Abstract
Context
Approximately 22% of U.S. young adults (aged 18–24 years) are smokers.1 Young adults typically display an interest in quitting,1,2 but it is unknown whether the evidence-based cessation programs designed for adults will be equally effective for young adults.
Purpose
This meta-analysis investigated the efficacy of smoking-cessation programs for this population.
Evidence acquisition
In 2009–2011, studies published between 2004 and 2008 that investigated smoking cessation were first found through the DHHS Clinical Practice Guidelines for Treating Tobacco Use and Dependence3 as well as a PubMed search (2009–2010) and were then subjected to a rigorous inclusion process. Authors were contacted to glean young adult raw data. Fourteen studies provided data that were coded for descriptive information and aggregated using the Comprehensive Meta-Analysis Version 2.04.
Evidence synthesis
Among young adults, any type of intervention was more effective in producing successful smoking cessation than the control. This was the case for intent-to-treat analyses as well as complete cases. When interventions were effective for the larger adult sample, they were also effective for the younger adult sample.
Conclusions
While young adults tend to underutilize evidence-based cessation treatments, the current meta-analysis showed that these treatments should be as effective for young adults as they are for the general adult population. Thus, it may be useful to focus on motivating young adults to seek cessation treatment to increase utilization.
Context
Historically, almost all adult smokers begin smoking as adolescents,5 but smoking becomes more entrenched during young adulthood (i.e., ages 18–24 years),6–8 and initiation into smoking is also not uncommon during this developmental period. This combination of initiation and escalation of smoking produces smoking prevalence equivalent to that of older age groups; in 2009, 21.8% of those aged 18–24 years in the U.S. were current smokers.1
Despite the increasing prevalence of smoking from adolescence into young adulthood, young adult smokers are interested in stopping smoking, and young adults are more likely to make a quit attempt than are older adults.1 In 2007, slightly more than half of current everyday smokers attempted to quit in the past 12 months.2 Yet young adults appear to differ in their methods of quitting smoking compared to older adults. For example, Solberg et al.9 found that young adults (aged 18–24 years) were less likely than older adults (aged 25–65 years) to use medication or to receive help from medical sources during the quit attempts, even in a sample that had access to assistance and medication. Curry et al. 10 similarly found in a national sample that young adults underutilized smoking-cessation treatments and rarely received advice from health providers concerning how to quit. What is unknown, however, is whether evidence-based treatments that benefit the general population are helpful for young adults also.
The DHHS Clinical Practice Guidelines for Treating Tobacco Use and Dependence3 identified multiple effective methods for smoking cessation among adults. The guidelines did not, however, specifically address the special population of young adults. Young adults' smoking patterns differ substantially from those of older adults.8 For example, young adults are more likely to be nondaily smokers and lighter smokers than are older adults.9,11 Among young adult smokers, Fagan et al.11 found that factors associated with quit attempts vary for daily and nondaily smokers, such that nicotine dependence may be more of a factor among daily smokers and sociodemographic factors may be more important among nondaily smokers. These different patterns of smoking as well as different factors associated with attempts at quitting bring into question whether cessation methods that are effective for adults in general will be equally effective for young adult smokers.
Villanti, et al.12 conducted a systematic qualitative review of smoking-cessation interventions for young adults (aged 18–24 years) in the U.S. They identified 12 RCCTs and two nonrandomized studies for review. Their analysis focused exclusively on interventions designed to meet the needs of young adults in the U.S., and not ones geared toward the general adult smoking population. All of the interventions included in the review focused on individual-level strategies for behavior change; there were no studies included of pharmacologic interventions in young adults. Of the 14 studies included, only two had significant results beyond 6 months follow-up. These authors conclude that there is limited evidence to date for the efficacy of smoking-cessation interventions for young adults in the U.S., but also note that the evidence base is limited by the methodologic variability in the studies, the lack of consistency of standard measures of smoking for this age group, and the limited diversity of smokers included in their sample of studies.
The overall goal of the current paper was to conduct a meta-analysis examining the efficacy of general adult smoking-cessation interventions in young adults. Smoking-cessation trials have generally not focused on young adults. When they are included in intervention trials, their outcomes typically are pooled with older or younger participants, making it difficult to assess whether particular interventions are effective for those aged 18–24 years.
