Abstract
Current models of care emphasize the importance of including patients’ values in the decision making process. This is particularly important for decisions for which there are few data supporting a clear strategy or treatment choice. Constructing preferences for complex decisions requires that patients be able to consider multiple trade-offs between specific risks and benefits. Several marketing research techniques have been recently applied to heath care settings to facilitate this process. Most can be programmed to generate patients’ preferences or priorities, which can then be used to improve patient-physician communication. In this paper, we will describe some of the currently available approaches that have been successfully used in the healthcare setting. We provide case examples to illustrate the potential value of adopting each of these approaches in clinical practice.
Keywords: Conjoint Analysis, Best Worst Scaling, Point-of-Care Clinical Decision Support
Bringing the Patient into the Decision Process
Incorporation of individual, informed, patient preferences is recognized as an essential component of treatment planning. Ideally, when faced with difficult decisions, all patients should be: a) aware that there is a choice; b) effectively informed of the expected outcomes associated with each option; and c) given the opportunity to discuss their views with their physician regardless of their desire to make or to avoid making a decision.
Patient participation can vary significantly—from assenting to a physician’s recommendation to engaging in an in-depth discussion comparing the specific trade-offs related to all available options (Whitney, McGuire, & McCullough, 2004). The former usually suffices in situations for which there is a clear, low risk, dominant strategy (e.g., starting aspirin after a myocardial infarction). However, more complex decisions (e.g., treatment for prostate cancer) frequently require additional efforts to support patient participation (Woolf et al., 2005). While patient participation is always important, it is vital in the latter, where the correct treatment choice can only be made once patients are informed and given the opportunity to vocalize their values and preferences.
While effectively informing patients is always challenging, it is even more difficult in situations where there is limited evidence underlying specific treatment strategies. Notable examples include treatment decisions for uncommon diseases for which there are few controlled studies available, chronic diseases which are treated based on outcome data generated from short-term randomized controlled trials, and patients with multiple co-morbidities in whom risks are likely to differ significantly from subjects included in controlled trials.
Several approaches have been used to quantify patient preferences under uncertainty. Utilities for policy decisions have long been measured using one or a combination of the following three techniques: standard gamble, time trade-off, or rating scales. All three modalities quantify preferences for defined health states that can then be incorporated into decision models to calculate quality-adjusted outcomes (such as quality-adjusted life years). However, there are limitations associated with these techniques including poor inter-method agreement, susceptibility to biases, and task-related difficulty associated with the standard gamble and time trade-off methods (Badia, Roset, & Herdman, 1999; Cook et al., 2001; Lenert & Kaplan, 2000; Souchek et al., 2000; Tsevat et al., 1998). These issues limit the use of these techniques in support of decision-making at the individual patient level. As a result, alternative approaches are increasingly used to measure patient preferences for decisions related to health care (Phillips, Maddala, & Johnson, 2002; Ryan & Gerard, 2003).
Conjoint Analysis
Conjoint analysis (CA) is a well-validated tool used to understand consumer preferences and help design new products. When faced with complex decisions, consumers evaluate a number of attributes and then make trade-offs to arrive at a final choice. CA evaluates these trade-offs to determine which combination of attributes is most preferred by consumers. The data generated from CA studies can be used to measure preferences for available options, as well as the relative importance that respondents assign to specific attributes (Green & Srinivasan, 1990; Orme, 2010; Wittink & Bergenstuen, 2001). For example, CA can determine 1) whether consumers prefer a hospital at close distance with fewer services to one that is farther away with a greater number of services, and 2) the incremental distance consumers are willing to travel to obtain specific services. CA can evaluate preferences over a range of probability estimates, thereby providing both patients and physicians an estimate of each individual patient’s sensitivity to varying risks and benefits in situations where precise individual probability estimates are not available.
