Abstract
Background and Objectives
The influence of the type of anesthesia on perioperative outcomes after bilateral total knee arthroplasty (BTKA) remains unknown. Therefore, we examined a large sample of BTKA recipients, hypothesizing that neuraxial anesthesia would favorably impact on outcomes.
Methods
We identified patient entries indicating elective BTKA between 2006 and 2010 in a national database; subgrouped them by type of anesthesia: general (G), neuraxial (N), or combined neuraxial-general (NG); and analyzed differences in demographics and perioperative outcomes.
Results
Of 15,687 identified procedures, 6.8% (n = 1066) were performed under N, 80.1% (n = 12,567) under G, and 13.1% (n = 2054) under NG. Comparing N to G and NG, patients in group N were, on average, younger (63.9, 64.6, and 64.8 years; P = 0.030) but did not differ in overall comorbidity burden. Patients in group N required blood product transfusions significantly less frequently (28.5%, 44.7%, 38.0%; P < 0.0001). In-hospital mortality, 30-day mortality, and complication rates tended to be lower in group N, without reaching statistical significance. After adjusting for covariates, N and NG were associated with 16.0% and 6.0% reduction in major complications compared with G, but only the reduced odds for the requirement of blood transfusions associated with N reached statistical significance (N vs G: odds ratio, 0.52 [95% CI, 0.45–0.61], P < 0.0001; NG vs G: odds ratio, 0.77 [95% CI, 0.69–0.86], P < 0.0001).
Conclusions
Neuraxial anesthesia for BTKA is associated with significantly lower rates of blood transfusions and, by trend, decreased morbidity. Although by itself the effect may be limited, N might be used within a multimodal approach to reduce complications after BTKA.
Simultaneous bilateral total knee arthroplasty (BTKA) is an increasingly used treatment modality for end-stage osteoarthritis in both knee joints.1 Although BTKA is believed to confer a number of advantages, such as reduced overall cost, reduced recovery time, and increased patient convenience,2 multiple studies have concluded that despite these advantages, the simultaneous bilateral approach is associated with increased perioperative morbidity and mortality.1,3,4 In an attempt to reduce adverse events, we recently proposed appropriate patient selection based on risk factor analysis.5 Although careful selection of appropriate candidates for this procedure may be one approach to improving outcomes, the identification of possible interventions capable of decreasing the associated risk remains critical yet largely unstudied to date.6 In this context, potential impacts of the anesthetic technique used during the procedure have not been evaluated. Previous publications have suggested that a potential benefit of regional anesthesia over general anesthesia may be present in the orthopedic surgical setting.7 Although some studies suggest beneficial results in terms of reduced overall morbidity and mortality, lower rates of thromboembolic phenomena, and reduced blood loss and operating time, the validity of these findings have been challenged because of limitations of small sample sizes and inclusion of historical data and data from only preferentially academic institutions.7–13 However, the low incidence of most studied complications renders randomized controlled studies at an institutional level relatively impractical, because of the extremely large sample size that would be required to capture low-incidence complications and to reach satisfactory statistical power. Therefore, we chose to access a large national database aiming to (1) analyze the utilization of different anesthesia techniques among patients undergoing BTKA and (2) examine, by means of comparative effectiveness research, whether there were differences between types of anesthesia in outcomes such as mortality, major in-hospital complications, need for blood product transfusion or mechanical ventilation, and length of hospitalization.
We hypothesized that the use of neuraxial anesthesia would favorably impact these outcomes in patients undergoing BTKA.
METHODS
Data Source, Ethics Approval
We obtained data for the period between 2006 and 2010 from Premier Perspective, Inc (Charlotte, North Carolina), an administrative database containing discharge information from approximately 400 acute care hospitals located throughout the United States. This database aggregates information on coding histories, patient billing, and hospital cost from approximately 45 million inpatient visits (at the time this study was conducted). All data received from hospitals undergo a rigorous 7-step validation process, involving approximately 100 validity and integrity assurance cross-checks before being incorporated in the Premier data warehouse.14 Data included are compliant with the Health Insurance Portability and Accountability Act15; this project was therefore exempt from requirements for consent by our institutional review board. Rigorous quality assurance and data validation checks are performed by the provider before distribution to ensure accuracy of entries.
Study Sample
We queried the database for entries with 2 occurrences of the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) procedure code for total knee arthroplasty (81.54) showing the same date of service, which has been considered a valid approach for identifying BTKA records in numerous studies.1,3,5,16 Only records indicating routine admission for elective surgery were included. The entries were separated into 3 groups using billing data: those for patients who underwent the procedure under general (G), neuraxial (N), or combined general and neuraxial anesthesia (NG). Records without indication of type of anesthesia were included in a separate group for the purpose of missing data analysis. The impact of missing data was assessed by performing a sensitivity analysis that consisted of (1) the inclusion and (2) the exclusion of patients with missing entries for type of anesthesia.17 No significant differences in results were observed.