The present meta-analysis differs from the Villanti et al.12 review in several ways. First, the present meta-analysis did not focus on interventions specifically designed for young adults, but rather, addressed the question of whether smoking-cessation interventions found effective for all adults would be effective for young adult smokers. Second, in addition to behavioral counseling studies, the present review included studies with a variety of pharmacotherapies as well as interventions found effective in special populations (e.g., pregnant women, hospitalized smokers). Finally, the analytic approaches were somewhat different, with the present study conducting a more formal meta-analysis. Only one study included in the previous review12 overlaps with the current study's meta-analysis.13 Thus, the present study complements the review of Villanti et al. and by more specifically addressing the question: Do smoking-cessation interventions geared for the general adult population show efficacy specifically for young adults?
Evidence Acquistion
Selection Process/Inclusion Criteria
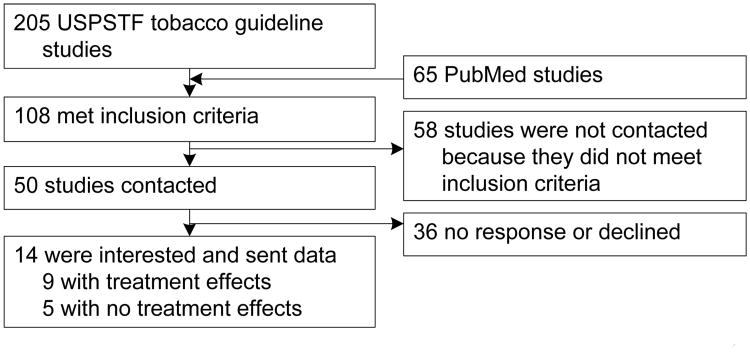
Search, selection and coding of relevant studies followed the PRISMA checklist.14 In 2009, potential studies initially were identified through two routes: (1) systematically reviewing studies included in the Clinical Practice Guidelines latest revision,3 which included comprehensive meta-analyses of peer-reviewed, RCCTs of tobacco use interventions with a minimum of 5 months follow-up for studies published between 2004 and 2008; and (2) a systematic literature search for relevant studies published between 2008 and 2010. Because the Clinical Practice Guidelines included well over 8,000 studies, only those studies published after 2004 were included, given the increase in methodologic rigor in study reporting more recently and the possibility of cohort effects for this age group given recent historic changes in tobacco marketing and smoking patterns.
Each study identified through the 2008 Clinical Practice Update (n = 205) was coded according to whether it had passed the following inclusion criteria: (1) some of the study participants were likely to be aged 18–24 years; (2) the total sample size was greater than 50 and a description of the study's sample suggested that that at least five participants aged 18–24 years were included in each condition (although an n of 5 is small, it was judged to be sufficient to compute an effect-size estimate). The coders' assessments were based on the descriptions of the samples in the studies' method sections, the total sample size, and the mean age and SD and/or range reported (if a study presented a range that did not include those aged 18–24 years, it was excluded from further consideration). If the study authors only reported the mean age or SD, the coders individually made judgments about the likelihood that a minimum of five participants between the ages of 18 and 24 years were included. If the coders did not concur, consensus was reached through discussion; (3) a reported smoking-cessation outcome(s) (e.g., cotinine, self-reported abstinence) rather than smoking reduction. Finally, it was determined whether a significant treatment effect (i.e., a difference between the intervention and control groups) was found. This information was used to prioritize the contacting of study authors for raw data (see below).
To acquire studies published after the Guideline's 2008 update, two of the authors conducted a literature search via the PubMed search engine. Search terms were “young adult” and “smoking cessation.” Search limits were placed to restrict articles to only RCTs and young adults aged 18– 24 years. Articles published in the years after the Guideline's publication (2008–2009) were then reviewed to avoid duplication and capture recent studies. Thirty studies were identified in this phase, yielding a total of 103 studies (73 from the 2008 Guidelines and 30 from PubMed search). In June 2011, this search process was repeated (also using PubMed) for studies published during 2010; ten new studies were identified as potentially relevant.
Author Contact for Data
Authors of the relevant published studies were contacted to obtain data (raw data or n's, means, and SDs) for participants aged 18–24 years (see Figure 1). Authors (contacted in 2010) were offered an honorarium of $200 to provide their data, which often involved their conducting new analyses by age group. Authors were supplied an Excel template to input effect sizes or raw data for the means or ORs between the experimental and control groups only for participants aged 18–24 years. Contacted authors also supplied various descriptive data about the study methods (e.g., length of counseling session, ethnicity of participants) and outcomes (e.g., point-prevalence data, expired CO data), which were imported to data files for the current study. The specific data requested can be found in Table 1.