CA assumes that each product is a composite of different attributes, and that each attribute is specified by a number of levels. In treatment decisions, attributes typically include specific treatment characteristics, such as route of administration, risk of adverse effects, probability and magnitude of benefit, and risk of toxicity. Levels refer to the range of estimates of each characteristic. In a CA survey designed to ascertain preferences for knee arthritis medications, levels for the attribute route of administration might include pills, creams and intra-articular injections.
The choice of which levels to include for risks and benefits is more challenging. This is especially true when evidence is lacking. Data from high quality randomized controlled trials or long-term prospective cohort studies are not available for many of the decisions encountered in clinical practice. Moreover, even when such studies exist, outcome probabilities for individual patients with varying co-morbidities are difficult to estimate. CA can contend with these challenges by examining patients’ preferences over a range of plausible outcome probabilities. For example, treatment preferences for hepatitis C are strongly influenced by patients’ willingness to accept the risk of toxicity associated with a prolonged course of antiviral therapy. Patients’ experiences on therapy, however, are highly variable (Cotler et al., 2001). We used CA to examine patients’ preference for chronic hepatitis C treatment under two conditions: risk of mild side effects versus risk of more severe side effects (L. Fraenkel, Chodkowski, Lim, & Garcia-Tsao, 2009). Subjects who are more concerned about the risk of side effects and less certain whether they wish to undergo therapy will have preferences that differ markedly between the two scenarios. In contrast, those whose preferences cluster together are less sensitive to the risk of side effects and have stronger treatment preferences (whether for or against treatment). The data generated from this type of survey can be used to facilitate patient-physician communication so that discussions address individual patient concerns and treatment plans are developed based on patient values and priorities.
There are several different forms of CA. Some studies have used the full-profile approach in which respondents are presented with complete descriptions of hypothetical products that include a specified level for each attribute. (Hawley et al., 2008) reported success in using a full profile CA survey to assess patient preferences for colorectal cancer screening. The same study also demonstrated the feasibility of using CA in patients with diverse educational backgrounds. The main advantage of this technique is that it provides respondents with the most realistic descriptions of the products being evaluated. However, Wright (Wright, 1975) and (Srinivasan & Park, 1997) demonstrated that respondents tend to employ simplifying tactics to compensate for information overload when presented with full profiles using as few as four attributes. Therefore, full profile CA is best used for treatment options having a limited number of attributes relevant to the decision process.
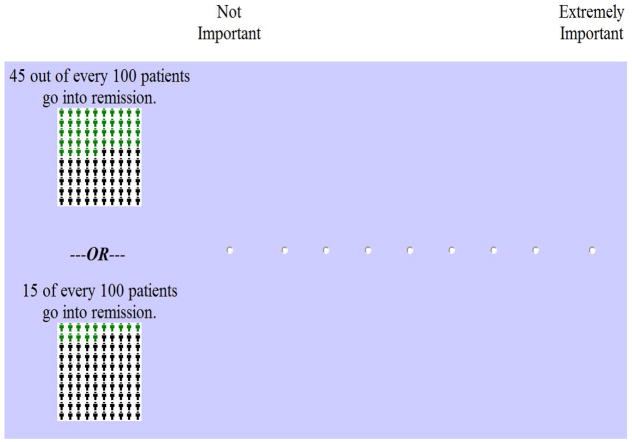
Adaptive Conjoint Analysis (ACA) generates preference data using a series of different question formats. Subjects are first asked to rank the levels for each attribute. This step can be omitted for attributes having a priori ordering, such as cost or probabilities of risks and benefits (Orme). The second set of questions involves rating the relative importance of each attribute (see example in Figure 1). This set of questions is often problematic, because subjects have difficulty incorporating the range of levels into their ratings. The importance questions can be omitted from the survey without impacting the accuracy of the results if the data are analyzed using Hierarchical Bayes models (Orme). However, this approach cannot be used for investigators requiring output in real time.
Figure 1.
Example of ACA relative importance question
In order to overcome this difficulty, we developed a modified version of the ACA importance questions (L. Fraenkel, 2010). In the first question, subjects are presented with a list of all the attributes and asked to choose the one that is most important to them. The second question asks subjects to rate, using a numeric scale, the importance of the remaining attributes on a grid relative to the one chosen as most important. This modified version of the ACA importance questions appears to perform as well as or better than the original version.
