Abstract
Sulfur mustard (SM) inhalation causes the rare but life-threatening disorder of plastic bronchitis, characterized by bronchial cast formation, resulting in severe airway obstruction that can lead to respiratory failure and death. Mortality in those requiring intubation is greater than 80%. To date, no antidote exists for SM toxicity. In addition, therapies for plastic bronchitis are solely anecdotal, due to lack of systematic research available to assess drug efficacy in improving mortality and/or morbidity. Adult rats exposed to SM analog were treated with intratracheal tissue plasminogen activator (tPA) (0.15–0.7 mg/kg, 5.5 and 6.5 h), compared with controls (no treatment, isoflurane, and placebo). Respiratory distress and pulse oximetry were assessed (for 12 or 48 h), and arterial blood gases were obtained at study termination (12 h). Microdissection of fixed lungs was done to assess airway obstruction by casts. Optimal intratracheal tPA treatment (0.7 mg/kg) completely eliminated mortality (0% at 48 h), and greatly improved morbidity in this nearly uniformly fatal disease model (90–100% mortality at 48 h). tPA normalized plastic bronchitis–associated hypoxemia, hypercarbia, and lactic acidosis, and improved respiratory distress (i.e., clinical scores) while decreasing airway fibrin casts. Intratracheal tPA diminished airway-obstructive fibrin–containing casts while improving clinical respiratory distress, pulmonary gas exchange, tissue oxygenation, and oxygen utilization in our model of severe chemically induced plastic bronchitis. Most importantly, mortality, which was associated with hypoxemia and clinical respiratory distress, was eliminated.
Keywords: plastic bronchitis, tissue plasminogen activator, airway obstruction, sulfur mustard, fibrin
Clinical Relevance
Sulfur mustard inhalation causes an often life-threatening disorder called plastic bronchitis, characterized by formation of branching bronchial casts that can obstruct the tracheobronchial tree. All current therapies available are based on anecdotal evidence, as there are no standardized guidelines for treatment available. While mortality rate due to respiratory failure is great, it is not known if any drug treatments can improve either morbidity and/or mortality. This study demonstrates that intra-airway administration of optimal tissue plasminogen activator (tPA) regimen will prevent mortality in rats with plastic bronchitis induced by toxic inhalation of a sulfur mustard analog compound. We also demonstrate that the use of tPA, particularly at higher doses (0.7 mg/kg), administered directly into the airways will improve several morbidity factors, including a decrease in airway obstruction from fibrin casts, an improvement of clinical signs of respiratory distress, and a correction of defects in oxygenation, ventilation, and tissue acidosis seen in this model of severe plastic bronchitis.
Sulfur mustard (bis(2-chloroethyl)sulfide; SM) is a vesicant and chemical weapon used in warfare during much of the twentieth century, and which remains in the stockpiles of multiple nations today (Syria, Iran, North Korea, Libya, the United States, and possibly others). SM exposure affects the eyes, skin, upper airways, and lungs. After a brief latent period, respiratory failure and death can develop within 12 to 48 hours. Despite a century of study, the mechanisms responsible for SM’s toxic effects remain unsolved, and clinically effective rescue therapies or antidotes are not available.
Initial reports of human SM inhalation toxicity in the early 1900s described the presence of airway-obstructive necrotic debris/mucosa, or “pseudomembranes,” in the large airways of victims, and these were more recently confirmed in the victims of the Iran–Iraq war (1, 2). Such severe lesions have been reported to lead to respiratory compromise, with need for artificial ventilation, and death in 80% of those needing intubation (2). Furthermore, chronic conducting airway lesions, such as bronchiolitis obliterans, tracheal/bronchial stenosis, and chronic bronchitis, are commonly found in survivors of SM inhalation months to years after exposure (2, 3), whereas chronic alveolar or parenchymal injury is less frequent. Therefore, airway injury predominates during both the acute and chronic phases of SM-induced lung injury.
Airway obstruction from fibrin casts represents one form of a disorder commonly referred to as plastic bronchitis. This is a rare condition characterized by formation of branching bronchial casts that partially or completely obstruct the tracheobronchial tree, often leading to life-threatening respiratory failure. Even though inhalation of chemicals, such as SM, can lead to the development of plastic bronchitis, it can also occur due to causes other than chemical inhalation. Although occasionally seen in adults (4, 5), plastic bronchitis not due to chemical inhalation is a disorder affecting mostly children. It can develop after Fontan surgery for congenital cyanotic heart diseases (6–12), or after various bronchopulmonary diseases, such as asthma (13, 14), cystic fibrosis (15, 16), acute chest syndrome, or sickle cell disease (17), viral lower respiratory tract infections, including H1N1 (18), neoplasms, such as lymphoma (19), chemical or thermal inhalation injuries (5, 20–22), or idiopathic causes (23). Presenting symptoms include wheezing, coughing, expectoration of rubbery casts, chest pain, hypoxemia, and/or frank respiratory distress (11, 24). Examination of patients with plastic bronchitis, regardless of etiology, reveals wheezing or absent breath sounds over affected regions, whereas chest radiographs can show segmental lung collapse or bilateral patchy consolidations often misdiagnosed as pneumonia (24). Diagnosis is made either by a history of cast expectoration or by bronchosopic or chest computed tomography findings of casts within airways (5, 6). Mortality from plastic bronchitis occurs due to respiratory failure related to central airway obstruction, and is more severe in those with underlying cardiac abnormalities (mortality rate of 44–60%) (11, 24, 25).
Treatment of plastic bronchitis, regardless of etiology, has been based primarily on anecdotal evidence reported from individual affected patients. Previously tried medications have included inhaled or systemic corticosteroids (26), mucolytics (5), antibiotics (27), pulmonary vasodilators (28), and anticoagulants, such as heparin (29). Nonpharmaceutical treatments have included cast removal via bronchoscopy (30), vest therapy, fenestration of the Fontan circuit (31), thoracic duct ligation (32), atrioventricular synchronization (33), extracorporeal membrane oxygenation (13), and heart transplantation for patients with severe cardiac conditions (34). In more recent case reports, tissue plasminogen activator (tPA), a potent fibrinolytic agent, has shown promise in ameliorating cast burden in plastic bronchitis of non-SM causes (7–10), but only anecdotally. tPA is currently used as first-line therapy in several clot-associated diseases, such as stroke (35, 36) and myocardial infarction (37), and can improve survival with these life-threatening entities. In regard to plastic bronchitis, no placebo-controlled clinical trials have been done to evaluate the effects of tPA. For this reason, it is unknown if tPA can truly alter mortality and/or morbidity measures associated with plastic bronchitis. Furthermore, the proper dose required for tPA efficacy has not yet been assessed in vivo.
We previously described a rat model of acute life-threatening plastic bronchitis (22), where bronchial fibrinous cast formation was induced by inhalation of the toxic SM analog, 2-chloroethyl ethyl sulfide (CEES). In the present study, we used this CEES inhalation model producing airway casts and obstruction to evaluate the effects of escalating doses of intratracheally administered tPA on quantifiable measures of outcome, including airway morphometry, and compared those findings to both placebo-treated and untreated groups. The study was designed to help assess/confirm the mechanism responsible for the respiratory compromise and death seen after mustard exposure (by assessing whether dissolution of airway casts would eliminate respiratory morbidity and mortality), and also to help systematically evaluate tPA as a therapeutic modality in this nearly uniformly fatal plastic bronchitis model.
Materials and Methods
An expanded description of methods can be found in the online supplement.
Chemicals
CEES was obtained from TCI America (Portland, OR). tPA was purchased from Genentech (Roche, San Francisco, CA).
