Abstract
Periostin is an extracellular matrix protein that is up-regulated by T helper cell type 2 cytokines in the asthmatic airway and implicated in mouse studies as promoting eosinophil recruitment. We asked whether periostin modulates eosinophil adhesion and motility in vitro. Periostin adsorbed to polystyrene supported adhesion of purified human blood eosinophils stimulated by IL-5, IL-3, or granulocyte/macrophage colony–stimulating factor, but did not support adhesion of eosinophils treated with IL-4 or IL-13. The degree of adhesion depended on the concentrations of periostin during coating and activating cytokine during the adhesion assay. Both full-length periostin and alternatively spliced periostin, lacking C-terminal exons 17, 18, 19, and 21, supported adhesion. Adhesion was inhibited by monoclonal antibody to αM or β2 integrin subunits, but not by antibodies to other eosinophil integrin subunits. Adsorbed periostin also supported αMβ2-dependent random motility of IL-5–stimulated eosinophils with optimal movement at an intermediate coating concentration. In the presence of IL-5, eosinophils adherent on periostin formed punctate structures positive for filamentous actin, gelsolin, and phosphotyrosine. These structures fit the criteria for podosomes, highly dynamic adhesive contacts that are distinct from classical focal adhesions. The results establish αMβ2 (CD11b/CD18, Mac-1) as an adhesive and promigratory periostin receptor on cytokine-stimulated eosinophils, and suggest that periostin may function as a haptotactic stimulus able to guide eosinophils to areas of high periostin density in the asthmatic airway.
Keywords: eosinophils, periostin, integrins, αMβ2 (CD11b/CD18, Mac-1), adhesion
Clinical Relevance
We demonstrate that the extracellular matrix protein, periostin, supports adhesion and random motility of cytokine-stimulated eosinophils via αMβ2 integrin, and that motility is optimal at an intermediate periostin coating concentration. We propose that periostin in the asthmatic airway promotes eosinophil recruitment to areas of high periostin density. The periostin–αMβ2 interaction may be a possible therapeutic target in asthma.
Infiltration with eosinophils is characteristic of allergic asthma and is associated with airway remodeling and changes in the extracellular matrix (ECM) (1–6). These observations raise the issue of how eosinophils become enriched in the asthmatic airway. Integrins play key roles in adhesion of leukocytes to walls of blood vessels associated with inflammation and in movement of leukocytes through inflamed tissues (7, 8). Eosinophils possess a unique repertoire of seven integrin heterodimers, α4β1, α6β1, αLβ2, αMβ2, αXβ2, αDβ2, and α4β7, each of which interacts with counter receptors or ligands on other cells or ligands deposited in the ECM (9). Studies of β2-deficient and conditionally α4-deficient mice indicate that α4 and β2 integrins contribute to trafficking of eosinophils to lung in models of allergen-induced acute and chronic asthma (10, 11). Integrins exist in inactive and active conformations, and are activated by signals triggered through other receptors and mediated by cytoplasmic factors, so-called “inside-out” signaling (7, 8, 12). Engagement of integrins by ligands, in turn, can activate cytoplasmic signaling pathways: “outside-in” signaling (12, 13). Whether a given pair of integrin and ligand participates in the complex two-way signaling that results in adhesion and migration depends on: (1) the cell surface density of the integrin; (2) the density of the ligand; and (3) the activation state of the integrin (14, 15).
Activation of β1 integrins on blood eosinophils correlates with decreased lung function in subjects with mild asthma, is triggered by P-selectin in vitro, is likely a result of association with P-selectin–bearing activated platelets in vivo, and leads to enhanced adhesion of eosinophils to the α4β1 ligand vascular cell adhesion molecule (VCAM)-1, which is up-regulated on lung endothelium in response to T helper cell (Th) type 2 mediators, presumably facilitating arrest in the airway (16–18). Less is known about eosinophil integrin–ligand interactions that provide interstitial guides used by eosinophils after extravasation to move about and persist in the ECM of the airway of patients with asthma. Eosinophil integrins have the potential to mediate migration on VCAM-1 via α4β1, α4β7, αMβ2, and αDβ2; intercellular adhesion molecules via αMβ2, αLβ2, and αDβ2; fibronectin via α4β1 and α4β7; laminin via α6β1; fibrinogen/fibrin via αMβ2 and αXβ2; and vitronectin via αMβ2 (9). These ligands are variably present in the airway wall; collagen is more widespread, but eosinophils lack the collagen-binding integrins, α1β1, α2β1, α10β1, and α11β1 (9). Regarding eosinophil integrin activation within the lung, the most is known about eosinophils recovered by bronchoalveolar lavage after segmental antigen challenge. These cells have highly activated αMβ2 and up-regulated αDβ2 in addition to activated β1 integrins (19, 20). In vitro, activation of αMβ2 can be induced by IL-5 (9, 17, 19, 21). Treatment of subjects with asthma with blocking monoclonal antibody (mAb) to IL-5 attenuates activation of β2 integrins on blood eosinophils, but does not diminish activation of αMβ2 on bronchoalveolar lavage eosinophils, suggesting that there are other stimulators of activation in the lung (22).
Periostin is an ECM protein (23, 24) that is up-regulated by Th2 cytokines IL-4 and IL-13 in bronchial epithelial cells and lung fibroblasts, deposited widely in the airway of subjects with asthma and animals provoked to model asthma (25–29), as well as in atopic dermatitis and an allergic skin inflammation animal model (30). Periostin is particularly highly expressed in patients with asthma identified as Th2-high subjects (31, 32), and high levels of serum periostin are associated with high numbers of sputum and tissue eosinophils in asthma (32). Mice lacking periostin respond to lung challenge with significantly decreased numbers of eosinophils in the lung and increased numbers in blood (33, 34), and have reduced inflammation in the allergic skin inflammation model (30). Some studies showed that periostin-deficient mice also have increased serum IgE and peripheral Th2 responses, airway resistance, and mucus production, and decreased lung transforming growth factor (TGF)-β production (35, 36), although another study showed decreased mucous metaplasia and decreased IL-4 expression (34). Taken together, the human and mouse data indicate that periostin secreted and deposited in allergy-induced lesions is required for eosinophil recruitment, but in a way that may modulate allergic inflammation and possibly protect the lung from adverse consequences of antigen exposure.
It is unknown if and how eosinophils interact with periostin. Eosinophils lack αV integrins (9), which have been demonstrated to mediate adhesion to periostin in other cell types (24, 37–40). We report herein that periostin supports robust adhesion and random motility of IL-5–treated eosinophils mediated by αMβ2 integrin, and speculate that the αMβ2–periostin interaction provides important guidance for eosinophils in Th2-conditioned ECM.
Materials and Methods
Cells
Eosinophils were purified from heparinized blood of donors with allergy and/or asthma as before (17). The studies were approved by the University of Wisconsin—Madison Health Sciences Institutional Review Board. Informed written consent was obtained from each subject before participation.
Antibodies, Adhesive Ligands, Cytokines, and ELISA
Antibodies, adhesive ligands, and cytokines used, and a direct ELISA for adsorbed periostin, are described in the online supplement.
Cell Adhesion
Eosinophil adhesion was assayed by cell content of eosinophil peroxidase, and results are expressed as percentage of input cells, as previously described (17, 19, 41). Briefly, wells in polystyrene 96-well plates (non-tissue-culture-treated, catalog no. 351172; BD Labware, Franklin Lakes, NJ) were coated with 100 μl of the relevant protein in Tris-buffered saline (pH 8.0) for 2 hours at 37°C, decanted, and blocked with 100 μl neat FBS. Control wells were coated with only FBS. Eosinophils (100 μl of 105 cells/ml) were added in the absence or presence of recombinant IL-5, IL-3, granulocyte/macrophage colony–stimulating factor (GM-CSF), IL-4, IL-13, or soluble periostin. After 1 hour, wells were washed three times with Tris-buffered saline and absorbance of the colored eosinophil peroxidase product was measured at 492 nm in a Genios Pro plate reader (Tecan, Männedorf, Switzerland). In some experiments, suspended eosinophils were preincubated with mAbs for 5 minutes before addition to the wells. To normalize data of some experiments, cells adherent under a given condition were expressed as percentage of cells adhering to a coating of FBS alone in the presence of IL-5 (10 ng/ml) or to periostin (or fibronectin) in the absence of soluble stimulus.
