Abstract
Syndecan-1 (sdc-1) is a cell surface proteoglycan that mediates the interaction of cells with their matrix, influencing attachment, migration and response to growth factors. In keratinocytes, loss of sdc-1 delays wound healing, reduces migration, and increases TGFβ1 expression. In this study we show that sdc-1 expression is significantly reduced in basal cell, squamous cell, and metastatic human skin cancers compared to normal human skin. In experimental mouse skin tumor induction, compared to wildtype (wt) BALB/c mice, papilloma formation in sdc-1 null mice was reduced by 50% and the percent of papillomas converting to squamous cell carcinoma (SCC) was enhanced. Sdc-1 expression on wildtype mouse papillomas decreased as they converted to SCC. Furthermore, papillomas forming on sdc-1 null mice expressed suprabasal α3 and β4 integrins; suprabasal β4 integrin is a marker of a high risk for progression. While the proliferative response to TPA did not differ among the genotypes, sdc-1 null mice had an enhanced inflammatory response and retained higher levels of total TGFβ1 within their skin after TPA treatment. Sdc-1 null keratinocytes, transduced in vitro by oncogenic rasHa, expressed higher levels of β4 integrin and had enhanced pSmad2 signaling and reduced senescence when compared to wildtype rasHa transduced keratinocytes. When rasHa transduced cells of both genotypes were grafted onto nude mice, null tumors converted to SCC with higher frequency confirming the skin painting experiments. These data indicate that sdc-1 is important both early in the development of skin tumors and in progression of skin cancers suggesting that reduced expression of sdc-1 could be a useful marker for progression in neoplastic skin lesions.
Keywords: skin carcinogenesis, keratinocytes, syndecan-1, integrin, laminin 332, TGFβ1, ras oncogene
INTRODUCTION
Changes in the expression of sdc-1 during carcinogenesis have been reported in various tissues including breast, prostate, head and neck, uterine, and colon cancer (reviewed in 1,2). Sdc-1 has been reported to be both up-regulated (breast cancer and head and neck cancers) or down-regulated (uterine and colon cancers) during carcinogenesis. The loss of sdc-1 can occur by transcriptional downregulation or constitutive or MMP-induced shedding of the sdc-1 ectodomain and in most cases the mechanism of loss of sdc-1 has not been determined.
We have shown previously that the loss of sdc-1 impacts dermal and corneal wound healing in vivo and alters migration and integrin functions of epidermal keratinocytes (3) and dermal fibroblasts (4) in vitro. Sdc-1 null keratinocytes are more adhesive and less migratory as they rely primarily on α6β4 to mediate their migration. In contrast, sdc-1 null fibroblasts show increased rates of cell migration. In null keratinocytes and fibroblasts, the differences in integrin function and expression are accompanied by altered TGFβ1 signaling.
Expanded expression of α6β4 within papillomas undergoing premalignant progression arising from 2-stage chemical carcinogenesis is associated with differences in the interaction of cells with laminin in their matrix (5,6). Otiz-Urda and colleagues (7) showed that the development of human epidermal squamous cell carcinomas required the interaction between the two extracellular matrix molecules laminin 332 (LM332) and type VII collagen and recent studies show that this interaction is mediated by signaling through integrin mediated adhesions. Sdc-1 associates with the α3 chain of LM332 via α3β1 integrin (8) and with the short arm of the laminin γ2 chain via α6β4 integrin (9).
The region of sdc-1 that associates with α3β1 and α6β4 integrins is called the co-receptor binding domain. Sdc-1 can also activate αv integrin function in mouse mammary tumor cells (10); sdc-1 null fibroblasts have reduced activation of αv integrins on their surface (4) which may contribute to delayed wound healing in sdc-1 null mice since several αv integrin heterodimers including αvβ5, αvβ6, and αvβ8 mediate TGFβ1 activation. The latent domain of TGFβ1 has an αv integrin binding site and studies have suggested that αv integrins sequester latent TGFβ1 to the cell surface and control its activation locally (11).
TGFβ1 is known for its ability to function as a pro-oncogenic factor late in cancer development and also function to inhibit tumor formation early in cancer development (12–15). Evidence for the importance of sdc-1 in epithelial homeostasis is shown by the fact that sdc-1 expression in epithelial cells is regulated via TGFβ1 mediated cell signaling (16). It is critical that we develop a more complete understanding of the dual nature of the cell signaling events regulated by both sdc-1 and TGFβ1 in epithelial cells.