Animal Care
The Institutional Animal Care and Use Committee of the National Jewish Health Center approved this study. Adult male (300–350 g) Sprague-Dawley rats (Harlan Co., Indianapolis, IN) were used.
Inhalation Exposure to CEES
CEES (10%) inhalation exposure was conducted as previously described (22).
Respiratory Distress Clinical Scoring
Respiratory quality, wheezing/stridor, and activity depression were assessed (Table 1), and the individual scores (0–3, higher being worse) were added to obtain a cumulative score (maximum, 9).
TABLE 1.
RESPIRATORY CLINICAL DISTRESS SCORE CRITERIA
| Score | Respiratory Quality | Stridor | Activity |
| 0 | Normal | Normal | Normal |
| 1 | Mild abdominal breathing with tachypnea (>100) | Stridor with activity only, mild | Mildly depressed activity |
| 2 | Moderate abdominal breathing, possibly with mild gasping | Stridor at rest, mild to moderate | Moderately depressed activity (movement with stimulation) |
| 3 | Severe abdominal breathing, severe gasping, and low respiratory rate (<60) | Stridor at rest, severe | Obtunded, no movement with stimulation; or severe agitation with stimulation |
Criteria for Early Termination
Animals were terminated if oxygen saturation was less than 70% and there was a respiratory distress score of 7 or greater. Animals were terminated by pentobarbital overdose (Sleepaway; Fort Dodge Animal Health, Fort Dodge, IA) (22).
Lung Fixation
Tracheas were cannulated, and lungs were fixed at 20 cm H2O with 4% paraformaldehyde in PBS for 30 minutes and then surgically removed.
Airway Cast Scoring
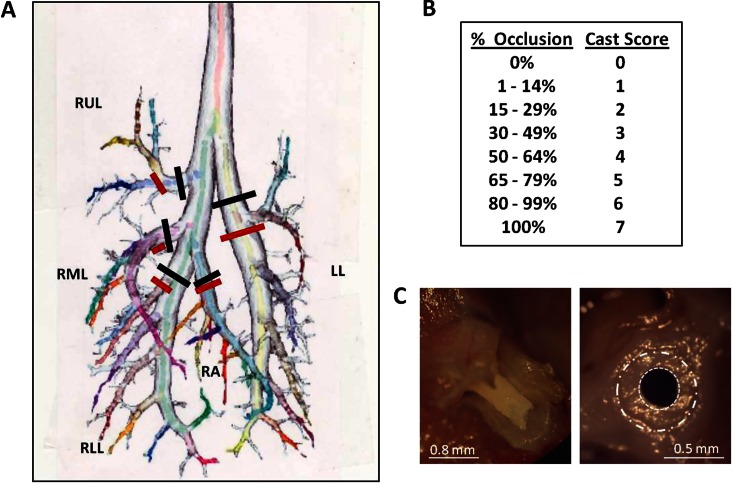
Cast scoring was developed based on previously described microdissection techniques developed by our group (22). Fixed lung was separated into five lobes by cross-sectioning each lobar bronchus at site of take-off from central airway bronchus (Figure 1A, black lines). To obtain “main airway cast score,” each lobe was positioned with the main lobar bronchus perpendicular to the microdissecting scope lens. A “dependent airway cast score” was also obtained for first major gravity-dependent lobar bronchi that were the most occluded in this model. To obtain this score, microdissection was performed on this first gravity-dependent (ventrally oriented) daughter branch, which was then cross-sectioned at its take-off position (Figure 1A, red lines) and then aligned perpendicularly under the dissecting scope. A digital picture (by Olympus C-750 camera; Olympus Imaging America, Inc., Hauppauge, NY) was obtained of each lobar opening (Figure 1C). The Image-J program (1.44p; National Institutes of Health, Bethesda, MD) was used to assess percent airway occlusion from cast. To obtain a quantitative score, percent occlusion for each bronchus was converted to a nominal score on a scale from 0 (no occlusion) to 7 (complete occlusion) (Figure 1C), and each score was weighted based on volumetric differences of rat lobes to total lung (38) (raw cast score obtained from a particularly lobe was multiplied by the ratio of that lobe's volume to the whole lung's volume) (see Table E1 in the online supplement). The five separate lobe weighted scores were then added to obtain the “main” or “dependent” composite cast score for each animal (total score of 0–7).
Figure 1.
Description of cast scoring to assess degree of airways obstruction in plastic bronchitis. (A) Map of the rat lower respiratory tract. During microdissection, extent of airway obstruction was determined at five lobar (main) branches (black lines), and at five major gravity-dependent branches (red lines). (B) Nominal cast scoring chart for a given percentage of airway occlusion present. (C) A branching bronchial cast present in the lobar bronchus of the right middle lobe (left panel). Also shown is the typical appearance of a cast partially obstructing a central airway visualized en face during microdissection (right panel). The dashed white circle (large) denotes normal airway wall perimeter, while the dotted circle (small) denotes the reduced airway lumen size due to cast attachment to the surrounding airway wall. The image shown in (A) was modified and reproduced by permission from the National Alliance for Medical Image Computing. Image available at: http://www.na-mic.org/Wiki/index.php/File:Small66ratLungModel_labelled.jpg.
Noninvasive Oxygen Saturation Measurements
MouseOx small animal oximeter (Starr Life Sciences, Oakmont, PA) in unanesthetized rats was used with XL CollarClip Sensor (Fisher Scientific, Pittsburgh, PA).
Arterial Blood Gas Measurements
Descending aorta blood was collected, placed into a precalibrated EPOC-BGEM Test Card, and analyzed using the EPOC-Vet Blood Analysis system (both from Epocal Inc., Ottawa, ON, Canada).
Statistical Analysis
Prism 5.01 software (GraphPad, La Jolla, CA) was used, with one-way ANOVA (ANOVA) plus Tukey’s post hoc analysis or Kruskal-Wallis plus Dunn’s post hoc analysis, depending on distribution of data. A P value less than 0.05 was significant.
Results
Optimal tPA Treatment Regimen Improves Survival
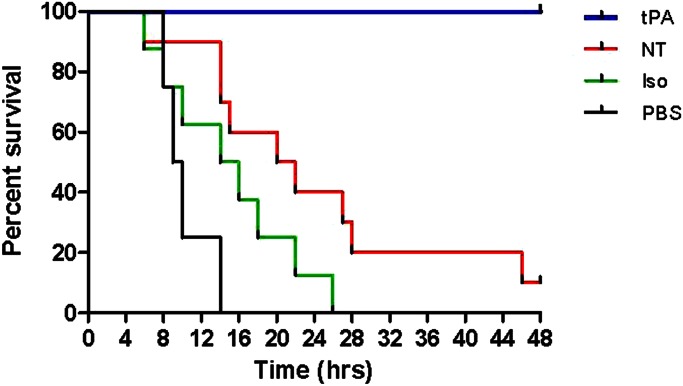
We measured survival over 48 hours after high-level (10%) CEES inhalation in rats with and without intratracheal tPA (0.7 mg/kg). Three control groups were also evaluated. These included: (1) rats given no drug, but exposed to CEES (NT); (2) rats receiving no drug treatment, exposed to CEES, and given isoflurane anesthesia (Iso); and (3) rats exposed to CEES, isoflurane, and intratracheally administered diluent for tPA (PBS, placebo). High-level CEES exposure caused high mortality at 48 hours in all control groups, including 90% mortality with NT, 100% with Iso, and 100% with PBS (Figure 2). Remarkably, tPA completely eliminated mortality in this model. Deaths in control groups occurred mainly between 8 and 28 hours after CEES exposure. Sham/placebo (PBS) and sham treatment alone (Iso) both hastened mortality (see Figures E1A and E1B in the online supplement), and these were studied in limited fashion thereafter.