Demographic Variables
We compared the characteristics of patients undergoing surgery under the different types of anesthesia. Patient-related characteristics were analyzed. Patient demographics included age, sex, and race (white, black, Hispanic, and other). The prevalence of comorbidities and overall comorbidity burden were assessed using the method described by Deyo et al.18
Complication Variables
For each group, the proportions of patients with major complications were computed by identifying cases that had ICD-9-CM diagnosis codes listed, consistent with such diagnosis (Appendix 1). The incidence of in-hospital as well as 30-day mortality was computed. Complications analyzed included wound infection, pulmonary embolism, cerebrovascular event, pulmonary compromise, cardiac complications (except myocardial infarction), pneumonia, other infectious complications, acute renal failure, gastrointestinal complications, and acute myocardial infarction.
Furthermore, the incidence of the use of blood product transfusion and mechanical ventilation was recorded using ICD-9 and billing codes (Appendix 1). Differences in length of hospital stay were analyzed. The length of hospital stay was also dichotomized based on 75th percentile. Entries above the 75th percentile were categorized as prolonged hospitalization.
Statistical Analysis
The study goal was to analyze whether the type of anesthesia is associated with differences in perioperative outcomes. All statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, North Carolina). The weighting procedure developed by Centers for Medicare and Medicaid Services and made available by Premier was utilized to derive nationally representative estimates from the available data.14 To facilitate analysis of weighted data, SAS procedures SURVEYMEANS, SURVEYFREQ, SURVEYREG, and SURVEYLOGISTIC were utilized for descriptive analyses and modeling efforts.
Univariate Analysis
Weighted means and percentages were described for continuous and categorical variables, respectively. For variables that had a skewed distribution, median and interquartile range were estimated. For a continuous variable, 95% confidence intervals (CIs) were shown as a measure of variability. χ2 Test was performed to evaluate the association of 2 categorical variables. One-way analysis of variance F test was used to compare means for a continuous variable between more than 2 groups. The logistic regression was performed to assess the association between anesthesia type and outcomes, and unadjusted odds ratio (OR), 95% CI, and P values were reported.
Multivariate Regression Analysis
Binary outcomes of incidence of complications, use of blood product transfusion, mechanical ventilation, and prolonged length of hospital stay as defined above were considered. For the purpose of the evaluation of major complications, a combined outcome variable was created, which included the complications listed above. For each outcome, logistic regression was used to evaluate its association with the type of anesthesia, while controlling for age, sex, race, and comorbidity burden. Adjusted ORs, 95% CI, and P values were reported. A test of model discrimination using the C-statistic and a test of model calibration using the Hosmer-Lemeshow (H-L) test were performed for each model.19 The conventional threshold of statistical significance (ie, 2-sided P G 0.05) was used to determine significance of variables.
RESULTS
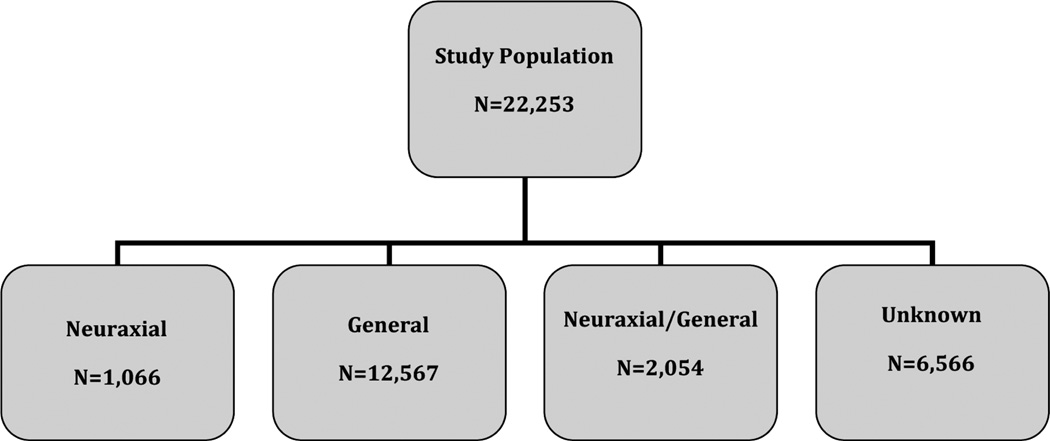
We identified 22,253 entries of patients undergoing simultaneous BTKA and indicating routine admission: 29.5% (n = 6566) did not have information on anesthesia type (Fig. 1). Of the remaining 70.5% (n = 15,687), 6.8% (n = 1066) were performed under N, 80.1% (n = 12,567) under G, and 13.1% (n = 2054) under N+G anesthesia, respectively. Table 1 details the patient and health care systemYrelated demographics of patients by anesthesia type utilized.
FIGURE 1.