The third set of questions is comprised of paired comparison tasks (Figure 2). ACA constructs pairs by examining all the possible ways the levels can be combined, pairing options for which it expects respondents to be indifferent (based on previous responses). Each question involves choosing one option from a pair in which one is superior in one attribute and the opposing option is superior in the other. The program uses the information obtained from each paired comparison to update the estimates of each respondent’s values and to select the next pair of options. As a result, each respondent answers a customized set of questions. Because it is interactive, ACA allows a large number of attributes to be evaluated without resulting in information overload or respondent fatigue. This is an important feature, since complex treatment decisions often require multiple trade-offs between competing risks and benefits.
Figure 2.
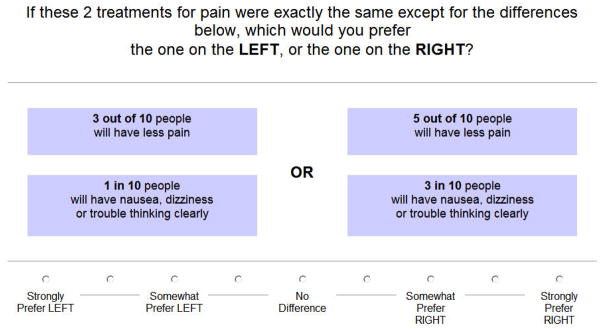
Example of a paired comparison task
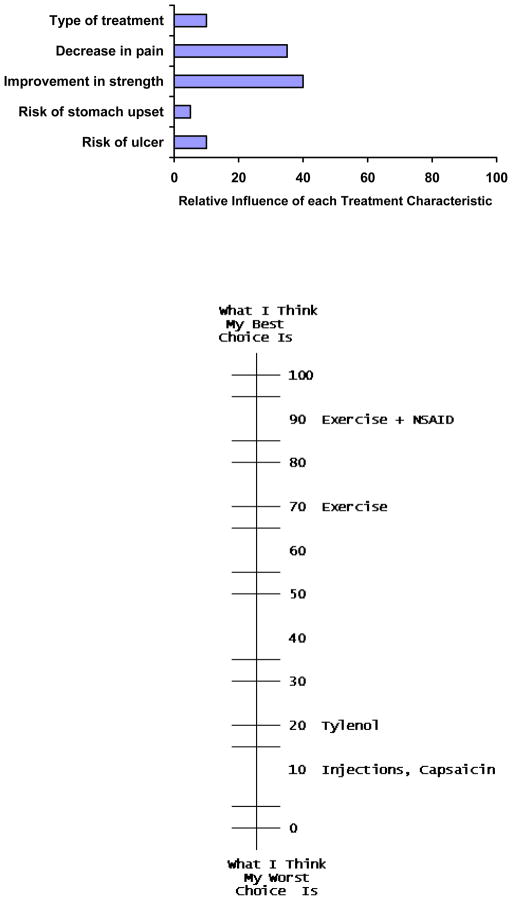
The potential value of ACA as a decision support tool was examined by this author and colleagues in two studies. In one, patients with knee pain were randomized to receive an information pamphlet or to complete an ACA survey before seeing their physician (L. Fraenkel, Rabidou, Wittink, & Fried, 2007). After performing the ACA survey, participants were given a handout illustrating their preferences (Figure 3). The results indicated that 74% of respondents randomized to perform the ACA task felt that the ACA task was “very easy” to do. Decisional self-efficacy, preparedness to participate in decision-making, and arthritis self-efficacy were greater in participants randomized to the intervention arm compared to participants receiving the information pamphlet (p<0.05 for all comparisons). 68% felt that the bar graph “very much” reflected their values.
Figure 3.