Figure 2.
Effect of tissue plasminogen activator (tPA) on mortality after sulfur mustard analog inhalation. Survival curves for rats exposed to 2-chloroethyl ethyl sulfide (CEES) and given: (1) no treatment (NT; n = 10; red line); (2) “sham” treatment with isoflurane anesthesia (Iso) only (n = 8; green line); (3) placebo treatment (Iso plus intratracheally administered PBS; n = 4; purple line); or (4) tPA treatment (Iso plus intratracheally administered tPA; n = 12; blue line). All three control groups had significantly lower survival than did rats given tPA treatment (P < 0.0001 for Iso or PBS versus tPA, and P < 0.001 for NT versus tPA, via Log-rank, Mantel-Cox test).
Initial tPA dosing was delayed until 5.5 hours after exposure. This dosing time was based on our previous observations that: (1) casts begin to form in airways at 4 hours (22); and (2) oxygen saturations decreased below 90% at 5 hours after exposure (see Figure E1A). Preliminary experiments indicated: a second tPA dose 1 hour after initial dosing was superior to only one dose, with consistently greater improvement in oxygen saturation (>90% within <1 h with two doses, versus 6 h with one dose); more consistently satisfactory oxygenation; higher peak oxygen saturation shortly after administration (96% with 2 doses versus 92% with one); and elimination of mortality only with two initial doses (see Figures E1C and E1D). Redosing of tPA was done using a similar two-dose regimen, with drug readministration when oxygen saturation reached 85% and clinical distress worsened. Again, the two-dose regimen (1 h apart) was superior to only one dose given at redosing. In summary, the optimal two-dose tPA regimen (0.7 mg/kg, given twice), administered intratracheally, was highly effective, completely eliminating mortality.
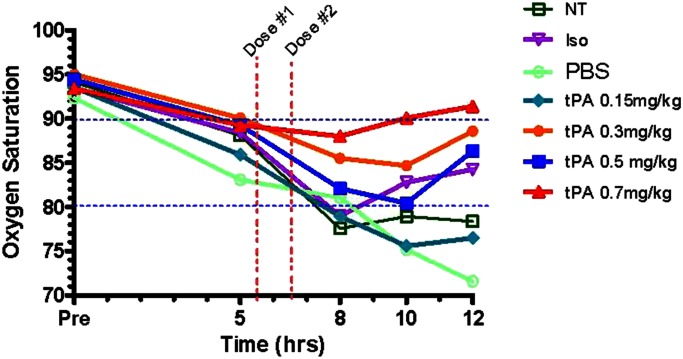
Oxygen Saturation Improves with tPA treatment
To determine if there was a dose effect of intratracheal tPA on oxygen saturation (SpO2) in rats with plastic bronchitis after CEES inhalation, we tested four tPA doses (0.15, 0.3, 0.5, and 0.7 mg/kg), given as a one-time, two-dose regimen at 5.5 and 6.5 hours, and performed pulse oximetry for 12 hours. We found a dose-dependent improvement in SpO2 with tPA treatment as compared with all three controls tested (Figure 3). Improvement was noted within 2 hours after giving tPA, with SpO2 measurements in tPA groups progressively diverging from controls over the next 4 hours. Optimal oxygenation occurred with 0.7 mg/kg tPA, with improved mean SpO2 at 12 hours versus controls (mean SpO2 of 91.4% in 0.7 mg/ml tPA group versus 78.4% in NT-CEES–alone group; P = 0.025; see Table E2). Improved SpO2 also occurred with 0.3 and 0.5 mg/kg, but to a lesser extent (mean SpO2 of 88.6 and 86.4%, respectively). We noted no improvement in oxygenation versus controls with 0.15 mg/kg. However, an impressive survival benefit was seen at 12 hours with every tPA dose. We observed no mortality at 12 hours in all tPA groups, versus 12.5% with NT, 30% with Iso, and 75% with PBS. PBS and Iso accelerated mortality. Due to high mortality in controls, SpO2 data collected at 12 hours in controls (particularly PBS) contained only data from rats remaining alive (i.e., least affected by CEES exposure). Therefore, oxygenation data reported at 12 hours underrepresents the real morbidity of this injury. Nevertheless, tPA remarkably corrected SpO2 at 12 hours in a dose-dependent fashion relative to surviving controls.
Figure 3.
Effect of tPA on tissue oxygenation in unanesthetized rats for 12 hours after CEES inhalation. Noninvasively acquired oxygen saturations measured by pulse oximetry (SpO2) over 12 hours in rats exposed to CEES and given: (1) NT (n = 16); (2) Iso (10); (3) Iso plus intratracheal PBS (PBS; n = 4) or Iso plus intratracheal tPA at (4) 0.15 mg/kg (n = 6), (5) 0.3 mg/kg (n = 6), (6) 0.5 mg/kg (n = 6), or (7) 0.7 mg/kg (n = 16) doses. Vertical red dashed lines indicate time interval during which the initial anesthetic, with tPA or PBS placebo, was administered (5.5 and 6.5 h). A dose-dependent improvement in SpO2 was noted with tPA relative to controls (P = 0.0003), with only the highest tPA (0.7 mg/kg) used resulting in near-baseline oxygen saturations (>90%) at 12 hours. Values represent mean SpO2, analyzed by ANOVA for repeated measures, with Tukey’s post hoc analysis (see detailed data in Table E2).
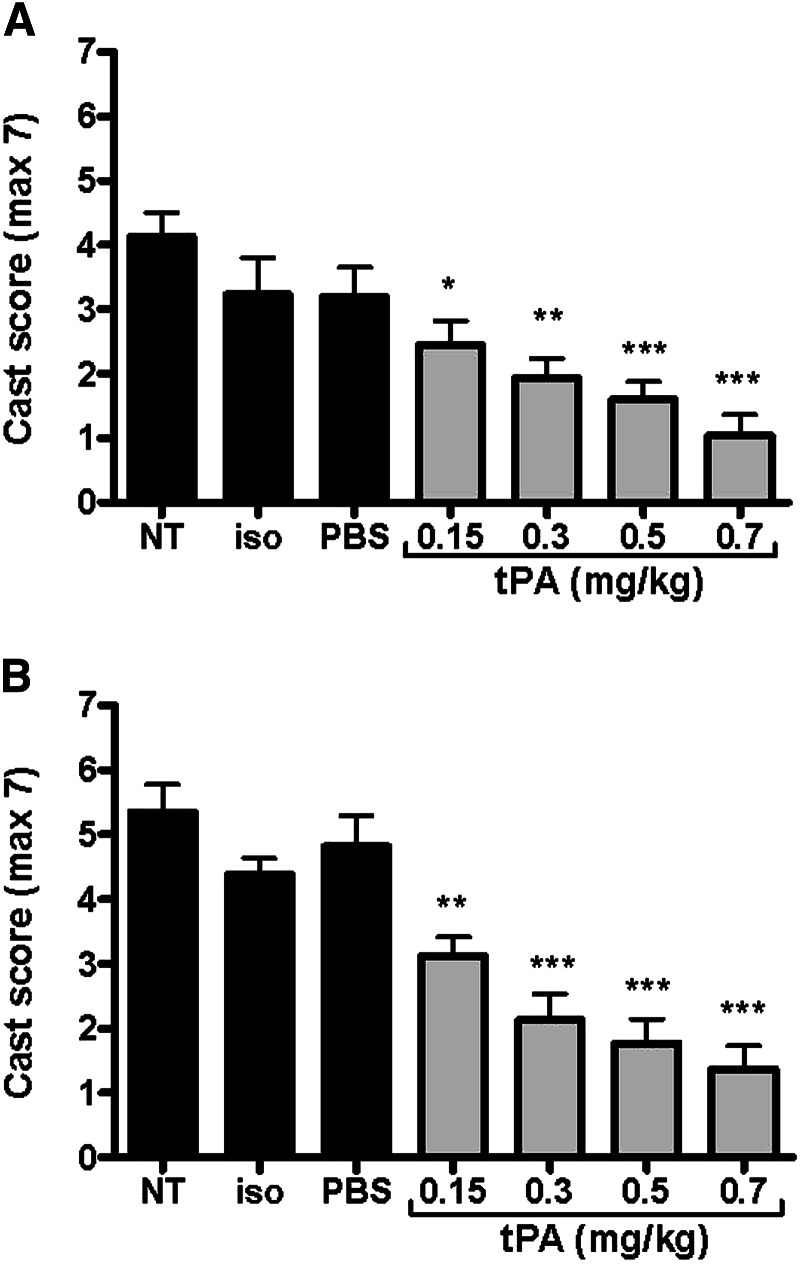
Reduction of Airway Obstruction by Fibrin Casts with tPA Treatment
