Summary
Phosphoinositides have been implicated in synaptic vesicle recycling largely based on studies of enzymes that regulate phosphoinositide synthesis and hydrolysis. One such enzyme is Synaptojanin1, a multifunctional protein conserved from yeast to humans, which contains two phospho-inositol phosphatase domains and a proline-rich domain. Genetic ablation of Synaptojanin1 leads to pleiotropic defects in presynaptic function, including accumulation of free clathrin-coated vesicles and delayed vesicle re-availability, implicating this enzyme in post-endocytic uncoating of vesicles. To further elucidate the role of Synaptojanin1 at nerve terminals, we performed quantitative synaptic vesicle recycling assays in synj1−/− neurons. Our studies show that Synaptojanin1 is also required for normal vesicle endocytosis. Defects in both endocytosis and post-endocytic vesicle re-availability can be fully restored upon reintroduction of Synaptojanin1. However, expression of Synaptojanin1 with mutations abolishing catalytic activity of each phosphatase domain reveals that the dual action of both domains is required for normal synaptic vesicle internalization and re-availability.
Introduction
Phosphorylated derivatives of phosphatidylinositol (phosphoinositides) have been implicated in many aspects of cell physiology, including signal transduction, organelle trafficking, cytoskeletal dynamics, and the regulation of membrane permeability. More specifically, phosphatidylinositol-(4,5)-bisphosphate (PI(4,5)P2), has been shown to be critically implicated in endocytosis (Di Paolo and De Camilli, 2006). PI(4,5)P2 has been shown to be essential for the recruitment of a variety of endocytic proteins and endocytic clathrin adaptors, including AP-2 and AP180, to the plasma membrane (Haucke, 2005; Brett and Traub, 2006; Zoncu et al., 2007). The biosynthesis and dephosphorylation of PI(4,5)P2 have been implicated in the recycling of vesicles at the presynaptic terminal, where phosphatidylinositol phosphate kinase type Iγ (PIPKIγ) and Synaptojanin 1 account for the majority of these two activities respectively (Cremona et al., 1999; Di Paolo et al., 2004; McPherson et al., 1996; Wenk et al., 2001). Absence of the PIPKIγ enzyme results in slower endocytosis in cultured cortical neurons (Di Paolo et al., 2004), suggesting that the biosynthesis of PI(4,5)P2 is necessary in order to maintain normal endocytic rates. However, the consequences of inadequate Synaptojanin 1-mediated dephosphorylation of phosphoinositides on the synaptic vesicle cycle are less clear. Synaptojanin was first identified as an interactor for dynamin binding proteins, and thus proposed to be a functional neighbor of dynamin (McPherson et al., 1994a and b). In mammals, Synj1 occurs as two alternatively spliced isoforms, a 145 kDa (Synj1-145) and a 170 kDa (Synj1-170) species. While both isoforms are expressed in different tissues, the Synj1-145 (hereafter simply referred to as Synj1) is expressed at very high levels in the nervous system, where its concentration increases in parallel with synapse development and maturation (McPherson et al., 1996; Ramjaun and McPherson, 1996). Synj1 contains three functional domains: a suppressor of actin1 (Sac-1)-like phosphatase domain, an inositol-5-phosphatase domain, and a proline-rich domain (PRD) (McPherson et al., 1996). The 5-phosphatase domain specifically hydrolyzes phosphates at the 5′ position of the inositol ring (Tsujishita et al., 2001; Whisstock et al., 2002), and the Sac1-like domain harbors less selective phosphoinositide phosphatase activity (Guo et al., 1999). The proline-rich domain can bind to cognate SH3 domains on partners such as endophilin and amphiphysin (McPherson et al., 1996; Micheva et al., 1997; Ringstad et al., 1997). Another synaptojanin isoform Synaptojanin 2 (Synj2) has a similar domain structure but more widespread tissue distribution (Nemoto et al., 1997; Khvotchev and Sudhof, 1998). Synj2 is expressed at much lower concentrations in the nervous system, where its function may partially overlap with that of Synj1 (Nemoto et al., 2001).
In mice, deletion of the synj1 gene causes early post-natal lethality, elevation of steady state PI(4,5)P2 in cortical cultures, accumulation of clathrin-coated vesicles at nerve terminals, greater synaptic depression (Cremona et al., 1999; Lüthi et al., 2001), and a delay in post-endocytic vesicle re-availability or “repriming” (Kim et al., 2002). These observations suggested that the role of Synj1 in dephosphorylation of phosphoinositides such as PI(4,5)P2, which would allow shedding of clathrin coat components after disruption of the clathrin lattice following fission, may underlie the pleiotropic defects in the Synj1 KO. The critical role of Synaptojanin in synapse function was further corroborated by the isolation of synj mutants with related phenotypes in forward genetic screens for synaptic transmission in C. elegans (Harris et al., 2000; Schuske et al., 2003), Drosophila (Verstreken et al., 2003; Dickman et al., 2005), and zebrafish (Van Epps et al., 2004).
Although the importance of Synaptojanin in the process of synaptic vesicle recycling is well recognized, the extent to which Synj1 is involved at distinct steps of the vesicle cycle, such as exocytosis, endocytosis, and post-endocytic vesicle re-availability, is unclear. It is also not known what role each of the functional domains of Synaptojanin plays in the vesicle cycle, and how their absence may contribute to the various defects observed in mice and other species. Here using a combination of synapto-pHluorin (spH, Sankaranarayanan and Ryan, 2000) and FM 4-64 dye uptake and re-release assays (Ryan & Smith 1995), we probed the kinetics of vesicle exocytosis, endocytosis, re-availability (repriming), and the size of the total recycling vesicle pool in synj1−/− synapses. Our results indicate that in addition to its previously described role in uncoating (Cremona et al., 1999; Gad et al., 2000) and re-availability (Kim et al., 2002) of newly internalized vesicles, Synj1 is critical for the endocytic step itself, over a broad range of stimuli. Our findings also reveal that the 5-phosphatase activity of Synj1 is required in endocytosis for both brief and prolonged stimuli. The Sac1-like activity and the Synj1-endophilin interaction are important for endocytosis of brief stimuli and prolonged stimuli respectively. Thus, our findings identify a role for Synaptojanin1 in vesicle internalization and indicate how different Synj1 domains enable its participation in endocytosis. Finally, our findings also indicate that both the Sac1-like and 5-phosphatase activities of Synj1 are required for normal post-endocytic re-availability of vesicles.
Results
Slowed endocytosis during persistent activity in synj1−/− neurons
We used spH to measure endocytic rates during action potential firing at 10 Hz. spH is a fusion protein consisting of a pH-sensitive mutant of GFP (pKa ~7.1, Sankaranarayanan et al., 2000) fused to the lumenal end of integral vesicle membrane protein VAMP-2 (Miesenböck et al, 1998). spH fluorescence is quenched when exposed to the internal vesicle pH (~5.5), but undergoes a ~25 fold increase upon exocytosis and exposure to the external buffer (pH ~7.4). The fluorescence is quenched again upon endocytosis and re-acidification (Sankaranarayanan and Ryan, 2000). During repetitive stimulation, when vesicles concurrently undergo fusion and retrieval, the fluorescence trace represents the net accumulation of spH on the synaptic surface, arising from the difference between exocytosis, and endocytosis and re-acidification. The rate of exocytosis can be resolved by blocking vesicle re-acidification with Bafilomycin A1 (Baf), a v-type ATPase blocker (Sankaranarayanan and Ryan, 2001). Thus, the difference between the spH response in the presence or absence of Bafilomycin A1 indicates the extent of endocytosis (Schweizer & Ryan, 2006), provided there is only a negligible contribution to fluorescence from internalized, non re-acidified vesicles. This was verified using acid quenching (Figure S1). Using this approach, we found that endocytosis occurring during repetitive stimulation at 10 Hz for 30 s was reduced ~2 fold in synj1−/− neurons as compared to wt (Figure 1A1, 1A2, and 1B). Similar results were also obtained in heterozygous synj1 +/− neurons (Figure S6A, B) where the Synj1 expression level is ~50% of wt levels (G. Di Paolo, unpublished results). We confirmed the presence of an endocytic defect in synj1−/− neurons using an alternate approach with FM 4-64 labeling (Figure 1C, 1D). Here the FM dye was applied during the 30 s stimulus and then rapidly washed using an FM dye chelator, so as to only label vesicles internalized during the stimulus period (Figure 1C). The amount of dye internalization is defined as the amount of dye re-released with a further round of maximal stimulation. Thus, synj1−/− neurons demonstrate a specific defect in vesicle endocytosis during repetitive stimulation.
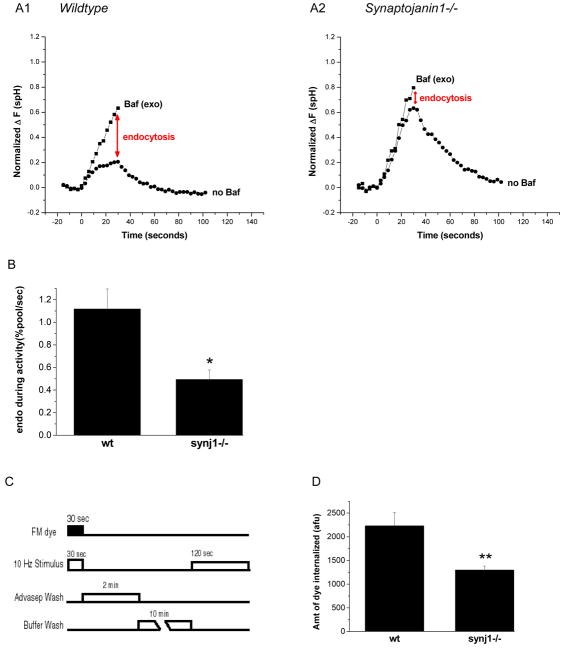
Figure 1. Endocytosis is slowed during persistent activity in synj1−/− neurons.
