Thus, a two-dimensional finite element analysis was performed using commercial software (ABAQUS 6.7–3; Dassault Systèmes Americas Corp., Waltham, MA, USA). A full model was developed for the beam to simulate the resulting stress and strain distribution. The beam was subjected to flexural loading according to the experimental configuration in Fig. 1(c) via simulated pins. The pins were defined as rigid body shells with frictionless contact between the pins and beam surfaces. The model beam was defined having three regions (Fig. 2(a)) including the resin composite, resin adhesive and enamel, and meshed with approximately 1200 type CPE4 plane strain elements. The materials were treated as linear elastic with elastic modulus (E) and Poisson’s ratio (3) defined for resin composite (6.0 GPa, 0.26) [AP-X, Kuraray USA], resin adhesive (4.4 GPa, 0.24) [Magni et al., 2010] and enamel (E=35 GPa, ν=0.29) [Ang et al., 2010; Bechtle et al., 2010]. The elastic modulus assigned for enamel is based on a macroscopic scale characterization and smaller than that typically found using nanoindentation (roughly 70 to 100 GPa) [Park et al., 2008; Cuy et al., 2002].
Figure 2.
Results of the finite element analysis for flexural loading of the bonded interface specimen are shown in Fig. 2. Specifically, the normal strain (εx) and normal stress (σx) distributions within the region of constant bending moment are shown in Fig. 2(b) and Fig. 2(c), respectively. These distributions result from a flexure load of 10 Newtons, which is within the range of applied experimental loads. As evident in Fig. 2(b), there is a concentration in normal strain within the adhesive, which is greatest near the tensile and compressive surfaces. The largest strain develops within the resin adhesive, and is substantially larger than that within the adjacent materials (up to a factor of 7x). In addition, there is a stress concentration in the composite that develops at the point of contact loading, which is apparent in Fig. 2(c).
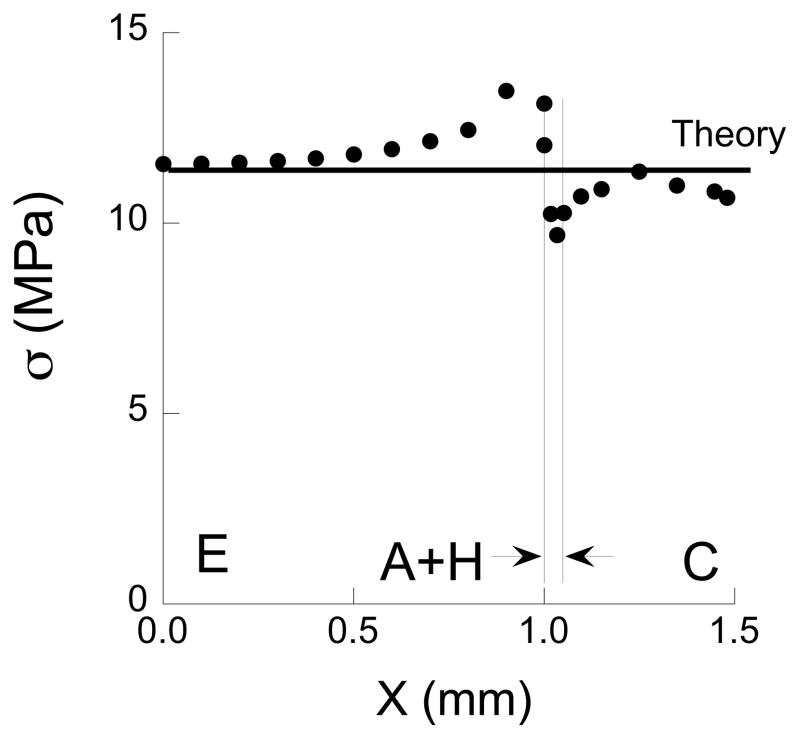
The maximum normal stress obtained from the model occurs on the tensile side of the flexure specimen and is plotted as a function of distance from the specimen’s axis of symmetry in Fig. 3. For comparison, the normal stress predicted using beam theory for a homogeneous specimen with identical geometry and loading configuration is presented in this figure as well. It is important to highlight that although the interfaces are located within one millimeter of the two interior loading pins, the tensile stress distribution is not influenced substantially by the contact stresses. Nevertheless, the normal stress is not constant between the two central loading pins as a result of the adhesive interface. There is a concentration of stress that develops in the enamel, adjacent to the bonded interface, which is caused by the elastic modulus mismatch. Based on a comparison of the numerical results with beam theory, the largest stress develops in the enamel and is approximately 16% greater than that predicted using beam theory. For convenience, all of the stresses reported at failure in the experiments are those estimated using beam theory. The actual stress in enamel is up to 16% greater, depending on distance from the interface.
Figure 3.
RESULTS
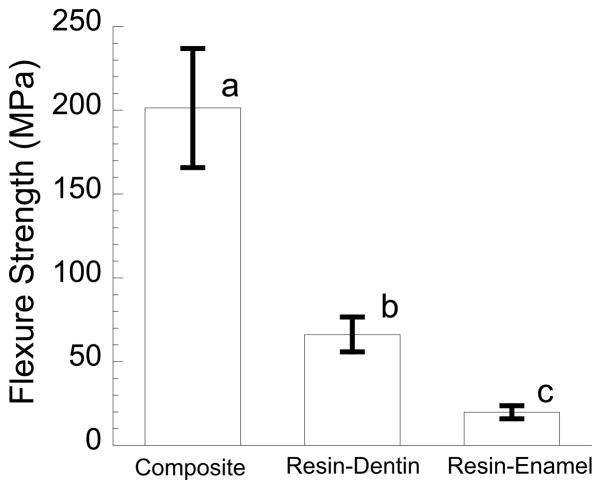
The strength distributions of the resin-enamel interface, resin composite control and resin-dentin bonded interface specimens obtained from monotonic loading to failure are shown in Fig. 4. All three responses are shown in terms of the average and standard deviation and were obtained using the exact same specimen configuration and loading conditions. According to the one-way ANOVA, the average flexural strength of the resin-enamel interface (19.9 ± 3.9 MPa) was significantly lower (p ≤ 0.000) than the resin composite controls (201.4 ± 35.6 MPa), as well as that reported for the resin-dentin interface (66.2 ± 10.4 MPa).