To assess effects of tPA on fibrin casts within airways, we developed a quantitative scoring system of total airway obstruction by casts (Figure 1). We compared 12-hour composite cast scores from main and first dependent bronchi from four tPA treatment groups (0.15–0.7 mg/kg, given at 5.5 and 6.5 h) with three control groups (NT, Iso, PBS). Lungs from animals terminated at any point during the study were included. tPA treatment greatly decreased airway obstruction by fibrin casts in a dose-dependent fashion (Figures 4A and 4B, respectively). No airway bleeding or pulmonary hemorrhage was noted at necropsy in any tPA-treated animals. Cast scores among the three control groups were not different, with each being greatly elevated. Controls showed a mean main bronchi cast score of >3.2 (corresponding to >46% total airway occlusion by casts), and a mean first dependent branch cast score of >4.4 (corresponding to >63% total airway occlusion). The greatest reduction of cast scores was noted with 0.7 mg/kg tPA, where a fourfold reduction occurred in both main bronchi cast scores (score of 1.3) and first dependent branch cast scores (score of 1.0) compared with controls (P < 0.001). With 0.5 mg/kg tPA, we found a threefold reduction in both main and dependent bronchi cast scores (scores of 1.6 and 1.7, respectively; P < 0.001), whereas, with the 0.3 mg/kg dose, we found a 2.5-fold reduction in these two respective scores (scores of 1.9 and 2.1, with P < 0.001 and P < 0.01, respectively). The 0.15 mg/kg tPA dose was less effective. An individual main branch cast score of >3.5 (corresponding to a >50% total airway occlusion) was associated with nonsurvival. Indeed, all rats dying before 12 hours had very severe airway obstruction by casts, ranging from cast scores of 3.5–5.7 (50–81% total airway occlusion). Thus, optimal tPA treatment had a remarkable effect in decreasing airway obstruction by fibrin casts.
Figure 4.
Effect of tPA on airway obstruction by fibrin-containing casts at 12 hours after CEES inhalation. Casts were revealed by airway microdissection of (A) main bronchi and (B) first dependent bronchi (gravity dependent) of all lobes. Airway obstruction in rats given NT or placebo (Iso or PBS) was significantly different than that observed at any dose of tPA (P < 0.0001 for both main and first dependent bronchi), with a dose-dependent improvement in obstruction found with tPA. Values represent means (±SEM), with data analyzed by ANOVA, followed by Tukey’s post hoc analysis. *P < 0.05; **P ≤ 0.01; ***P < 0.001 denoting significance relative to NT (n = 6 for all groups, except Iso and PBS, where n = 3).
Respiratory Clinical Scoring Improvement after tPA Administration
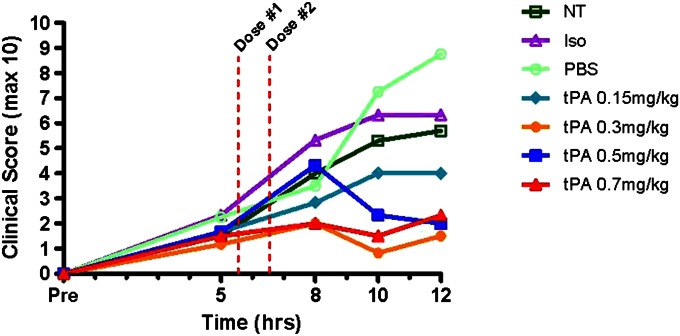
We developed a clinical respiratory distress scoring system based upon signs of distress in rats due to plastic bronchitis, including: (1) respiratory quality (i.e., work of breathing); (2) level of activity; and (3) degree of stridor (Table 1). The three higher tPA doses tested, but not 0.15 mg/kg, significantly improved respiratory clinical scores at 12 hours compared with controls (P = 0.004; Figure 5). Although controls had greatly elevated clinical scores of >5.7 at 12 hours (on a scale of 0–10, higher scores implying worse distress), clinical scores with tPA (≥0.3 mg/kg doses) were <2.3, which is a 2.5-fold reduction in distress. Moreover, these higher tPA dose groups had 12-hour clinical scores (all <2.3) near baseline (pre-exposure) levels. Meanwhile, the controls (NT, Iso, PBS) and 0.15 mg/kg tPA group had progressively worsening scores over 12 hours. All differed significantly from baseline (zero) by the conclusion of the experiment (5.7 for NT, P < 0.0001; 6.3 for Iso, P = 0.0009; 8.8 for PBS, P < 0.0001; and 4.0 for 0.15 mg/kg tPA, P = 0.008; see Table E3). The placebo (PBS) group had the worst mean clinical score at 10 and 12 hours, representing the group with the greatest distress or clinical morbidity. Intratracheal tPA, at higher doses, was quite beneficial in reducing clinical distress.
Figure 5.
Effect of tPA on respiratory distress for 12 hours after CEES inhalation. Respiratory clinical distress was scored on the basis of quality of respirations (0–3), stridor (0–3), and physical activity (0–3), with highest numbers indicating more severe clinical distress (see Table 1 for further details). Respiratory clinical scores for the groups given the highest three tPA doses (>0.3 mg/kg) were significantly different from each of the three control groups (P = 0.004), whereas 0.15 mg/kg tPA did not differ from controls. Values represent mean clinical scores, analyzed by ANOVA for repeated measures, with Tukey’s post hoc analysis (n = 10 in NT; n = 3 for PBS; n = 4 for Iso; and n = 6 for all tPA groups; see detailed data in Table E2).