Figure 1. Flowchart of study selection and author contact procedure USPSTF, U.S. Preventive Services Task Force.
Table 1. Descriptive information about study subsamples of those aged 18–24 years.
| Study | Young adult sampl e size | Intervention type | Control type | Length of follow-up | Cessation measure | Outcome confirmed? | Effect size (complete cases, final time point) | Effect size (intent to treat, final time point) | Treatment effect for parent study? |
|---|---|---|---|---|---|---|---|---|---|
| Rabius, (2004)13 | 355 | Five telephone counseling sessions based on motivational interviewing + self-help booklets | Mailed self-help booklets | 6 months | Point prevalence | No; self-report | OR=1.60, 95% CI=0.46, 5.52 | OR=3.37, 95% CI=1.21, 9.39 | Yes |
| Cooper, (2005)19 | 41 | 1. Nicotine replacement therapy (NRT) gum + cognitive behavioral (CB) counseling (e.g.., self monitoring, problem solving, social support, relapse prevention)2. Phenyl propanolamine (PPA) gum + CB counseling | Placebo gum + CB counseling | 12 months | Point prevalence | Yes; expired CO concentration | ORNRT=0.43, 95% CI=0.01, 14.08 ORPPA=3.46, 95% CI=0.13, 90.68 | ORNRT=0.33, 95% CI=0.01, 8.93 ORPPA=6.10, 95% CI=0.29, 128.95 | No |
| Hennrikus, (2005)20 | 97 | 1. Brief advice + usual care2. Brief advice and extended counseling (one bedside session and up to six telephone sessions) | Usual care (smoking-cessation manuals and list of community resources) | 12 months | Point prevalence | Yes; saliva cotinine concentration | ORadvice=0.35, 95% CI=0.08, 1.59 ORcounsel=0.91, 95% CI=0.25, 3.29 | ORadvice=0.33, 95% CI=0.08, 1.44 ORcounsel=0.74, 95% CI=0.21, 2.55 | No |
| Dornelas, (2006)21 | 51 | One therapy session (focused on emotional distress and smoking) with bimonthly supportive follow-up telephone calls | Usual care (standard smoking-cessation treatment guidelines, personalized quit message) and cessation manual | 12 months | Point prevalence | Yes; expired CO concentration | OR=4.44, 95% CI=0.88, 22.34 | OR=4.44, 95% CI=0.88, 22.34 | Yes |
|
| |||||||||
| Gilbert, (2006)22 | 169 | Five proactive telephone counseling calls (based on motivational principles and relapse prevention) + brief counseling during participant call to a quitline and offer of an information pack | Brief counseling during participant call to a quitline and offer of an information pack | 12 months | Prolonged abstinence | No; self-report | OR=0.84, 95% CI=0.11, 6.37 | OR=0.88, 95% CI=0.12, 6.38 | No |
| Aveyard, (2008)23 | 43 | Nicotine replacement therapy (NRT) + Nortryptyline | NRT + Placebo | 12 months | Prolonged abstinence | Yes; saliva cotinine | OR=2.29, 95% CI=0.19, 27.99 | OR=3.06, 95% CI=0.26, 36.42 | No |
| Borland, (2008)24 | 122 | In-practice management (assessment of quit readiness, possible pharmacotherapy or quitline referral) | Usual practice | 12 months | Prolonged abstinence | concentration or exhaled CO concentration No; self-report | OR=3.53, 95% CI=0.16, 76.64 | OR=2.94, 95% CI=0.14, 62.50 | Yes |
| Cropsey, (2008)25 | 85 | NRT patch plus 10-week group counseling (mood management training) | Waitlist | 6 months | Point prevalence | Yes; expired CO concentration | OR=3.74, 95% CI=0.72, 19.50 | OR=3.94, 95% CI=0.77, 20.06 | Yes |
|
| |||||||||
| McCarthy, (2008)26 | 70 | 1. sustained-released bupropion2. Individual counseling (preparation for quit day, coping/problem solving, relapse prevention, intratreatment social support | 1. placebo2. no counseling | 12 months | Point prevalence and prolonged abstinence | Yes; saliva cotinine concentration and expired CO concentration | ORcounsel=1.08*, 95% CI=0.16, 7.42 ORncounsel=0.67 *, 95% CI=0.17, 24.10 | ORcounsel=1.91*, 95% CI=0.12, 7.41 ORncounsel=0.59 *, 95% CI=0.16, 19.34 | Yes |
| Meyer, (2008)27 | 544 | 1. Brief advice by general practitioner (based on health behavior change counseling)2. Tailored letters to support quitting (included normative feedback and ipsative feedback) | Usual care | 24 months | Point prevalence | No; self-report | ORadvice=1.57, 95% CI=0.81, 3.03 ORletter=1.80. 95% CI=0.92, 3.51 | ORadvice=1.57, 95% CI=0.91, 3.25 ORletter=1.80, 95% CI=0.76, 2.71 | Yes |