Given the association between sdc-1 and integrin activation, binding of α3 integrin to LM332 and its role in carcinoma development (2, 6), and the altered regulation of integrin and TGFβ1 signaling in sdc-1 null keratinocytes (3,17), we hypothesized that sdc-1 null mice would show altered susceptibility to skin cancer. Here we report that human skin cancers lose sdc-1 and confirm that similar events occur in the mouse using 2-step chemical carcinogenesis protocols. The loss of sdc-1 in mice reduces the number of papillomas forming after 2-step chemical carcinogenesis yet the malignant conversion frequency to squamous cell cancers (SCCs) is greater. Additional data are presented that assess the response of sdc-1 null skin to TPA treatment and the ability of rasHa transduced sdc-1 null keratinocytes to convert to SCCs when grafted onto the backs of nude mice. Taken together, our data add needed insight into sdc-1-integrin mediated cell signaling during skin carcinogenesis and indicate that sdc-1 is important both early in the development of skin tumors as well as in progression of skin cancers.
MATERIALS AND METHODS
Antibodies used
For immunoblots, the following antibodies were used: actin (MAB1501R; Chemicon International, Temecula, CA), LM332 (Jonathan Jones, Northwestern University, Chicago, IL), polyclonal antibody #3101S against the pSmad2 465/467 phosphorylation sites and polyclonal antibody #3102 for total Smad2 (Cell Signaling Technology Danvers, MA). The β4 and α3 antibodies used for blotting were rabbit polyclonals against cytoplasmic domain peptides (18). For immunofluorescence microscopy, the same antibodies listed above were used except for 346-11A for β4 integrin (BD Pharmingen). Other antibodies used include K8 TROMA-I (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), α5 integrin polyclonal (AB1928; Chemicon International), 281-2 for mouse sdc-1 (BD Pharmingen) and MCA2459GA (AbD Serotec.IS) for sdc-1 staining of human tissues.
Human skin tumor array
Human sdc-1 was detected in a human skin cancer and normal tissue macroarray (US Biomax, Inc. Cat# SK208) and immunostained with mouse anti-human CD138. The tissue array was baked for 2 hours at 60°C for paraffin removal and underwent 7 minutes of high heat citrate bath for antigen retrieval (Vector Laboratories). Bound antibody was detected with biotinylated antimouse secondary antibody and the Vectastain Elitekit (Vector Laboratories).
Mouse procedures and treatments
Mouse 2-stage chemical carcinogenesis and grafting experiments were performed under a protocol approved by the National Cancer Institute (NCI) and the NIH Animal Care and Use Committee. Experiments using acute and chronic TPA treatments were conducted under IACUC protocols approved by the GWUMC. The backs of age matched (8 wk old) wildtype and sdc-1 null mice were shaved and the following day TPA (#524400; phorbol-12-myristate-13 acetate, Calbiochem, SanDiego, CA), dissolved in acetone, was applied to the backs of mice at a concentration of 5 μg/200 μl.
Tumor induction experiments
The backs of age matched wildtype and sdc-1 null mice backcrossed 7 generations onto the BALB/cJ genetic background were shaved 2 d prior to initiation with a single topical application of 5 μg DMBA in 100 μl acetone. Promoter treatments with TPA [5 μg/100 μl once per week] was started 1 week after initiation and continued for 20 weeks. Mice were sacrificed at 52 weeks after initiation and skin tumors induced by the initiation protocol were bisected and either placed in tissue embedding medium for frozen section or fixed in formalin. Chemical carcinogenesis experiments were repeated twice. Tumor type [squamous papilloma or SCC] was verified by examination of H&E stained sections of each tumor.
Nude mouse grafts
Primary keratinocytes from wt or sdc-1 null mice were placed in culture and on day 3 were infected with the v-rasHa retrovirus and used for grafting on day 8 as described previously (19). After trypsinization, 2 ×106 wt or sdc-1 null ras-transduced keratinocytes were mixed with 6 ×106 BALB/c primary dermal fibroblasts cultured for 1 week, and the mixture was grafted onto the backs of nude mice on a prepared skin graft site.
v-rasHa retroviral construct
The v-rasHa replication defective ecotropic retrovirus was prepared using ψ2 producer cells as previously described (19). Retrovirus titers were routinely 1×107 virus particles per ml. Keratinocytes were infected with the v-rasHa expression vector on day 3 at a multiplicity of infection of 1 in medium containing 4 μg/ml Polybrene (Sigma Aldrich). For time lapse and immunoblot experiments studying cell migration, v-rasHa transduced keratinocytes were used on day 5.
MPO activity assay
8 mm skin punch biopsies were obtained from skin after treatment with acetone and/or TPA and stored frozen at −80°C. MPO activity was assessed as described in Cataisson and colleagues (20). No fewer than 5 mice were used per time point for studies involving MPO analyses.