Fluorescence Microscopy
Fluorescent staining and microscopy were performed as previously described (42), with the following modifications. Eosinophils (3 × 105) were added to each coated coverslip. For phosphotyrosine staining, 1 mM Na3VO4 was present throughout the washes with PBS–3% BSA and the primary and secondary antibody staining steps, as we have done before (43, 44). Coverslips were mounted with Immumount (Thermo Scientific, Pittsburgh, PA). Representative eosinophils were photographed as before (17).
Cell Motility Assay
Cell motility was assessed with a microbead monolayer assay (45). Wells were coated and blocked with FBS as for the adhesion assay. Yellow 1-μm-diameter Polybeads (200 μl; Polysciences, Warrington, PA) diluted 1:200 in RPMI were added to the wells, the plates were centrifuged, and 150 μl supernatant was aspirated, as described previously (45). A total of 50 μl of 20,000 eosinophils/ml in RPMI–20% FBS were then added in the absence or presence of IL-5 (10 ng/ml) and incubated for 20 hours at 37°C. Wells were viewed in a Diaphot inverted microscope (Nikon, Melville, NY) and photographed using a digital camera. Results were quantified by tracing the migratory paths using ImageJ (http://imagej.nih.gov/ij/).
Statistical Analysis
Two-tailed t test was used to compare data between two groups. ANOVA with Dunnett’s post test was used to compare data among several groups. A P value of 0.05 or less was considered significant. Analyses were performed using Prism (GraphPad Inc., San Diego, CA) or UsableStats (http://www.usablestats.com/calcs.php).
Results
Eosinophils Adhere to Periostin
Eosinophil adhesion assays were performed in microtiter plates in which wells were coated with the relevant protein and postcoated with neat FBS; control wells were coated only with neat serum. This approach allows the protein of interest to have “first crack” to occupy the protein binding sites in the wells, after which the sites are blocked with a vast excess (50–70 mg/ml) of protein, including 30–40 mg/ml albumin, in the serum. Cells were in Hanks’ balanced salt solution containing 1.3 mM Ca2+ and 0.8 mM Mg2+.
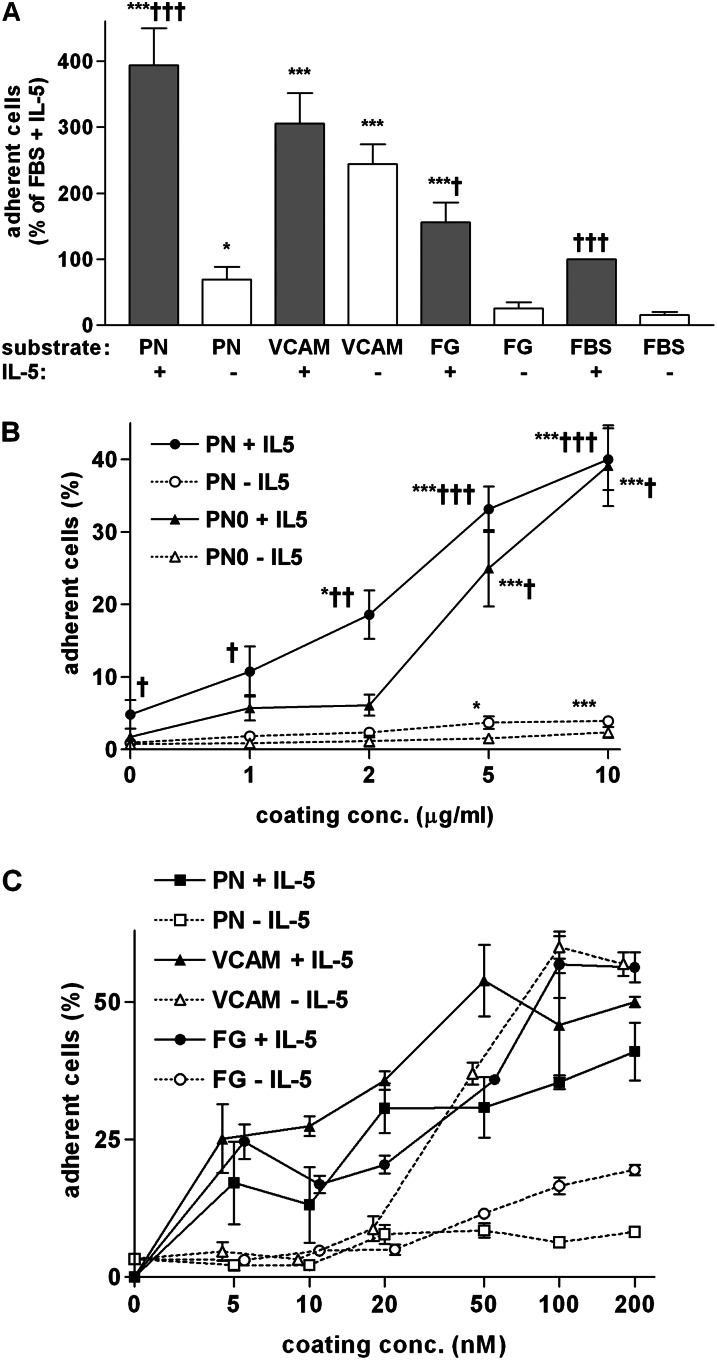
In 12 experiments on cells from different subjects, 25–65% of the 104 eosinophils stimulated with 10 ng/ml IL-5 adhered to the 0.32-cm2 (3.2 × 107-μm2) wells coated with 10 μg/ml (110 nM) full-length periostin. Such adhesion was higher than to wells coated with 10 μg/ml extracellular portion of 7-domain VCAM-1 or fibrinogen, and roughly fourfold higher than to wells coated with serum alone (Figure 1A). Adhesion to periostin was less when IL-5 was absent, similar to the diminished adhesion to fibrinogen without IL-5, but roughly fourfold higher than adhesion to serum without IL-5 (Figure 1A). In contrast, omitting IL-5 diminished adhesion to VCAM-1 only slightly (Figure 1A).
Figure 1.
Eosinophil adhesion to periostin (PN) compared with other proteins. Adhesion of purified blood eosinophils incubated for 1 hour in the absence or presence of IL-5 (10 ng/ml) in wells of microtiter plates. (A) Wells were coated with the following proteins at 10 μg/ml: full-length PN (10 μg/ml = 110 nM); 7-domain extracellular domain of vascular cell adhesion molecule (VCAM)-1 (10 μg/ml = 130 nM); and fibrinogen (FG; 10 μg/ml = 56 nM). Wells were postcoated with FBS or coated with FBS alone (FBS). Data shown are means (±SEM) (n = 12 donors for PN ± IL-5, 8 for VCAM ± IL-5, and 3 for FG ± IL-5). Each individual assay was performed in duplicate. Means of the duplicates were calculated and normalized to adhesion to FBS + IL-5 before pooling experiments. ***P ≤ 0.001 versus FBS; *P ≤ 0.05 versus FBS; †††P ≤ 0.001 versus no IL-5; †P ≤ 0.05 versus no IL-5 (t test). (B) Wells were coated with various concentrations of PN lacking sequences from exons 17, 18, 19, and 21 (PN0, triangles) compared with full-length PN including sequences from exons 17, 18, 19, and 21 (PN, circles). Adhesion was determined in the absence (open symbols) or presence (closed symbols) of IL-5. Data shown are means (±SEM) (n = 5 donors for PN, 3 donors for PN0). ANOVA for curves of: PN + IL-5, P = 0.0003; PN − IL-5, P = 0.01; PN0 + IL-5, P < 0.0001; and PN0 − IL-5 P = 0.35. Post test: ***P ≤ 0.001 versus no coating of PN; *P ≤ 0.05 versus no coating of PN. t test of the effect of IL-5: †††P ≤ 0.001 versus no IL-5; ††P ≤ 0.01 versus no IL-5; †P ≤ 0.05 versus no IL-5. (C) Comparisons of dose responses of adhesion to full-length PN (squares), VCAM-1 (triangles), and FG (circles) in the absence (open symbols) or presence (closed symbols) of IL-5. Wells were coated with the same range of molar concentrations. Data shown are means (±SEM) of two wells with cells from one donor. For clarity, symbols that overlap have been displaced slightly laterally from one another.