(A1, A2) Representative examples of wt and synj1−/− cortical neuron responses to 300 stimuli. Neurons transfected with synaptopHluorin were subject to field stimulation at 10 Hz in the absence (circles) or presence (squares) of bafilomycin A1. The responses are normalized to the size of the total recycling vesicle pool, i.e. the maximum, saturating response to 1200 AP in the presence of Baf (see ‘Methods’). The difference between the two traces measures the extent of endocytosis during activity (red arrows), and is reduced in synj1−/− neurons.
(B) The endocytic rate during 30 sec of activity is ~2-fold lower in synj 1−/− neurons as compared to wt neurons. (*P<0.01; n=10 each) Error bars are SEM.
(C) Protocol for FM dye loading as a measure of endocytosis during activity. Neurons were stimulated in the presence of FM dye, which was rapidly removed at the end of the stimulus by a 2 min wash with 1 mM Advasep-7, a dye chelator. This was followed by a 10 min wash and rest period. Neurons were then stimulated to release loaded dye, and this change in fluorescence intensity was quantified.
(D) FM dye loading during 30 s activity is reduced in synj1−/− neurons as compared to wt neurons, indicating slower endocytosis. (**P<0.005; wt n=11, ko n=12) Error bars are SEM.
Slowed endocytosis after short stimuli
For periods of prolonged stimulation, the endocytic recovery time grows with increasing accumulation of synaptic vesicles on the synaptic surface (Sankaranarayanan & Ryan, 2000, Sun et al., 2002). Endocytosis following brief stimuli, however, appears to occur with an invariant time constant (Granseth et al., 2006), indicating that endocytosis behaves differently in different stimulus regimes. We therefore investigated whether the absence of Synj1 also affected endocytosis for smaller numbers of stimuli (50–100 AP at 10 Hz). The endocytic rate during activity cannot be accurately measured for these stimuli using the Bafilomycin technique, since the stimulus duration of 5–10 s is not significantly greater than the ~4 s time constant for vesicle re-acidification (Atluri and Ryan, 2006). We therefore measured the decay in fluorescence after short stimuli and obtained the time constant (τ) for endocytosis by fitting the traces to an equation derived from a two-step sequential model for endocytosis and re-acidification (Granseth et al., 2006, and Methods). Over the 50–100 AP range, we found the time constant in wt mouse cortical neurons to be invariant (average 23.0 ± 2.0 s, Figure 2A1–4, B), modestly greater than the τ previously reported for rat hippocampal neurons (~17 s, Granseth et al., 2006). This difference may reflect biological differences between the species and cell types, or be due to differences in the reporter molecules used. Nevertheless, the τ in synj1−/− neurons was also invariant but elevated ~1.6 fold (average 36.7 ± 3.0 s, Figure 2A1–4, B). Furthermore, while spH accumulated on the surface due to the stimulus is completely retrieved in wt neurons, ~25% of the accumulated surface spH is not immediately recovered in synj1−/− neurons (Figure 2A4, C). Instead, this fraction is slowly retrieved over several minutes, with spH fluorescence eventually recovering to pre-stimulus levels. Accordingly, the steady state surface fraction of spH is not significantly different between genotypes (18.5 ± 3.8% in wt and 21.0 ± 6.3% in synj1−/− neurons, n=5 each). These findings indicate that the endocytic defect in synj1−/− neurons is prevalent for both short stimuli and persistent activity. The defect after a short stimulus was not evident in a previous FM dye assay for vesicle endocytosis in synj1−/− neurons (Kim et al., 2002), probably because of limited time resolution (time points spaced by 15 s, as opposed to 0.5–3 s in the spH measurements), and due to dye uptake unrelated to the stimulus: in the previous study (Kim et al., 2002), approximately 30% of the total dye uptake occurred 80–170 s after a 100 AP stimulus in wt neurons, even though stimulus-related endocytosis is nearly complete by that time (Figure 2A3). Interestingly, we did not find any significant defect in endocytosis following brief stimulation in synj1+/− neurons (Figure S6C, D, and E), suggesting that unlike for prolonged stimulation, the ~50% Synj1 protein remaining in these neurons is sufficient for normal handling of small endocytic loads.
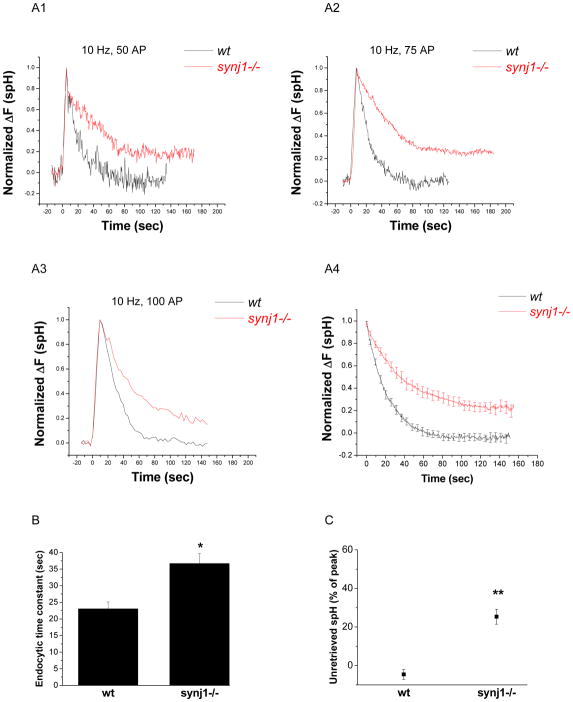
Figure 2. The time constant for endocytosis and the amount of unretrieved spH following small stimuli are increased in synj1−/− neurons.
(A1–3) Representative examples of endocytosis after 50 AP (A1), 75 AP (A2), and 100 AP (A3) at 10 Hz in wt (black) and synj1−/− (red) neurons. Traces are normalized to the peak of the change in fluorescence.
(A4) The average endocytic time course for 50–100 AP in wt (black) and synj1−/− neurons (red).
(B) The endocytic time constant is increased in synj1−/− neurons as compared to wt neurons. Time constants were obtained by fitting spH traces to an equation that derives from a two-step chemical reaction model for endocytosis and re-acidification (Granseth et al., 2006, and Methods). *P<0.002
(C) The percentage of spH that remained unretrieved after 125 s is higher in synj1−/−neurons as compared to wt neurons. **P<0.000001
Averages from 19 wt and 30 synj1−/− expts. Error bars are SEM.
No change in the rate of exocytosis or size of the recycling vesicle pool
We next wanted to determine whether the slower endocytic rate was related to a perturbation in exocytosis. The size of the readily releasable vesicle pool was shown to m scale with PI(4,5)P2 levels in chromaffin cells (Gong et al., 2005; Milosevic et al., 2005). Higher plasma membrane PI(4,5)P2 levels in synj1−/− neurons could in principle cause more efficient exocytosis, leading to an increased load on the endocytic machinery and therefore a slower endocytic rate. To investigate this possibility, we determined the rate of exocytosis from the spH response in the presence of Bafilomycin. The normalized response to a stimulus that turns over the entire recycling pool (10 Hz 120 s) did not differ significantly between wt and synj1−/− neurons (Figure 3A). The exocytic time constants obtained by first order exponential fits to the traces were accordingly similar (Figure 3B). Thus, slowed endocytosis during persistent activity in synj1−/− neurons was not due to an alteration in exocytosis.
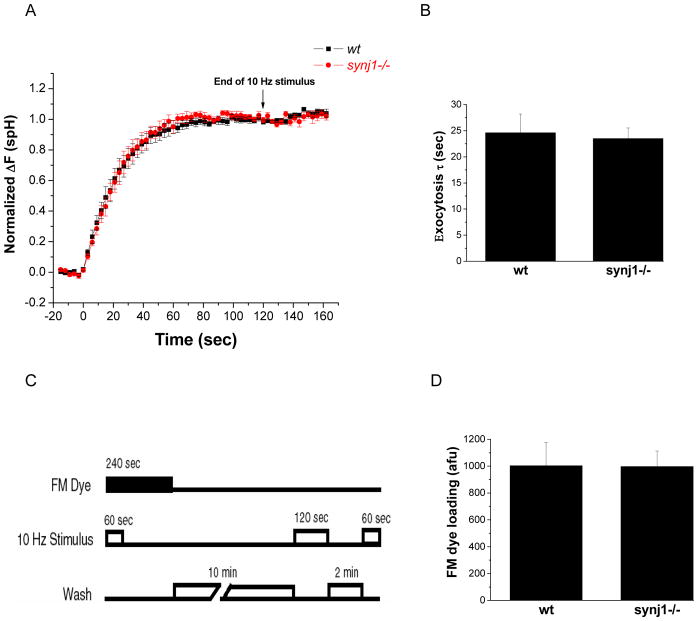
Figure 3. The rate of exocytosis and the size of the recycling pool of vesicles in synj1−/− neurons are similar to wt neurons.
(A) The normalized response to 10 Hz stimulation in the presence of Baf is similar in wt (black squares) and synj1−/− neurons (red circles). Responses are the average behavior from 9 wt and 11 synj1−/− neurons. Error bars are SEM.
(B) The time constant for exocytosis was obtained by fitting Baf response traces to a first order exponential. The average τ in synj1−/− neurons is not significantly different from that in wt neurons. (wt n=9, ko n=11). Error bars are SEM.