The flowchart depicts the study population and subgroup distribution.
TABLE 1.
Patient and Health Care System–Related Characteristics by Anesthesia Type
| Variable | Category | Neuraxial | General | Neuraxial/General | P |
|---|---|---|---|---|---|
| Unweighted n | 1066 | 12567 | 2054 | — | |
| % of total | 6.8% | 80.1% | 13.1% | — | |
| Average comorbidity index (CI) | 0.5 (0.5–0.6) | 0.6 (0.5–0.6) | 0.5 (0.5–0.6) | 0.9911 | |
| Deyo comorbidity index categories, % | 0 | 68.8 | 66.8 | 68.3 | 0.3142 |
| 1 | 15.4 | 15.0 | 15.6 | 0.3142 | |
| 2 | 12.5 | 14.3 | 12.5 | 0.3142 | |
| ≥3 | 3.4 | 3.9 | 3.6 | 0.3142 | |
| Average age (CI), y | 63.9 (63.3–64.5) | 64.6 (64.4–67.8) | 64.8 (64.3–65.3) | 0.0298 | |
| Age group, % | ≤44 y | 1.2 | 1.4 | 2.0 | 0.032 |
| 45–54 y | 14.9 | 13.4 | 12.8 | 0.032 | |
| 55–64 y | 37.0 | 34.2 | 31.9 | 0.032 | |
| 65–74 y | 32.5 | 34.4 | 36.6 | 0.032 | |
| ≥75 y | 14.3 | 16.5 | 16.8 | 0.032 | |
| Sex, % | Female | 58.1 | 57.9 | 55.9 | 0.3141 |
| Male | 41.9 | 42.1 | 44.1 | 0.3141 | |
| Race, % | White | 77.8 | 75.9 | 74.5 | <0.0001 |
| Black | 5.6 | 5.4 | 4.6 | <0.0001 | |
| Other | 0.3 | 5.6 | 0.6 | <0.0001 | |
| Missing data | 16.3 | 13.1 | 20.4 | <0.0001 |
Patients in group N were, on average, younger than those operated on under G or N+G. There were no significant differences in overall comorbidity burden or the prevalence of individual comorbidities (Table 2).
TABLE 2.
Prevalence of Preexisting Comorbidities Among Patients in the Different Types of Anesthesia Groups
| Neuraxial, % | General, % | Neuraxial/General, % | P | |
|---|---|---|---|---|
| Myocardial infarction | 2.4 | 2.5 | 3.4 | 0.0985 |
| Peripheral vascular disease | 0.9 | 1.2 | 1.3 | 0.6998 |
| Dementia | 0.2 | 0.1 | 0.1 | 0.2759 |
| Chronic obstructive pulmonary disease | 12.3 | 11.6 | 11.3 | 0.7097 |
| Uncomplicated diabetes | 14.5 | 16.8 | 15.0 | 0.0619 |
| Complicated diabetes | 0.7 | 1.1 | 0.5 | 0.0623 |
| Cancer | 1.2 | 1.5 | 1.9 | 0.2702 |
Table 3 depicts the incidence of in-hospital and 30-day mortality, major in-hospital complications, incidence of blood product transfusion, mechanical ventilation, and the median length of stay. No difference was found in regard to in-hospital mortality or 30-day mortality between groups. Despite trends toward lower morbidity for studied complications, no significant differences in the unadjusted rates of major in-hospital complications were recorded across the groups. However, patients in group N required blood product transfusion significantly less frequently. Although the requirement for mechanical ventilation was lower in patients in the N and N+G groups, the difference did not reach statistical significance. Compared with G, N and N+G were associated with shorter length of stay.
TABLE 3.