tPA Normalizes Arterial Blood Gas Abnormalities
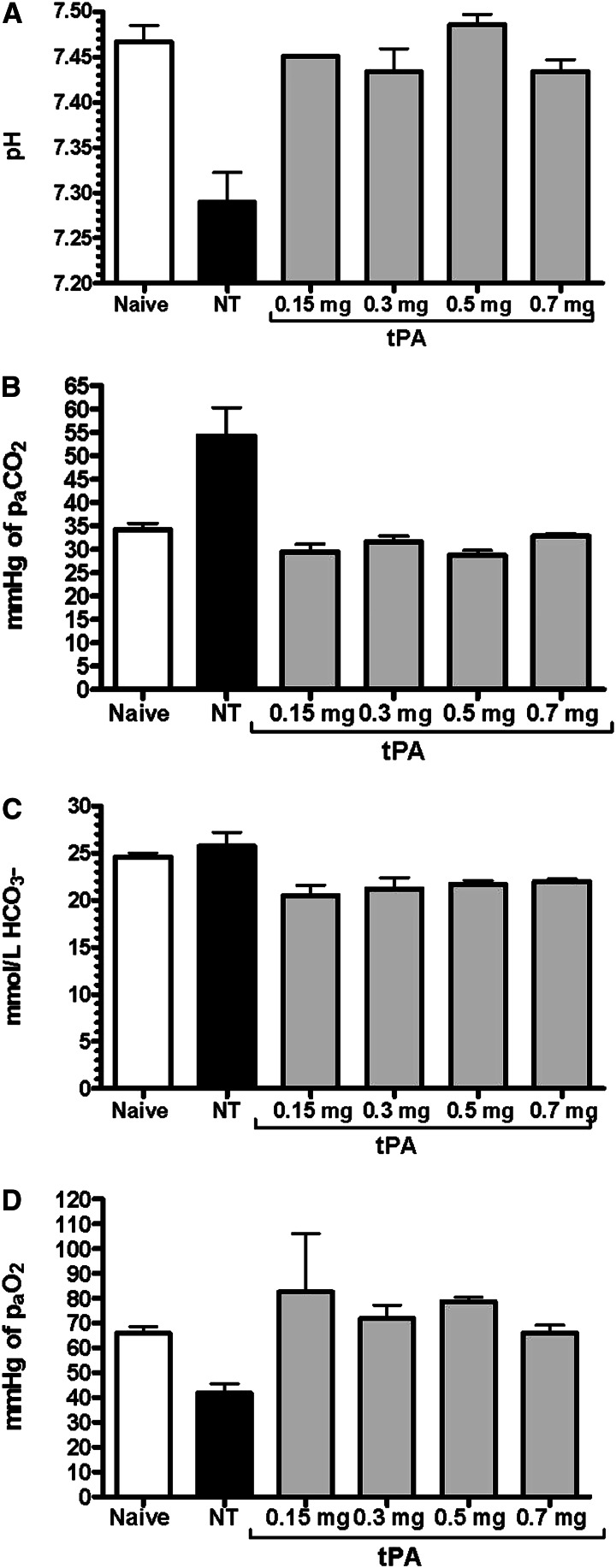
We obtained arterial blood gases (ABGs) 12 hours after CEES exposure in rats receiving escalating tPA doses (0.15–0.7 mg/kg) at 5.5 and 6.5 hours, and compared these results to those from an untreated CEES-exposed group (NT), as well as an unexposed naive group (at Denver altitude, 5,292 ft, barometric pressure 624 mm Hg; Figures 6A–6D). CEES exposure caused significant acidosis by 12 hours (mean pH of 7.29 in NT versus 7.47 in naive animals; P = 0.004), and all doses of tPA normalized pH levels to >7.4 (P = 0.026; Figure 6A). CEES exposure also impaired ventilation, resulting in elevation of arterial carbon dioxide pressure (PaCO2; mean of 54 mm Hg in NT versus 34 mm Hg in naive animals; P = 0.012). All tPA doses improved ventilation (PaCO2 means < 33 mm Hg; P = 0.016; Figure 6B). Although bicarbonate (HCO3−) levels appeared normal with NT (mean HCO3− of 25.7 mmol/L), all tPA groups had a slight decrease in mean HCO3− (20.5–21.9 mmol/L; P = 0.024; Figure 6C). Invasive arterial oxygenation measurements via arterial oxygen pressure (PaO2) analysis confirmed earlier noninvasive SpO2 findings, with decreased PaO2 levels at 12 hours due to CEES inhalation (mean PaO2 of 42 mm Hg in NT versus 66 mm Hg in naïve animals; P = 0.003). Impressively, all tPA treatments, regardless of dose, returned PaO2 levels to those comparable to naive animals (PaO2 means > 66 mm Hg in all tPA groups; P = 0.041; Figure 6D).
Figure 6.
Effect of tPA on pulmonary gas exchange via arterial blood gas (ABG) measurements in rats 12 hours after CEES inhalation. Data for (A) arterial pH, (B) arterial carbon dioxide pressure (PaCO2), (C) bicarbonate (HCO3−), and (D) arterial oxygen pressure (PaO2) were compared in rats given tPA versus those given NT, and naive rats at Denver altitude. All tPA doses effectively normalized CEES-induced acidosis (P = 0.026), hypercarbia (P = 0.016), and hypoxemia (P = 0.041) at 12 hours. Values represent means (±SEM) (n = 4 per group, except in 0.15 mg/kg tPA, where n = 2). Data were analyzed using the Kruskal-Wallis test for not normally distributed data.
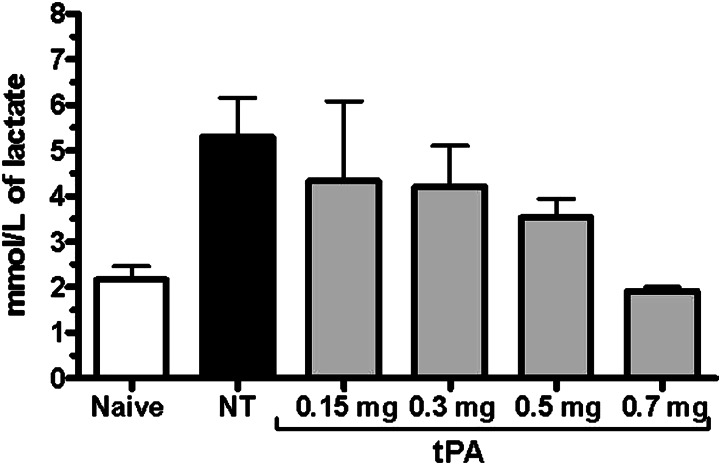
Finally, we did observe a dose-dependent improvement in blood lactate levels with tPA (Figure 7). CEES exposure caused an increase in lactate by 12 hours compared with naive levels (mean lactate of 6.5 mmol/L in NT versus 2.1 mmol/L in naïve animals; P = 0.011). tPA (0.7 mg/kg) was able to normalize lactate completely (mean lactate of 1.9 mmol/L; P = 0.006), whereas lesser doses of tPA were progressively less effective at correcting this marker of tissue acidosis (lactate means of 3.5, 4.3, and 4.4 mmol/L with 0.5, 0.3, and 0.15 mg/kg tPA, respectively). In summary, whereas ABGs collected under anesthesia were substantially improved by any tPA dose tested, the metabolic acidosis marker, lactate, was only optimally improved at the highest tPA dose (0.7 mg/kg).
Figure 7.
Effect of tPA on tissue acidosis marker of arterial blood lactate at 12 hours after CEES inhalation. Data for lactate were compared in rats given tPA versus NT, as well as naive rats. An inverse dose relationship between blood lactate and tPA dose was noted (P = 0.016), with only the highest 0.7 mg/kg tPA normalizing lactate back to naive levels. Values represent means (±SEM) (n = 4 per group, except in 0.15 mg/kg tPA, where n = 2). Data were analyzed using the Kruskal-Wallis test for nonnormally distributed data.
Discussion
In this study, we found that optimal intratracheal tPA treatment for CEES-induced severe plastic bronchitis in rats eliminated mortality in this almost uniformly fatal disease model, and that tPA also greatly improved other morbidity outcome measures often associated with plastic bronchitis. We found that a tPA dose of 0.7 mg/ml delivered intratracheally via a two-dose regimen in which doses were given 1 hour apart resulted in 0% mortality by 48 hours after plastic bronchitis induction with CEES inhalation compared with 90% mortality with no treatment, and 100% mortality in both PBS and isoflurane controls. Furthermore, improved morbidity with tPA was evidenced by normalization of plastic bronchitis–associated hypoxemia, hypercarbia, and acidosis, as well as by dose-dependent improvements in both respiratory distress and airway fibrin casts (i.e., main and dependent bronchi composite cast scores) after treatment. Using a systematic approach for experimental design in this severe injury model, we demonstrated the first in vivo dosimetric assessment of intratracheal tPA efficacy on survival and morbidity in plastic bronchitis, induced by SM analog inhalation.