| Schumann, (2008)28 | 28 | 1. computer-tailored letters and self-help booklets2. Health Behavior Change counseling + computer-tailored letters and self-help booklets | Assessment only | 24 months | Point prevalence | No; self-report | ORcomputer=1.50, 95% CI=0.18, 12.78 ORcounsel=1.00, 95% CI=0.07, 13.64 | ORcomputer=0.60, 95% CI=0.09, 3.99 ORcounsel=0.50, 95% CI=0.05, 5.51 | No |
| Ellerbeck, (2009)29 | 42 | 1. Nicotine patch and sustained-release bupropion2. Up to six counseling calls (based on motivational interviewing and relapse prevention) and a newsletter every 6 months | Medication only (patch and bupropion) | 24 months | Point prevalence | No; self-report | OR6calls=1.09, 95% CI=0.21, 5.73 OR3calls=1.40, 95% CI=0.23, 8.46 | OR6calls=0.80, 95% CI=0.17, 3.80 OR3calls=1.50, 95% CI=0.27, 8.45 | Yes |
|
| |||||||||
| Cinciripini, (2010)30 | 128 | 10-week depression-focused counseling (Cognitive Behavioral Analysis System of Psychotherapy-CBASP) | Health and wellness counseling | 6 months | Prolonged abstinence | Yes; expired CO concentration | --- | OR= 1.19, 95% CI=0.44, 3.24 | Yes |
| Reitzel, (2010)31 | 151 | Seven counseling sessions based on motivation and skills training (Motivation and Problem Solving; MAPS) | Self-help materials and brief advice | 6 months | Prolonged abstinence | Yes; saliva cotinine concentration and expired CO concentration | OR=1.74, 95% CI=0.65, 4.66 | OR=1.79, 95% CI=0.68, 4.72 | Yes |
Because of budget constraints and wanting to maximize yield from studies most likely to be able to contribute to the analyses, studies were prioritized for contacting authors. Contact was made with studies (n=45) where each study group (experimental and control group) contained at least 50 participants. This decision-rule was based on the expectation that there were likely to be at ≥5 participants in each study group aged 18–24 years in a group of such size. The information about the participants and the mean age reported in the study's Method section was also used to prioritize contact. Of the 45 studies contacted, 35 reported significant treatment effects and ten demonstrated no overall treatment effect. Studies without treatment effects were included to avoid the “file-drawer problem,”15 which suggests that studies tend to be published only if they show effects or, conversely, researchers only submit papers which show effects. This omission of nonsignificant effects can bias the results of a meta-analysis. Of the 45 study authors contacted, 13 were interested and sent raw data while 32 either did not respond or did not produce data.
To ascertain whether studies for which raw data were received from the authors (n=13) differed from those who opted not to participate, studies were coded for funding source (i.e., government, pharmaceutic company, foundation), time since publication (1–2 years, 3–4 years, or 5–6 years), and type of intervention (counseling, pharmacotherapy, both). Although the number of studies was too small to conduct chi-square analyses, there were three trends. Authors were less likely to participate if their study had been published 5–6 years previously. As researchers leave institutions and move onto new projects, this trend is unsurprising.
Authors of studies involving support from the pharmacologic industry were also less likely to be responsive (nine declined/did not respond vs zero followed-up). A similarly related trend was that authors of studies involving only pharmacotherapy (six declined vs three followed-up) or pharmacotherapy plus counseling (12 declined vs four followed-up) were less likely to send their data. A response from one of the investigators who declined probably provides the explanation for both trends. This author noted that pharma funding would require getting the company's permission to release the data, which would be time-consuming (and might not be approved).