Example of handout illustrating a hypothetical respondent’s preferences
In a second study, this author and colleagues used ACA to help patients with chronic hepatitis C construct their treatment preferences (L. Fraenkel et al., 2009). Data were collected on the day subjects were scheduled to talk to their physician about treatment, and subjects were asked to come in 90 minutes before their appointment to provide preference data. In order to ensure that subjects were presented with individualized estimates of potential benefits, we created eight versions of the ACA questionnaire for patients with varying degrees of hepatitis-related fibrotic changes on biopsy (none, mild, moderate or cirrhosis) and virus genotype (1 or 2). In this study, we found significant variability in preferences for treatment of hepatitis C amongst patients with mild to moderate disease. In contrast, almost all patients with more severe fibrosis had a strong preference for treatment. Thus, our results reaffirm the need for decision support to enable patients with mild to moderate disease to construct their preferences For patients with severe disease and awareness of the level of health risk it poses, informed consent for treatment may be sufficient.
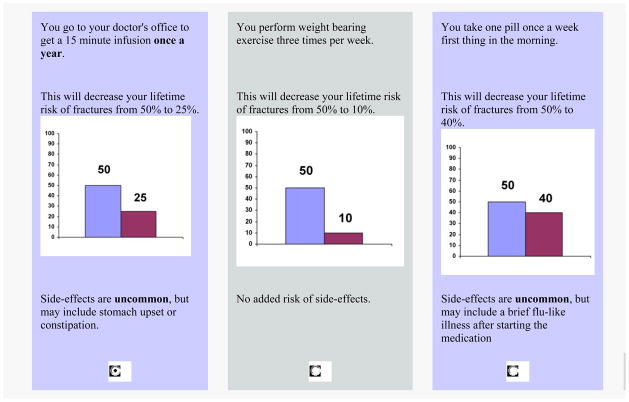
Choice-based CA (CBCA) is the most common form of CA used today in both marketing and health services research. This method derives preference data based on respondents’ answers to a series of choice tasks (Figure 4). CBCA is an attractive option because choice tasks are generally easier to perform than rating tasks and more closely mirror decision making in the real world. Moreover, CBCA surveys can be programmed to include a “None” option, thus enabling researchers to measure the proportion of subjects opting out of all available options. This option may be valuable for choices where deferral or refusal is common such as in elective surgical procedures. The main limitation of this approach is that, as with full profile CA, because all attributes are included in each task, CBCA is not appropriate when evaluating a large number of attributes.
Figure 4.
Example of a Choice Based Conjoint Analysis task
Many CBCA studies have been used to examine patients’ preferences for processes of care and treatment settings, as well as treatment choices (Burge, Devlin, Appleby, Rohr, & Grant; Christofides, Muirhead, Jewkes, Penn-Kekana, & Conco; Darba et al.; Hill, Foss, Tu, & Feasby; Hodgkins et al.; Howard et al.; King et al.; Schwappach & Strasmann). Yet, based on the absence of reports in the literature, this approach has not been used to facilitate decision making at the individual patient level in real time. This may be because, unlike with ACA or the full profile approach, commercially available software is not able to generate reliable preference data based on the responses of a single patient. New advances in CA (e.g., Complete Conjoint and Adaptive CBCA) have been developed recently and may provide even greater flexibility for researchers to construct decision support tools in healthcare settings that yield results more closely aligned with actual patient preferences.
Best Worst Scaling
Best Worst Scaling (BWS), first described by Finn and Louviere (Finn & Louviere, 1992), was applied to health care issues in 2002 (McIntosh & Louviere, 2002). Surveys using this approach ask respondents to choose the best (or the most important) item and the worst (or least important) item from a series of sets containing different combinations of items from a master list. For example, the choice set displayed in Figure 5 was obtained from a survey designed to enable patients to consider the factors most important to them when considering colorectal cancer screening. The BWS question format is easy to understand, and respondents from diverse sociodemographic backgrounds have been shown to be able to provide reliable data. BWS can handle a large set of attributes, and unlike CA, BWS can be administered over the telephone (Solomon, Rabidou, Kulkarni, Formica, & Fraenkel, 2010). Scale-related response bias is not a concern because respondents make choices instead of indicating their preference using rating scales. Because each task generates more information about respondents’ preferences, BWS is also more efficient than rating scales. For example, consider the attribute set in Figure 5. A respondent who chooses “The need to have a second test” as most important and “The need to take a day off work” as least important tells us that A>B, A>C, A>D, B>C, D>C where the attributes are labeled A through D.