BrdU cell proliferation analyses
For BrdU analyses after TPA treatment of mouse back skin, mice were injected with 25 μl per gm of body weight with a 1 mg/ml solution of BrdU 2 hrs prior to sacrifice. BrdU positive cells were determined in skin sections that had been fixed in 100% MeOH, paraffin embedded, sectioned, and subjected to staining using the in situ BrdU labeling kit fluorescence (Roche, # 11 296 736 001) as per the manufacturers instructions.
Cellular senescence assay
For the measurement of senescence, we used the Senescence β-Galactosidase Staining Kit (#9860; Cell Signaling Technologies) with the following modifications: To increase the intensity of the β-gal staining, fixed, stained cells were left in a dry 37°C oven for 48 h. Three independent sets of wt and sdc-1 null mock and rasHa-transduced keratinocytes were assayed and the number of total cells and the % of blue cells per field in 3 separate fields per well with 3 wells were counted per experimental variable.
Primary mouse keratinocyte cell culture
Wild type (wt) mice were obtained from NCI-Frederick (Frederick, MD). Tissue culture media, stocks, and buffers were obtained from Gibco/Invitrogen (Carlesbad, CA) unless otherwise indicated. Construction of sdc-1 deficient mice was described previously (18); mice have been backcrossed into a BALB/c genetic background (22,23). Primary mouse keratinocytes were grown as described (3). For studies of pSmad2, 500 pg/ml TGFβ1 was added per well and cells were extracted and used for determination of pSmad2 levels as described (21).
Immunoblotting and immunofluorescence
Wt or sdc-1 null keratinocytes were cultured for 3 days, ras transfected at day 3, and extracted for immunoblotting at day 5. For immunofluorescence microscopy, unfixed frozen sections were stained as described previously (17) except that TBST was substituted for PBS in the blocking buffers and for all antibody dilutions. Images were taken on a Nikon Eclipse 600 fluorescent microscope using a SPOT RT Slider cooled CCD camera (Diagnostic Instruments, Inc) and processed using Adobe Photoshop 7.0. No-primary antibody controls were included in each immunofluorescence experiment to assure specificity of antibody staining.
Statistics
Data were subjected to statistical analysis using Graphpad Instat software. ANOVA was used unless otherwise indicated in the text. For the data on skin painting studies, a Chi-squared analysis was used and for the grafting experiments, data were subjected to the Mann-Whitney test. Data were deemed to be statistically significant if the p values were < 0.05.
RESULTS
Sdc-1 is lost in human skin cancers
We used a human skin cancer tissue macroarray to determine the expression of sdc-1 in a series of phenotypically different non-melanoma cancers. Presented in Figure 1A are representative images showing sdc-1 in normal skin and in tumors identified as squamous cell carcinoma (SCC), basal cell carcinoma (BCC), and metastatic adenocarcinoma. In normal human skin, sdc-1 is abundant in the stratum spinosum and granulosum, less abundant in the basal cell layer, and absent in the stratum corneum. Sections from 75 individual human skin tumors or normal skin were assessed for sdc-1 staining based on a scale of 0 to 3 (3 being the maximum level observed in normal skin). Sdc-1 was reduced or absent in SCCs, BCCs, and metastatic adenocarcinomas as quantified in Figure 1B showing the mean value for all tumors analyzed of a particular phenotype.
Figure 1. Sdc-1 is reduced in human skin tumors of several phenotypes.
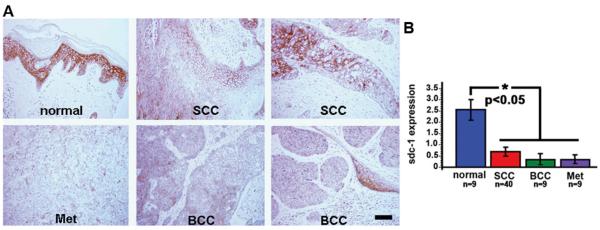
A human tumor array was used to assess the expression of sdc-1 in human cancers. A. Shown are representative IHC images showing sdc-1 in normal skin and in tumors identified as SCC, BCC, and metastatic adenocarcinomas. B. Values assigned to sdc-1 positive tumors were averaged for the human tumor macroarray. Sdc-1 is significantly reduced in SCCs, basal cell carcinomas, and metastatic adenocarcinomas. Bar = 60 μm.