Human periostin comprises an emilin module, four fasciclin-1 modules, and an alternatively spliced C-terminal region (46). The recombinant full-length periostin used in Figure 1A includes sequences coded by the differentially spliced exons 17, 18, 19, and 21, as described in the online supplement, and is hereafter called full-length periostin. To determine whether sequences encoded by the differentially spliced C-terminal exons are necessary for periostin’s ability to support adhesion of eosinophils and to test protein from an alternate source, we produced recombinant periostin lacking exons 17, 18, 19, and 21 (periostin-0) in the baculovirus system. Analyzing cells from five donors on wells coated with 1–10 μg/ml full-length periostin, adhesion of eosinophils in the presence of IL-5 increased in proportion to the concentration used to coat the wells, with significantly enhanced adhesion with coating concentrations of 2 μg/ml (22 nM) and higher, and a suggestion of the beginning of a plateau at the highest coating concentration of 10 μg/ml (110 nM) (Figure 1B). Periostin-0 coated at 5 or 10 μg/ml supported eosinophil adhesion to a similar degree as did full-length periostin (Figure 1B). A direct ELISA with an mAb that recognizes full-length periostin and periostin-0 demonstrated similar increases in signals for the two forms of periostin coated at concentrations between 0.1 and 10 μg/ml, including a similar signal at a coating concentration of 2 μg/ml (data not shown), for which adhesion was lower to periostin-0 than to full-length periostin (Figure 1B). We conclude that sequences encoded by the C-terminal alternatively spliced exons are not required for periostin’s activity toward eosinophils. However, the sequences may influence whether periostin presented to the surface at low concentration adsorbs in conformations that are active in adhesion. We therefore used the active concentrations of both full-length periostin and periostin-0.
As an alternative “head-to-head” test of adhesive activity, we compared adhesion to periostin (∼90 kD) to VCAM-1 (∼76 kD) and fibrinogen (∼180 kD for the αβγ half molecule) coated at increasing molar concentrations in the range of 5–200 nM, and quantified adhesion of eosinophils in the presence or absence of IL-5 (Figure 1C). In the presence of IL-5, the three proteins had roughly equal molar dose–response curves. In the absence of IL-5, adhesion to periostin and fibrinogen was lower than in the presence of IL-5 at all coating concentrations (Figure 1C). In contrast, adhesion to VCAM-1 without IL-5 was similar to that with IL-5 (Figure 1C). The IL-5–induced shift to the left of the curve of eosinophil adhesion versus coating concentration of VCAM-1 (Figure 1C) is similar to what has been described previously (41).
Eosinophil Adhesion to Periostin Is Stimulated by IL-3 or GM-CSF, but Not by IL-4 or IL-13
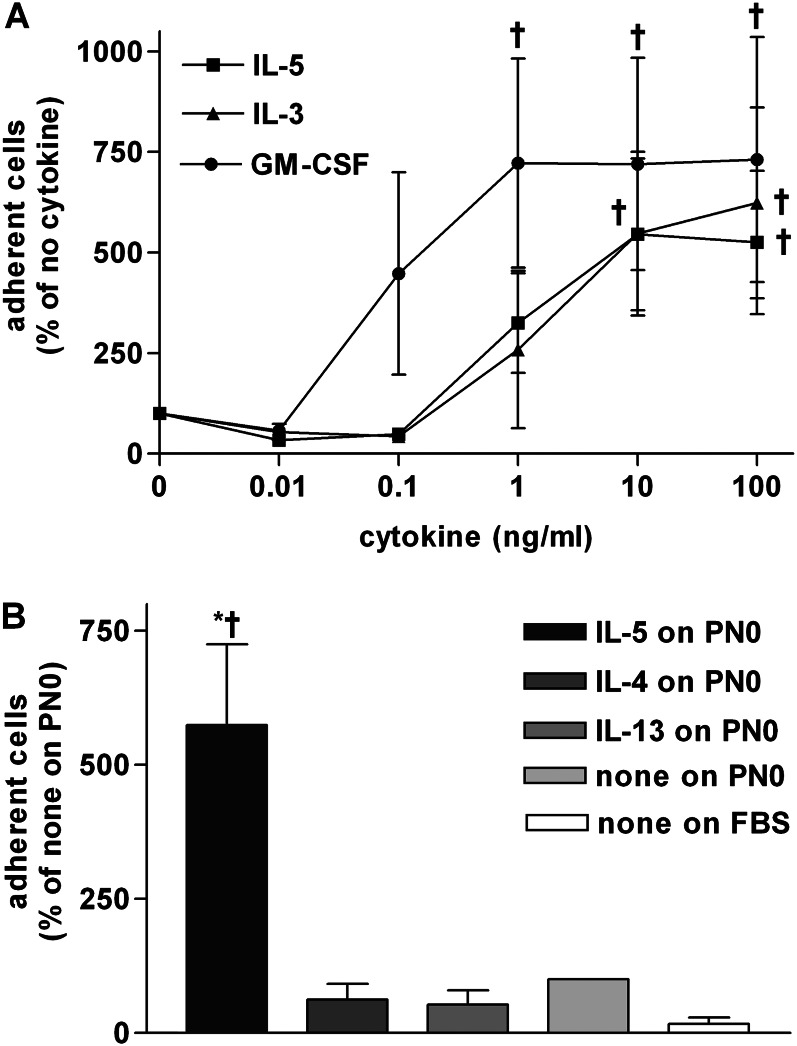
The stimulating effect of IL-5 was dose dependent, with an intermediate effect at 1 ng/ml and the greatest effect at 10 ng/ml (Figure 2A). IL-5–related cytokines IL-3 and GM-CSF also stimulated adhesion to periostin, up to similar maximal levels as IL-5, although the dose–response curve of GM-CSF was shifted compared with that of IL-5, with a maximum at 1 ng/ml (Figure 2A). IL-4 and IL-13, which up-regulate periostin expression in epithelial cells and fibroblasts (25, 26, 28, 29), did not stimulate eosinophil adhesion to periostin at 10 ng/ml (Figure 2B), nor at higher or lower concentrations (data not shown). The same batches of IL-13 and IL-4 that did not stimulate eosinophil adhesion to periostin were active, at 5–20 ng/ml, in replicating the reported increase in the amount of periostin, detected by immunoblotting, in fibroblasts (26), and activation of STAT6 in eosinophils and synergism with TNF-α to increase eosinophil secretion of CCL17 and CCL22 (47) (S. Esnault, personal communication).
Figure 2.