Figure 5.
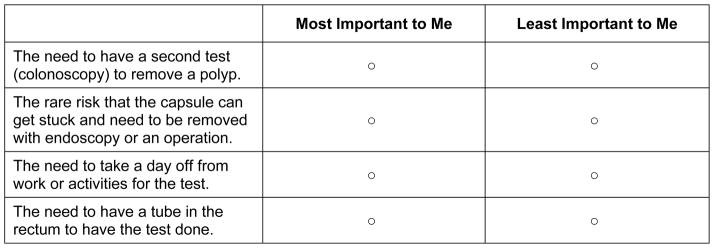
Example of a Best Worse Scaling question
BWS generates ratio data and provides estimates of the absolute importance, as well the relative importances, of all the items included in the survey. Unlike CA, BWS is not well suited to examining values attached to differences in probabilities of specific risks or benefits, nor does it generate preferences for competing options. BWS is, however, very valuable for helping patients to prioritize attributes. Therefore, BWS may be particularly helpful in situations where informed choice requires that patients understand the salient differences between available treatment options as opposed to the precise differences in the probabilities of specific outcomes.
We recently piloted a BWS survey in the waiting room of primary care clinics to determine if patients could complete this type of survey independently before seeing their physician (Imaeda, D., & L., 2010). The tool was developed to evaluate how patients value the characteristics of colorectal cancer screening tests. Unlike other decision aids, we did not focus on specific options, but rather asked patients to consider general features, including: the sensitivity of test, risk of an intestinal tear, need for a second test, risk of a large capsule taken by mouth getting stuck, need for sedation, risk of pain, need for colon prep, need for a tube in the rectum, need to smear stool on a card, need for a ride, need to swallow a capsule, and need to miss work. The output of the tool included a bar graph illustrating the rank ordering of the relative importance of each feature. Patients reported that the tool was easy to use (95%) and helpful (92%). Among the respondents, 85% who had previously refused screening, stated that they were willing to be screened if offered their preferred test.
In a more recent study, we used BWS to elicit patients’ priorities after completing a web-based educational tool for rheumatoid arthritis (L. Fraenkel, Peters, Charpentier, & al., 2012). The tool was developed to enable patients to generate a rank ordering of their priorities after they had been effectively informed of the pros and cons related to therapy. In a pre-post test study we found that objective knowledge, perceived knowledge, and value clarity significantly improved among members of the study group. Most importantly, the proportion of subjects making an informed, value-concordant choice increased substantially from 35% to 64%.
These studies suggest that tools may be most helpful if they educate patients effectively and produce an output that can be used to facilitate physician-patient communication. In such cases, the application of the tool need not result in a choice per se. In fact, tools generating an actual choice may be less acceptable to both patients and physicians. This is particularly true for patients who are reluctant to assume responsibility for making the final decision.
Current Landscape
Communicating complex medical information to patients in a manner which allows them to consider all salient trade-offs is difficult. This is particularly true in situations for which there are multiple risks and benefits to consider and/or uncertain probability estimates. CA and BWS surveys enable patients to construct their preferences based on how they value the risks and benefits related to all options under consideration. The optimal approach will vary depending on the needs of the specific research project and clinical scenario. Table 1 provides a comparison of the main features relevant to a researcher considering one of these approaches.
Table 1.