We have previously reported that plastic bronchitis can result from acute inhalation of toxic chemicals, such as the SM analog CEES, via a mechanism involving damage to the bronchial circulation, with resultant leakage of plasma contents into airways (22). We have reported that fibrin-containing casts occluded conducting airway generations 3–15, the region of bronchial artery distribution, and that, indeed, the entire bronchial circulation had greatly increased permeability (shown with Monastral blue pigment labeling of permeable bronchial vessels). Herein we show that the resulting airway obstruction from casts leads to impaired gas exchange and tissue oxygenation, respiratory distress, and often death. In addition, we show that tPA, even when treatment is delayed for several hours after injury, can reverse airway fibrin deposition, resulting in improved gas exchange and tissue oxygenation, eliminating respiratory distress and death in this relevant animal model of toxic chemical inhalation. This is a considerable advance relative to our previously reported findings, as it supports the concept that, not only do airway-obstructive lesions stain positively by fibrin, but, furthermore, that the airway-obstructive lesions are responsible for the gas exchange abnormalities, clinical respiratory distress, and mortality in this model. Thus, fibrin is not just immunologically detectable, but, more importantly, fibrin is structurally responsible for the pathology in our model for this disorder.
Development of airway-obstructive lesions containing fibrin in conducting airways has also been noted with authentic SM, regardless of route of entry, both in humans and in various animal models using either neat or ethanolic SM vapor to produce injury (2). Since the early 1900s, human victims of mustard gas injury have been reported to contain “fibrinous pseudomembranes” within their airways, at times resulting in death by suffocation from complete blockage of bronchial or tracheal passages by these pseudomembranes (1). More recent reports of human SM exposures from the Iran–Iraq war (1984–1986) have given detailed descriptions of the deleterious effects of these airway casts, reporting death in 80% of patients needing intubation for respiratory insufficiency due to severe airway obstruction (2). These human reports correlate with our findings presented here, where we used a nose inhalation model of CEES in rats, with pseudomembranes further defined as fibrin-containing casts of plastic bronchitis. In regard to other animal models, when ethanolic SM vapor was delivered to glass catheter–intubated rats, the same type of injury in the conducting airways occurred, with fibrin-containing casts present in the bronchi and bronchioles (39). In the porcine SM model, where high-dose SM was delivered as a neat vapor via nasal inhalation, gas exchange abnormalities mirroring our findings were noted, but more importantly, airway-obstructive lesions caused mortality in 40% of the pigs in less than 6 hours from initial SM exposure (40). Thus, an injury affecting the conducting airways has been reported in both humans and animal models of SM inhalation, just as we have found in our CEES inhalation model.
The selectivity for injury of the conducting airways with SM or CEES, particularly to the areas of bronchial (systemic) circulation, is of interest. Although SM is more reactive and toxic than CEES, they both tend to cause injury very near their point of entry, with dissipation down the airways. We believe that the more proximal conducting airways are injured because: (1) there is a heightened bronchial circulation susceptibility to mustard injury; (2) the agent is highly reactive as it dissipates while traversing down the airways; and (3) the size of particles created by either CEES aerosol or SM vapor is suitable for distribution in the affected airways. With respect to particle size in our model, the count median diameter for our CEES-ethanolic aerosol is 0.6 μm, and the mass median diameter of the aerosol is 3.87 μm. This small particle size assures that the particles can reach all lung compartments, including both distal airways and alveoli. Certainly, a small amount of the CEES-ethanolic particles can agglomerate and become deposited more proximally into the uppermost airways, as we have previously reported in a publication showing extensive nasal injury after CEES inhalation (41). Nevertheless, a pulmonary injury selective to the proximal conducting airways is the dominating feature of acute mustard inhalation, despite the ability of particles to reach and deposit within alveolar surfaces.
Over the past three decades, tPA has become accepted therapy for a spectrum of intravascular events, including acute myocardial infarction (42), thrombotic stroke (43), acute pulmonary embolism, and severe deep vein thromboses (44). It also can be useful in removing clots from blocked catheters and grafts (arteriovenous shunts) (45), treatment of unusual acute thrombotic events (46), treatment of empyemas and pleural effusions (47), and to limit amputation in frostbite (48). tPA can also be useful in animal models for treating a variety of extravascular fibrin deposition disorders, including prevention of intra-abdominal adhesions (49) and clearance of airways after burns and smoke inhalation (50). In addition, it has been reported anecdotally as beneficial therapy for patients with plastic bronchitis after Fontan procedure in congenital heart disease (7, 8, 10).
In our model, intratracheal administration of tPA completely eliminated CEES exposure–related mortality from plastic bronchitis, even when rescue treatment was delayed almost 6 hours after injury, and yet was uniformly effective, provided that two consecutive doses were given once dosing was initiated. This effect, despite delay of treatment, is of important clinical relevance, because patients with plastic bronchitis often do not present early in their clinical course, but rather present to an acute care facility when their cast obstruction is severe enough to cause significant morbidity (such as respiratory distress and hypoxemia). Moreover, victims of SM poisoning are often unaware that they have been exposed until several hours later, when skin blisters and respiratory symptoms arise as their airways begin to form obstructive bronchial casts. Thus, emergency medical care can be significantly delayed, yet tPA treatment remains extremely effective as “late rescue” therapy for fibrin-related plastic bronchitis.
We noted dose-related improvements in multiple parameters related to CEES inhalation injury after tPA treatment. First, we found that tissue oxygen delivery, as measured via pulse oximetry at 12 hours, was improved in a dose-dependent fashion. Second, we also observed dose-related reduction in percent airway obstruction by airway casts, as assessed morphometrically during airway microdissection. This effect was most profound with the highest tPA dose used (0.7 mg/kg), and no bleeding-associated side effects were observed. Third, we recognized dose-related reduction in clinical symptoms of respiratory distress, confirming the likely relationship between tissue oxygenation, airway obstruction by casts, and clinical respiratory distress. In addition, we also found a greatly beneficial effect of tPA treatment on pulmonary gas exchange and tissue acidosis. All tPA doses tested resulted in significant improvements of CEES-associated gas exchange abnormalities (as reflected by normalization of PaO2, PaCO2, and arterial blood pH), whereas plasma lactate showed normalization only with the highest dose (0.7 mg/kg). These nonuniform, dose-related morbidity measure findings are most likely due to the varying severity of inadequate tissue oxygen delivery seen with lower tPA doses, corresponding to a lesser efficacy of cast lysis, and thus a diminished capacity for respiratory compensation at higher degrees of airway obstruction. Therefore, it is most likely that a threshold level of airway obstruction from casts exists, below which normal ventilation and arterial oxygenation is still maintained, whereas tissue oxygenation is already compromised.
In summary, we report that intratracheal delivery of the fibrinolytic drug, tPA, relieved airway obstruction due to fibrin-containing casts seen after SM analog exposure. In addition, tPA treatment in this model improved pulmonary gas exchange, tissue oxygenation, and oxygen utilization. More importantly, mortality, which was associated with hypoxemia and clinical respiratory distress, was eliminated. These findings provide further support for the concept that the physiologic abnormalities and death resulting from acute mustard inhalation do, indeed, result from airway fibrin deposition and resultant airway obstruction. Furthermore, based on these findings, as well as reports of similar fibrin-containing airway lesions present in lungs of humans after SM exposure, and those of many patients with non-SM plastic bronchitis, we speculate that tPA could effectively decrease airway obstruction in a similar manner in human subjects. Although our plastic bronchitis animal model was useful in demonstrating a dose response with intratracheal tPA, as well as a profound survival benefit, further research is needed to examine effects of tPA in the clinical setting. A systematic approach using endpoints analogous to those assessed here is needed before further recommendations for routine intra-airway tPA administration can be made for plastic bronchitis of any etiology, particularly for that produced by SM inhalation.