Cytokine concentration dependencies of eosinophil adhesion to PN. (A) Adhesion to full-length PN coated at 5 μg/ml followed by postcoating with FBS of purified blood eosinophils in the absence or presence of different concentrations of IL-5 (squares), IL-3 (triangles), or granulocyte/macrophage colony–stimulating factor (GM-CSF) (circles). Data shown are means (±SEM) (n = 3 donors). ANOVA for curves of: IL-5, P = 0.005; IL-3, P = 0.02; and GM-CSF, P = 0.02. Post test: †P ≤ 0.05 versus no cytokine. (B) Adhesion of purified blood eosinophils in the absence or presence of 10 ng/ml IL-5, IL-4, or IL-13 to PN lacking exons 17, 18, 19, and 21 (PN0) coated at 10 μg/ml followed by postcoating with FBS or FBS alone. Data shown are means (±SEM) (n = 3 donors). †P ≤ 0.05 versus no cytokine on PN0; *P ≤ 0.05 versus FBS (t test).
Eosinophil Adhesion to Periostin Is Mediated by αMβ2 Integrin
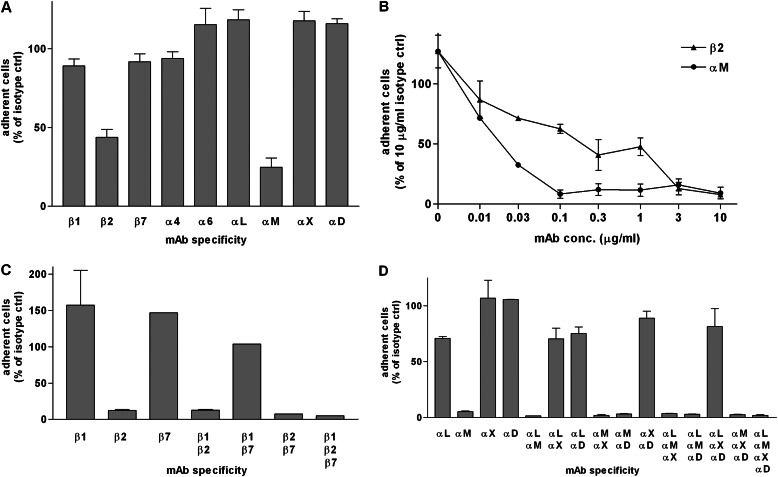
Preincubation of IL-5–treated eosinophils with inhibitory mAbs to the different integrin subunits known to be expressed on eosinophils demonstrated that mAbs 2LPM19c and TS1/18 to αM and β2, respectively, but not mAbs to other subunits, inhibited eosinophil adhesion to periostin (Figure 3A). Dose–response experiments showed that anti-αM mAb 2LPM19c was strongly inhibitory at a concentration as low as 0.1 μg/ml, whereas anti-β2 mAb TS1/18 had a shallower dose–response curve, but was strongly inhibitory at concentrations of 3 μg/ml or higher (Figure 3B). It has been shown previously that αMβ2 is the major eosinophil receptor for fibrinogen (19), whereas adhesion of IL-5–stimulated eosinophils to VCAM-1 is inhibited by mAbs to both α4β1 and αMβ2 (41). To address whether, as for adhesion to VCAM-1, there is involvement in adhesion to periostin of eosinophil integrins in addition to αMβ2, we tested various combinations of anti-β and anti-α mAbs. Strong inhibition was only seen with combinations that included anti-β2 or anti-αM (Figures 3C and 3D). Analyzing the different experiments with anti-integrin mAbs together by ANOVA and post test, it was determined that only anti-β2 or anti-αM significantly inhibited adhesion of IL-5–stimulated eosinophils to periostin (P < 0.001; n = 5 donors for anti-β2 and anti-αM). Thus, taking these data together, we conclude that αMβ2 is the integrin adhesive receptor for periostin on eosinophils.
Figure 3.
Effects of anti-integrin monoclonal antibodies (mAbs) on adhesion to PN of purified blood eosinophils preincubated with mAb to eosinophil integrin subunits in the presence of IL-5 (10 ng/ml). Results are expressed as percentage of adhesion obtained with control Ig(s) of the same isotype(s). (A) Adhesion to full-length PN, coated at 2 μg/ml, of cells preincubated with mAb (10 μg/ml) to the indicated eosinophil integrin subunit. Data shown are means (±SEM) of three wells, representative of two experiments. (B) Adhesion to PN0, coated at 10 μg/ml, of cells preincubated with different concentrations of mAb to β2 or αM integrin subunit. Data shown are means (±SEM) of two wells, representative of two experiments. (C) Adhesion to PN0, coated at 10 μg/ml, of cells preincubated with mixtures of mAbs (10 μg/ml each) to the different eosinophil β integrin subunits. Data shown are means (±SEM) of two wells (except one well with mixtures including β7 mAb). (D) Adhesion to PN0, coated at 10 μg/ml, of cells preincubated with mixtures of mAbs (10 μg/ml each) to the different eosinophil α integrin subunits that partner with β2. Data shown are means (±SEM) of two wells.
Effect of Soluble Periostin on Adhesion to Various Proteins
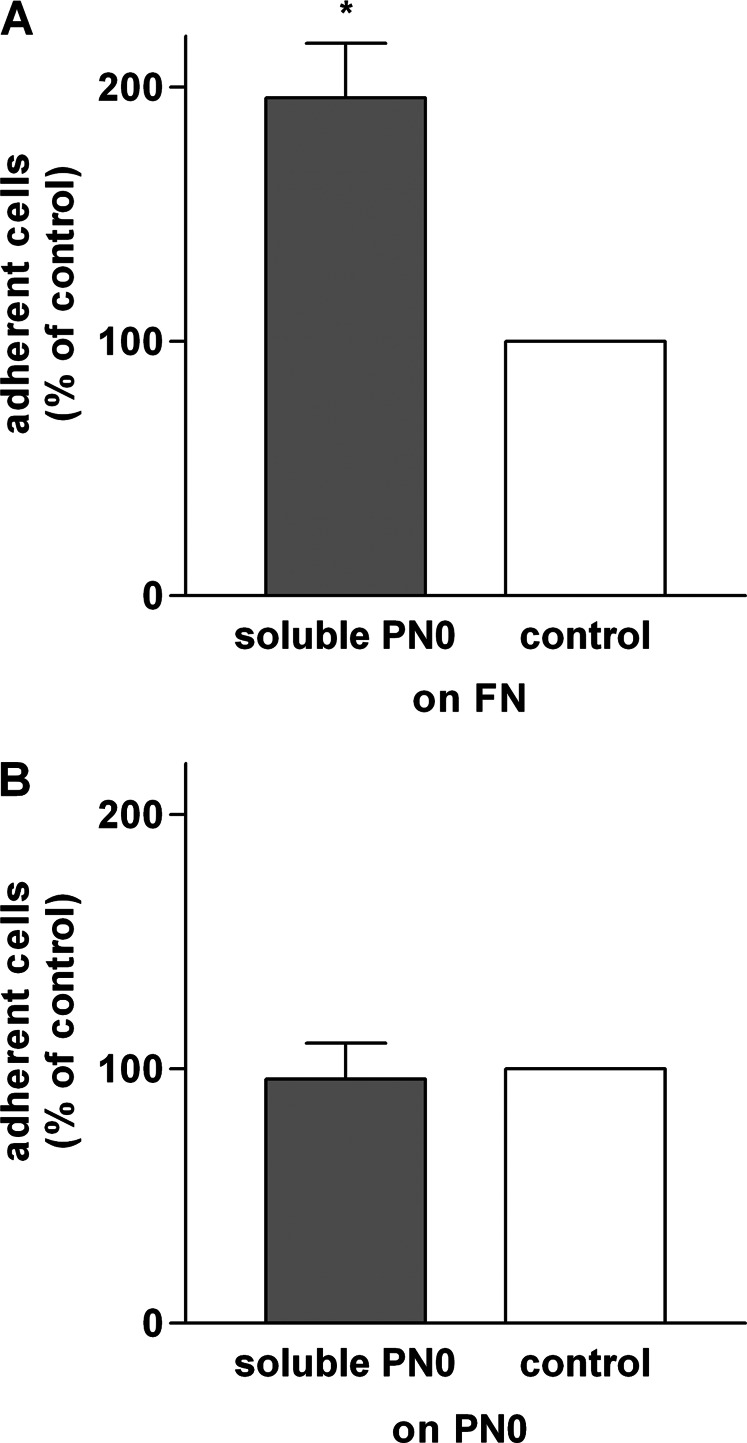
Soluble periostin has been shown to induce adhesion of eosinophils from IL-5–transgenic mice to adsorbed fibronectin (33), which otherwise is poorly supportive of adhesion of unstimulated or IL-5–stimulated eosinophils (19). We found a similar effect in that 10 μg/ml soluble periostin significantly stimulated adhesion to human fibronectin coated at 10 μg/ml (Figure 4A). Soluble periostin, in contrast, did not enhance or diminish adhesion to a coating of periostin (Figure 4B).