Figure 4.
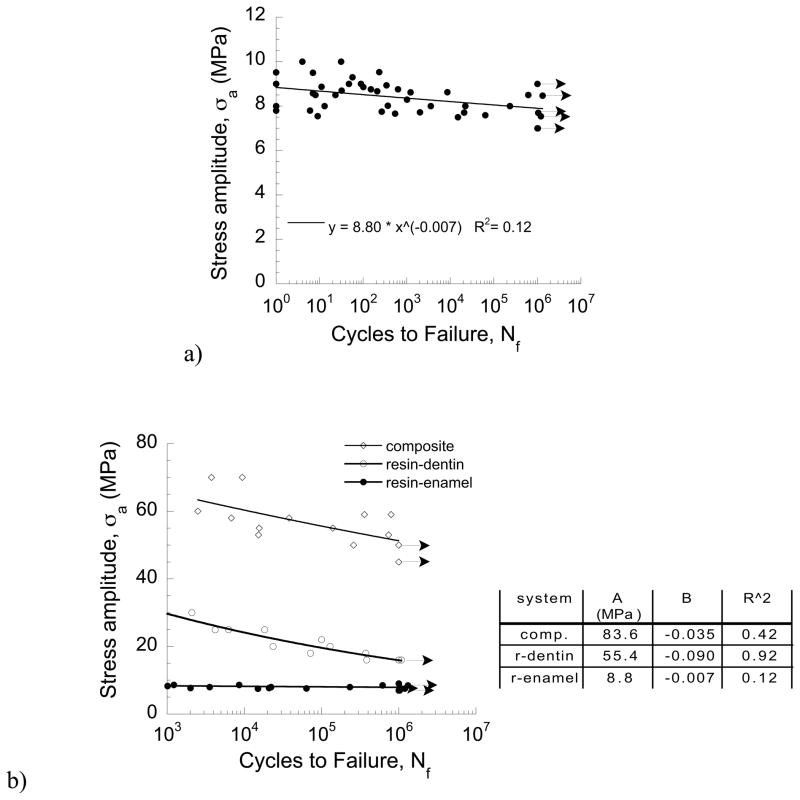
A fatigue life diagram with results from cyclic loading of the resin-enamel bonded interface specimens is shown in Fig. 5(a). This diagram presents results for all of the specimens evaluated with lives range from 1 to over 1 million cycles. Data points presented with arrows represent specimens in which failure did not take place within 1,200 kcycles and the test was discontinued. A power law model describing the mean fatigue response was developed according to Eqn. 1 and is presented in this figure as well. There is a large degree of variability in the fatigue performance, and that at the lower range in cyclic stresses the number of cycles to failure ranged from 1 to over 1 million cycles. Results for the resin-enamel specimens with lives of Nf ≥ 1 kcycles, i.e. commonly regarded as the stress-life regime, are compared with those for the resin composite and resin-dentin interface specimens in Fig. 5(b). Experimental results for the resin-dentin interface are from Mutluay et al., [2012a]. Note that the resin-enamel bonded interface exhibited the lowest fatigue strength over the entire fatigue life regime. The fatigue strengths of the resin composite (Z = −5.55; p ≤ 0.0001) and the resin-dentin bonded interface (Z = −6.19; p ≤ 0.0001) were significantly greater than the resin-enamel interface. Power law models were developed to describe the mean fatigue responses and the model parameters (A, B) are presented in Fig. 5(b). When defined at 1×107 cycles, the “apparent” endurance limit estimated for the resin-enamel bonded interface is 7.9 MPa. That value is more than 80% lower than the apparent endurance limit of the resin composite (48 MPa) and nearly 40% lower than that of the resin-dentin interface (13.0 MPa) reported in Mutluay et al., [2012a].
Figure 5.
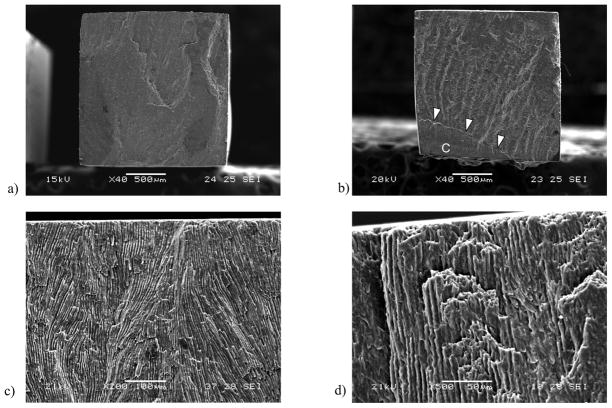
The fracture surfaces were examined using a scanning electron microscope and micrographs detailing the features of selected interface specimens are shown in Fig. 6. For both quasi-static and cyclic loading fracture of the interface initiated on the tensile side of the specimen and within the enamel, as shown in Fig. 6(a) and Fig. 6(b). In most specimens the entire fracture surface was comprised of enamel (Fig. 6(a)), whereas in some specimens fractured proceeded in the adhesive interface or within the composite (Fig. 6(b)), on the compressive side of the neutral axis. Nevertheless, none of the bonded interface specimens showed details indicating that failure initiated in the resin adhesive or the resin composite. At higher magnification it was apparent that the enamel of the tensile region generally exhibited parallel straight prisms (i.e. without decussation) as evident in Fig. 6(c). Fracture occurred along the interfaces of adjacent prisms (Fig. 6(d)). Indeed, the enamel cubes were prepared and arranged for cyclic loading such that the occlusal aspect was oriented toward the tensile surface. Closer to the neutral axis and continuing towards the compressive region, the enamel microstructure transitioned into a decussated pattern comprised of interwoven parazone and diazone regions that are evident towards the lower half of the fracture surface in Fig. 6(b).
Figure 6.