An updated search for relevant studies published after 2009 was conducted in 2011 with PubMed using the same search techniques as adopted previously. Because extramural support was no longer available, authors were contacted as before, but no remuneration was offered. Of the ten studies identified as relevant, five met the inclusion criteria. Authors of two of the five studies responded to the request for data regarding those aged 18–24 years. At face value, no differences were detected between the authors who responded to the request for data and those that did not. All five studies were funded by a government source. Of the nonresponders, two studies involved counseling-based interventions and the other had a pharmacologic intervention. Both studies for which data were provided involved counseling-based interventions.
Data Analysis
Of the 18 authors who indicated a willingness to share data, 14 completed the Excel spreadsheets. All received data were cleaned and then entered into the computer program, Comprehensive Meta-Analysis (CMA) Version 2.0. 4
Studies were coded for the descriptive information provided in Table 1 (with disagreements settled in discussion or arbitrated by a third coauthor). The individual study effect sizes (comparing treatment condition with control) were computed for the first time point, which ranged from the end of treatment to 12 months, and then aggregated according to the Hedges and Olkin24 procedures in the Comprehensive Meta-Analysis program, version 2.0.4 The random effects model was chosen as heterogeneity existed between studies, and random effect is considered to be the more conservative model.17,18 Next, the same analyses were conducted on the study effect sizes for the last time point measured in each study, which ranged from 6 months to 24 months. In addition to calculating the mean OR and 95% CIs, the software also produced two indices of heterogeneity among study effect sizes: Q and I2.
Evidence Synthesis
Table 1 presents relevant information (i.e., study authors, publication date, sample size, nature of cessation treatment, ORs for comparison of treatment with control) about the 14 studies (with 20 total effect sizes), which were included for analysis. Some studies involved multiple treatment conditions, resulting in more outcomes than studies, which introduces non-independence in the meta-analyses. According to Hunter and Schmidt,32 however, a violation of independence is unlikely to cause systematic bias when estimating the mean effect sizes. As an extra precaution, the analyses were rerun by aggregating effect sizes at the study level first. Point estimates did not differ by more than 0.04, indicating there is no systematic bias.
Meta-Analytic Results for All Studies
Table 2 shows the relevant meta-analytic statistics represented by the mean OR, the 95% CI, and the heterogeneity statistics. For ORs, a CI that does not include 1.00 is significant at p< 0.05, meaning that the treatment condition is associated with greater odds of successful smoking cessation than the control group. The nonsignificant Q statistic and a small I2 (< 25 = low heterogeneity) indicate that there is little to no variation among the individual study effect sizes, suggesting that the calculated summary effect can be considered an accurate estimate of the average magnitude of the efficacy of the treatment compared to the controls. 33
Table 2. Meta-analytic results for all studies.
| All studies | |||||
|---|---|---|---|---|---|
|
| |||||
| Number of study outcomes aggregated | Mean OR | 95% CI | Q-test for heterogeneity of study sample outcomes | I2 test for heterogeneity of study sample outcomes | |
| First time point, ITT | 20 | 1.55* | 1.16, 2.06 | 15.83 (ns) | 0.00 |
| First time point, | 19 | 1.76* | 1.26, 2.46 | 12.01 (ns) | 0.00 |
| Complete Cases | |||||
| Last time point | 20 | 1.48* | 1.11, 1.97 | 16.25 (ns) | 0.00 |
| Last time point, | 19 | 1.54* | 1.12, 2.11 | 9.32 (ns) | 0.00 |
| Complete Cases | |||||
Note: I2 test is a more conservative test of heterogeneity. Small numbers indicate little variability among study treatment outcomes.
= average OR is significantly different from 1.00, p< 0.05 (i.e., the effects of treatment were more effective than control ITT, intent to treat; ns, nonsignificant
For all relevant studies, intent-to-treat outcomes (i.e., based on all those who were enrolled regardless of whether they completed the study) for smoking cessation at the first time point measured were aggregated. The results (see Table 2) indicate that any type of intervention was associated with a greater odds of cessation (OR = 1.55, 95% CI= 1.16, 2.06). This was also shown when the complete cases (i.e., all participants who actually completed the study through all time points) were investigated (OR = 1.76, 95% CI=1.26, 2.46).
For both the intent-to-treat and complete case analyses, the individual study effect sizes were homogenous, based on the Q-values and the more conservative I2 index. The intent to treat outcomes at the last time point measured also yielded an effect with an average OR of 1.48 (95% 95% CI = 1.11, 1.97); individual study effect sizes did not differ, based on the nonsignificant Q-test and the I2. Similar values (OR = 1.54, 95% CI=1.12, 2.11) were found for the complete cases at the last time point. In sum, aggregation of all smoking-cessation outcome results for the subsamples aged 18–24 years suggest that interventions (of any sort) were associated with higher odds of cessation than control conditions.