Incidences of Complications, Use of Mechanical Ventilation, and Blood Product Transfusion, as Well as Median Length of Stay by Type of Anesthesia
| Neuraxial | General | Neuraxial/General | P | |
|---|---|---|---|---|
| In-hospital mortality, % | 0.1 | 0.1 | 0.1 | 0.9260 |
| 30-d Mortality, % | 0.1 | 0.1 | 0.1 | 0.8222 |
| Wound infection, % | 0.1 | 0.1 | 0.1 | 0.6429 |
| Pulmonary embolism, % | 1.5 | 0.9 | 0.6 | 0.0998 |
| Cerebrovascular event, % | 0.1 | 0.3 | 0.2 | 0.6001 |
| Pulmonary compromise, % | 0.5 | 0.8 | 0.9 | 0.4865 |
| Cardiac (non–myocardial infarction), % | 5.2 | 5.9 | 5.5 | 0.5589 |
| Pneumonia, % | 0.7 | 0.9 | 0.8 | 0.6789 |
| All infections, % | 3.2 | 4.5 | 4.6 | 0.1515 |
| Acute renal failure, % | 1.9 | 2.7 | 2.3 | 0.2396 |
| Gastrointestinal complication, % | 1.1 | 1.3 | 1.3 | 0.8475 |
| Acute myocardial infarction, % | 0.2 | 0.4 | 0.4 | 0.6062 |
| Blood transfusion, % | 28.5 | 44.7 | 38.0 | <0.0001 |
| Mechanical ventilation, % | 0.5 | 0.9 | 0.7 | 0.2171 |
| Length of stay, median (interquartile range), d | 2.8 (2.1–4.1) | 3.1 (2.4–4.3) | 3.1 (2.5–4.2) | <0.0001 |
Tables 4 and 5 show the results of the univariate and multivariate regression analyses, respectively. In the logistic regressions, unadjusted and adjusted ORs of anesthesia type were similar. Specifically, in the multivariate analysis, N and NG were associated with lower, although insignificantly reduced, odds for major complications compared with G. However, the adjusted risk for blood product transfusion was significantly lower in patients receiving N or N+G (OR, 0.52 [CI, 0.45–0.61] for N vs G, P < 0.0001; OR, 0.77 [CI, 0.69–0.86] for NG vs G, P < 0.0001). Moreover, a trend toward decreased risk for mechanical ventilation became apparent in N and N+G. The odds for increased length of hospitalization (above the 75th percentile) were not different between groups. When analyzing outcomes by including the group with unknown anesthesia type, the results did not change significantly.
TABLE 4.
Results From the Univariate Regression, ORs, and 95% CIs (Reference = 1)
| Cumulative Complications (C-Statistics = 0.64, H-L = 0.30) |
Transfusion (C-Statistics = 0.62, H-L = 0.38) |
Mechanical Ventilation (C-Statistics = 0.63, H-L = 0.34) |
Length of Stay >75th Percentile (C-Statistics = 0.61, H-L = 0.94) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Category | Comparison | Reference | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P |
| Anesthesia technique | N | G | 0.86 (0.69–1.07) | 0.1710 | 0.49 (0.42–0.57) | <0.0001 | 0.49 (0.19–1.24) | 0.1309 | 0.91 (0.77–1.07) | 0.2472 |
| NG | G | 0.99 (0.85–1.15) | 0.8822 | 0.76 (0.68–0.84) | <0.0001 | 0.76 (0.42–1.38) | 0.3641 | 0.97 (0.86–1.09) | 0.5803 | |
Cumulative complications comprise the following events: in-hospital mortality, 30-day mortality, wound infections, pulmonary embolism, cerebrovascular event, cardiac complications including myocardial infarction, pneumonia, all other infections, acute renal failure, and gastrointestinal complications.
N indicates neuraxial anesthesia; G, general anesthesia; NG, neuraxial and general anesthesia (combined).
TABLE 5.
Results From the Multivariate Regression, ORs, and 95% CIs (Reference = 1)
| Cumulative Complications (C-Statistics = 0.64, H-L = 0.2174) |
Transfusion (C-Statistics = 0.62, H-L = 0.0241) |
Mechanical Ventilation (C-Statistics = 0.63, H-L = 0.0994) |
Length of Stay >75th percentile (C-statistics = 0.61, H-L = 0.0025) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Category | Comparison | Reference | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P |
| Anesthesia technique | N | G | 0.87 (0.70–1.08) | 0.2014 | 0.52 (0.45–0.61) | <0.0001 | 0.47 (0.18–1.21) | 0.1161 | 1.07 (0.91–1.26) | 0.4100 |
| NG | G | 0.94 (0.81–1.10) | 0.4432 | 0.77 (0.69–0.86) | <0.0001 | 0.71 (0.39–1.29) | 0.2555 | 1.09 (0.97–1.23) | 0.1583 | |
| Age | 55–64 y | ≤45 | 1.84 (1.07–3.19) | 0.0288 | 1.40 (1.02–1.94) | 0.039 | 0.38 (0.10–1.43) | 0.1544 | 1.01 (0.70–1.47) | 0.9427 |
| 65–74 y | ≤45 | 2.56 (1.48–4.41) | 0.0008 | 2.02 (1.47–2.79) | <0.0001 | 0.47 (0.13–1.75) | 0.2594 | 1.19 (0.82–1.72) | 0.3615 | |
| ≥75 y | ≤45 | 4.80 (2.78–8.31) | <0.0001 | 3.09 (2.23–4.29) | <0.0001 | 0.46 (0.12–1.76) | 0.2541 | 1.69 (1.16–2.46) | 0.0064 | |
| Sex | Female | Male | 0.73 (0.66–0.81) | <0.0001 | 1.55 (1.44–1.68) | <0.0001 | 0.96 (0.65–1.41) | 0.8259 | 1.02 (0.94–1.11) | 0.6084 |
| Ethnicity | Black | White | 1.03 (0.81–1.30) | 0.8287 | 1.06 (0.91–1.24) | 0.4329 | 1.09 (0.47–2.32) | 0.9077 | 1.02 (0.85–1.21) | 0.8438 |
| Hispanic | White | 0.66 (0.49–0.90) | 0.0074 | 5.21 (4.20–6.46) | <0.0001 | 0.18 (0.03–1.33) | 0.0928 | 12.12 (9.74–15.10) | <0.0001 | |
| Other | White | 1.29 (1.11–1.50) | 0.0008 | 1.78 (1.59–1.99) | <0.0001 | 1.36 (0.79–2.34) | 0.2746 | 1.47 (1.30–1.65) | <0.0001 | |