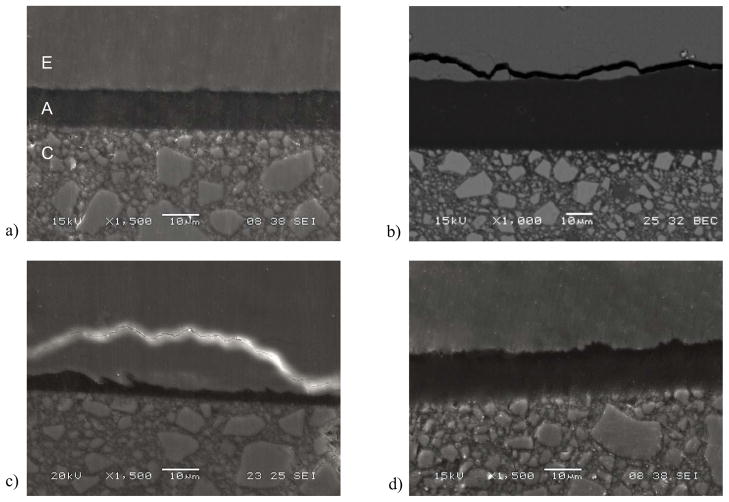
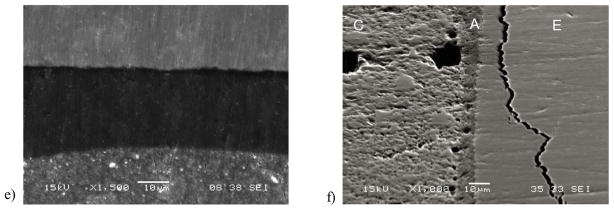
The bonded interface of a specimen not subjected to cyclic loading is shown in Fig. 7(a). Note that there are distinct boundaries between the resin composite and adhesive in this micrograph, as well as between the adhesive and enamel. However, there are no defects apparent that may have resulted from the light curing process or the fixation technique used in preparation for the SEM evaluation. This image does not clearly show resin tags as the level of magnification is not enough to observe those that develop in enamel bonding [Peumans et al., 1999, Shinoahara et al., 2006]. One advantage of the twin interface design is that after fatigue failure of the specimens there is an unbroken interface available for further evaluation. Representative unbroken interfaces from specimens that failed by fatigue are shown in Fig. 7(b) and 7(c), and were obtained after comparatively low and high cycle fatigue lives, respectively. Both micrographs were obtained from the tensile side of the specimen that resulted from flexural loading and exhibit cracks within the enamel; most clear in Fig. 7(b). In contrast, the compressive side of the specimens did not exhibit evidence of cracks or other forms of degradation, as shown in Fig. 7(d). Micrographs obtained from bonded interface specimens that endured 1.2×106 cycles without fatigue failure are shown in Fig. 7(e) and 7(f); both were obtained from the tensile surface. While fatigue related damage was not evident in some of these specimens (Fig. 7(e)), the majority had sub-critical cracks within the enamel as apparent in Fig. 7(f), that were located roughly 5 μm to 50 μm from the adhesive layer. The cracks follow the boundary of adjacent enamel prisms, which is most evident in Fig. 7(b) and Fig. 7(f). The demonstrative value of the SEM imaging is clearly apparent. Yet, although these specimens were dehydrated using the descending ethanol treatment to minimize the introduction of artifacts, the preparation methods could potentially magnify signs of fatigue.
Figure 7.
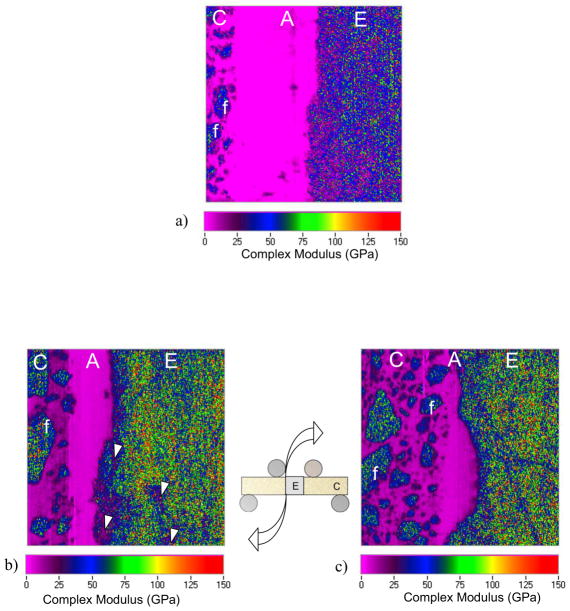
The elastic behavior of the adhesive interface and the potential changes resulting from cyclic loading were evaluated using scanning mode nanoDMA. The complex modulus distribution for a control specimen (no cyclic loading) is shown in Fig. 8(a). Evident in the property distributions are the enamel (E), resin adhesive (A) and resin composite (C), as well as the composite filler particles (f). The map in this figure represents a scan window of 50 μm × 50 μm. Representative property maps for the complex modulus distribution near the tensile and compressive surfaces of a selected specimen are shown in Fig. 8(b) and 8(c), respectively. These maps were obtained from an interface specimen that achieved 1,200 kcycles of loading and the testing was stopped. All of the scans in Fig. 8 were taken from the side of the specimen (parallel to the loading axis) as noted. The complex modulus for the enamel ranged from roughly 60 to 120 GPa (average±std dev = 79±48 GPa). The monomer and filler particles of the resin composite exhibited complex moduli of 9.8±2.2 GPa and 69.9±38.4 GPa, respectively. There was no significant difference in the complex moduli of the constituents from the control specimen and those subjected to cyclic loading. In regards to the adhesive, the average complex modulus ranged between 4.5 and 6.5 MPa. The tensile side of the resin adhesive generally exhibited a higher complex modulus than in compression, but the differences were not significant (p > .05). The average complex modulus for the control was 5.77±0.66 GPa.
Figure 8.
In examining the nanoDMA property maps there was damage evident within the enamel in many specimens, and concentrated nearest the tensile surface of the fatigued interfaces (noted by arrows in Fig. 8(b)). The damage was generally oriented parallel to the interface. Microcracks were not identified near the neutral axis or in the compressive region of the enamel. Neither were there signs of damage or defects within the adhesive, on either side of the neutral axis, or along the interface boundaries. Lastly, the interface thickness (i.e. width of the resin adhesive) appeared to be elongated on the tensile side of the neutral axis with respect to the compressive side. Note the larger interfacial thickness on the tensile side (Fig. 8(b)) in comparison to that in compression (Fig. 8(c)).