Figure 4.
Effects of soluble PN on eosinophil adhesion to fibronectin or PN. Experiments were performed with PN0. Adhesion of purified blood eosinophils in the presence of IL-5 (10 ng/ml) with or without soluble PN (10 μg/ml) to fibronectin (FN), coated at 10 μg/ml (A) or PN, coated at 2 μg/ml (B). Data shown are means (±SEM) (n = 3 donors). *P ≤ 0.05 versus control (no soluble PN) (t test).
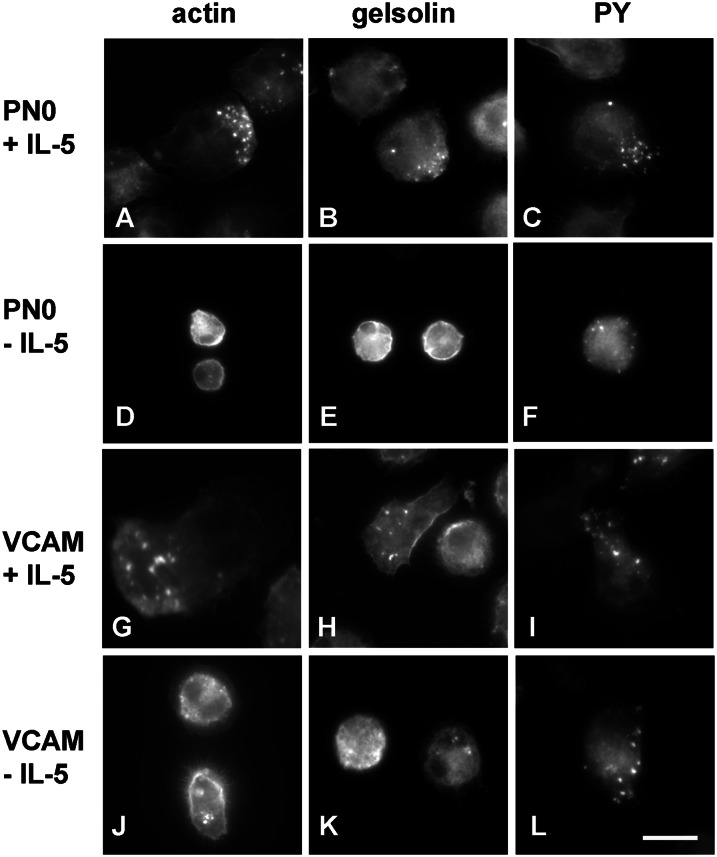
IL-5–Stimulated Eosinophils Adherent to Periostin Form Podosomes
To learn whether eosinophil adhesion to periostin triggers outside-in signaling and formation of adhesive structures, we stained eosinophils attached to periostin in the presence or absence of IL-5 in comparison to eosinophils attached to VCAM-1, which we have studied previously (19, 42). Eosinophils adherent on periostin in the presence of IL-5 were flat and well spread and formed punctate structures positive for filamentous actin (F-actin), gelsolin, and phosphotyrosine-containing proteins (Figures 5A–5C). Such a staining pattern is indicative of podosomes, highly dynamic adhesive contacts of many cells, including cancer cells, macrophages, osteoclasts, dendritic cells, and vascular smooth muscle cells; gelsolin is not present in classical focal adhesions of normal fibroblasts (42, 48–51). The podosomes were often found in one region or at one end of the IL-5–stimulated eosinophils (Figures 5A–5C). In the absence of IL-5, the few eosinophils that attached were round and less spread (Figures 5D–5F) than in the presence of IL-5. Without IL-5, F-actin (Figure 5D) and gelsolin (Figure 5E) were mostly in a cortical distribution at the cell periphery, whereas phosphotyrosine was found in small punctate structures (Figure 5F). As these cells did not have prominent F-actin– and gelsolin-containing podosomes (Figures 5D and 5E), the phosphotyrosine-positive structures in the absence of IL-5 may be a different type of structure, or possibly a precursor to podosomes formed in response to adhesion to periostin without exposure to cytokine. Eosinophils adherent on VCAM-1 in the presence of IL-5 (Figures 5G–5I) were similar to those on periostin in the presence of IL-5. In the absence of IL-5, more eosinophils were found on VCAM-1 than on periostin. Such cells (Figures 5J and 5K) were more spread and less round than on periostin without IL-5, but less spread than on VCAM-1 with IL-5. On VCAM-1 without IL-5, cells had some F-actin– and gelsolin-positive and phosphotyrosine-positive podosomes (Figures 5J–5L), as described previously (42). We also attempted to stain for focal adhesion kinase, which is a known component of focal adhesions in nonhematopoietic cells, and its paralog, proline-rich tyrosine kinase 2, which is expressed in hematopoietic cells (52). We saw little staining with mAbs to focal adhesion kinase and proline-rich tyrosine kinase 2 in eosinophils adherent to periostin (data not shown), even though there are reports indicating that these proteins are expressed and functional in eosinophils (53–55).
Figure 5.
Fluorescent localization of filamentous actin (F-actin), gelsolin, and phosphotyrosine (PY) in eosinophils adhered to PN compared with eosinophils adhered to VCAM-1. Micrographs of eosinophils adhered for 1 hour in the presence (A–C and G–I) or absence (D–F and J–L) of IL-5 (10 ng/ml) to adsorbed PN0 (A–F) or VCAM-1 (G–L), both coated at 5 μg/ml, analyzed by visualization of F-actin with rhodamine-phalloidin (A, D, G, and J) or by immunofluorescent staining with mAb to gelsolin (B, E, H, and K) or PY (C, F, I, and L) and rhodamine-conjugated secondary antibody. Bar, 10 μm. Representative fields are shown from experiments with four donors.