Some Differences between CA Approaches and BWS
| Full Profile Conjoint Analysis | Adaptive Conjoint Analysis | Choice Based Conjoint Analysis | Best Worst Scaling | |
|---|---|---|---|---|
| Delivery | Paper or Computer | Computer Only | Paper or Computer | Paper or Computer |
| Type of survey questions | Rating | Rating | Choice | Choice |
| Can be administered over the telephone | No | No | No | Yes |
| Able to generate individual respondent data in real time | Yes | Yes | No | Yes |
| Able to handle numerous attributes/levels | No | Yes | No | Yes |
| Able to predict preferences for specified options | Yes | Yes | Yes | No |
| Well suited to examining trade-offs between differences in probabilities | Yes | Yes | Yes | No |
| Possible for patients to do on their own | Yes | Unlikely | Yes | Yes |
These approaches to decision support hold several advantages over more traditional decision aids. CA and BWS surveys are relatively easy to update as advances in medicine become available. They can be formatted to present patients with individualized estimates of risks and benefits. All can be administered by computer, which minimizes provider and interviewer biases and facilitates data collection, management, and analyses. For subjects having limited access to computers, or difficulty using computers, full profile, CBCA and BWS surveys can be administered on paper. Adaptive approaches, however, all require the use of a computer. In addition, depending on the complexity of the survey, some patients may need assistance to complete these tools. Data generated from CA surveys can be used to examine the magnitude of importance respondents place on specific treatment characteristics. Some ACA, full profile, and BWS also enable patients to receive immediate feedback. This feature allows physicians to gain insight into the reasons underlying their patients’ preferences. CA surveys also provide simulation capability, allowing the investigator to assess the impact of varying specific treatment characteristics on choice. For example, researchers can determine how much benefit patients require before accepting the risk of drug toxicity, whether decreasing the burden or inconveniences of therapy might increase patients’ acceptance of treatment, or how consistent available treatment is with each patient’s values. CA may be particularly helpful in decisions where there are limited data available, because of its ability to observe the degree to which preferences vary across a plausible range of estimates. These data may help physicians and their patients choose treatment options that encompass each patient’s expectations of benefit and risk tolerance.
While considerable evidence is now available supporting the potential value of these approaches in healthcare, randomized controlled trials demonstrating an incremental benefit of these tools in clinical practice is lacking, and there is no current repository of CA-based decision support tools. Consequently, CA and BWS may be currently best viewed as promising techniques in need of further research. Through publications like one, it is hoped that awareness of these techniques will be heightened among a multidisciplinary cadre of investigators who will take up the challenges of further development, testing, refinement of these methods so that their full potential can realized in fostering patient-centered health care.
Acknowledgments
Dr. Fraenkel is supported by the NIAMS K24 AR060231-01.
The Eisenberg Conference Series 2011 Meeting on Differing Levels of Clinical Evidence: Exploring Communication Challenges in Shared Decision Making was conducted by the John M. Eisenberg Center for Clinical Decisions and Communications Science at Baylor College of Medicine, Houston, Texas under contract to the Agency for Healthcare Research and Quality Contract No. HHSA290200810015C, Rockville, MD. The authors of this article are responsible for its content. No statement may be construed as the official position of the Agency for Healthcare Research and Quality of the U.S. Department of Health and Human Services.