DISCUSSION
An experimental evaluation on the strength and durability of the resin-enamel bonded interface was conducted. Although restored teeth undergo cyclic loading during mastication, few studies have assessed the fatigue behavior of enamel bonds [De Munck et al., 2005; Erickson et al., 2006; 2008; 2009a; Barkmeier et al., 2009]. Moreover, previous evaluations on this topic have been limited to 1×105 cycles of loading and less. With the average daily routine involving between 1000 and 2000 cycles of mastication [Anusavice, 2003; Sakaguchi and Powers, 2012], the bonded interface between the restorative material and tooth structure will experience well over 250k cycles of loading per year. Consequently, this investigation is the first to evaluate the interface durability over a period that exceeds one year of oral function.
When evaluated by quasi-static loading to failure (Fig. 4) the resin-enamel interface exhibited a strength of approximately 20 MPa, which was only 10% that of the resin composite and only 30% that of the resin-dentin interface [Mutluay et al., 2012a]. Furthermore, the apparent endurance limit of the enamel bonds (7.9 MPa) was less than 20% that for the resin composite, and approximately 60% of the value achieved in dentin bonding (Fig. 5(b)) when evaluated in the same manner. Perhaps more importantly, the estimated endurance limit is lower than the magnitude of stresses that develop in the enamel of restored teeth during simulated mastication [Arola et al., 2001; Asmussen and Peutzfeldt; 2008].
One common measure of the relative resistance to fatigue degradation is given by the ratio of strength in cyclic and static loading conditions. Using the Goodman model [Stephens et al., 2001] to correct for the mean stress that results from a cyclic stress ratio of 0.1, the equivalent “fully reversed” endurance limit of the resin-enamel interface is 15.4 MPa. Using that estimate, the ratio of fully reversed endurance limit to the ultimate strength is 0.77. This value is substantially greater than that for the resin composite (69 MPa/201 MPa = 0.35) and the resin-dentin bonded interface (17 MPa/66 MPa = 0.26) obtained using the same approach. According to these ratios, cyclic tensile stresses cause substantially more degradation to the strength of resin-dentin adhesive bonds than those involving enamel. De Munck et al [2005] also found that enamel bonds exhibited a higher ratio of fatigue to static strengths in comparison to those achieved in dentin bonding. Nevertheless, if the bonded interface is subjected to cyclic tension, results of the experiments show that the resin-enamel interface possesses the lowest fatigue strength overall. Therefore, prior to degradation of the resin-dentin interface by hydrolytic [Breschi et al., 2008; Liu et al., 2011] or enzymatic [Pashley et al., 2011] processes, the resin-enamel interface appears to be a critical weak link of adhesive bonds to tooth structure.
With the exception of the study by De Munck et al., [2005] the previous studies on durability of resin-enamel bonds adopted cyclic shear loading to evaluate the interface [Ruse et al., 1995; Erickson et al., 2006; 2008; 2009a; Barkmeier et al., 2009]. In comparing the findings from these studies to that presented herein, there are some important observations. Most evident are the differences in magnitude of the fatigue strength in cyclic shear with respect to that determined in cyclic tension. Ruse et al., [1995] reported an apparent endurance limit of 10 MPa in cyclic shear loading of enamel bonds prepared by etching (15 sec) and application of Scotch Bond Multi Purpose (primer and adhesive). In the studies reported by Erickson et al [2006, 2008, 2009a] and Barkmeir et al [2009] the shear bond fatigue strength ranged from 8.4±0.5 MPa to 29.6±1.8 MPa. Using the power law model for the enamel in Fig. 5(a) to estimate fatigue strengths at the defined fatigue limits in those previous studies (40 kcycles to 100 kycles), results in values of between 8.1 and 8.2 MPa, respectively. These values are lower than nearly all estimates of the reported fatigue strengths in cyclic shear. There are differences in the resin adhesive and bonding procedures, both of which can contribute to the strength of enamel bonds [e.g. Rotta et al., 2007; Ando et al., 2008; Erickson et al., 2009b; Torres et al., 2009; Caneppele et al., 2012]. Size effects are potentially also relevant due to differences in the bonded interface area [Ghassemieh, 2008]. Nevertheless, comparing results from the two loading modes suggests that the resin-enamel interface is least durable under cyclic tensile stresses. It is worthwhile noting that the bonded interface is subjected to cyclic tensile stresses during mastication via cuspal deflections arising from lateral excursions of the mandible [Schuurs, 2013].
Under both static and cyclic loading conditions, failure did not occur within the adhesive bond. Rather, the initiation of failure developed in tension within the organic-rich interfaces of the enamel prisms located adjacent to the interface (Fig. 6). Occlusal enamel exhibits a comparatively low resistance to the initiation and growth of fatigue cracks and low fracture toughness with respect to the decussated region located closer to the dentin enamel junction [Bajaj and Arola, 2009a; 2009b; Yahyazadehfar et al., 2012]. That suggests that the estimated strengths reported here are dominated by the inferior fatigue strength of enamel, and that the adhesive fatigue strength to enamel is greater than the estimated resin-enamel interface strength. Nevertheless, the present loading configuration includes an enamel and restorative composite layer that is not supported by the underlying dentin. Most restorations include an underlying dentin layer that supports the enamel during function and contributes to the structural integrity of the restored tooth as a whole. Additional issues of clinical relevance are the prism orientation and the technique used for preparing the cavity walls. Changes in the prism orientation would be expected to influence the mode of interface failure and apparent bond strength [Shimada and Tagami, 2003]. Beveling is usually recommended for purposes of esthetics and durability. Such a beveling could influence the outcome of the present evaluation by changing the interface stress distributions. This concern is reserved for future study.