Meta-Analytic Results for Studies in Which the Parent Study Obtained Significant or No Treatment Effects
Nine parent studies (reporting 12 outcomes, because of multiple treatment conditions), from which the young adult data were gleaned, had reported an overall treatment effect, and five parent studies (n= 7) had not found an overall treatment effect. The analyses reported above were replicated but outcomes were partitioned by whether the parent study had found a treatment effect (p < 0.05). For the intent-to-treat analyses, results for the subsample of those aged 18–24 years from the parent studies with significant effects followed those of the parent study for both the first time point (OR = 1.75, 95% CI = 1.26, 2.44) and the last time point (OR=1.58; 95% CI = 1.16, 2.15). Analyses of the subsamples of those aged 18–24 years from the parent studies in which no significant treatment effect was found for the intent to treat analyses similarly found no treatment effects (all CIs included 1.0).
When examining the complete cases, the same pattern emerged. In the subsample of those aged 18–24 years from parent studies with a treatment effect, there was similarly an effect at both the first time point (OR=1.99, 95 % CI=1.17, 3.37) and the last time point (OR = 1.66, 95% CI=1.11, 2.48), and no effects were found for the subsamples from parent studies that did not find an overall treatment effect (all CIs include 1.0). Thus, in the subset of studies in which an overall treatment effect was obtained for all ages studied, there was also a treatment effect for the participants aged 18–24 years. Accordingly, effects were not significant in the subsets of those aged 18–24 years when the parent study did not obtain a treatment effect.
Discussion
Aggregating all study outcomes indicated that interventions (versus controls) were associated with higher odds of smoking cessation in young adults. The results for subsamples of those aged 18–24 years followed those of the parent studies: in cases where there was an overall treatment effect for all ages in the parent study, there was similarly an effect for the young adult group, but no treatment effect for the subsample of young adults when the parent study found no treatment effect. The treatments involved pharmacotherapies (nicotine replacement or bupropion) and/or cognitive behavior therapy, counseling and social support. However, there were too few studies to investigate the distinctive benefits for one particular intervention.
National data indicate that although young adults are motivated to quit, they underutilize evidence-based treatments. These findings suggest that effective smoking-cessation treatments for adults in general are also likely to be effective for young adults. Thus, the findings suggest that the development of effective interventions to increase the use of evidence-based treatments among motivated young adult smokers could have an important public health impact.
The current findings may be contrasted with those of Villanti et al.,12 whose qualitative review concluded that interventions geared specifically for young adults have been ineffective. As noted above, their review had more limited inclusion criteria (focusing specifically on studies designed for young adults and more limited representation of population than the present meta-analysis). From the present study results, it is concluded that interventions that are generally effective for the general adult population work as well for young adults. The difference may be in driving or attracting young adults to an intervention versus what is delivered once the young adult is ready to engage in treatment. Perhaps more work is needed to motivate young adults to seek evidence-based treatments. Some effective tools to help young adults quit have been developed, but interventions may need to be tailored to mobilize them to seek appropriate help.
This project implemented a relatively novel methodologic approach as the focus was on identifying and aggregating results from particular subsets of participants (i.e., those aged 18–24 years) in each study. This approach was adopted because rarely were results reported separately for the participants in this age range in the original publication. Identification and cooperation of relevant study authors is labor-intensive but optimizes the use of data already collected.
These meta-analyses are limited by a low rate of participation by authors of studies with experimental and control groups of at least 50 participants. However, obtaining raw data or other relevant statistics for subsamples from larger published studies, which had pooled the data, is a relatively novel enterprise. Although the approach attempted to make additional use of data already collected, research teams are not accustomed to such requests, and some team members relocate. Recent NIH policies about data sharing will probably make the kinds of requests made of researchers in the current study more common and anticipated. In any case, a follow-up project with additional resources for contact/follow-up would help address the rate of participation.
There were limitations in the ability to look at differences among young adults by gender, ethnicity, comorbidities, and other clinical or behavioral characteristics. Therefore, the possibility of interaction of any characteristics by age could not be assessed. On the other hand, heterogeneity across studies was low, suggesting that the average effect size estimates are appropriate indices of the efficacy of smoking-cessation treatments.