Eosinophils Display Random Motility on Periostin
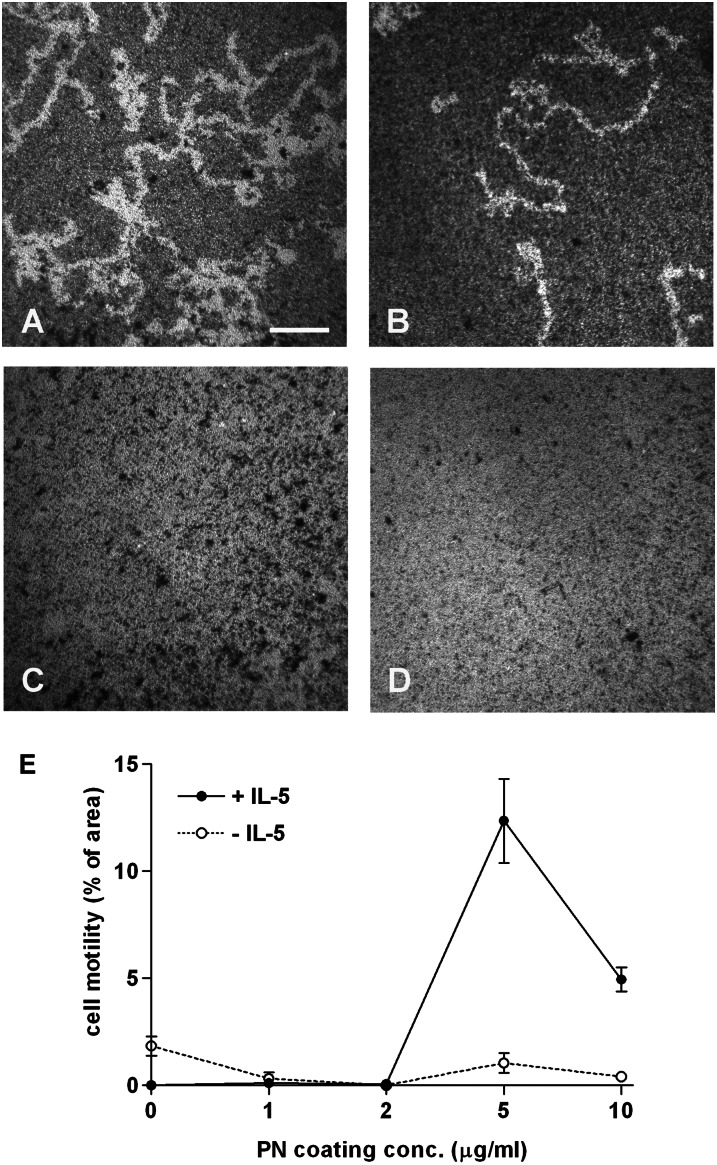
Podosomes are common in migratory cells (48, 50, 51). The effect of periostin on surface-dependent motility (haptokinesis) of eosinophils was assessed with a microbead monolayer assay (45). After protein coating and blocking, microbeads were added at a concentration that, after centrifugation of the plate, forms a nearly confluent “lawn” of beads. In Figure 6, white areas cleared of microbeads represent paths of migration in contrast to the dark bead monolayer background. Immobilized periostin supported motility of blood eosinophils (Figures 6A and 6B) compared with the serum-blocking control (Figures 6C and 6D). Long tracks were cleared. More tracks formed in the presence (Figure 6A) than in the absence (Figure 6B) of IL-5. Motility was quantified by tracing the migratory paths using ImageJ software. Maximal motility was bimodal, with the most motility at a periostin coating concentration of 5 μg/ml, and lesser motility on surfaces coated with 2 and 10 μg/ml (Figure 6E). Experiments with cells from three donors showed that motility on 5 μg/ml periostin was significantly higher than motility on 10 μg/ml periostin; motility on 5 μg/ml was 200 (±30)% (mean ± SEM; range, 150–250%) of that on 10 μg/ml (P = 0.02, t test). The anti-αM and β2 integrin mAbs abolished eosinophil motility on periostin (data not shown), demonstrating that the random motility is mediated by αMβ2-mediated interaction of IL-5–treated eosinophils with periostin.
Figure 6.
Eosinophil motility on PN. Paths of purified blood eosinophils migrating in the presence (A and C) or absence (B and D) of IL-5 (10 ng/ml) on full-length PN coated at 5 μg/ml (A and B) or FBS alone (C and D) were revealed by perturbation of a monolayer of 1 μm diameter latex beads. Wells were photographed after 20 hours. Bar, 100 μm. (E) Morphometric analysis of track areas as a function of PN coating concentration. Data shown are means (±SEM) of two wells, representative of experiments with three donors.
Discussion
We report that the ECM protein, periostin, which is up-regulated in the asthmatic airway by Th2 mediators and has been implicated in mouse and human studies as promoting eosinophil recruitment (25–29, 31–34), supports specific adhesion of blood eosinophils stimulated by IL-5 or the related cytokines, IL-3 and GM-CSF. In contrast, the Th2 cytokines, IL-4 and IL-13, did not induce adhesion to periostin. IL-5 and GM-CSF activate STAT3 and STAT5 in blood eosinophils (56), whereas IL-4 activates STAT6 (47). Thus, even though these cytokines activate JAK/STAT pathways, the IL-5 family cytokine- and the IL4/13–stimulated pathways are distinct in that only the IL-5 family–triggered pathway stimulates eosinophil adhesion to periostin.
Adsorbed periostin is roughly equivalent to VCAM-1 or fibrinogen in mediating adhesion of IL-5–stimulated eosinophils. Eosinophil adhesion to periostin was dependent on the periostin coating concentration and cytokine dose, and did not require the presence of the alternatively spliced C-terminal exons of periostin. Furthermore, eosinophil adhesion was mediated by αMβ2 integrin, which is activated by IL-5 on eosinophils (9, 17, 19, 21). To our knowledge, αMβ2 has not been reported previously to be a periostin receptor. Instead, for other cell types, αV integrins, which are absent on eosinophils (9), have been identified as adhesive receptors for periostin (24, 37–40).
Immobilized periostin supported αMβ2-dependent random motility of IL-5–stimulated eosinophils. In contrast to adhesion, which increased in proportion to the periostin coating concentration over the concentration range studied, motility was optimal at a periostin coating concentration of 5 μg/ml, with lower motility at a coating concentration of 10 μg/ml. We propose that periostin deposited in the airway under Th2 immunity conditions promotes eosinophil recruitment and serves as a guide for eosinophil migration in the ECM of the asthmatic airway. Thus, a gradient of periostin may complement exposure of eosinophils to gradients of chemotactic agents. Furthermore, cells may arrest at a critical density of periostin. The interaction with periostin may also affect eosinophil survival and mediator release, and lead to modulation of allergic inflammation and airway remodeling.
Our motility results are reminiscent of fibrinogen-supported random motility in a model system of αIIbβ3-transfected Chinese hamster ovary cells in which motility was found to be optimal at 5 μg/ml fibrinogen, with lower motility at 1 and 10 μg/ml (14). Such data indicate that the strength of adhesion, as modulated by adhesive substrate concentration, is an important regulator of the cell migration rate. Thus, optimal motility occurs at an intermediate coating concentration, possibly because, at a higher ligand density, release at the rear of the migrating cell is inhibited (14). However, migration of the transfected Chinese hamster ovary cells may not be a good paradigm for eosinophils adherent on periostin. Eosinophils form podosomes, highly dynamic adhesive contacts found in cancer cells, and several normal cell types that are distinct from classical focal adhesions of fibroblasts (42, 48–51). Podosomes provide a mechanism for cells to degrade ECM locally (48, 50, 51). It has been suggested that the formation of podosomes is required for or facilitates migration of many cell types, although a detailed understanding of the mechanism by which this occurs is not yet available (51). Future studies are needed to address if and how podosome formation in eosinophils is associated with motility on periostin, turnover of periostin, and migration through the ECM.
A periostin paralog, TGF-β–induced protein (TGFBI), which is expressed in corneal epithelium, and mutations of which are associated with corneal dystrophies (57), has been shown to support αMβ2-mediated monocyte adhesion and motility (58). In a preliminary experiment, we found that recombinant TGFBI (also from R&D Systems and made in mouse NSO myeloma cells) supports adhesion of IL-5–stimulated eosinophils, albeit to a lower degree than does periostin (data not shown). In addition, by using a myeloperoxidase assay (59), we observed that neutrophils stimulated with GM-CSF adhere to periostin, again to a lower degree than do IL-5– or GM-CSF–stimulated eosinophils (data not shown). More detailed comparisons of the adhesive and promigratory activities of periostin and TGBFI for αMβ2-bearing leukocytes are needed to learn the relative impacts of the two proteins on trafficking and enrichment of various types of αMβ2-bearing leukocytes in tissues.