Previous evaluations of the resin-enamel interface reported fatigue failure in the adhesive or at the bonded interface, not in enamel [De Munck et al., 2005; Erickson et al., 2006; 2008; 2009a; Barkmeier et al., 2009]. But that is a consequence of the enamel rod orientation, as most previous studies involving bonding to labial surfaces, and/or due to shear loading of the interface. Differences in the modes of failures between shear and tension distinguishes that there is one advantage of adopting the cyclic shear configuration for evaluating the interface durability, i.e. that failures occur at the adhesive interface and are less often due to cohesive failures. But that comment conflicts with the previous findings of Della Bona and Van noort [1995] in comparing microtensile and microshear tests for characterizing bond strength to ceramics. However, if the forces of mastication or changes in temperature promote cyclic tension of the bonded interface, then there is greater likelihood that failure will initiate within the supporting enamel. That may increase the need for introducing a remineralization strategy to maintain the integrity of enamel and performing “in situ repair” of these defects to prevent bond degradation [Liu et al., 2011].
Results of the finite element analysis revealed that the compliant adhesive layer causes a stress concentration in the enamel, adjacent to the interface (Fig. 3). Previous finite element evaluations of the stress distribution in micro-tensile testing have identified the development of a non-uniform stress distribution at the adhesive interface and a stress concentration as well [e.g. Van Noort et al., 1989; Phrukkanon et al., 1998]. While a uniform stress across the interface may be considered more desirable, the stress concentration within the tooth structure is clinically relevant. Finite element studies of restored teeth show that the concentration of stress develops at the restoration margins [Arola et al., 2001; Ausiello et al., 2002; Asmussen and Peutzfeldt, 2008]. Failure of the bonded interface via fracture of enamel is a critical issue, and to the authors’ knowledge, one that has not been addressed in previous evaluations. Even in specimens that endured more than one million cycles, cracks were found aligned with the interface in the enamel (Fig. 7(f)). Some of these cracks were found to extend within the enamel towards the neutral axis until reaching the region of decussation (not shown). Although cyclic loading did not cause failure, these specimens are more susceptible to bacterial penetration. This may be another important mechanism contributing to degradation of the interface and the low service life of bonded resin composite restorations [Bernardo et al., 2007; Demarco et al., 2012].
The present study applied a novel approach to evaluate the durability of resin-enamel adhesive bonds and identified an important mechanism contributing to fatigue failure of the bonded interface. Nevertheless, there are limitations to the evaluation. One limitation is the absence of polymerization shrinkage stress in the chosen testing configuration. These stresses have a deleterious effect on the interface integrity [Ferracane, 2008], and would be expected to cause additional degradation of the interface beyond that estimated in the present evaluation. Stresses of this type can be accounted for by modifying the stress ratio, and will be addressed in future studies. Furthermore, the results still should be evaluated cautiously as the effect of long-term aging of on the bonded interface was not taken into account. A water aging pretreatment could be applied to the protocol, but would likely lack the synergism of water movement that takes place at the interface during cyclic loading [Wood et al., 2008]. Furthermore, a water aging treatment is potentially less important here than in evaluations concerning dentin bonding.
Another important limitation to the evaluation is that it did not include the effects from pH variations that occur from the presence of biofilms. Secondary caries at the tooth-restoration margins arise from the acid production of biofilms [Sakaguchi, 2005; ten Cate, 2006] and are the primary cause for failure of resin composite restorations [Deligeorgi et al., 2001; Brunthaler et al., 2003; Sunnegårdh-Grönberg et al., 2009]. A recent study on the influence of biofilm activity to the durability of resin-dentin bonds reported a 30% reduction in apparent endurance limit within 14 days of exposure [Mutluay et al., 2012a]. The effects of an exposure of this type on the bond strength to enamel should be studied to examine the effects of the biofilm environment. Indeed, these studies are presently underway to evaluate the influence of water aging and oral bacteria on the durability of resin-enamel bonds. Despite the aforementioned concerns, the results serve as an important foundation towards exploring the mechanisms contributing to the degradation of adhesive bond durability to tooth structure in the oral environment.
CONCLUSION
An experimental evaluation of the strength and durability of resin-enamel bonds was conducted under monotonic and cyclic normal stresses using a specially designed bonded interface specimen. The apparent endurance limit of the resin-enamel interface resulting from bonding with a commercial self-etch adhesive and resin composite was less than 20% that of the resin composite, and just over 60% of the value achieved in dentin bonding when evaluated in the same manner. Failure of the bonded interface specimens occurred by fracture within the enamel, and initiated from microcracks that developed adjacent to the adhesive interface. If the adhesive interface supporting resin composites in restored teeth is subjected to cyclic tensile stresses, then fatigue of enamel is a potential mechanism of degradation that should be considered.
Acknowledgments
Support for the following investigation was provided in part by the National Institutes of Dental and Craniofacial Research (DE016904).