(C) Protocol for FM dye loading. In the presence of FM4-64, neurons were stimulated to turn over the entire recycling pool (10 Hz, 60 sec). The dye remained in the bath for 3 minutes after the end of the stimulus to allow complete endocytosis in both genotypes. Following a 10 minute wash, neurons were maximally stimulated and the total amount of dye released was measured as an indicator of recycling vesicle pool size.
(D) FM dye loading with a 10 Hz, 600 AP stimulus is similar in wt and synj1−/− neurons. (wt n=7; ko n=6) Error bars are SEM.
We also measured the size of the recycling pool of vesicles, using maximal FM dye loading and prolonged post-stimulus dye exposure to ensure labeling of all vesicles despite slower endocytic rates (Figure 3C). The amount of dye internalized was quantified by re-releasing it with a maximal stimulus (120 s at 10 Hz). These experiments indicate that the recycling pool size in synj1−/− and wt nerve terminals are similar (Figure 3D), in agreement with ultrastructural observations (Cremona et al. 1999). Previous measurements in synj1−/− synapses indicated a reduction in the recycling vesicle pool size (Kim et al. 2002); we surmise that the dye loading periods in those experiments were too short to escape complications due to incomplete endocytosis.
Rescue of the endocytosis defects by acute Synaptojanin1 expression
We examined whether post-natal expression of wildtype Synj1 (WT-Synj1) in synj1−/− neurons could correct the observed endocytic defects despite prolonged absence of the protein during embryonic development in these animals. We found that Flag or HA-tagged WT-Synj1 expressed in synj1−/− neurons was transported to presynaptic terminals (Figure S3), and completely restored the endocytic rate to wt levels during persistent activity (Figure 4A, B). The time constant for endocytosis after brief 50–100 AP stimuli (average 23.3 ± 2.2 s, Figure 4C1–4, D) and the extent of spH retrieval (Figure 4E) were also found to be restored to wt levels in these experiments. Exocytosis kinetics remained unaltered under these conditions (Figure S2), indicating that the rescue of endocytosis was not due to an additional perturbation of the exocytic pathway. These findings indicate that the endocytic defects in synj1−/− neurons can be fully restored by expression of WT-Synj1.
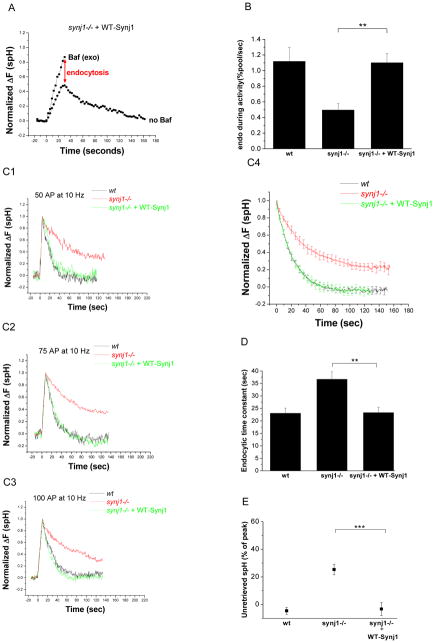
Figure 4. The endocytosis defects in synj1−/− neurons are corrected by the expression of wildtype Synj1.
(A) Synj1−/− neurons were co-transfected with spH and HA-tagged wildtype Synj1 (WT-Synj1). Representative example of the responses to a 10 Hz, 30 sec stimulus is shown here. The red arrow indicates the extent of endocytosis during persistent activity.
(B) The endocytic rate during 30 s activity in synj1−/− neurons transfected with WT-Synj1 is restored to wt levels. (n=8 for synj1−/− + WT-Synj1) **P<0.005, Error bars are SEM.
(C1–3) The endocytic time constant is restored by the expression of WT-Synj1. Representative examples of endocytosis after 50 AP (C1), 75 AP (C2), and 100 AP (C3) at 10 Hz in wt (black), synj1−/− (red), and synj1−/− + WT-Synj1 (green) conditions. Traces are normalized to the peak of the change in fluorescence.
(C4) The average endocytic time course for 50–100 AP in wt (black), synj1−/− (red), and synj1−/− + WT-Synj1 (green) conditions.
(D) The endocytic time constant for small stimuli is completely restored by the expression of WT-Synj1. **P<0.005
(E) The percentage of spH that remained unretrieved after 125s is restored to wt levels upon expression of WT-Synj1. ***P<0.0001
Averages from 19 wt, 30 synj1−/−, and 16 synj1−/− + WT-Synj1 expts. Error bars are SEM.
Critical role of the 5-phosphatase activity in endocytosis
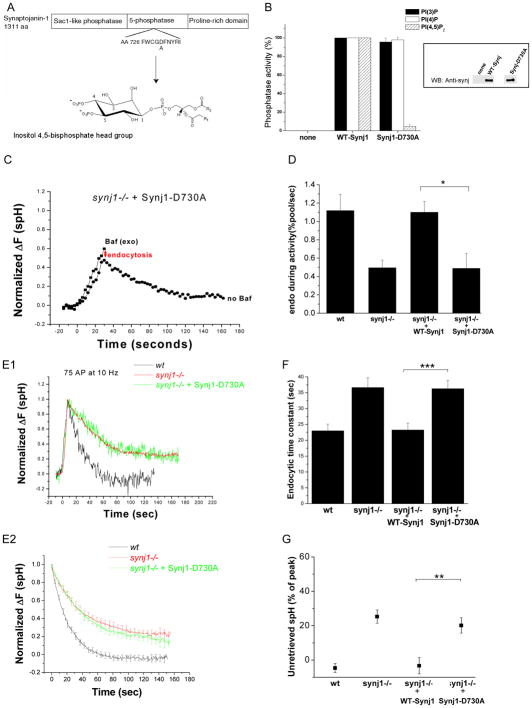
We used the ability to rescue the endocytic defect in synj1−/− synapses with exogenously expressed protein to investigate the potential roles of different domains of Synj1 in the vesicle cycle. We first tested the ability of a 5-phosphatase activity-deficient point mutant form of Synj1 to rescue the endocytic defects in synj1−/− neurons. All known inositol 5-phosphatases contain the consensus sequence of the form XWXGDXN(F/Y)R, wherein the aspartate residue is known to be important for catalysis (Jefferson and Majerus, 1996; Whisstock et al., 2000, Tsujishita et al., 2001). The corresponding mutation in Synj1 (Synj1-D730A) nearly completely eliminated 5-phosphatase activity, but did not affect the Sac1-like phosphatase activity (Figure 5A and 5B). When expressed in synj1−/− neurons, Synj1-D730A was transported to the presynaptic terminal (Figure S3) and did not perturb the rate of exocytosis (Figure S2). However, it was unable to restore endocytosis both during persistent activity (Figure 5C and 5D) and after brief stimuli (Figure 5E–G), indicating that metabolism of inositol-5-phosphates by Synj1 is a critical regulator of endocytosis during both strong and weak synaptic activity.
Figure 5. The 5-phosphatase activity of Synj1 controls both the endocytic rate during persistent activity and endocytosis for small stimuli.
(A) Design of the 5-phosphatase activity deficient mutant (Synj1-D730A). An aspartate residue conserved in all mammalian inositol-5-phosphatases and previously shown to be important for catalytic activity (Whisstock et al., 2000; Whisstock et al., 2002) was mutated to alanine.
(B) The D730A mutation inhibits 5-phosphatase activity of Synj1. Flag-tagged Synj1 (WT or D730A) was incubated with the phosphoinositide substrate, and the free phosphate generated was measured using the Malachite green phosphatase assay (Harder et al., 1994; Lee, et al, 2004). Phosphatase activities are normalized with wildtype Synj1 set to 100%. Mean and SEM of 3–4 expts are presented. (Inset) Equal amounts of immunoprecipitated Synj was tested by Western blotting with the polyclonal α-Synj1 antibody.
(C) Representative example of the response to a 10 Hz, 30 sec stimulus. The red arrow indicates the extent of endocytosis during activity.
(D) The endocytic rate during activity is reduced in Synj1-D730A-expressing neurons as compared to those expressing WT-Synj1. *P<0.01, n=8 for Synj1-D730A. Error bars are SEM.
(E1, 2) Endocytosis after short stimuli is not restored by expression of Synj1-D730A. Representative examples of endocytosis after 75 AP (E1), and the average endocytic time course for 50–100 AP (E2) in wt (black), synj1−/− (red), and synj1−/−+Synj1-D730A (green) conditions.
(F) The endocytic time constant for 50–100 stimuli is not restored by the expression of Synj1-D730A. ***P<0.001.
(G) The percentage of unretrieved spH remains elevated despite expression of Synj1-D730A. **P<0.002
n=14 for Synj1-D730A. Error bars are SEM.