References
- Badia X, Roset M, Herdman M. Inconsistent responses in three preference-elicitation methods for health states. Social Science & Medicine. 1999;49(7):943–950. doi: 10.1016/s0277-9536(99)00182-3. [DOI] [PubMed] [Google Scholar]
- Burge P, Devlin N, Appleby J, Rohr C, Grant J. Do patients always prefer quicker treatment?: a discrete choice analysis of patients’ stated preferences in the London Patient Choice Project. [Research Support, Non-U.S. Gov’t] Applied Health Economics & Health Policy. 3(4):183–194. doi: 10.2165/00148365-200403040-00002. [DOI] [PubMed] [Google Scholar]
- Christofides NJ, Muirhead D, Jewkes RK, Penn-Kekana L, Conco DN. Women’s experiences of and preferences for services after rape in South Africa: interview study. [Multicenter Study Research Support, Non-U.S. Gov’t] BMJ. 332(7535):209–213. doi: 10.1136/bmj.38664.482060.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook KF, Ashton CM, Byrne MM, Brody B, Geraci J, Giesler RB, Wray NP. A psychometric analysis of the measurement level of the rating scale, time tradeoff, and standard gamble. Social Science & Medicine. 2001;53(10):1275–1285. doi: 10.1016/s0277-9536(00)00409-3. [DOI] [PubMed] [Google Scholar]
- Cotler SJ, Patil R, McNUtt RA, Speroff T, Banaad-Omiotek G, Ganger DR, Jensen DM. Patients’ values for health states associated with hepatitis C and physicians’ estimates of those values. Am J Gastroenterol. 2001;96:2730–2736. doi: 10.1111/j.1572-0241.2001.04132.x. [DOI] [PubMed] [Google Scholar]
- Darba J, Restovic G, Kaskens L, Balbona MA, Carbonell A, Cavero P, Padro I. Patient preferences for osteoporosis in Spain: a discrete choice experiment. [Research Support, Non-U.S. Gov’t] Osteoporosis International. 22(6):1947–1954. doi: 10.1007/s00198-010-1382-3. [DOI] [PubMed] [Google Scholar]
- Finn A, Louviere J. Determining the Appropriate Response to Evidence of Public Concern: The Case of Food Safety. J Public Policy Mark. 1992;11:12– 25. [Google Scholar]
- Fraenkel L. Feasibility of Using Modified Adaptive Conjoint Analysis Importance Questions. The Patient: Patient-Centered Outcomes Research. 2010;3:209–215. doi: 10.2165/11318820-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel L, Chodkowski D, Lim J, Garcia-Tsao G. Patients’ preferences for treatment of hepatitis C. Med Decis Making, Med Decis Making. 2009 Jul 27; doi: 10.1177/0272989X09341588. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel L, Peters E, Charpentier P, et al. A Decision Tool to Improve the Quality of Care in Rheumatoid Arthritis. Arthritis Care Res. 2012 doi: 10.1002/acr.21657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel L, Rabidou N, Wittink DR, Fried T. Improving informed decision-making for patients with knee pain. J Rheumatol. 2007;34:1894–1898. [PubMed] [Google Scholar]
- Green PE, Srinivasan V. Conjoint analysis in marketing: new developments with implications for research and practice. J Marketing. 1990;54:3–17. [Google Scholar]
- Hawley ST, Volk RJ, Krishnamurthy P, Jibaja-Weiss M, Vernon SW, Kneuper S. Preferences for Colorectal Cancer Screening Among Racially/Ethnically Diverse Primary Care Patients. Medical Care. 2008;46(9):S10–S16. doi: 10.1097/MLR.1090b1013e31817d31932e. [DOI] [PubMed] [Google Scholar]
- Hill MD, Foss MM, Tu JV, Feasby TE. Factors influencing the decision to perform carotid endarterectomy. [Research Support, Non-U.S. Gov’t] Neurology. 62(5):803–805. doi: 10.1212/01.wnl.0000113791.42995.4b. [DOI] [PubMed] [Google Scholar]
- Hodgkins P, Swinburn P, Solomon D, Yen L, Dewilde S, Lloyd A. Patient preferences for first-line oral treatment for mild-to-moderate ulcerative colitis: a discrete-choice experiment. The Patient: Patient-Centered Outcomes Research. 5(1):33–44. doi: 10.2165/11595390-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Howard K, Salkeld G, Pignone M, Hewett P, Cheung P, Olsen J, Roberts-Thomson IC. Preferences for CT colonography and colonoscopy as diagnostic tests for colorectal cancer: a discrete choice experiment. [Research Support, Non-U.S. Gov’t] Value in Health. 14(8):1146–1152. doi: 10.1016/j.jval.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaeda ADB, LF What is most important to patients when deciding about colorectal screening? J Gen Intern Med. 2010;25:688–693. doi: 10.1007/s11606-010-1318-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MT, Viney R, Smith DP, Hossain I, Street D, Savage E, Armstrong BK. Survival gains needed to offset persistent adverse treatment effects in localised prostate cancer. [Research Support, Non-U.S. Gov’t] British Journal of Cancer. 106(4):638–645. doi: 10.1038/bjc.2011.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenert L, Kaplan RM. Validity and interpretation of preference-based measures of health-related quality of life.[comment] Medical Care. 2000;38(9 Suppl):II138–150. doi: 10.1097/00005650-200009002-00021. [DOI] [PubMed] [Google Scholar]
- McIntosh E, Louviere J. Separating weight and scale value: an exploration of best-attribute scaling in health economics. Health Economists’ Study Group meeting 2002 [Google Scholar]
- Orme B. http://www.sawtoothsoftware.com/download/techpap/acatech.pdf Retrieved May 1, 2008.