Conclusion
It is encouraging to find that young adults can benefit from existing treatments for smoking cessation. Encouraging a greater proportion of young adults to seek out these evidence-based treatments and perhaps more-tailored marketing of these effective treatments to this population may help to further reduce the prevalence of smoking among this age group.
Acknowledgments
The authors extend their thanks to Kristie Taylor and Jocelyn Fraum who assisted with study author contact and data collection.
This study was supported by the Robert Wood Johnson Foundation grant 61337 (SC and RM) and the National Cancer Institute grant 5R01CA134861 (SC and RM).
Footnotes
No financial disclosures were reported by the authors of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jerry M. Suls, Department of Psychology, College of Public Health, University of Iowa, Iowa City, Iowa.
Tana M. Luger, Department of Psychology, College of Public Health, University of Iowa, Iowa City, Iowa.
Susan J. Curry, Department of Psychology, College of Public Health, University of Iowa, Iowa City, Iowa.
Amy K. Sporer, Institute for Health Research and Policy, University of Illinois at Chicago, Chicago, Illinois.
Larry C. An, University of Michigan Medical School, Ann Arbor, Michigan.
References
- 1.MMWR. Vital signs: Current cigarette smoking among adults aged > 18 years—U S 2009. 2010;59:1135–1140. [PubMed] [Google Scholar]
- 2.MMWR. Cigarette smoking among adults—2007. 2008;57:1221–1226. [PubMed] [Google Scholar]
- 3.Fiore FC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ. Clinical Practice Guideline. DHHS Public Health Service; Rockville, MD: May, 2008. Treating Tobacco Use and Dependence: 2008 Update. [PubMed] [Google Scholar]
- 4.Bornstein M, Rothstein H. Englewood, NJ. Biostat; 2009. Comprehensive meta-analysis: a computer program for research synthesis. [Google Scholar]
- 5.DHHS. Preventing tobacco use among young people: a report of the surgeon general. DHHS, Public Health Service, CDC, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, Georgia: 1994. [Google Scholar]
- 6.Bachman JG. The decline of substance use in young adulthood: changes in social activities, roles, and beliefs. Lawrence Erlbaum Associates; Mahwah, NJ: p. 2002. [Google Scholar]
- 7.Flora DB, Chassin L. Changes in drug use during young adulthood: the effects of parent alcoholism and transition into marriage. Psychol Addict Behav. 2005;19:352–62. doi: 10.1037/0893-164X.19.4.352. [DOI] [PubMed] [Google Scholar]
- 8.Hammond D. Smoking behaviour among young adults: beyond youth prevention. Tob Control. 2005;14:181–185. doi: 10.1136/tc.2004.009621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solberg LI, Boyle RG, McCarty M, Asche SE, Thoele MJ. Young adult smokers: Are they different? Am J Manag Care. 2007;13:626–632. [PubMed] [Google Scholar]
- 10.Curry SJ, Sporer AK, Pugach O, Campbell RT, Emery T. Use of tobacco cessation treatments among young adult smokers: 2005 national health interview survey. Am J Public Health. 2007;97:1464–69. doi: 10.2105/AJPH.2006.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fagan P, Augustson E, Backinger C, O'Connell ME, Vollinger RE, Kaufman A, Gibson JT. Quit attempts and intentions to quit cigarette smoking among young adults in the U.S. Am J Public Health. 2007;97:1412–1420. doi: 10.2105/AJPH.2006.103697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villanti AC, McKay HS, Abrams DB, Holtgrave DR, Bowie JV. Smoking-cessation interventions for U.S. young adults: a systematic review. Am J Prev Med. 2010;39:564–574. doi: 10.1016/j.amepre.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Rabius V, McAlister AL, Geiger A, Huang P, Todd R. Telephone counseling increases cessation rates among young smokers. Health Psychol. 2004;23:539–541. doi: 10.1037/0278-6133.23.5.539. [DOI] [PubMed] [Google Scholar]
- 14.Mohr D, Liberati A, Tetzlaff J, Altman DG the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. J Clin Epid. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Rosenthal R. The file-drawer problem and tolerance for null results. Psychol Bull. 1979;86:638–641. [Google Scholar]
- 16.Hedges LV, Olkin I. Statistical methods for meta-analysis. Academic Press; New York: p. 1985. [Google Scholar]