This study was supported in part by a seed grant from the University of Maryland Baltimore County (D. Arola) and an award from the National Institutes of Health (NIDCR R01 DE016904; Arola). The authors also gratefully acknowledge Kuraray America for their generous donation of bonding supplies and resin composite, and Prof. Frederick Rueggeberg of the Georgia Health Sciences University for kindly supplying and calibrating the light curing unit.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ando S, Watanabe T, Tsubota K, Yoshida T, Irokawa A, Takamizawa T, Kurokawa H, Miyazaki M. Effect of adhesive application methods on bond strength to bovine enamel. J Oral Sci. 2008;50 (2):181–6. doi: 10.2334/josnusd.50.181. [DOI] [PubMed] [Google Scholar]
- Ang SF, Bortel EL, Swain MV, Klocke A, Schneider GA. Size-dependent elastic/inelastic behavior of enamel over millimeter and nanometer length scales. Biomaterials. 2010;31 (7):1955–63. doi: 10.1016/j.biomaterials.2009.11.045. [DOI] [PubMed] [Google Scholar]
- Anusavice KJ. Phillips Science of Dental Materials. 11. Philadelphia: Saunders; 2003. pp. 90–91. [Google Scholar]
- Armstrong S, Geraldeli S, Maia R, Raposo LH, Soares CJ, Yamagawa J. Adhesion to tooth structure: a critical review of “micro” bond strength test methods. Dent Mater. 2010;26 (2):e50–62. doi: 10.1016/j.dental.2009.11.155. [DOI] [PubMed] [Google Scholar]
- Arola D, Galles LA, Sarubin MF. A comparison of the mechanical behavior of posterior teeth with amalgam and composite MOD restorations. J Dent. 2001;29 (1):63–73. doi: 10.1016/s0300-5712(00)00036-1. [DOI] [PubMed] [Google Scholar]
- Arola D, Reprogel R. Effects of aging on the mechanical behavior of human dentin. Biomaterials. 2005;26 (18):4051–61. doi: 10.1016/j.biomaterials.2004.10.029. [DOI] [PubMed] [Google Scholar]
- Arola DD, Reprogel RK. Tubule orientation and the fatigue strength of human dentin. Biomaterials. 2006;27 (9):2131–40. doi: 10.1016/j.biomaterials.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Asmussen E, Peutzfeldt A. Class I and Class II restorations of resin composite: an FE analysis of the influence of modulus of elasticity on stresses generated by occlusal loading. Dent Mater. 2008;24 (5):600–5. doi: 10.1016/j.dental.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Ausiello P, Apicella A, Davidson CL. Effect of adhesive layer properties on stress distribution in composite restorations--a 3D finite element analysis. Dent Mater. 2002;18 (4):295–303. doi: 10.1016/s0109-5641(01)00042-2. [DOI] [PubMed] [Google Scholar]
- Bajaj D, Arola D. On the R-Curve Behavior of Human Tooth Enamel. Biomaterials. 2009a;30 (23–24):4037–4046. doi: 10.1016/j.biomaterials.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj D, Arola D. Role of prism decussation on fatigue crack growth and fracture of human enamel. Acta Biomat. 2009b;5 (8):3045–3056. doi: 10.1016/j.actbio.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkmeier WW, Erickson RL, Latta MA. Fatigue limits of enamel bonds with moist and dry techniques. Dent Mater. 2009;25 (12):1527–31. doi: 10.1016/j.dental.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Bechtle S, Ang SF, Schneider GA. On the mechanical properties of hierarchically structured biological materials. Biomaterials. 2010;31 (25):6378–85. doi: 10.1016/j.biomaterials.2010.05.044. [DOI] [PubMed] [Google Scholar]
- Belli R, Baratieri LN, Braem M, Petschelt A, Lohbauer U. Tensile and bending fatigue of the adhesive interface to dentin. Dent Mater. 2010;26 (12):1157–65. doi: 10.1016/j.dental.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Bernardo M, Luis H, Martin MD, Leroux BG, Rue T, Leitão J, DeRouen TA. Survival and reasons for failure of amalgam versus composite posterior restorations placed in a randomized clinical trial. J Am Dent Assoc. 2007;138 (6):775–83. doi: 10.14219/jada.archive.2007.0265. [DOI] [PubMed] [Google Scholar]
- Braga RR, Meira JB, Boaro LC, Xavier TA. Adhesion to tooth structure: a critical review of “macro” test methods. Dent Mater. 2010;26 (2):e38–49. doi: 10.1016/j.dental.2009.11.150. [DOI] [PubMed] [Google Scholar]
- Breschi L, Mazzoni A, Ruggeri A, Cadenaro M, Di Lenarda R, De Stefano Dorigo E. Dental adhesion review: aging and stability of the bonded interface. Dent Mater. 2008;24 (1):90–101. doi: 10.1016/j.dental.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Brunthaler A, König F, Lucas T, Sperr W, Schedle A. Longevity of direct resin composite restorations in posterior teeth. Clin Oral Investig. 2003;7 (2):63–70. doi: 10.1007/s00784-003-0206-7. [DOI] [PubMed] [Google Scholar]
- Buonocore MG. A simple method of increasing the adhesion of acrylic filling materials to enamel surfaces. J Dent Res. 1955;34 (6):849–53. doi: 10.1177/00220345550340060801. [DOI] [PubMed] [Google Scholar]
- Caneppele TM, Torres CR, Sassaki A, Valdetaro F, Fernandes RS, Prieto de Freitas C, Batista GR. Effects of surface hydration state and application method on the bond strength of self-etching adhesives to cut enamel. J Adhes Dent. 2012;14 (1):25–30. doi: 10.3290/j.jad.a22091. [DOI] [PubMed] [Google Scholar]
- Collins JA. Fatigue of Metals in Mechanical Design. John Wiley and Sons; 1981. Fatigue Testing Procedures and Statistical Interpretations of Data. [Google Scholar]
- Cuy JL, Mann AB, Livi KJ, Teaford MF, Weihs TP. Nanoindentation mapping of the mechanical properties of human molar tooth enamel. Arch Oral Biol. 2002;47 (4):281–91. doi: 10.1016/s0003-9969(02)00006-7. [DOI] [PubMed] [Google Scholar]
- Deligeorgi V, Mjör IA, Wilson NH. An overview of reasons for the placement and replacement of restorations. Prim Dent Care. 2001;8 (1):5–11. doi: 10.1308/135576101771799335. [DOI] [PubMed] [Google Scholar]