Sac1-like phosphatase activity is required for normal endocytosis after short stimuli
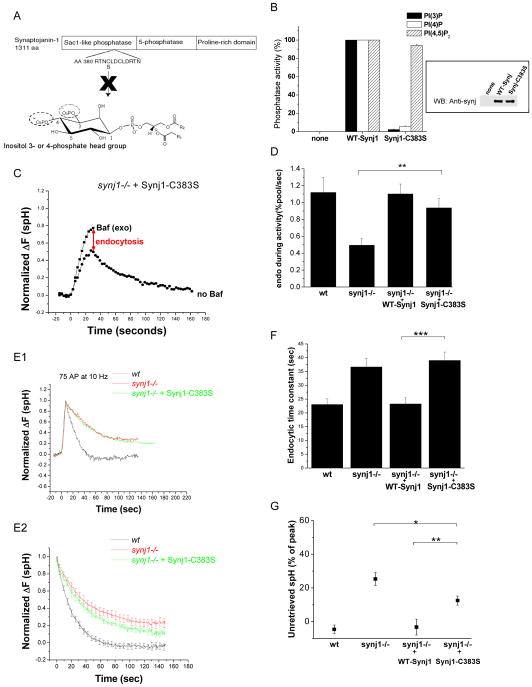
We tested the ability of a Sac1-phosphatase activity-deficient point mutant of Synj1 to rescue the endocytic defects in synj1−/− neurons. Sac1-like phosphatases contain a consensus sequence of the form C(X5)RT/S, with the cysteine residue thought to be important for catalytic activity (Hughes et al., 2000). Accordingly, the C383S mutation in Synj1 almost entirely eliminated 3-phosphatase and 4-phosphatase activity, but did not affect the 5-phosphatase activity (Figure 6A and 6B). When expressed in synj1−/− neurons, the Synj1-C383S protein was transported to the presynaptic terminal (Figure S3) and did not perturb the rate of exocytosis (Figure S2). Expression of the Sac1-like domain mutant restored endocytosis during persistent activity (Figure 6C and 6D), although there was a slight trend towards less efficient rescue. However, Synj1-C383S did not restore the endocytic time constant for small stimuli (Figure 6E, F), even though it partially reduced the fraction of unretrieved spH (Figure 6G). These results indicate that Sac1-like phosphatase activity of Synj1, like its 5-phosphatase activity, controls endocytosis during weak synaptic activity.
Figure 6. The Sac1-like phosphatase activity of Synj1 controls endocytosis for small stimuli, but not the endocytic rate during persistent activity.
(A) Design of the Sac1-like phosphatase activity deficient mutant (Synj1-C383S). The first conserved cysteine residue in the putative catalytic motif RXNCXDCLDRTN (Hughes et al., 2000) was mutated to serine.
(B) The C383S mutation inhibits Sac1-like phosphatase activity of Synaptojanin1 (n=3). Error bars represent SEM. (Inset) Equal amounts of immunoprecipitated Synj1 was tested by Western blotting with the polyclonal α-Synj1 antibody.
(C) Representative example of the response to a 10 Hz, 30 sec stimulus. The red arrow indicates the extent of endocytosis during activity.
(D) The endocytic rate during activity in Synj1-C383S-expressing neurons is similar to those expressing WT-Synj1. **P<0.01, n=7 for Synj1-C383S. Error bars are SEM.
(E1, 2) Endocytosis after short stimuli is not restored by expression of Synj1-C383S. Representative examples of endocytosis after 75 AP (E1), and the average endocytic time course for 50–100 AP (E2) in wt (black), synj1−/− (red), and synj1−/−+Synj1-C383S (green) conditions.
(F) The endocytic time constant for 50–100 stimuli is not restored by the expression of Synj1-C383S. ***P<0.0005
(G) The percentage of unretrieved spH is partly reduced upon expression of Synj1-C383S. *P<0.05 compared to synj1−/− and **P<0.01 compared to synj1−/− +WT-Synj1 neurons.
n=16 for Synj1-C383S. Error bars are SEM.
The Synj1-endophilin interaction controls endocytic rate during persistent activity
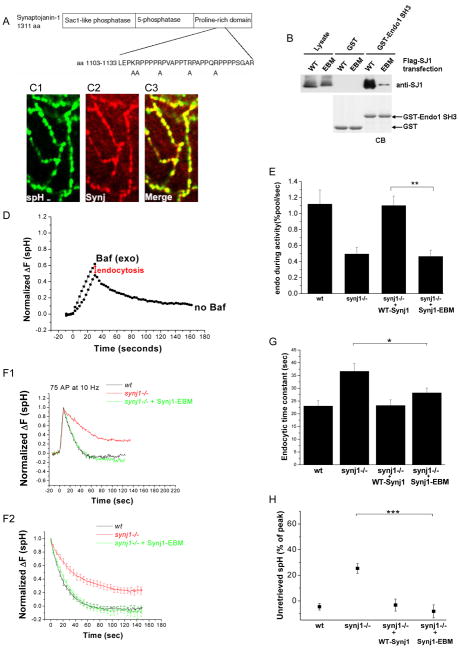
Studies in the lamprey reticulospinal axon (Gad et al., 2000), at the neuro-muscular junction of Drosophila (Verstreken et al., 2003), and in C. elegans (Schuske et al., 2003) suggested that the interaction of Synj and endophilin is necessary for appropriate localization of Synaptojanin. We therefore examined the importance of the binding of Synj1 to endophilin1 at mammalian presynaptic terminals. In order to specifically disrupt this interaction, we generated an endophilin-binding mutant form of Synaptojanin1 (Synj1-EBM). Five point mutations (Figure 7A) were introduced in a ~30 aa region of the PRD which contains motifs previously shown to be important for endophiln binding (Cestra et al., 1999; Gad et al., 2000; Ringstad et al., 2001). These mutations did not alter the ability of Synj1 to hydrolyze phosphoinositides (Figure S4). The interaction with amphiphysin1, another partner whose binding site is thought to be in close proximity to the endophilin-binding region (Cestra et al., 1999), was also unaltered (Figure S4). However, the mutations specifically and drastically reduced the ability of Synj1 to bind to the SH3 domain of endophilin1 (Figure 7B). Synj1-EBM was appropriately localized to the presynaptic terminal (Figure 7C1–3) upon expression in synj1−/− neurons. Its expression level was sufficient to restore the endocytic defect after short stimuli (Figure 7F, G, and H) without perturbing the rate of exocytosis (Figure S2). However, Synj1-EBM expression did not restore the endocytic defect during persistent activity (Figure 7D and 7E), suggesting that the interaction with endophilin is required during increased demand on the endocytic machinery.
Figure 7. The interaction of Synj1 and endophilin controls endocytic rate during persistent activity, but not endocytosis for small stimuli.
(A) Design of the endophilin binding-deficient mutant (Synj1-EBM). Five positively charged residues were mutated to alanine in a region shown to be important for endophilin binding in mammalian Synj1 (Cestra et al., 1999; Ringstad et al., 2001) and lamprey Synj (Gad et al., 2000).
(B) Synj1-EBM shows reduced binding to the endophilin1 SH3 domain. Wildtype or mutant Flag-tagged Synj1 was incubated with glutathione beads coupled with the GST-Endo1 SH3 domain. Pull-down material was analyzed by western blotting with anti-Synj1 antibody. The CB (Coomassie blue) staining indicated the same amounts of bait proteins for pull-down. Representative example from 3 similar experiments is shown.
(C1, C2, C3) Synj1-EBM (red) co-localizes with synaptopHluorin (green) at the presynaptic terminal. Scale bar, 1 μm.
(D) Representative example of the response to a 10 Hz, 30 sec stimulus. The red arrow indicates the extent of endocytosis during activity.
(E) The endocytic rate during activity is reduced in Synj1-EBM-expressing neurons as compared to those expressing WT-Synj1. **P<0.005, n=5 for Synj1-EBM. Error bars are SEM.
(F1, 2) The endocytic time constant is restored by the expression of Synj1-EBM. Representative examples of endocytosis after 75 AP (F1), and the average endocytic time course for 50–100 AP (F2) in wt (black), synj1−/− (red), and synj1−/−+Synj1-EBM (green) conditions.
(G) The endocytic time constant for 50–100 stimuli is restored by the expression of Synj1-EBM. *P<0.05.
(H) The percentage of spH that remained unretrieved after 125s is restored to wt levels upon expression of Synj1-EBM. ***P<0.0001
n=29 for Synj1-EBM. Error bars are SEM.
Sac1-like and 5-phosphatase activities control the re-availability of newly internalized vesicles
In synj1−/− neurons, vesicle re-availability after endocytosis is significantly delayed (Kim et al., 2002), likely due to impaired clathrin uncoating (Cremona et al., 1999). We therefore investigated the role of each domain of Synj1 in the assay for post-endocytic vesicle re-availability (repriming), which measures the time course for re-release of FM dye internalized during a given stimulus train (Ryan and Smith, 1995; Figure 8A). Since the amount of dye re-released is normalized to the amount of initial dye loading in each genotype, it is not confounded by differences in endocytic rates between comparison groups. We detected a delay in re-release of FM dye in synj1−/− neurons, confirming a large decrease in vesicle re-availability in this genotype (Figure 8B). Expression of WT-Synj1 restored vesicle re-availability, and Synj1-EBM partially rescued it (Figure 8C). However, expression of neither Synj1-C383S nor Synj1-D730A corrected the defect (Figure 8D). These results indicate that the post-endocytic re-availability of vesicles is controlled by both the 5-phosphatase and the Sac1-like phosphatase activities of Synj1, and partially regulated by endophilin binding.
Figure 8. The re-availability of vesicles following endocytosis is controlled by both phosphatase activities of Synj1, and partially determined by the Synj1-endo interaction.
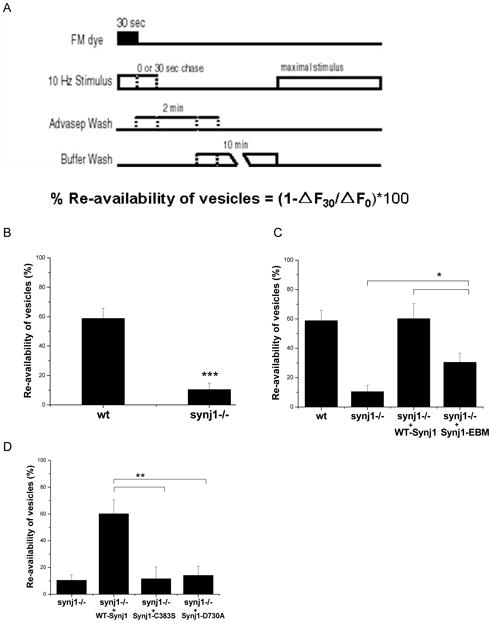
(A) Protocol to measure re-availability of vesicles (Ryan and Smith, 1995). Neurons were stimulated for 30 sec at 10 Hz in the presence of FM dye, which was rapidly removed at the end of the stimulus by a 2 min wash with 1 mM Advasep-7, a dye chelator. Cells were then either subject to another 30 sec stimulus at 10 Hz, or left unstimulated. After a 10 min wash, the cells were maximally stimulated to release loaded dye, and the change in fluorescence intensity was quantified. The amount of dye retained after a 30 sec chase stimulus relative to dye retained in the absence of a chase stimulus was used to determine the percent re-availability of vesicles.