Acknowledgments
The authors thank the following people for their contributions to our study: Brian Day, Ph.D., from the Department of Medicine at National Jewish Health, for his expert advice on inhalation exposures; Anna Forssen from the Division of Biostatistics and Bioinformatics at National Jewish Health for her help in statistical analysis of the presented data; and Robin Deterding, M.D., from the Department of Pediatrics, Pulmonary Medicine, at the University of Colorado in Denver for her guidance in regard to the clinical aspects of the plastic bronchitis disease process in human patients. They also thank their External Advisory Committee members from the Countermeasures Against Chemical Threats Research Network, for their support and advice during development of this model and testing of tissue plasminogen activator as rescue therapy for sulfur mustard toxicity. The authors also thank the National Alliance for Medical Image Computing (NAMIC), funded by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant U54 EB005149, for granting permission for use the rat airway diagram image used to create Figure 1A in this manuscript. Information on the National Centers for Biomedical Computing can be obtained from http://nihroadmap.nih.gov/bioinformatics.
Footnotes
This research is supported by the CounterACT Program, National Institutes of Health (NIH), Office of the Director, and the National Institute of Environmental Health Sciences (NIEHS) Grant Number U54 ES015678.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2012-0177OC on December 20, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Eisenmenger W, Drasch G, von Clarmann M, Kretschmer E, Roider G. Clinical and morphological findings on mustard gas [bis(2-chloroethyl)sulfide] poisoning. J Forensic Sci 1991;36:1688–1698 [PubMed] [Google Scholar]
- 2.Willems JL. Clinical management of mustard gas casualties. Ann Med Mil Belg 1989;3(suppl 1):1–61 [Google Scholar]
- 3.Ghanei M, Tazelaar HD, Chilosi M, Harandi AA, Peyman M, Akbari HM, Shamsaei H, Bahadori M, Aslani J, Mohammadi A. An international collaborative pathologic study of surgical lung biopsies from mustard gas–exposed patients. Respir Med 2008;102:825–830 [DOI] [PubMed] [Google Scholar]
- 4.Watanabe K, Ishida T, Sugawara A, Tachihara M, Munakata M. An adult case of plastic bronchitis. Intern Med 2008;47:1549. [DOI] [PubMed] [Google Scholar]
- 5.Eberlein MH, Drummond MB, Haponik EF. Plastic bronchitis: a management challenge. Am J Med Sci 2008;335:163–169 [DOI] [PubMed] [Google Scholar]
- 6.Goo HW, Jhang WK, Kim YH, Ko JK, Park IS, Park JJ, Yun TJ, Seo DM. CT findings of plastic bronchitis in children after a Fontan operation. Pediatr Radiol 2008;38:989–993 [DOI] [PubMed] [Google Scholar]
- 7.Do TB, Chu JM, Berdjis F, Anas NG. Fontan patient with plastic bronchitis treated successfully using aerosolized tissue plasminogen activator: a case report and review of the literature. Pediatr Cardiol 2009;30:352–355 [DOI] [PubMed] [Google Scholar]
- 8.Costello JM, Steinhorn D, McColley S, Gerber ME, Kumar SP. Treatment of plastic bronchitis in a Fontan patient with tissue plasminogen activator: a case report and review of the literature. Pediatrics 2002;109:e67. [DOI] [PubMed] [Google Scholar]
- 9.Heath L, Ling S, Racz J, Mane G, Schmidt L, Myers JL, Tsai WC, Caruthers RL, Hirsch JC, Stringer KA. Prospective, longitudinal study of plastic bronchitis cast pathology and responsiveness to tissue plasminogen activator. Pediatr Cardiol 2011;32:1182–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wakeham MK, Van Bergen AH, Torero LE, Akhter J. Long-term treatment of plastic bronchitis with aerosolized tissue plasminogen activator in a Fontan patient. Pediatr Crit Care Med 2005;6:76–78 [DOI] [PubMed] [Google Scholar]
- 11.Brogan TV, Finn LS, Pyskaty DJ, Jr, Redding GJ, Ricker D, Inglis A, Gibson RL. Plastic bronchitis in children: a case series and review of the medical literature. Pediatr Pulmonol 2002;34:482–487 [DOI] [PubMed] [Google Scholar]
- 12.Seear M, Hui H, Magee F, Bohn D, Cutz E. Bronchial casts in children: a proposed classification based on nine cases and a review of the literature. Am J Respir Crit Care Med 1997;155:364–370 [DOI] [PubMed] [Google Scholar]
- 13.Tonan M, Hashimoto S, Kimura A, Matsuyama H, Kinose H, Sawada M, Shime N, Tokuhira N, Kato Y, Sasaki M, et al. Successful treatment of severe asthma-associated plastic bronchitis with extracorporeal membrane oxygenation. J Anesth 2012;26:265–268 [DOI] [PubMed] [Google Scholar]
- 14.Pawar SS, Chun RH, Rao AR, Kerschner JE. Management of plastic bronchitis in a child with mild intermittent asthma. Ann Otol Rhinol Laryngol 2011;120:697–699 [DOI] [PubMed] [Google Scholar]
- 15.Mateos-Corral D, Cutz E, Solomon M, Ratjen F. Plastic bronchitis as an unusual cause of mucus plugging in cystic fibrosis. Pediatr Pulmonol 2009;44:939–940 [DOI] [PubMed] [Google Scholar]
- 16.Waring WW, Brunt CH, Hilman BC. Mucoid impaction of the bronchi in cystic fibrosis. Pediatrics 1967;39:166–175 [PubMed] [Google Scholar]
- 17.Moser C, Nussbaum E, Cooper DM. Plastic bronchitis and the role of bronchoscopy in the acute chest syndrome of sickle cell disease. Chest 2001;120:608–613 [DOI] [PubMed] [Google Scholar]
- 18.Deng J, Zheng Y, Li C, Ma Z, Wang H, Rubin BK. Plastic bronchitis in three children associated with 2009 influenza A(H1N1) virus infection. Chest 2010;138:1486–1488 [DOI] [PubMed] [Google Scholar]
- 19.Kuperman T, Wexler ID, Shoseyov D, Weintraub M, Revel-Vilk S, Kerem E. Plastic bronchitis caused by neoplastic infiltrates in a child. Pediatr Pulmonol 2006;41:893–896 [DOI] [PubMed] [Google Scholar]
- 20.Pruitt BA., Jr Complications of thermal injury. Clin Plast Surg 1974;1:667–691 [PubMed] [Google Scholar]
- 21.Cox RA, Burke AS, Soejima K, Murakami K, Katahira J, Traber LD, Herndon DN, Schmalstieg FC, Traber DL, Hawkins HK. Airway obstruction in sheep with burn and smoke inhalation injuries. Am J Respir Cell Mol Biol 2003;29:295–302 [DOI] [PubMed] [Google Scholar]
- 22.Veress LA, O’Neill HC, Hendry-Hofer TB, Loader JE, Rancourt RC, White CW. Airway obstruction due to bronchial vascular injury after sulfur mustard analog inhalation. Am J Respir Crit Care Med 2010;182:1352–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krenke K, Krenke R, Krauze A, Lange J, Kulus M. Plastic bronchitis: an unusual cause of atelectasis. Respiration 2010;80:146–147 [DOI] [PubMed] [Google Scholar]