- Della Bona A, van Noort R. Shear vs. tensile bond strength of resin composite bonded to ceramic. J Dent Res. 1995;74 (9):1591–6. doi: 10.1177/00220345950740091401. [DOI] [PubMed] [Google Scholar]
- Demarco FF, Corrêa MB, Cenci MS, Moraes RR, Opdam NJ. Longevity of posterior composite restorations: not only a matter of materials. Dent Mater. 2012;28 (1):87–101. doi: 10.1016/j.dental.2011.09.003. [DOI] [PubMed] [Google Scholar]
- De Munck J, Braem M, Wevers M, Yoshida Y, Inoue S, Suzuki K, Lambrechts P, Van Meerbeek B. Micro-rotary fatigue of tooth-biomaterial interfaces. Biomaterials. 2005;26 (10):1145–53. doi: 10.1016/j.biomaterials.2004.04.013. [DOI] [PubMed] [Google Scholar]
- De Munck J, Mine A, Poitevin A, Van Ende A, Cardoso MV, Van Landuyt KL, Peumans M, Van Meerbeek B. Meta-analytical review of parameters involved in dentin bonding. J Dent Res. 2012;91 (4):351–7. doi: 10.1177/0022034511431251. [DOI] [PubMed] [Google Scholar]
- Drummond JL, Sakaguchi RL, Racean DC, Wozny J, Steinberg AD. Testing mode and surface treatment effects on dentin bonding. J Biomed Mater Res. 1996;32 (4):533–41. doi: 10.1002/(SICI)1097-4636(199612)32:4<533::AID-JBM6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Erickson RL, De Gee AJ, Feilzer AJ. Fatigue testing of enamel bonds with self-etch and total-etch adhesive systems. Dent Mater. 2006;22 (11):981–7. doi: 10.1016/j.dental.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Erickson RL, De Gee AJ, Feilzer AJ. Effect of pre-etching enamel on fatigue of self-etch adhesive bonds. Dent Mater. 2008;24 (1):117–23. doi: 10.1016/j.dental.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Erickson RL, Barkmeier WW, Kimmes NS. Fatigue of enamel bonds with self-etch adhesives. Dent Mater. 2009a;25 (6):716–20. doi: 10.1016/j.dental.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Erickson RL, Barkmeier WW, Latta MA. The role of etching in bonding to enamel: a comparison of self-etching and etch-and-rinse adhesive systems. Dent Mater. 2009b;25 (11):1459–67. doi: 10.1016/j.dental.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Ferracane JL. Buonocore Lecture. Placing dental composites--a stressful experience. Oper Dent. 2008;33 (3):247–57. doi: 10.2341/07-BL2. [DOI] [PubMed] [Google Scholar]
- Ferracane JL. Resin composite--state of the art. Dent Mater. 2011;27 (1):29–38. doi: 10.1016/j.dental.2010.10.020. [DOI] [PubMed] [Google Scholar]
- Frankenberger R, Krämer N, Petschelt A. Fatigue behaviour of different dentin adhesives. Clin Oral Investig. 1999;3 (1):11–7. doi: 10.1007/s007840050072. [DOI] [PubMed] [Google Scholar]
- Frankenberger R, Strobel WO, Krämer N, Lohbauer U, Winterscheidt J, Winterscheidt B, Petschelt A. Evaluation of the fatigue behavior of the resin-dentin bond with the use of different methods. J Biomed Mater Res B Appl Biomater. 2003;67 (2):712–21. doi: 10.1002/jbm.b.10052. [DOI] [PubMed] [Google Scholar]
- Frankenberger R, Pashley DH, Reich SM, Lohbauer U, Petschelt A, Tay FR. Characterisation of resin-dentine interfaces by compressive cyclic loading. Biomaterials. 2005;26 (14):2043–52. doi: 10.1016/j.biomaterials.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Ghassemieh E. Evaluation of sources of uncertainties in microtensile bond strength of dental adhesive system for different specimen geometries. Dent Mater. 2008;24 (4):536–47. doi: 10.1016/j.dental.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Kelly JR, Benetti P, Rungruanganunt P, Bona AD. The slippery slope: critical perspectives on in vitro research methodologies. Dent Mater. 2012;28 (1):41–51. doi: 10.1016/j.dental.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Liu Y, Tjäderhane L, Breschi L, Mazzoni A, Li N, Mao J, Pashley DH, Tay FR. Limitations in bonding to dentin and experimental strategies to prevent bond degradation. J Dent Res. 2011;90 (8):953–68. doi: 10.1177/0022034510391799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magni E, Ferrari M, Hickel R, Ilie N. Evaluation of the mechanical properties of dental adhesives and glass-ionomer cements. Clin Oral Investig. 2010;14 (1):79–87. doi: 10.1007/s00784-009-0259-3. [DOI] [PubMed] [Google Scholar]
- Mutluay MM, Ryou H, Zhang K, Yahyazadefar M, Majd H, Xu HHK, Arola D. On the durability of resin-dentin bonds after Degradation by Biofilm. J Mech Behav Biomed Mater. 2013a doi: 10.1016/j.jmbbm.2012.10.019. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutluay MM, Yahyazadefar M, Ryou H, Majd H, Arola D. Fatigue of the resin-dentin interface: A new approach for evaluating the durability of dentin bonds. Dent Mater. 2013b doi: 10.1016/j.dental.2013.01.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalla RK, Kinney JH, Ritchie RO. On the fracture of human dentin: is it stress- or strain-controlled? J Biomed Mater Res A. 2003;67 (2):484–95. doi: 10.1002/jbm.a.10079. [DOI] [PubMed] [Google Scholar]
- Park S, Wang DH, Zhang D, Romberg E, Arola D. Mechanical properties of human enamel as a function of age and location in the tooth. J Mater Sci: Mater Med. 2008;19 (6):2317–24. doi: 10.1007/s10856-007-3340-y. [DOI] [PubMed] [Google Scholar]
- Pashley DH, Carvalho RM, Sano H, Nakajima M, Yoshiyama M, Shono Y, Fernandes CA, Tay FR. The microtensile bond test: a review. J Adhes Dent. 1999;1 (4):299–309. [PubMed] [Google Scholar]
- Pashley DH, Tay FR, Breschi L, Tjäderhane L, Carvalho RM, Carrilho M, Tezvergil-Mutluay A. State of the art etch-and-rinse adhesives. Dent Mater. 2011;27 (1):1–16. doi: 10.1016/j.dental.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phrukkanon S, Burrow MF, Tyas MJ. The influence of cross-sectional shape and surface area on the microtensile bond test. Dent Mater. 1998;14 (3):212–21. doi: 10.1016/s0109-5641(98)00034-7. [DOI] [PubMed] [Google Scholar]
- Popov EP. Mechanics of Materials. 2. Prentic Hall Inc; New Jersey: 1978. [Google Scholar]