- Orme B. Getting started with conjoint analysis: strategies for product design and pricing research. 2. Madison: Research Publishers LLC; 2010. Market simulators for conjoint analysis. [Google Scholar]
- Phillips KA, Maddala T, Johnson FR. Measuring preferences for health care interventions using conjoint analysis: an application to HIV testing. Health Services Research. 2002;37(6):1681–1705. doi: 10.1111/1475-6773.01115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan M, Gerard K. Using discrete choice experiments to value health care programs: current practice and future research reflections. Applied Health Econ Health Policy. 2003;2:55–64. [PubMed] [Google Scholar]
- Schwappach DLB, Strasmann TJ. Does location matter? A study of the public’s preferences for surgical care provision. Journal of Evaluation in Clinical Practice. 13(2):259–264. doi: 10.1111/j.1365-2753.2006.00688.x. [DOI] [PubMed] [Google Scholar]
- Solomon DA, Rabidou N, Kulkarni S, Formica R, Fraenkel L. Accepting a donor kidney: an evaluation of patients’ and transplant surgeons’ priorities. Clin Transplant. 2010 doi: 10.1111/j.1399-0012.2010.01342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souchek J, Stacks JR, Brody B, Ashton CM, Giesler RB, Byrne MM, Wray NP. A trial for comparing methods for eliciting treatment preferences from men with advanced prostate cancer: results from the initial visit. Medical Care. 2000;38(10):1040–1050. doi: 10.1097/00005650-200010000-00008. [DOI] [PubMed] [Google Scholar]
- Srinivasan V, Park CS. Surprising robustness of the self-explicated approach to customer preference structure measurement. J Market Res. 1997;34:286–291. [Google Scholar]
- Tsevat J, Dawson NV, Wu AW, Lynn J, Soukup JR, Cook EF, Phillips RS. Health values of hospitalized patients 80 years or older. HELP Investigators. Hospitalized Elderly Longitudinal Project.[comment] JAMA. 1998;279(5):371–375. doi: 10.1001/jama.279.5.371. [DOI] [PubMed] [Google Scholar]
- Whitney SN, McGuire AL, McCullough LB. A Typology of Shared Decision Making, Informed Consent, and Simple Consent. Annals of Internal Medicine. 2004;140(1):54–59. doi: 10.7326/0003-4819-140-1-200401060-00012. [DOI] [PubMed] [Google Scholar]
- Wittink DR, Bergenstuen T. Forecasting with conjoint analysis. In: Armstrong JS, editor. Principles of forecasting: a handbook for researchers and practitioners. Norwell, MA: Kluwer Academic Publishers; 2001. [Google Scholar]
- Woolf SH, Chan ECY, Harris R, Sheridan SL, Braddock CH, Kaplan RM, Tunis S. Promoting Informed Choice: Transforming Health Care To Dispense Knowledge for Decision Making. Annals of Internal Medicine. 2005;143(4):293–300. doi: 10.7326/0003-4819-143-4-200508160-00010. [DOI] [PubMed] [Google Scholar]
- Wright P. Consumer choice strategies: simplifying vs. optimizing. J Market Res. 1975;12:60–67. [Google Scholar]