Soluble periostin circulates at concentrations of ∼10–40 ng/ml in subjects with asthma (32). We confirmed the report (33) that microgram per milliliter concentrations of soluble periostin enhance eosinophil adhesion to fibronectin. The trace concentrations of periostin in blood presumably come from a pool of more concentrated periostin in tissues. Thus, it may be that periostin secreted in response to Th2 cytokines has a dual function in eosinophil adhesion, with soluble periostin driving adhesion to ECM fibronectin and ECM periostin directly supporting adhesion and migration. Future studies are needed to identify the receptor that recognizes soluble periostin to enhance adhesion of IL-5–stimulated eosinophils to fibronectin and the receptor on periostin-stimulated eosinophils that recognizes fibronectin.
Acknowledgments
The authors thank Paul Fichtinger and Elizabeth Schwantes for the purification of eosinophils, Steven Akiyama for monoclonal antibody (mAb) 13, Icos Corporation for mAbs 217I and 240I, Steven Barthel for advice on the motility assay, Mary Gillis for help with performing motility experiments and the purification of fibronectin, Greg Wiepz for help with photography, Manav Khanna and Kavya Nallamothu for help with motility and adhesion experiments, Stephane Esnault and Monica Gavala for discussion, and Michael Evans for advice on statistics.
Footnotes
This work was supported by National Institutes of Health Program Project grant P01 HL088594 (N. N. Jarjour and D.F.M.) and Clinical and Translational Science Award grant UL1 RR025011 (M. K. Drezner).
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2012-0150OC on January 10, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Busse WW, Lemanske RF., Jr Asthma. N Engl J Med 2001;344:350–362 [DOI] [PubMed] [Google Scholar]
- 2.Kay AB, Phipps S, Robinson DS. A role for eosinophils in airway remodelling in asthma. Trends Immunol 2004;25:477–482 [DOI] [PubMed] [Google Scholar]
- 3.Wills-Karp M, Karp CL. Eosinophils in asthma: remodeling a tangled tale. Science 2004;305:1726–1729 [DOI] [PubMed] [Google Scholar]
- 4.Scott KA, Wardlaw AJ. Eosinophilic airway disorders. Semin Respir Crit Care Med 2006;27:128–133 [DOI] [PubMed] [Google Scholar]
- 5.Nissim Ben Efraim AH, Levi-Schaffer F. Tissue remodeling and angiogenesis in asthma: the role of the eosinophil. Ther Adv Respir Dis 2008;2:163–171 [DOI] [PubMed] [Google Scholar]
- 6.Busse WW, Ring J, Huss-Marp J, Kahn JE. A review of treatment with mepolizumab, an anti–IL-5 mAb, in hypereosinophilic syndromes and asthma. J Allergy Clin Immunol 2010;125:803–813 [DOI] [PubMed] [Google Scholar]
- 7.Evans R, Patzak I, Svensson L, De Filippo K, Jones K, McDowall A, Hogg N. Integrins in immunity. J Cell Sci 2009;122:215–225 [DOI] [PubMed] [Google Scholar]
- 8.Hogg N, Patzak I, Willenbrock F. The insider’s guide to leukocyte integrin signalling and function. Nat Rev Immunol 2011;11:416–426 [DOI] [PubMed] [Google Scholar]
- 9.Barthel SR, Johansson MW, McNamee DM, Mosher DF. Roles of integrin activation in eosinophil function and the eosinophilic inflammation of asthma. J Leukoc Biol 2008;83:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banerjee ER, Jiang Y, Henderson WR, Jr, Scott LM, Papayannopoulou T. Alpha4 and beta2 integrins have nonredundant roles for asthma development, but for optimal allergen sensitization only alpha4 is critical. Exp Hematol 2007;35:605–617 [DOI] [PubMed] [Google Scholar]
- 11.Banerjee ER, Jiang Y, Henderson WR, Jr, Latchman Y, Papayannopoulou T. Absence of alpha 4 but not beta 2 integrins restrains development of chronic allergic asthma using mouse genetic models. Exp Hematol 2009;37:715–727e713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Margadant C, Monsuur HN, Norman JC, Sonnenberg A. Mechanisms of integrin activation and trafficking. Curr Opin Cell Biol 2011;23:607–614 [DOI] [PubMed] [Google Scholar]
- 13.Harburger DS, Calderwood DA. Integrin signalling at a glance. J Cell Sci 2009;122:159–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huttenlocher A, Ginsberg MH, Horwitz AF. Modulation of cell migration by integrin-mediated cytoskeletal linkages and ligand-binding affinity. J Cell Biol 1996;134:1551–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Askari JA, Buckley PA, Mould AP, Humphries MJ. Linking integrin conformation to function. J Cell Sci 2009;122:165–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johansson MW, Barthel SR, Swenson CA, Evans MD, Jarjour NN, Mosher DF, Busse WW. Eosinophil beta(1) integrin activation state correlates with asthma activity in a blind study of inhaled corticosteroid withdrawal. J Allergy Clin Immunol 2006;117:1502–1504 [DOI] [PubMed] [Google Scholar]
- 17.Johansson MW, Mosher DF. Activation of {beta}1 integrins on blood eosinophils by P-selectin. Am J Respir Cell Mol Biol 2011;45:889–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johansson MW, Han ST, Gunderson KA, Busse WW, Jarjour NN, Mosher DF. Platelet activation, P-selectin, and eosinophil beta1-integrin activation in asthma. Am J Respir Crit Care Med 2012;185:498–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barthel SR, Jarjour NN, Mosher DF, Johansson MW. Dissection of the hyperadhesive phenotype of airway eosinophils in asthma. Am J Respir Cell Mol Biol 2006;35:378–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansson MW, Kelly EA, Busse WW, Jarjour NN, Mosher DF. Up-regulation and activation of eosinophil integrins in blood and airway after segmental lung antigen challenge. J Immunol 2008;180:7622–7635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu X, Munoz NM, Kim KP, Sano H, Cho W, Leff AR. Cytosolic phospholipase A2 activation is essential for beta 1 and beta 2 integrin–dependent adhesion of human eosinophils. J Immunol 1999;163:3423–3429 [PubMed] [Google Scholar]
- 22.Johansson MW, Gunderson KA, Kelly EAB, Denlinger LC, Jarjour NN, Mosher DF. Anti–IL-5 attenuates activation and surface density of β2-integrins on circulating eosinophils after segmental antigen challenge. Clin Exp Allergy 2013;43:292–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamilton DW. Functional role of periostin in development and wound repair: implications for connective tissue disease. J Cell Commun Signal 2008;2:9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norris RA, Moreno-Rodriguez R, Hoffman S, Markwald RR. The many facets of the matricellular protein periostin during cardiac development, remodeling, and pathophysiology. J Cell Commun Signal 2009;3:275–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuyama N, Davies DE, Akaiwa M, Matsui K, Hamasaki Y, Suminami Y, Yoshida NL, Maeda M, Pandit A, Lordan JL, et al. Analysis of novel disease-related genes in bronchial asthma. Cytokine 2002;19:287–296 [DOI] [PubMed] [Google Scholar]
- 26.Takayama G, Arima K, Kanaji T, Toda S, Tanaka H, Shoji S, McKenzie AN, Nagai H, Hotokebuchi T, Izuhara K. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol 2006;118:98–104 [DOI] [PubMed] [Google Scholar]