(B) The re-availability of vesicles was reduced in synj1−/− neurons as compared to wt neurons. ***P<0.001, n=7 wt and n=5 synj1−/−. Error bars are SEM.
(C) The re-availability of vesicles is fully restored by the expression of WT-Synj1, and partially restored by the expression of Synj1-EBM. *P<0.03, n=4 WT-Synj1 and n=6 Synj1-EBM. Error bars are SEM.
(D) The re-availability of vesicles following endocytosis is not restored by expression of phosphatase-deficient mutants of Synj1. **P<0.01 n=5 Synj1-C383S and n=6 Synj1-D730A. Error bars are SEM.
Discussion
This study demonstrates the importance of Synj1 not only for post-endocytic steps in synaptic vesicle recycling (Cremona et al., 1999; Kim et al., 2002) but also in endocytosis itself. In contrast, the rate of exocytosis and size of the recycling vesicle pool are unaltered in the absence of Synj1. Our study also examines the extent to which each functional domain of Synaptojanin is involved in endocytosis and post-endocytic recycling of vesicles. The conservation of Synaptojanin proteins from yeast to humans, and the unusual juxtaposition of two distinct phosphatase activities within a single protein suggest that the ability to hydrolyze phosphates at separate positions on the inositol head group is critical for its biological function. Our data provide a direct test of this hypothesis for the neuronal isoform of Synaptojanin, Synj1, and show that the enzymatic activity of each phosphatase domain is required for efficient endocytic retrieval of synaptic vesicles. This study further demonstrates the importance of the catalytic activity of both phosphatase domains in controlling the progression of newly internalized synaptic vesicles to the releasable pool of vesicles. Furthermore, although the absence of Synj1 results in defects in endocytosis for both brief and prolonged stimulation, the requirement of individual domains appears to vary with different stimulus conditions. The Synj1-endophilin interaction is necessary during prolonged stimulation but not for brief stimuli. This phenotype is similar to that of heterozygous synj1+/− neurons with ~50% Synj1 protein levels (Figure S6) and suggests that maintenance of normal endocytic rates, especially during persistent activity with its greater endocytic load, requires an adequate concentration of Synj1 to be available at endocytic sites. Reduction in this concentration by deletion of one synj1 gene copy or disruption of the Synj1-endophilin interaction therefore results in an activity-dependent effect on endocytic rate. Finally, the activity of the Sac1-like domain appears important for briefer stimuli, suggesting that the molecular interactions utilized in clathrin-mediated endocytosis can vary depending on the total endocytic load, which in turn is regulated by activity.
The synaptic vesicle cycle in synj1−/− neurons
The absence of Synj1 results in a defect in endocytosis for stimuli ranging from 50–300 AP (Figures 1 and 2). We investigated this range of stimuli for two reasons: first, the presumed diversity in firing rates and times of neurons obtained from the entire cortex; and second, in hippocampal neurons, retrieval for brief stimuli was recently found to be clathrin-dependent (Granseth et al., 2006). Hence, endocytic retrieval for short stimuli might also require accessory proteins such as Synaptojanin. Indeed, we found that the endocytic time constant for 50–100 AP stimuli was slowed in synj1−/− neurons and retrieval was incomplete (Figure 2). While we cannot rule out that the requirement for Synj1 is variable across regions of the cortex, reduced uptake of FM dye (Figure 1C, 1D), which is taken up by all nerve terminals, indicates that the defect is not specific to neurons that express spH. Given the absence of changes in the rate of exocytosis and the size of the recycling vesicle pool (Figure 3), the defects in endocytosis and in post-endocytic vesicle re-availability can account for the greater synaptic depression and slower recovery observed in both excitatory (Cremona et al., 1999) and inhibitory (Lüthi et al., 2001) synj1−/− neurons.
Endocytic control by the phosphatase activities of Synj1
Biochemical, genetic, and cell biological studies indicate that PI(4,5)P2 serves as a signal for the recruitment of a number of endocytic factors to the plasma membrane (Beck and Keen, 1991; Hao et al., 1997; Cremona et al., 1999; Ford et al., 2001; Honing et al., 2005; Zoncu et al., 2007). Our present study indicates that dephosphorylation by Synj1 is also essential for normal endocytic function. The 5-phosphatase activity of Synj1 is required for normal endocytosis over a broad range of stimuli (Figure 5), and its Sac1-like phosphatase activity is required at least for brief stimuli. (Figure 6). The less stringent requirement for the Sac1-like phosphatase activity of Synj1 during prolonged stimulation may reflect compensatory or overlapping action of other phosphatases during stronger activity. It is also possible that the Sac1-like domain, via its binding to phosphoinositides, may contribute to the recruitment of Synj1 to the membrane. During sustained activity on the other hand, recruitment may be accomplished primarily by an interaction with endophilin and/or other proteins that bind to the COOH-terminal region of Synj1. Indeed, prolonged activity can increase the Ca2+-dependent dephosphorylation of Synj1, which is expected to strongly enhance its interaction with endophilin (Lee et al., 2004).
At present it is unclear what molecular step in endocytosis requires Synj1 phosphatase activities. It is possible that efficient growth of the endocytic bud requires constant turnover of PI(4,5)P2, potentially allowing greater flexibility in the organization of the clathrin coat as curvature is generated. Indeed, when expressed in a fibroblast cell line, a 5-phosphatase mutant of the Synj-170 isoform froze the dynamics of clathrin coated pits (Perera et al., 2006). Depletion of Synj2 in a lung carcinoma cell line also resulted in reduced clathrin coated pit formation (Rusk et al., 2003). Finally, in yeast, the Synj homolog Sjl2 tagged with GFP localizes to cortical actin patches (Stefan et al., 2005) as they become dynamic (Sun et al, 2007). These studies collectively suggest that Synaptojanin has an active role in the budding process. Given that a number of endocytic factors, including dynamin, bind to PI(4,5)P2 (Zheng et al., 1996; Itoh and De Camilli, 2006; Takenawa and Suetsugu, 2007), it is also possible that dephosphorylation of PI(4,5)P2 may have a role in the fission reaction itself. Alternatively, reduced phosphatase activity of Synj1 may increase membrane levels of phosphoinositides such as PI(4,5)P2 and PI(3,4,5)P3, which can bind to endocytic proteins (Gaidarov et al., 1996; Schiavo et al., 1996; Haucke, 2005). This could result in ectopic nucleation or impaired recycling of endocytic factors, leading to slowed endocytosis. Reduced phosphatase activity of Synj1 may also reduce membrane levels of PI(4)P, a phosphoinositide important in budding from the trans-Golgi network (Godi et al., 2004; Shin and Nakayama, 2004) that may similarly participate in synaptic vesicle internalization. Finally, one cannot rule out that the effects of impaired inositol phosphatase activity may be mediated by accumulation of soluble inositol phosphates such as I(1,4,5)P3 and IP6 (Beck and Keen, 1991; Chang et al., 1993). However, the physiological importance of the ability of Synj1 to dephosphorylate soluble inositol polyphosphates (McPherson et al., 1996) remains unclear.
Activity-dependent involvement of the endophilin binding function of synj1
Disruption of the Synj1-endophilin interaction does not prevent Synj1 localization at the presynaptic terminal but inhibits endocytosis selectively during persistent activity (Figure 7). Since endophilin has been shown to recruit Synj1 to clathrin-coated pits (Perera et al., 2006), we propose the following scenario: the concentration of Synj1 established at the membrane by other interactions is sufficient to complete endocytosis after weak stimuli. A larger stimulus which imposes a greater load on the endocytic machinery requires enhanced recruitment of Synj1 to the plasma membrane, a task accomplished, at least in part, by endophilin. This scenario is consistent with the reported constitutive phosphorylation of Synj1 at rest, which inhibits its interaction with endophilin (Lee et al., 2004; Irie et al., 2005), and with its stimulus-driven dephosphorylation (McPherson et al., 1994). Importantly, disruption of the endophilin-binding site in Synj1 does not disrupt other interactions of this protein, such as that with amphiphysin (Figure S4). Thus, the inability of the Synj1 mutant lacking the endophilin binding site to rescue the defect in endocytosis during a large stimulus provides further evidence for the hypothesis that endophilin is a preferred and critically important physiological partner of Synj1 at the synapse (de Heuvel et al., 1997; Ringstad et al., 1997; Gad et al., 2000; Schuske et al., 2003; Verstreken et al., 2003).
The role of Synj1 in the uncoating and re-availability of vesicles
Perturbation of the Sac1 and 5-phosphatase activities of Synj1 dramatically reduces the re-availability of vesicles following internalization. Our results support a mechanism wherein the hydrolysis of PI(4,5)P2 to PI, which requires the actions of both the Sac1-like and 5-phosphatase domains, can facilitate shedding of coat components and progression of the newly re-formed vesicles to sites of release. While the Hsc70-Auxilin interaction appears responsible for disruption of clathrin triskelia (Ungewickell et al., 1995; Morgan et al., 2001), it is plausible that release of the clathrin adaptors and other endocytic factors requires PI(4,5)P2 dephosphorylation, because PI(4,5)P2 is implicated in their membrane recruitment (Haucke, 2005). Finally, binding of Synj1 to endophilin also plays a role in this post-endocytic trafficking step, although only a partial one. This is most likely due to the role of other interactors of Synj1 whose function may overlap with that of endophilin.