| Deyo index group | 1 | 0 | 1.46 (1.27–1.68) | <0.0001 | 1.12 (1.01–1.24) | 0.0357 | 1.90 (1.22–3.24) | 0.0059 | 1.19 (1.06–1.34) | 0.0027 |
| 2 | 0 | 1.39 (1.20–1.60) | <0.0001 | 1.13 (1.02–1.26) | 0.0224 | 1.77 (1.04–3.01) | 0.0341 | 1.47 (1.32–1.66) | <0.0001 | |
| ≥3 | 0 | 1.74 (1.36–2.23) | <0.0001 | 1.15 (0.95–1.39) | 0.1405 | 3.14 (1.54–6.39) | 0.0016 | 1.49 (1.21–1.83) | 0.0001 | |
Cumulative complications comprise the following events: in-hospital mortality, 30-day mortality, wound infections, pulmonary embolism, cerebrovascular event, cardiac complications including myocardial infarction, pneumonia, all other infections, acute renal failure, and gastrointestinal complications.
N indicates neuraxial anesthesia; G, general anesthesia; NG, neuraxial and general anesthesia (combined).
The C-statistics were 0.64, 0.62, 0.63, and 0.61, and P values for H-L goodness of fit were 0.21, 0.02, 0.10, and 0.01 for cumulative complication, transfusion, mechanical ventilation, and prolonged length of stay, respectively.
DISCUSSION
When considering a large national patient sample, the utilization of neuraxial anesthesia for BTKA is associated with trends toward decreased rates and risk of perioperative major complications, without reaching statistical significance, however. We found decreased rates and odds for perioperative blood product transfusion among neuraxial anesthesia recipients compared with those receiving general anesthesia. There was no impact of the choice of anesthetic technique on the need for mechanical ventilation or the length of hospitalization.
The potential impact of anesthetic technique on complication incidence seems limited in our sample. However, our results indicate an overall trend toward beneficial impact of neuraxial anesthesia over the general approach. This might indicate that, despite the comparatively large number of subjects included, an even larger sample size would have been required to achieve statistical significance. Although to our knowledge, no study has focused on this issue in the setting of BTKA, some evidence indicates advantages of the neuraxial technique with regard to complication incidence and mortality. Two of the largest studies, a meta-analysis by Rodgers et al7 including 141 trials with a total of 9559 patients, and an administrative database analysis by Wijeysundera et al,8 retrospectively examining 259,037 patient records, found various small overall benefits for regional anesthesia compared with general anesthesia. Whereas Rodgers et al7 detected a mortality reduction by about one third in patients receiving epidural anesthesia, the difference in the second study mentioned was not significant. Unfortunately, despite the large sample sizes, these studies were not adequately powered to allow for subgroup analysis toward specific surgical specialties or procedures.7,8 In addition, the latter did not analyze the incidence of perioperative complications, except the need for mechanical ventilation.
Two meta-analyses by Mauermann et al10 and Hu et al9 subsequently limited their scope to outcomes after elective hip and, respectively, hip or knee replacement only. Although they were able to demonstrate advantages of neuraxial anesthesia with regard to perioperative complications (most notably reduced incidence of thromboembolic phenomena, reduced operating time, and lower blood loss), criticisms about these studies include issues regarding publication bias, small sample sizes, inclusion of data from small specialized centers, analysis of limited outcomes, and inclusion of studies that do not reflect current medical practice.9,10
The majority of patients in our sample received general anesthesia during their surgeryVonly 6.8% of patients underwent surgery under neuraxial anesthesia alone, and 13.1% received a combination of neuraxial and general anesthesia. Interestingly, the odds for most outcomes of the combined N+G group lie between those of G only and N only, suggesting an intrinsic positive effect of neuraxial anesthesia. However, it must be noted that some complication rates are unchanged or minimally higher in the N+G group. Yet, these observations raise the question of whether the observed benefits in outcome can be associated with the performance of neuraxial anesthesia itself, the avoidance of general anesthesia, or a combination of both. The positive impact of neuraxial anesthesia can probably be partly attributed to some of its described beneficial effects. With regard to reduced rates of thromboembolic complications, including myocardial infarction, deep venous thrombosis, or pulmonary embolism, antithrombotic properties of regional anesthesia come to mind, especially in light of the predominance of this complication entity among the orthopedic population. 10,19 Conversely, neuraxial anesthesia appears capable of reducing not only thromboembolism but also bleeding and thus the demand for blood product transfusion.