- 27.Hayashi N, Yoshimoto T, Izuhara K, Matsui K, Tanaka T, Nakanishi K. T helper 1 cells stimulated with ovalbumin and IL-18 induce airway hyperresponsiveness and lung fibrosis by IFN-gamma and IL-13 production. Proc Natl Acad Sci USA 2007;104:14765–14770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woodruff PG, Boushey HA, Dolganov GM, Barker CS, Yang YH, Donnelly S, Ellwanger A, Sidhu SS, Dao-Pick TP, Pantoja C, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci USA 2007;104:15858–15863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sidhu SS, Yuan S, Innes AL, Kerr S, Woodruff PG, Hou L, Muller SJ, Fahy JV. Roles of epithelial cell–derived periostin in TGF-beta activation, collagen production, and collagen gel elasticity in asthma. Proc Natl Acad Sci USA 2010;107:14170–14175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masuoka M, Shiraishi H, Ohta S, Suzuki S, Arima K, Aoki S, Toda S, Inagaki N, Kurihara Y, Hayashida S, et al. Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J Clin Invest 2012;122:2590–2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, Koth LL, Arron JR, Fahy JV. T-helper type 2–driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med 2009;180:388–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jia G, Erickson RW, Choy DF, Mosesova S, Wu LC, Solberg OD, Shikotra A, Carter R, Audusseau S, Hamid Q, et al. Periostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patients. J Allergy Clin Immunol 2012;130:647–654e610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blanchard C, Mingler MK, McBride M, Putnam PE, Collins MH, Chang G, Stringer K, Abonia JP, Molkentin JD, Rothenberg ME. Periostin facilitates eosinophil tissue infiltration in allergic lung and esophageal responses. Mucosal Immunol 2008;1:289–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bentley JK, Linn MJ, Lei J, Comstock A, Zhao Y, Hershenson MB. Periostin knockout mice are protected from house dust mite allergen-induced lung inflammation [abstract]. Am J Respir Crit Care Med 2012;185:A6873 [Google Scholar]
- 35.Sehra S, Yao W, Nguyen ET, Ahyi AN, Tuana FM, Ahlfeld SK, Snider P, Tepper RS, Petrache I, Conway SJ, et al. Periostin regulates goblet cell metaplasia in a model of allergic airway inflammation. J Immunol 2011;186:4959–4966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gordon ED, Sidhu SS, Wang ZE, Woodruff PG, Yuan S, Solon MC, Conway SJ, Huang X, Locksley RM, Fahy JV. A protective role for periostin and TGF-beta in IgE-mediated allergy and airway hyperresponsiveness. Clin Exp Allergy 2012;42:144–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gillan L, Matei D, Fishman DA, Gerbin CS, Karlan BY, Chang DD. Periostin secreted by epithelial ovarian carcinoma is a ligand for alpha(V)beta(3) and alpha(V)beta(5) integrins and promotes cell motility. Cancer Res 2002;62:5358–5364 [PubMed] [Google Scholar]
- 38.Butcher JT, Norris RA, Hoffman S, Mjaatvedt CH, Markwald RR. Periostin promotes atrioventricular mesenchyme matrix invasion and remodeling mediated by integrin signaling through Rho/PI 3-kinase. Dev Biol 2007;302:256–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wallace DP, Quante MT, Reif GA, Nivens E, Ahmed F, Hempson SJ, Blanco G, Yamaguchi T. Periostin induces proliferation of human autosomal dominant polycystic kidney cells through alphaV-integrin receptor. Am J Physiol Renal Physiol 2008;295:F1463–F1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li G, Jin R, Norris RA, Zhang L, Yu S, Wu F, Markwald RR, Nanda A, Conway SJ, Smyth SS, et al. Periostin mediates vascular smooth muscle cell migration through the integrins alphaVbeta3 and alphaVbeta5 and focal adhesion kinase (FAK) pathway. Atherosclerosis 2010;208:358–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barthel SR, Annis DS, Mosher DF, Johansson MW. Differential engagement of modules 1 and 4 of vascular cell adhesion molecule-1 (CD106) by integrins alpha4beta1 (CD49d/29) and alphaMbeta2 (CD11b/18) of eosinophils. J Biol Chem 2006;281:32175–32187 [DOI] [PubMed] [Google Scholar]
- 42.Johansson MW, Lye MH, Barthel SR, Duffy AK, Annis DS, Mosher DF. Eosinophils adhere to vascular cell adhesion molecule-1 via podosomes. Am J Respir Cell Mol Biol 2004;31:413–422 [DOI] [PubMed] [Google Scholar]
- 43.Johansson MW, Larsson E, Lüning B, Pasquale EB, Ruoslahti E. Altered localization and cytoplasmic domain-binding properties of tyrosine-phosphorylated β1 integrin. J Cell Biol 1994;126:1299–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sakai T, Jove R, Fässler R, Mosher DF. Role of the cytoplasmic tyrosines of β1A integrins in transformation by v-src. Proc Natl Acad Sci USA 2001;98:3803–3813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakai T, Zhang Q, Fassler R, Mosher DF. Modulation of beta1A integrin functions by tyrosine residues in the beta1 cytoplasmic domain. J Cell Biol 1998;141:527–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoersch S, Andrade-Navarro MA. Periostin shows increased evolutionary plasticity in its alternatively spliced region. BMC Evol Biol 2010;10:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu LY, Bates ME, Jarjour NN, Busse WW, Bertics PJ, Kelly EA. Generation of Th1 and Th2 chemokines by human eosinophils: evidence for a critical role of TNF-alpha. J Immunol 2007;179:4840–4848 [DOI] [PubMed] [Google Scholar]
- 48.Linder S, Aepfelbacher M. Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol 2003;13:376–385 [DOI] [PubMed] [Google Scholar]
- 49.Spinardi L, Marchisio PC. Podosomes as smart regulators of cellular adhesion. Eur J Cell Biol 2006;85:191–194 [DOI] [PubMed] [Google Scholar]
- 50.Linder S. The matrix corroded: Podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol 2007;17:107–117 [DOI] [PubMed] [Google Scholar]
- 51.Murphy DA, Courtneidge SA. The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Biol 2011;12:413–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci 2003;116:1409–1416 [DOI] [PubMed] [Google Scholar]
- 53.Yoon SW, Kim TY, Sung MH, Kim CJ, Poo H. Comparative proteomic analysis of peripheral blood eosinophils from healthy donors and atopic dermatitis patients with eosinophilia. Proteomics 2005;5:1987–1995 [DOI] [PubMed] [Google Scholar]
- 54.Cheung PF, Wong CK, Ip WK, Lam CW. FAK-mediated activation of ERK for eosinophil migration: a novel mechanism for infection-induced allergic inflammation. Int Immunol 2008;20:353–363 [DOI] [PubMed] [Google Scholar]
- 55.Zhu X, Boetticher E, Wang L, Duan Y, Learoyd J, Leff AR. Proline-rich tyrosine kinase 2 regulates spreading and migration of eosinophils after beta2-integrin adhesion. Am J Respir Cell Mol Biol 2008;39:263–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stout BA, Bates ME, Liu LY, Farrington NN, Bertics PJ. IL-5 and granulocyte-macrophage colony-stimulating factor activate STAT3 and STAT5 and promote Pim-1 and cyclin D3 protein expression in human eosinophils. J Immunol 2004;173:6409–6417 [DOI] [PubMed] [Google Scholar]
- 57.Munier FL, Frueh BE, Othenin-Girard P, Uffer S, Cousin P, Wang MX, Heon E, Black GC, Blasi MA, Balestrazzi E, et al. BIGH3 mutation spectrum in corneal dystrophies. Invest Ophthalmol Vis Sci 2002;43:949–954 [PubMed] [Google Scholar]
- 58.Kim HJ, Kim IS. Transforming growth factor-beta–induced gene product, as a novel ligand of integrin alphaMbeta2, promotes monocytes adhesion, migration and chemotaxis. Int J Biochem Cell Biol 2008;40:991–1004 [DOI] [PubMed] [Google Scholar]
- 59.Stark JM, Godding V, Sedgwick JB, Busse WW. Respiratory syncytial virus infection enhances neutrophil and eosinophil adhesion to cultured respiratory epithelial cells: roles of CD18 and intercellular adhesion molecule-1. J Immunol 1996;156:4774–4782 [PubMed] [Google Scholar]