In conclusion, our study demonstrates how the dual-phosphatase modules of Synaptojanin1 and its proline-rich COOH-terminal region enable its participation in two distinct steps of the synaptic vesicle cycle: endocytosis, and the post-endocytic re-availability of vesicles. Given the different relative roles of Synj1 domains during short or prolonged stimulus trains, our results suggest that the machinery for endocytosis responds to the context of activity and accordingly modulates the use of endocytic factors. The stimulus-dependent need for the Synj1-endophilin interaction may reflect the effects of available Synj1 concentration on endocytic rate, and the precise molecular reasons for the activity-dependent use of the Sac-1 like domain remain to be elucidated. This concentration dependence and activity-mediated use of an endocytic protein may represent important modulatory mechanisms in the synaptic vesicle cycle.
Experimental Procedures
Neuronal cultures and transfection
Cortical cultures were generated from one day-old Synj1 knockout and wild-type mice as described previously (Di Paolo et al., 2002). Neurons were transfected with the spH expression plasmid (a gift from Dr. James Rothman, Columbia University) alone, or co-transfected with spH and Flag- or HA-tagged human synaptojanin1 (145 kDa isoform) using standard calcium-phosphate mediated gene transfer at 7–8 DIV (days in-vitro). Experiments were typically performed at 14–30 DIV under previously described buffer, perfusion, and stimulus conditions (Di Paolo et al., 2002). FM4-64 dye (Invitrogen, CA) was used at a concentration of 15 μM, and Bafilomycin A1 (Calbiochem/EMD Biosciences, CA) at a concentration of 1 μM.
Confocal microscopy and analysis
Laser-scanning fluorescence images were acquired using a custom-built laser-scanning microscope, through a 40× 1.3 numerical aperture Zeiss Fluar objective. Specimens were illuminated with ~45 μwatts of the 488 nm line of an argon ion laser that was rapidly shuttered during all non data-acquiring periods using acousto-optic or electro-optic modulation. The sequential scanning time for a single frame was ~1 s, and the time courses of spH and FM responses were measured from time-lapse images taken every 3 s. Images were acquired for at least 15 s prior to the start of the stimulus (time 0 s in all experiments). Quantitative measurements of fluorescence intensity at individual presynaptic terminals were obtained by averaging square regions of interest (ROI) of side length 0.44 μm (4 × 4 pixels). Individual regions were selected by hand, and the optical center of mass used to center the measurement box was computed over a slightly larger area (typically 8 × 8 pixels). Each experiment represents the average behavior of 20–80 ROIs.
Wide-field microscopy and analysis
Images for the spH responses to 5–10 s stimuli were acquired with a highly sensitive, back-illuminated EM-CCD camera (iXon+ Model # DU-897E-BV, Andor Corp., CT). An epifluorescence microscope (Zeiss Axiovert 200) was modified to use laser illumination. The 488 nm argon ion laser beam was sufficiently expanded to get near uniform illumination, and was shuttered using acousto-optic modulation in all non-data acquiring periods. Fluorescence excitation and collection were done through a 40X 1.3 NA Fluar Zeiss objective using a 515–560 nm emission filter and a 510 nm dichroic filter. Images were acquired every 500 ms, with integration times of 50–80 ms. Circular ROIs with a diameter of 2.5 μm were used for measurement of fluorescence intensities at presynaptic varicosities. Only those regions where the spH response peak ΔF>2σ, where σ is the standard deviation of ~10–15 s of pre-stimulus baseline fluorescence, were used for analysis. Each experiment constitutes the average of 20–50 ROIs. We also measured the rate of photobleaching of surface spH by imaging spH-expressing neurons under typical illumination and acquisition conditions, but without any AP stimulus. The decline in spH fluorescence thus measured was 0.024 ± 0.003% per frame (n=10). We therefore corrected for photobleaching as follows:
Fluorescence decays were then fit to the following equation, derived from the previously described two-step chemical reaction model for vesicle endocytosis and re-acidification (Granseth et al., 2006).
where
F(t) = Fluorescence at any given time t
F(final) = Steady state fluorescence after recovery
A0 = Initial surface fluorescence
B0 = Initial fluorescence from internalized, non-reacidified vesicles
τe = Time constant for endocytosis
τr = Time constant for re-acidification
The re-acidification time constant was fixed as 4 s (Atluri and Ryan, 2006), and the endocytic time constant was obtained from the fit.
Fluorescence Normalization
The spH responses in experiments measuring endocytosis during 300 AP (for example, Figures 1A1, 1A2, and 4A) were normalized to the size of the recycling pool of vesicles, which was measured as the ΔF elicited by stimulation with 10 Hz, 1200 AP in the presence of Bafilomycin. The saturation of the spH response (Figure 3A) with this protocol indicates that all recycling vesicles have undergone at least one round of fusion; thus, the ΔF in this experiment offers a measure of the size of the recycling pool. The endocytic rates quantified in this manner can directly be compared between genotypes, provided the total recycling vesicle pool sizes are similar, as is the case here (Figure 3C, 3D). For brief stimuli (for example, Figures 2A1–4), responses were normalized to change in spH fluorescence, with the pre-stimulus baseline at 0 and the peak of the response being set to 1.
Immunocytochemistry
When neurons were co-transfected with spH and various forms of Synj1, they were initially identified for experimentation solely based on spH fluorescence. However, the expression of Synj1 was always verified after the experiment. Cultures were fixed for 15 min with PBS containing 4% paraformalydehye (EMS, PA) and 4% sucrose (w/v). The blocking/permeabilization solution for the rest of the procedure was Phosphate-buffered saline (PBS) containing 1% BSA (w/v) and 3 mg/mL saponin. After a 1 hour incubation with 1:50 dilution of anti-flag M2 or anti-HA (Covance, USA), coverslips were washed twice for 5 min each. Cells were then incubated for one hour with 1:500 dilutions of Alexa Fluor® 546 goat anti-mouse antibody and Alexa Fluor®488-conjugated rabbit anti-GFP (Invitrogen, CA), and washed 3 times for 5 min each prior to imaging. Chemicals were obtained from Sigma (St. Louis, MO), unless indicated otherwise.
Synaptojanin1 phosphatase assay
The mammalian expression constructs of Flag-tagged full-length wild-type and mutant Synj1 were transfected into HEK293 cells (20 μg plasmid per 10 cm dish). After 24 hrs, cells were scraped with 1 ml lysis buffer [20 mM Hepes (pH 7.4), 120 mM KCl, 5 mM NaF, 1 mM EDTA, 1 mM EGTA, 1 mM β-glycerophosphate, 1 mM Na3PO4, 1 mM Na3VO4, 1 mM DTT, and 1% TX-100 (v/v)], solubilized on ice for 20 min, and then centrifuged at 13000 rpm for 30 min at 4°C. Flag-Synj1 was immunoprecipitated by incubation of the resulting lysates (2–4 mg) with α-FLAG-M2-Agarose beads for 2 h at 4°C. The beads (40–50 μl bed volume) were washed with the lysis buffer three times and then twice with a Synj assay buffer (30 mM Hepes (pH 7.4), 100 mM KCl, 1 mM EGTA and 2 mM MgCl2). Aliquots of the beads (5–10 μl bed volume) were added to assay tubes containing 2 μg water-soluble phosphoinositide substrate (with a diC8-acyl chain, Echelon, Salt Lake City) in a final 60 μl volume. Assay mixtures were incubated for 30 min at 37°C with tapping every 2–3 min, and centrifuged for separating supernatant from beads. Aliquots (20 μl) of the supernatant were transferred to tubes containing 80 μl of malachite-green solution. Free phosphate generated was measured by a plate reader at 650 nm wavelength (Harder et al., 1994; Lee, et al, 2004). Equal amounts of immunoprecipitated Synj1 was tested by Western blotting with the polyclonal α-Synj antibody.
Endophilin1-SH3 or amphiphysin1-SH3 binding assay
To test the protein interaction of Synj1-EBM, a pulldown assay of the cell lysates from transfected HEK 293 cells was performed with GST-fusion proteins. The SH3 domain of endophilin1 or amphiphysin1 was purified as a GST-fusion protein for interaction with the Synj1-EBM mutant. Cell lysates (2–4 mg) were incubated for 2 h at 4°C with GST-SH3 or GST (20–40 μg) coupled to glutathione beads. The beads were washed with the lysis buffer five times and processed for SDS-PAGE to detect Synj by Western blotting.