In our study, neuraxial anesthesia was indeed associated with a highly significantly decreased incidence of perioperative blood product transfusion. Substantial blood loss is recognized as a very common complication during BTKA. Although various methods to reduce or compensate for blood loss have been proposed, including administration of erythropoietin, tranexamic acid, or topical hemostatic agents, the requirement for intraoperative and postoperative allogeneic or autologous blood transfusions remains disproportionately high when compared with unilateral procedures.20–23 In a previous study, the incidence of posthemorrhagic anemia was found to be as high as 28.6% after BTKA, compared with 15.3% after unilateral TKA.16 In the previously mentioned study by Rodgers et al,7 a comparable reduction in incidence of blood product transfusion was noticed.7 A meta-analysis by Guay,24 including 24 studies on surgical blood loss and blood transfusion requirements after different types of surgery, similarly concluded that neuraxial anesthesia confers a highly significant reduction in blood loss and transfusion incidence when utilized for total hip replacement and spinal fusion, but not for other procedures such as hip fracture surgery, lumbar disk surgery, peripheral vascular surgery, prostatectomy, cesarean delivery, or bowel surgery.24 Reasons for this finding have to remain speculative. Associations with improved analgesia, sympathicolysis, decreased vascular tone, and, subsequently, decreased blood pressure in the lower extremities seem intuitive, yet there is no conclusive evidence available to this date.
Large administrative databases are increasingly used to explore a wide variety of different questions in anesthesiology research. The specific advantage of the Premier Perspective database is its relatively high level of detail, allowing for the distinction of perioperative management techniques, including the type of anesthesia used. To ensure adequate validity, data undergo rigorous integrity inspection by the vendor before it is added to the data set.14 A large number of published studies across various medical specialties demonstrate the high level of confidence in this database.25–27 In addition, we performed extensive model diagnostics to ensure the validity of our methodology. Nonetheless, our study is limited by a number of factors that are related to secondary database analysis. First, we were not able to capture intraoperative events such as total amount of blood loss or medications administered to the subjects, including anticoagulants or cardioprotective agents. The outcomes are, however, evaluated under realistic settings rather than in an experimental setup and therefore reflect “real world” level of care as demanded by comparative effectiveness research standards. Second, because of the nature of the database, post-discharge events, except death within 30 days, cannot be taken into consideration because only events recorded during the index admission in which the surgery was performed are recorded. Third, as comorbidities and complications were identified using ICD-9-CM codes (see Appendix 1), there is a possibility that some of the events are not adequately captured because of coding errors or inconsistencies, despite quality checks by the vendor. However, this effect is presumably attenuated by the equal distribution of this coding bias across the entire data collection construct. Finally, we did not analyze anesthesia-related complications, including postdural puncture headache, blood vessel and nerve damage, damages to oropharyngeal structures during intubation, or failed intubation because they are not provided in the database. Although anesthesia-associated morbidity is known to be very low in comparison to other complications studied, contraindications to either method can certainly prevail and obviate the utilization of a particular technique, for instance, regional anesthesia in anticoagulated patients or general anesthesia in those suffering from pulmonary comorbidity.
In conclusion, to our best knowledge, our study is the first to analyze the impact of various types of anesthesia on perioperative outcomes after BTKA.We were able to depict some of the advantages of neuraxial or combined neuraxial-general versus general anesthesia alone during BTKA. Being considered a high-risk procedure, careful patient selection and perioperative management have proven useful tools to improve outcomes. Although by itself the impact of neuraxial anesthesia on outcomes may be limited, it may be considered as one part of various interventions to reduce complications after simultaneous BTKA. With approximately only 1 in 5 patients receiving some form of neuraxial anesthesia, a tremendous potential for growth becomes apparent. Our data support the promotion of the use of neuraxial anesthesia for the management of BTKA patients, but also point out the demand for further research to quantify the magnitude of its beneficial effects.
Acknowledgments
This work was supported with funds from The Kellen Clinician-Scientist Career Development Award and the Hospital for Special Surgery, Department of Anesthesiology (S.G.M.). Contribution of M.M., Y.-L.C., and X.S. on this project was supported, in part, by funds from the Clinical Translational Science Center, National Center for Advancing Translational Sciences (NCATS) grant no. UL1-RR024996, and the Center for Education, Research, and Therapeutics, Agency for Healthcare Research and Quality (AHRQ) grant no. U18 HSO16-75.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding sources, NCATS and AHRQ, which are based in Rockville, MD.
This work is attributed to the Department of Anesthesiology, Hospital for Special Surgery, Weill Medical College of Cornell University, New York, NY.