- 17.Hedges LV, Vevea JL. Fixed- and random-effects models in meta-analysis. Psychol Methods. 1998;3:486–504. [Google Scholar]
- 18.Egger M, Smith GD, Phillips AN. Meta-analysis: Principles and procedures. Br Med J. 1997;315:1610–14. doi: 10.1136/bmj.315.7121.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooper TV, Klesges RC, DeBon MW, Zbikowski SM, Johnson KC, Clemens L. The effects of PPA and nicotine gum on cessation rates and post cessation weight gain in women. Addict Behav. 2005;30:61–75. doi: 10.1016/j.addbeh.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Hennrikus DJ, Lando HA, McCarty MC, Klevan D, Holtan N, Huebsch JA, et al. The TEAM project: the effectiveness of smoking cessation intervention with hospital patients. Prev Med. 2005;40:249–258. doi: 10.1016/j.ypmed.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 21.Dornelas EA, Magnavita JJ, Beazoglou T, et al. Efficacy and cost-effectiveness tested in an ethnically diverse sample of pregnant smokers. Patient Educ Couns. 2006;64:342–349. doi: 10.1016/j.pec.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 22.Gilbert H, Sutton S. Evaluating the effectiveness of proactive telephone counseling for smoking cessation in a randomized controlled trial. Addiction. 2006;101:590–8. doi: 10.1111/j.1360-0443.2006.01398.x. [DOI] [PubMed] [Google Scholar]
- 23.Aveyard P, Johnson C, Fillingham S, Parsons A, Murphy M. A pragmatic randomized controlled trial of nortriptyline plus nicotine replacement versus placebo plus nicotine replacement for smoking cessation. BMJ. 2008;336:1223–27. doi: 10.1136/bmj.39545.852616.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borland R, Balmford J, Bishop N, et al. In-practice management versus quitline referral for enhancing smoking cessation in general practice: a cluster randomised trial. Fam Pract. 2008;25(5):382–389. doi: 10.1093/fampra/cmn046. [DOI] [PubMed] [Google Scholar]
- 25.Cropsey KL, Eldridge GD, Weaver MF, Villalobos GC, Stitzer ML, Best AM. Smoking cessation intervention for female prisoners: addressing an urgent public health need. Am J Public Health. 2008;98:1894–1901. doi: 10.2105/AJPH.2007.128207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCarthy DE, Piasecki TM, Lawrence DL, Jorenby DE, Shiffman S, Fiore MC, Baker TB. A randomized controlled clinical trial of bupropion SR and individual smoking cessation counseling. Nicotine Tob Res. 2008;10:717–729. doi: 10.1080/14622200801968343. [DOI] [PubMed] [Google Scholar]
- 27.Meyer C, Ulbricht S, Baumeister SE, Schumann A, Rüge J, Bischof G, et al. Proactive interventions for smoking cessation in general medical practice: a quasi-randomized controlled trial to examine the efficacy of computer-tailored letters and physician-delivered brief advice. Addiction. 2008;103:294–304. doi: 10.1111/j.1360-0443.2007.02031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schumann A, John U, Baumeister SE, Ulbricht S, Rumpf HJ, Meyer C. Computer-tailored smoking cessation intervention in a general population setting in Germany: outcome of a randomized controlled trial. Nicotine Tob Res. 2008;10:371–379. doi: 10.1080/14622200701825767. [DOI] [PubMed] [Google Scholar]
- 29.Ellerbeck EF, Mahnken JD, Cupertino AP, Cox LS, Greiner KA, Mussulman LM, et al. Effect of varying levels of disease management on smoking cessation. A randomized trial. Ann Intern Med. 2009;150:437–46. doi: 10.7326/0003-4819-150-7-200904070-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cinciripini PM, Blalock JA, Minnix JA, Robinson JD, Brown VL, Lam C, et al. Effects of an intensive depression-focused intervention for smoking cessation in pregnancy. J Consult Clin Psychol. 2010;78:44–54. doi: 10.1037/a0018168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reitzel LR, Vidrine JI, Businell MS, et al. Preventing postpartum smoking relapse among diverse low-income women: A randomized clinical trial. Nicotine Tob Res. 2010;12:326–335. doi: 10.1093/ntr/ntq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunter JE, Schmidt FL, editors. Methods of meta-analysis: Correcting error and bias in research findings. Thousand Oaks CA: Sage Publications; 2004. [Google Scholar]
- 33.Higgins JPT, Thompson S. Quantifying heterogeneity in meta-analysis. Stat in Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]