- 24.Madsen P, Shah SA, Rubin BK. Plastic bronchitis: new insights and a classification scheme. Paediatr Respir Rev 2005;6:292–300 [DOI] [PubMed] [Google Scholar]
- 25.Zahorec M, Kovacikova L, Martanovic P, Skrak P, Kunovsky P. The use of high-frequency jet ventilation for removal of obstructing casts in patients with plastic bronchitis. Pediatr Crit Care Med 2009;10:e34–e36 [DOI] [PubMed] [Google Scholar]
- 26.Wang G, Wang YJ, Luo FM, Wang L, Jiang LL, Mao B. Effective use of corticosteroids in treatment of plastic bronchitis with hemoptysis in Chinese adults. Acta Pharmacol Sin 2006;27:1206–1212 [DOI] [PubMed] [Google Scholar]
- 27.Shinkai M, Rubin BK. Macrolides and airway inflammation in children. Paediatr Respir Rev 2005;6:227–235 [DOI] [PubMed] [Google Scholar]
- 28.Haseyama K, Satomi G, Yasukochi S, Matsui H, Harada Y, Uchita S. Pulmonary vasodilation therapy with sildenafil citrate in a patient with plastic bronchitis after the Fontan procedure for hypoplastic left heart syndrome. J Thorac Cardiovasc Surg 2006;132:1232–1233 [DOI] [PubMed] [Google Scholar]
- 29.Desai MH, Mlcak R, Richardson J, Nichols R, Herndon DN. Reduction in mortality in pediatric patients with inhalation injury with aerosolized heparin/N-acetylcystine [correction of acetylcystine] therapy. J Burn Care Rehabil 1998;19:210–212 [DOI] [PubMed] [Google Scholar]
- 30.Silva RC, Simons JP, Chi DH, Yellon RF, Alper CM. Endoscopic treatment of plastic bronchitis. Arch Otolaryngol Head Neck Surg 2011;137:401–403 [DOI] [PubMed] [Google Scholar]
- 31.Wilson J, Russell J, Williams W, Benson L. Fenestration of the Fontan circuit as treatment for plastic bronchitis. Pediatr Cardiol 2005;26:717–719 [DOI] [PubMed] [Google Scholar]
- 32.Shah SS, Drinkwater DC, Christian KG. Plastic bronchitis: is thoracic duct ligation a real surgical option? Ann Thorac Surg 2006;81:2281–2283 [DOI] [PubMed] [Google Scholar]
- 33.Barber BJ, Burch GH, Tripple D, Balaji S. Resolution of plastic bronchitis with atrial pacing in a patient with fontan physiology. Pediatr Cardiol 2004;25:73–76 [DOI] [PubMed] [Google Scholar]
- 34.Laubisch JE, Green DM, Mogayzel PJ, Reid Thompson W. Treatment of plastic bronchitis by orthotopic heart transplantation. Pediatr Cardiol 2011;32:1193–1195 [DOI] [PubMed] [Google Scholar]
- 35.Barreto AD, Alexandrov AV, Lyden P, Lee J, Martin-Schild S, Shen L, Wu TC, Sisson A, Pandurengan R, Chen Z, et al. The argatroban and tissue-type plasminogen activator stroke study: final results of a pilot safety study. Stroke 2012;43:770–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kablau M, Alonso A, Hennerici MG, Fatar M. Treatment with tPA predicts better outcome even if MCA occlusion persists. Int J Stroke (In press) [DOI] [PubMed] [Google Scholar]
- 37.Fitchett DH, Theroux P, Brophy JM, Cantor WJ, Cox JL, Gupta M, Kertland H, Mehta SR, Welsh RC, Goodman SG. Assessment and management of acute coronary syndromes (ACS): a Canadian perspective on current guideline-recommended treatment—part 2: ST-segment elevation myocardial infarction. Can J Cardiol 2011. 27(Suppl A):S402–S412 [DOI] [PubMed] [Google Scholar]
- 38.Zeltner TB, Bertacchini M, Messerli A, Burri PH. Morphometric estimation of regional differences in the rat lung. Exp Lung Res 1990;16:145–158 [DOI] [PubMed] [Google Scholar]
- 39.Gao X, Anderson DR, Brown AW, Lin H, Amnuaysirikul J, Chua AL, Holmes WW, Ray P. Pathological studies on the protective effect of a macrolide antibiotic, roxithromycin, against sulfur mustard inhalation toxicity in a rat model. Toxicol Pathol 2011;39:1056–1064 [DOI] [PubMed] [Google Scholar]
- 40.Fairhall SJ, Jugg BJ, Read RW, Stubbs SJ, Rutter SJ, Smith AJ, Mann TM, Jenner J, Sciuto AM. Exposure-response effects of inhaled sulfur mustard in a large porcine model: a 6-h study. Inhal Toxicol 2010;22:1135–1143 [DOI] [PubMed] [Google Scholar]
- 41.O’Neill HC, Orlicky DJ, Hendry-Hofer TB, Loader JE, Day BJ, White CW. Role of reactive oxygen and nitrogen species in olfactory epithelial injury by the sulfur mustard analogue 2-chloroethyl ethyl sulfide. Am J Respir Cell Mol Biol 2011;45:323–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.The effects of tissue plasminogen activator, streptokinase, or both on coronary-artery patency, ventricular function, and survival after acute myocardial infarction. The GUSTO Angiographic Investigators. N Engl J Med 1993;329:1615–1622 [DOI] [PubMed] [Google Scholar]
- 43.Lansberg MG, O’Donnell MJ, Khatri P, Lang ES, Nguyen-Huynh MN, Schwartz NE, Sonnenberg FA, Schulman S, Vandvik PO, Spencer FA, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012;141:e601S–e636S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, Nelson ME, Wells PS, Gould MK, Dentali F, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012;141:e419S–e494S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hilleman D, Campbell J. Efficacy, safety, and cost of thrombolytic agents for the management of dysfunctional hemodialysis catheters: a systematic review. Pharmacotherapy 2011;31:1031–1040 [DOI] [PubMed] [Google Scholar]
- 46.Garcia A, Gander JW, Gross ER, Reichstein A, Sheth SS, Stolar CJ, Middlesworth W. The use of recombinant tissue-type plasminogen activator in a newborn with an intracardiac thrombus developed during extracorporeal membrane oxygenation. J Pediatr Surg 2011;46:2021–2024 [DOI] [PubMed] [Google Scholar]
- 47.Rahman NM, Maskell NA, West A, Teoh R, Arnold A, Mackinlay C, Peckham D, Davies CW, Ali N, Kinnear W, et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med 2011;365:518–526 [DOI] [PubMed] [Google Scholar]
- 48.Johnson AR, Jensen HL, Peltier G, DelaCruz E. Efficacy of intravenous tissue plasminogen activator in frostbite patients and presentation of a treatment protocol for frostbite patients. Foot Ankle Spec 2011;4:344–348 [DOI] [PubMed] [Google Scholar]
- 49.van Goor H, Bom VJ, van der Meer J, Sluiter WJ, Geerards S, van der Schaaf W, de Graaf JS, Bleichrodt RP. Pharmacokinetics of human recombinant tissue-type plasminogen activator, administered intra-abdominally, in a rat peritonitis model. Eur Surg Res 1996;28:287–294 [DOI] [PubMed] [Google Scholar]
- 50.Enkhbaatar P, Murakami K, Cox R, Westphal M, Morita N, Brantley K, Burke A, Hawkins H, Schmalstieg F, Traber L, et al. Aerosolized tissue plasminogen inhibitor improves pulmonary function in sheep with burn and smoke inhalation. Shock 2004;22:70–75 [DOI] [PubMed] [Google Scholar]