APPENDIX 1
ICD-9-CM Diagnosis Codes for Major Complications
| Complications | ICD-9-CM Diagnosis Codes |
|---|---|
| Wound infection | 998.5x |
| Pulmonary embolism | 415.1 |
| Cerebrovascular event | 433.01, 433.11, 433.21, 433.31, 433.81, 433.91, 434.01, 434.11, 434.91, 997.02 |
| Pulmonary compromise | 514, 518.4, 518.5, 518.81, 518.82 |
| Cardiac (non–myocardial infarction) | 426.0, 427.41, 427.42, 429.4, 997.1, 427.4, 427.3, 427.31, 427.32 |
| Pneumonia | 481, 482.00- 482.99, 483, 485, 486, 507.0, 997.31, 997.39 |
| All infections | 590.1, 590.10, 590.11, 590.8, 590.81, 590.2, 590.9, 595.0, 595.9, 599.0, 567.0 |
| 480, 480.0, 480.1, 480.2, 480.8, 480.9, 481, 482.0, 482.1, 482.2, 482.3, 482.30, 482.31, 482.32, 482.39, 482.4, 482.40, 482.41, 482.42, 482.49, 482.5, 482.8, 482.81, 482.82, 482.83, 482.84, 482.89, 482.9, 483, 483.0, 483.1, 483.8, 485, 486, 487, 997.31 | |
| 038, 038.0, 038.1, 038.10, 038.11, 038.12, 038.19, 038.2, 038.3, 038.4, 038.40, 038.41, 038.42, 038.43, 038.44, 038.49, 038.8, 038.9, 790.7 | |
| 998.0, 958.4, 998.5, 998.59, 998.89, 785, 785.50, 785.52, 785.59, 999.39, 999.31, 999.3 | |
| Acute renal failure | 584, 584.5, 584.9 |
| Gastrointestinal complication | 997.4, 560.1, 560.81, 560.9, 536.2, 537.3 |
| Acute myocardial infarction | 410.XX |
| Blood transfusion | 99.0, 99.01, 99.02, 99.03, 99.04, 99.05, 99.06, 99.07, 99.08, 99.09 (HCPCS codes) P9010, P9011, P9012, P9016, P9017, P9019, P9020, P9021, P9022, P9023, P9031, P9032, P9033, P9034, P9035, P9036, P9037, P9038, P9039, P9040 |
| Mechanical ventilation | 93.90, 96.7, 96.70, 96.71, 96.72 (CPT code) 94002, 94656, 94003, 94657 |
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Memtsoudis SG, Besculides MC, Reid S, Gaber-Baylis LK, Gonzalez Della Valle A. Trends in bilateral total knee arthroplasties: 153,259 discharges between 1990 and 2004. Clin Orthop Relat Res. 2009;467:1568–1576. doi: 10.1007/s11999-008-0610-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kovacik MW, Singri P, Khanna S, Gradisar IA. Medical and financial aspects of same-day bilateral total knee arthroplasties. Biomed Sci Instrum. 1997;33:429–434. [PubMed] [Google Scholar]
- 3.Memtsoudis SG, Ma Y, Gonzalez Della Valle A, et al. Perioperative outcomes after unilateral and bilateral total knee arthroplasty. Anesthesiology. 2009;111:1206–1216. doi: 10.1097/ALN.0b013e3181bfab7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parvizi J, Sullivan TA, Trousdale RT, Lewallen DG. Thirty-day mortality after total knee arthroplasty. J Bone Joint Surg Am. 2001;83-A:1157–1161. doi: 10.2106/00004623-200108000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Memtsoudis SG, Ma Y, Chiu YL, Poultsides L, Gonzalez Della Valle A, Mazumdar M. Bilateral total knee arthroplasty: risk factors for major morbidity and mortality. Anesth Analg. 2011;113:784–790. doi: 10.1213/ANE.0b013e3182282953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jules-Elysee K, Wilfred SE, Memtsoudis SG, et al. Steroid modulation of cytokine release and desmosine levels in bilateral total knee replacement: a prospective double-blind, randomized controlled trial. J Bone Joint Surg Am. 2012 doi: 10.2106/JBJS.K.00995. In press. [DOI] [PubMed] [Google Scholar]
- 7.Rodgers A, Walker N, Schug S, et al. Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: results from overview of randomised trials. BMJ. 2000;321:1493. doi: 10.1136/bmj.321.7275.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wijeysundera DN, Beattie WS, Austin PC, Hux JE, Laupacis A. Epidural anaesthesia and survival after intermediate-to-high risk non-cardiac surgery: a population-based cohort study. Lancet. 2008;372:562–569. doi: 10.1016/S0140-6736(08)61121-6. [DOI] [PubMed] [Google Scholar]
- 9.Hu S, Zhang ZY, Hua YQ, Li J, Cai ZD. A comparison of regional and general anaesthesia for total replacement of the hip or knee: a meta-analysis. J Bone Joint Surg Br. 2009;91:935–942. doi: 10.1302/0301-620X.91B7.21538. [DOI] [PubMed] [Google Scholar]
- 10.Mauermann WJ, Shilling AM, Zuo Z. A comparison of neuraxial block versus general anesthesia for elective total hip replacement: a meta-analysis. Anesth Analg. 2006;103:1018–1025. doi: 10.1213/01.ane.0000237267.75543.59. [DOI] [PubMed] [Google Scholar]