Supplementary Material
Acknowledgments
We would like to thank J. Balaji for the setup for wide-field microscopy, Ricky Kwan for technical contributions, and members of the Ryan and De Camilli labs for valuable discussions and critical reading of the manuscript. This project was supported by NIH-GM07739 (MM), NIH-NS036942 (TAR), NIH-NS056049 (GDP), NIH-NS036251, NIH-CA46128, and The G. Harold and Leila Y. Mathers Charitable Foundation (PDC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atluri PP, Ryan TA. The kinetics of synaptic vesicle reacidification at hippocampal nerve terminals. J Neurosci. 2006;26:2313–2320. doi: 10.1523/JNEUROSCI.4425-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck KA, Keen JH. Interaction of phosphoinositide cycle intermediates with the plasma membrane-associated clathrin assembly protein AP-2. J Biol Chem. 1991;266:4442–4447. [PubMed] [Google Scholar]
- Brett TJ, Traub LM. Molecular structures of coat and coat-associated proteins: function follows form. Curr Opin Cell Biol. 2006;18:395–406. doi: 10.1016/j.ceb.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Cestra G, Castagnoli L, Dente L, Minenkova O, Petrelli A, Migone N, Hoffmuller U, Schneider-Mergener J, Cesareni G. The SH3 domains of endophilin and amphiphysin bind to the proline-rich region of synaptojanin 1 at distinct sites that display an unconventional binding specificity. J Biol Chem. 1999;274:32001–32007. doi: 10.1074/jbc.274.45.32001. [DOI] [PubMed] [Google Scholar]
- Chang MP, Mallet WG, Mostov KE, Brodsky FM. Adaptor self-aggregation, adaptor-receptor recognition and binding of alpha-adaptin subunits to the plasma membrane contribute to recruitment of adaptor (AP2) components of clathrin-coated pits. EMBO J. 1993;12:2169–2180. doi: 10.1002/j.1460-2075.1993.tb05865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremona O, Di Paolo G, Wenk MR, Luthi A, Kim WT, Takei K, Daniell L, Nemoto Y, Shears SB, Flavell RA, McCormick DA, De Camilli P. Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell. 1999;99:179–188. doi: 10.1016/s0092-8674(00)81649-9. [DOI] [PubMed] [Google Scholar]
- de Heuvel E, Bell AW, Ramjaun AR, Wong K, Sossin WS, McPherson PS. Identification of the major synaptojanin-binding proteins in brain. J Biol Chem. 1997;272:8710–8716. doi: 10.1074/jbc.272.13.8710. [DOI] [PubMed] [Google Scholar]
- Di Paolo G, Sankaranarayanan S, Wenk MR, Daniell L, Perucco E, Caldarone BJ, Flavell R, Picciotto MR, Ryan TA, Cremona O, De Camilli P. Decreased synaptic vesicle recycling efficiency and cognitive deficits in amphiphysin 1 knockout mice. Neuron. 2002;33:789–804. doi: 10.1016/s0896-6273(02)00601-3. [DOI] [PubMed] [Google Scholar]
- Di Paolo G, Moskowitz HS, Gipson K, Wenk MR, Voronov S, Obayashi M, Flavell R, Fitzsimonds RM, Ryan TA, De Camilli P. Impaired PtdIns(4,5)P2 synthesis in nerve terminals produces defects in synaptic vesicle trafficking. Nature. 2004;431:415–422. doi: 10.1038/nature02896. [DOI] [PubMed] [Google Scholar]
- Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- Dickman DK, Horne JA, Meinertzhagen IA, Schwarz TL. A slowed classical pathway rather than kiss-and-run mediates endocytosis at synapses lacking synaptojanin and endophilin. Cell. 2005;123:521–533. doi: 10.1016/j.cell.2005.09.026. [DOI] [PubMed] [Google Scholar]
- Ford MG, Pearse BM, Higgins MK, Vallis Y, Owen DJ, Gibson A, Hopkins CR, Evans PR, McMahon HT. Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science. 2001;291:1051–1055. doi: 10.1126/science.291.5506.1051. [DOI] [PubMed] [Google Scholar]
- Gad H, Ringstad N, Low P, Kjaerulff O, Gustafsson J, Wenk M, Di Paolo G, Nemoto Y, Crun J, Ellisman MH, De Camilli P, Shupliakov O, Brodin L. Fission and uncoating of synaptic clathrin-coated vesicles are perturbed by disruption of interactions with the SH3 domain of endophilin. Neuron. 2000;27:301–312. doi: 10.1016/s0896-6273(00)00038-6. [DOI] [PubMed] [Google Scholar]
- Gaidarov I, Chen Q, Falck JR, Reddy KK, Keen JH. A functional phosphatidylinositol 3,4,5-trisphosphate/phosphoinositide binding domain in the clathrin adaptor AP-2 alpha subunit. Implications for the endocytic pathway. J Biol Chem. 1996;271:20922–20929. doi: 10.1074/jbc.271.34.20922. [DOI] [PubMed] [Google Scholar]
- Godi A, Di Campli A, Konstantakopoulos A, Di Tullio G, Alessi DR, Kular GS, Daniele T, Marra P, Lucocq JM, De Matteis MA. FAPPs control Golgi-to-cell-surface membrane traffic by binding to ARF and PtdIns(4)P. Nat Cell Biol. 2004;6:393–404. doi: 10.1038/ncb1119. [DOI] [PubMed] [Google Scholar]
- Gong LW, Di Paolo G, Diaz E, Cestra G, Diaz ME, Lindau M, De Camilli P, Toomre D. Phosphatidylinositol phosphate kinase type I gamma regulates dynamics of large dense-core vesicle fusion. @Proc Natl Acad Sci USA. 2005;102:5204–5209. doi: 10.1073/pnas.0501412102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granseth B, Odermatt B, Royle SJ, Lagnado L. Clathrin-mediated endocytosis is the dominant mechanism of vesicle retrieval at hippocampal synapses. Neuron. 2006;51:773–786. doi: 10.1016/j.neuron.2006.08.029. [DOI] [PubMed] [Google Scholar]
- Guo S, Stolz LE, Lemrow SM, York JD. SAC1-like domains of yeast SAC1, INP52, and INP53 and of human synaptojanin encode polyphosphoinositide phosphatases. J Biol Chem. 1999;274:12990–12995. doi: 10.1074/jbc.274.19.12990. [DOI] [PubMed] [Google Scholar]
- Hao W, Tan Z, Prasad K, Reddy KK, Chen J, Prestwich GD, Falck JR, Shears SB, Lafer EM. Regulation of AP-3 function by inositides. Identification of phosphatidylinositol 3,4,5-trisphosphate as a potent ligand. J Biol Chem. 1997;272:6393–6398. doi: 10.1074/jbc.272.10.6393. [DOI] [PubMed] [Google Scholar]
- Harder KW, Owen P, Wong LK, Aebersold R, Clark-Lewis I, Jirik FR. Characterization and kinetic analysis of the intracellular domain of human protein tyrosine phosphatase beta (HPTP beta) using synthetic phosphopeptides. Biochem J. 1994;298:395–401. doi: 10.1042/bj2980395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TW, Hartwieg E, Horvitz HR, Jorgensen EM. Mutations in synaptojanin disrupt synaptic vesicle recycling. J Cell Biol. 2000;150:589–600. doi: 10.1083/jcb.150.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haucke V. Phosphoinositide regulation of clathrin-mediated endocytosis. Biochem Soc Trans. 2005;33:1285–1289. doi: 10.1042/BST0331285. [DOI] [PubMed] [Google Scholar]
- Honing S, Ricotta D, Krauss M, Spate K, Spolaore B, Motley A, Robinson M, Robinson C, Haucke V, Owen DJ. Phosphatidylinositol-(4,5)-bisphosphate regulates sorting signal recognition by the clathrin-associated adaptor complex AP2. Mol Cell. 2005;18:519–531. doi: 10.1016/j.molcel.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Hughes WE, Cooke FT, Parker PJ. Sac phosphatase domain proteins. Biochem J. 2000;350(Pt 2):337–352. [PMC free article] [PubMed] [Google Scholar]
- Irie F, Okuno M, Pasquale EB, Yamaguchi Y. EphrinB-EphB signalling regulates clathrin-mediated endocytosis through tyrosine phosphorylation of synaptojanin 1. Nat Cell Biol. 2005;7:501–509. doi: 10.1038/ncb1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T, De Camilli P. BAR, F-BAR (EFC) and ENTH/ANTH domains in the regulation of membrane-cytosol interfaces and membrane curvature. Biochim Biophys Acta. 2006;1761:897–912. doi: 10.1016/j.bbalip.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Jefferson AB, Majerus PW. Mutation of the conserved domains of two inositol polyphosphate 5-phosphatases. Biochemistry. 1996;35:7890–7894. doi: 10.1021/bi9602627. [DOI] [PubMed] [Google Scholar]
- Khvotchev M, Sudhof TC. Developmentally regulated alternative splicing in a novel synaptojanin. J Biol Chem. 1998;273:2306–2311. doi: 10.1074/jbc.273.4.2306. [DOI] [PubMed] [Google Scholar]
- Kim WT, Chang S, Daniell L, Cremona O, Di Paolo G, De Camilli P. Delayed reentry of recycling vesicles into the fusion-competent synaptic vesicle pool in synaptojanin 1 knockout mice. Proc Natl Acad Sci USA. 2002;99:17143–17148. doi: 10.1073/pnas.222657399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Wenk MR, Kim Y, Nairn AC, De Camilli P. Regulation of synaptojanin 1 by cyclin-dependent kinase 5 at synapses. Proc Natl Acad Sci USA. 2004;101:546–551. doi: 10.1073/pnas.0307813100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthi A, Di Paolo G, Cremona O, Daniell L, De Camilli P, McCormick DA. Synaptojanin 1 contributes to maintaining the stability of GABAergic transmission in primary cultures of cortical neurons. J Neurosci. 2001;21:9101–9111. doi: 10.1523/JNEUROSCI.21-23-09101.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson PS, Takei K, Schmid SL, De Camilli P. p145, a major Grb2-binding protein in brain, is co-localized with dynamin in nerve terminals where it undergoes activity-dependent dephosphorylation. J Biol Chem. 1994a;269:30132–30139. [PubMed] [Google Scholar]