- Peumans M, Van Meerbeek B, Yoshida Y, Lambrechts P, Vanherle G. Porcelain veneers bonded to tooth structure: an ultra-morphological FE-SEM examination of the adhesive interface. Dent Mater. 1999;15(2):105–19. doi: 10.1016/s0109-5641(99)00020-2. [DOI] [PubMed] [Google Scholar]
- Roulet JF. Is in vitro research in restorative dentistry useless? J Adhes Dent. 2012;14 (2):103–4. doi: 10.3290/j.jad.a23445. [DOI] [PubMed] [Google Scholar]
- Rotta M, Bresciani P, Moura SK, Grande RH, Hilgert LA, Baratieri LN, Loguercio AD, Reis A. Effects of phosphoric acid pretreatment and substitution of bonding resin on bonding effectiveness of self-etching systems to enamel. J Adhes Dent. 2007;9 (6):537–45. [PubMed] [Google Scholar]
- Ruse ND, Shew R, Feduik D. In vitro fatigue testing of a dental bonding system on enamel. J Biomed Mater Res. 1995;29 (3):411–5. doi: 10.1002/jbm.820290316. [DOI] [PubMed] [Google Scholar]
- Ryou H, Niu LN, Dai L, Pucci CR, Arola DD, Pashley DH, Tay FR. Effect of biomimetic remineralization on the dynamic nanomechanical properties of dentin hybrid layers. J Dent Res. 2011;90 (9):1122–8. doi: 10.1177/0022034511414059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryou H, Romberg E, Pashley DH, Tay FR, Arola D. Nanoscopic dynamic mechanical properties of intertubular and peritubular dentin. J Mech Behav Biomed Mater. 2012;7 (spec iss):3–16. doi: 10.1016/j.jmbbm.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi RL. Review of the current status and challenges for dental posterior restorative composites: clinical, chemistry, and physical behavior considerations. Dent Mater. 2005;21 (1):3–6. doi: 10.1016/j.dental.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Sakaguchi RL, Powers JM. Craigs Restorative Dental Materials. 13. Mosby Publ; St Louis Missouri: 2012. pp. 87–88. [Google Scholar]
- Scherrer SS, Cesar PF, Swain MV. Direct comparison of the bond strength results of the different test methods: a critical literature review. Dent Mater. 2010;26 (2):e78–93. doi: 10.1016/j.dental.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Schuurs A. Pathology of the Hard Dental Tissues. 1. Wiley-Blackwell; UK: 2013. [Google Scholar]
- Shimada Y, Tagami J. Effects of regional enamel and prism orientation on resin bonding. Oper Dent. 2003;28 (1):20–7. [PubMed] [Google Scholar]
- Shinohara MS, de Oliveira MT, Di Hipólito V, Giannini M, de Goes MF. SEM analysis of the acid-etched enamel patterns promoted by acidic monomers and phosphoric acids. J Appl Oral Sci. 2006;14(6):427–35. doi: 10.1590/S1678-77572006000600008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soappman MJ, Nazari A, Porter JA, Arola D. A comparison of fatigue crack growth in resin composite, dentin and the interface. Dent Mater. 2007;23 (5):608–14. doi: 10.1016/j.dental.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Söderholm KJ. Time to abandon traditional bond strength testing? J Adhes Dent. 2012;14 (1):3–4. doi: 10.3290/j.jad.a23317. [DOI] [PubMed] [Google Scholar]
- Söderholm KJ, Geraldeli S, Shen C. What Do Microtensile Bond Strength Values of Adhesives Mean? J Adhes Dent. 2012;14(4):307–14. doi: 10.3290/j.jad.a22767. [DOI] [PubMed] [Google Scholar]
- Spencer P, Ye Q, Park J, Topp EM, Misra A, Marangos O, Wang Y, Bohaty BS, Singh V, Sene F, Eslick J, Camarda K, Katz JL. Adhesive/Dentin interface: the weak link in the composite restoration. Ann Biomed Eng. 2010;38 (6):1989–2003. doi: 10.1007/s10439-010-9969-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staninec M, Nguyen H, Kim P, Marshall GW, Rithchie RO, Marshall SJ. Four-point bending evaluation of dentin-composite interfaces with various stresses. Med Oral Patol Oral Cir Bucal. 2008;13(1):E81–4. [PubMed] [Google Scholar]
- Staninec M, Kim P, Marshall GW, Ritchie RO, Marshall SJ. Fatigue of dentin-composite interfaces with four-point bend. Dent Mater. 2008;24 (6):799–803. doi: 10.1016/j.dental.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens RI, Fatemi A, Stephens RR, Fuchs HO. Metal Fatigue in Engineering. 2. John Wiley and Sons, Inc; New York: 2001. [Google Scholar]
- Sunnegårdh-Grönberg K, van Dijken JW, Funegård U, Lindberg A, Nilsson M. Selection of dental materials and longevity of replaced restorations in Public Dental Health clinics in northern Sweden. J Dent. 2009;37 (9):673–8. doi: 10.1016/j.jdent.2009.04.010. [DOI] [PubMed] [Google Scholar]
- ten Cate JM. Biofilms, a new approach to the microbiology of dental plaque. Odontology. 2006;94 (1):1–9. doi: 10.1007/s10266-006-0063-3. [DOI] [PubMed] [Google Scholar]
- Torres CR, Barcellos DC, Pucci CR, de Lima GM, Rodrigues CM, Siviero M. Influence of methods of application of self-etching adhesive systems on adhesive bond strength to enamel. J Adhes Dent. 2009;11 (4):279–86. [PubMed] [Google Scholar]
- Van Meerbeek B, De Munck J, Yoshida Y, Inoue S, Vargas M, Vijay P, Van Landuyt K, Lambrechts P, Vanherle G. Buonocore memorial lecture. Adhesion to enamel and dentin: current status and future challenges. Oper Dent. 2003;28 (3):215–35. [PubMed] [Google Scholar]
- Van Noort R, Noroozi S, Howard IC, Cardew G. A critique of bond strength measurements. J Dent. 1989;17 (2):61–7. doi: 10.1016/0300-5712(89)90131-0. [DOI] [PubMed] [Google Scholar]
- Wood JD, Sobolewski P, Thakur V, Arola D, Nazari A, Tay FR, Pashley DH. Measurement of microstrains across loaded resin-dentin interfaces using microscopic moiré interferometry. Dent Mater. 2008;24 (7):859–66. doi: 10.1016/j.dental.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahyazadehfar M, Bajaj D, Arola DD. Hidden contributions of the enamel rods on the fracture resistance of human teeth. Acta Biomater. 2012 doi: 10.1016/j.actbio.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]