- 11.Brueckner S, Reinke U, Roth-Isigkeit A, Eleftheriadis S, Schmucker P, Siemens HJ. Comparison of general and spinal anesthesia and their influence on hemostatic markers in patients undergoing total hip arthroplasty. J Clin Anesth. 2003;15:433–440. doi: 10.1016/s0952-8180(03)00082-5. [DOI] [PubMed] [Google Scholar]
- 12.Planes A, Vochelle N, Fagola M, Feret J, Bellaud M. Prevention of deep vein thrombosis after total hip replacement. The effect of low-molecular-weight heparin with spinal and general anaesthesia. J Bone Joint Surg Br. 1991;73:418–422. doi: 10.1302/0301-620X.73B3.1670442. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell D, Friedman RJ, Baker JD, 3rd, Cooke JE, Darcy MD, Miller MC., 3rd Prevention of thromboembolic disease following total knee arthroplasty. Epidural versus general anesthesia. Clin Orthop Relat Res. 1991;(269):109–112. [PubMed] [Google Scholar]
- 14.Premier Inc. Premier Perspective Database. Charlotte, NC: Premier Inc; 2011. [Google Scholar]
- 15.US Department of Health and Human Services. OCR Privacy Brief: Summary of the HIPAA Privacy Rule. Washington, DC: Office for Civil Rights, HIPAA Compliance Assistance; 2003. [Google Scholar]
- 16.Memtsoudis SG, Gonzalez Della Valle A, Besculides MC, Gaber L, Sculco TP. In-hospital complications and mortality of unilateral, bilateral, and revision TKA: based on an estimate of 4,159,661 discharges. Clin Orthop Relat Res. 2008;466:2617–2627. doi: 10.1007/s11999-008-0402-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groenwold RH, Donders AR, Roes KC, Harrell FE, Jr, Moons KG. Dealing with missing outcome data in randomized trials and observational studies. Am J Epidemiol. 2012;175:210–217. doi: 10.1093/aje/kwr302. [DOI] [PubMed] [Google Scholar]
- 18.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 19.Hahnenkamp K, Theilmeier G, Van Aken HK, Hoenemann CW. The effects of local anesthetics on perioperative coagulation, inflammation, and microcirculation. Anesth Analg. 2002;94:1441–1447. doi: 10.1097/00000539-200206000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Cushner FD, Lee GC, Scuderi GR, Arsht SJ, Scott WN. Blood loss management in high-risk patients undergoing total knee arthroplasty: a comparison of two techniques. J Knee Surg. 2006;19:249–253. doi: 10.1055/s-0030-1248114. [DOI] [PubMed] [Google Scholar]
- 21.Sepah YJ, Umer M, Ahmad T, Nasim F, Chaudhry MU, Umar M. Use of tranexamic acid is a cost effective method in preventing blood loss during and after total knee replacement. J Orthop Surg Res. 2011;6:22. doi: 10.1186/1749-799X-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aktas E, Kaya AV, Deveci MA, Ozler K, Akdeniz H, Yildirim H. Polysaccharide hemostatic system reduces blood loss in high-body-mass-index patients undergoing simultaneous bilateral total knee arthroplasty. J Orthop Sci. 2012;17:432–436. doi: 10.1007/s00776-012-0221-0. [DOI] [PubMed] [Google Scholar]
- 23.Parvizi J, Chaudhry S, Rasouli MR, et al. Who needs autologous blood donation in joint replacement? J Knee Surg. 2011;24:25–31. doi: 10.1055/s-0031-1275404. [DOI] [PubMed] [Google Scholar]
- 24.Guay J. The effect of neuraxial blocks on surgical blood loss and blood transfusion requirements: a meta-analysis. J Clin Anesth. 2006;18:124–128. doi: 10.1016/j.jclinane.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 25.Stulberg JJ, Delaney CP, Neuhauser DV, Aron DC, Fu P, Koroukian SM. Adherence to surgical care improvement project measures and the association with postoperative infections. JAMA. 2010;303:2479–2485. doi: 10.1001/jama.2010.841. [DOI] [PubMed] [Google Scholar]
- 26.Blanchette CM, Wang PF, Joshi AV, Kruse P, Asmussen M, Saunders W. Resource utilization and costs of blood management services associated with knee and hip surgeries in US hospitals. Adv Ther. 2006;23:54–67. doi: 10.1007/BF02850347. [DOI] [PubMed] [Google Scholar]
- 27.Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353:349–361. doi: 10.1056/NEJMoa041895. [DOI] [PubMed] [Google Scholar]