- McPherson PS, Czernik AJ, Chilcote TJ, Onofri F, Benfenati F, Greengard P, Schlessinger J, De Camilli P. Interaction of Grb2 via its Src homology 3 domains with synaptic proteins including synapsin I. Proc Natl Acad Sci USA. 1994b;91:6486–6490. doi: 10.1073/pnas.91.14.6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson PS, Garcia EP, Slepnev VI, David C, Zhang X, Grabs D, Sossin WS, Bauerfeind R, Nemoto Y, De Camilli P. A presynaptic inositol-5-phosphatase. Nature. 1996;379:353–357. doi: 10.1038/379353a0. [DOI] [PubMed] [Google Scholar]
- Micheva KD, Kay BK, McPherson PS. Synaptojanin forms two separate complexes in the nerve terminal. Interactions with endophilin and amphiphysin. J Biol Chem. 1997;272:27239–27245. doi: 10.1074/jbc.272.43.27239. [DOI] [PubMed] [Google Scholar]
- Miesenbock G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- Milosevic I, Sorensen JB, Lang T, Krauss M, Nagy G, Haucke V, Jahn R, Neher E. Plasmalemmal phosphatidylinositol-4,5-bisphosphate level regulates the releasable vesicle pool size in chromaffin cells. J Neurosci. 2005;25:2557–2565. doi: 10.1523/JNEUROSCI.3761-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JR, Prasad K, Jin S, Augustine GJ, Lafer EM. Uncoating of clathrin-coated vesicles in presynaptic terminals: roles for Hsc70 and auxilin. Neuron. 2001;32:289–300. doi: 10.1016/s0896-6273(01)00467-6. [DOI] [PubMed] [Google Scholar]
- Nemoto Y, Arribas M, Haffner C, DeCamilli P. Synaptojanin 2, a novel synaptojanin isoform with a distinct targeting domain and expression pattern. J Biol Chem. 1997;272:30817–30821. doi: 10.1074/jbc.272.49.30817. [DOI] [PubMed] [Google Scholar]
- Nemoto Y, Wenk MR, Watanabe M, Daniell L, Murakami T, Ringstad N, Yamada H, Takei K, De Camilli P. Identification and characterization of a synaptojanin 2 splice isoform predominantly expressed in nerve terminals. J Biol Chem. 2001;276:41133–41142. doi: 10.1074/jbc.M106404200. [DOI] [PubMed] [Google Scholar]
- Perera RM, Zoncu R, Lucast L, De Camilli P, Toomre D. Two synaptojanin 1 isoforms are recruited to clathrin-coated pits at different stages. Proc Natl Acad Sci USA. 2006;103:19332–19337. doi: 10.1073/pnas.0609795104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramjaun AR, McPherson PS. Tissue-specific alternative splicing generates two synaptojanin isoforms with differential membrane binding properties. J Biol Chem. 1996;271:24856–24861. doi: 10.1074/jbc.271.40.24856. [DOI] [PubMed] [Google Scholar]
- Ringstad N, Nemoto Y, De Camilli P. The SH3p4/Sh3p8/SH3p13 protein family: binding partners for synaptojanin and dynamin via a Grb2-like Src homology 3 domain. Proc Natl Acad Sci USA. 1997;94:8569–8574. doi: 10.1073/pnas.94.16.8569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringstad N, Nemoto Y, De Camilli P. Differential expression of endophilin 1 and 2 dimers at central nervous system synapses. J Biol Chem. 2001;276:40424–40430. doi: 10.1074/jbc.M106338200. [DOI] [PubMed] [Google Scholar]
- Rusk N, Le PU, Mariggio S, Guay G, Lurisci C, Nabi IR, Corda D, Symons M. Synaptojanin 2 functions at an early step of clathrin-mediated endocytosis. Curr Biol. 2003;13:659–663. doi: 10.1016/s0960-9822(03)00241-0. [DOI] [PubMed] [Google Scholar]
- Ryan TA, Smith SJ. Vesicle pool mobilization during action potential firing at hippocampal synapses. Neuron. 1995;14:983–989. doi: 10.1016/0896-6273(95)90336-4. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan S, Ryan TA. Real-time measurements of vesicle-SNARE recycling in synapses of the central nervous system. Nat Cell Biol. 2000;2:197–204. doi: 10.1038/35008615. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan S, De Angelis D, Rothman JE, Ryan TA. The use of pHluorins for optical measurements of presynaptic activity. Biophys J. 2000;79:2199–2208. doi: 10.1016/S0006-3495(00)76468-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaranarayanan S, Ryan TA. Calcium accelerates endocytosis of vSNAREs at hippocampal synapses. Nat Neurosci. 2001;4:129–136. doi: 10.1038/83949. [DOI] [PubMed] [Google Scholar]
- Schiavo G, Gu QM, Prestwich GD, Sollner TH, Rothman JE. Calcium-dependent switching of the specificity of phosphoinositide binding to synaptotagmin. Proc Natl Acad Sci USA. 1996;93:13327–13332. doi: 10.1073/pnas.93.23.13327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuske KR, Richmond JE, Matthies DS, Davis WS, Runz S, Rube DA, van der Bliek AM, Jorgensen EM. Endophilin is required for synaptic vesicle endocytosis by localizing synaptojanin. Neuron. 2003;40:749–762. doi: 10.1016/s0896-6273(03)00667-6. [DOI] [PubMed] [Google Scholar]
- Schweizer FE, Ryan TA. The synaptic vesicle: cycle of exocytosis and endocytosis. Curr Opin Neurobiol. 2006;16:298–304. doi: 10.1016/j.conb.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Shin HW, Nakayama K. Dual control of membrane targeting by PtdIns(4)P and ARF. Trends Biochem Sci. 2004;29:513–515. doi: 10.1016/j.tibs.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Stefan CJ, Padilla SM, Audhya A, Emr SD. The phosphoinositide phosphatase Sjl2 is recruited to cortical actin patches in the control of vesicle formation and fission during endocytosis. Mol Cell Biol. 2005;25:2910–2923. doi: 10.1128/MCB.25.8.2910-2923.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JY, Wu XS, Wu LG. Single and multiple vesicle fusion induce different rates of endocytosis at a central synapse. Nature. 2002;417:555–559. doi: 10.1038/417555a. [DOI] [PubMed] [Google Scholar]
- Sun Y, Carroll S, Kaksonen M, Toshima JY, Drubin DG. PtdIns(4,5)P2 turnover is required for multiple stages during clathrin- and actin-dependent endocytic internalization. J Cell Biol. 2007;177:355–367. doi: 10.1083/jcb.200611011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenawa T, Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol. 2007;8:37–48. doi: 10.1038/nrm2069. [DOI] [PubMed] [Google Scholar]
- Tsujishita Y, Guo S, Stolz LE, York JD, Hurley JH. Specificity determinants in phosphoinositide dephosphorylation: crystal structure of an archetypal inositol polyphosphate 5-phosphatase. Cell. 2001;105:379–389. doi: 10.1016/s0092-8674(01)00326-9. [DOI] [PubMed] [Google Scholar]
- Ungewickell E, Ungewickell H, Holstein SE, Lindner R, Prasad K, Barouch W, Martin B, Greene LE, Eisenberg E. Role of auxilin in uncoating clathrin-coated vesicles. Nature. 1995;378:632–635. doi: 10.1038/378632a0. [DOI] [PubMed] [Google Scholar]
- Van Epps HA, Hayashi M, Lucast L, Stearns GW, Hurley JB, De Camilli P, Brockerhoff SE. The zebrafish nrc mutant reveals a role for the polyphosphoinositide phosphatase synaptojanin 1 in cone photoreceptor ribbon anchoring. J Neurosci. 2004;24:8641–8650. doi: 10.1523/JNEUROSCI.2892-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreken P, Koh TW, Schulze KL, Zhai RG, Hiesinger PR, Zhou Y, Mehta SQ, Cao Y, Roos J, Bellen HJ. Synaptojanin is recruited by endophilin to promote synaptic vesicle uncoating. Neuron. 2003;40:733–748. doi: 10.1016/s0896-6273(03)00644-5. [DOI] [PubMed] [Google Scholar]
- Wenk MR, Pellegrini L, Klenchin VA, Di Paolo G, Chang S, Daniell L, Arioka M, Martin TF, De Camilli P. PIP kinase Igamma is the major PI(4,5)P(2) synthesizing enzyme at the synapse. Neuron. 2001;32:79–88. doi: 10.1016/s0896-6273(01)00456-1. [DOI] [PubMed] [Google Scholar]
- Whisstock JC, Romero S, Gurung R, Nandurkar H, Ooms LM, Bottomley SP, Mitchell CA. The inositol polyphosphate 5-phosphatases and the apurinic/apyrimidinic base excision repair endonucleases share a common mechanism for catalysis. J Biol Chem. 2000;275:37055–37061. doi: 10.1074/jbc.M006244200. [DOI] [PubMed] [Google Scholar]
- Whisstock JC, Wiradjaja F, Waters JE, Gurung R. The structure and function of catalytic domains within inositol polyphosphate 5-phosphatases. IUBMB Life. 2002;53:15–23. doi: 10.1080/15216540210814. [DOI] [PubMed] [Google Scholar]
- Zheng J, Cahill SM, Lemmon MA, Fushman D, Schlessinger J, Cowburn D. Identification of the binding site for acidic phospholipids on the pH domain of dynamin: implications for stimulation of GTPase activity. J Mol Biol. 1996;255:14–21. doi: 10.1006/jmbi.1996.0002. [DOI] [PubMed] [Google Scholar]
- Zoncu R, Perera RM, Sebastian R, Nakatsu F, Chen H, Balla T, Ayala G, Toomre D, De Camilli PV. Loss of endocytic clathrin-coated pits upon acute depletion of phosphatidylinositol 4,5-bisphosphate. Proc Natl Acad Sci USA. 2007;104:3793–3798. doi: 10.1073/pnas.0611733104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.