Sdc-1 null mice develop fewer papillomas after chemical carcinogenesis but the frequency of conversion of papillomas to SCCs is higher
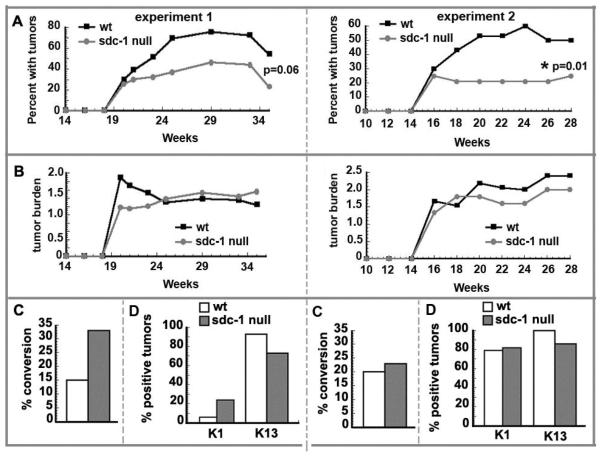
To determine how sdc-1 might influence tumor formation, we used a 2-step carcinogenesis protocol with DMBA as initiator and TPA as promoter. Given the delayed skin wound healing phenotype in sdc-1 null mice (18), care was taken to use low concentrations of DMBA and TPA to prevent the development of overt skin lesions in sdc-1 null mice during long term promotion with TPA. No such lesions developed. Figure 2A and B show the tumor frequency and burden data respectively from two independent experiments. The tumor incidence in the sdc-1 null mice is reduced to approximately 50% that of the wt mice in both experiments. While this reduction was not quite statistically significant for experiment 1 (p=0.06), for experiment 2, the number of mice used was increased and the results were highly significant (p=0.01). The tumor burdens are similar for both genotypes and range between 1 and 2 tumors per mouse.
Figure 2. Sdc-1 null mice develop fewer tumors than wildtype mice after chemical carcinogenesis.
Age matched (8 wk old) mice of sdc-1 null and wildtype genotypes were used for skin painting studies. A. The numbers of mice with tumors and the total numbers of tumors generated for both genotypes of mice were determined and the percentages of mice with tumors shown for 2 independent experiments. Experiment 1 utilized 30 wt and 23 sdc-1 null mice; experiment 2 utilized 33 wt and 43 sdc-1 null mice. Statistical significance was determined by the Chi-square test and found to be not quite significant at p=0.06 for experiment 1 and highly significant for experiment 2 at p=0.01.B. The tumor burden per mouse was determined by dividing the total numbers of tumors formed by the number of mice with tumors. C. The percentage of tumors that converted from papilloma to either CIS or SCC was determined by dividing the number of CIS carcinoma in situ/SCC tumors as determined by histopathology by the total number of tumors; too few SCCs were generated and results were not significant D. The percentage of tumors that were positive for either K1 or K13 was determined by immunohistochemistry.
In this model on a BALB/c genetic background, tumors develop as benign papillomas with generally low risk to progress to malignancy (22). The conversion frequency is then calculated on the number of carcinomas developing from the total tumor burden. Data presented in Figure 2C show that for sdc-1 null mice for experiment 1, there was a 2-fold increase whereas experiment 2 showed a slight increase in the % conversion which was not significant. Tumors (or squamous papilloma only) were also stained for Keratin1 and Keratin13, markers of benign or progessing papillomas respectively (Figure 2D), but these showed no difference among the genotypes.
Sdc-1 is lost from wildtype papillomas as they convert to SCC
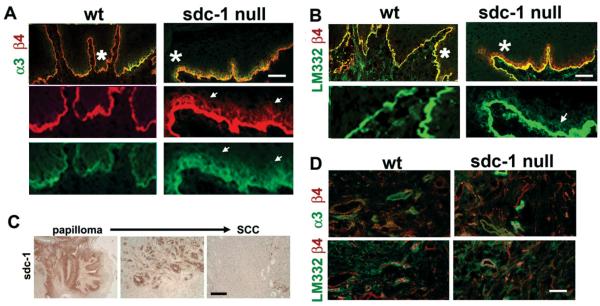
Figure 3A and B show that sdc-1 is expressed on the suprabasal cells of the papillomas that form from wt mice in regions that do not overlap with those that express α3 or β4 integrin or LM332. The localization of both α3 and β4 is enhanced within the suprabasal cells present above the LM332-rich basement membrane in the absence of sdc-1. In wt SCCs (Figure 3C and D), sdc-1 is lost but α3 and β4 integrins retained while these integrins are reduced in SCC from sdc-1 null mice. The expression of α5 integrin was increased in wt SCCs as was K8, a marker upregulated in mouse and human SCC. K8 was also seen in sdc-1 null SCC.
Figure 3. Integrin and laminin localization is altered in sdc-1 papillomas.
Unfixed frozen sections from wt and sdc-1 null mouse tumors were evaluated by immunoflourescence microscopy. Images from serial frozen sections of representative papillomas are presented in A and B whereas C and D show representative wt and sdc-1 null SCCs. The asterisks in the merged images in A and B indicate the region shown below after 3-fold magnification. Bars in A–D = 60 μm.
Chronic TPA treatment promotes cell proliferation, inflammation, and TGFβ1 accumulation in wt and sdc-1 null skin
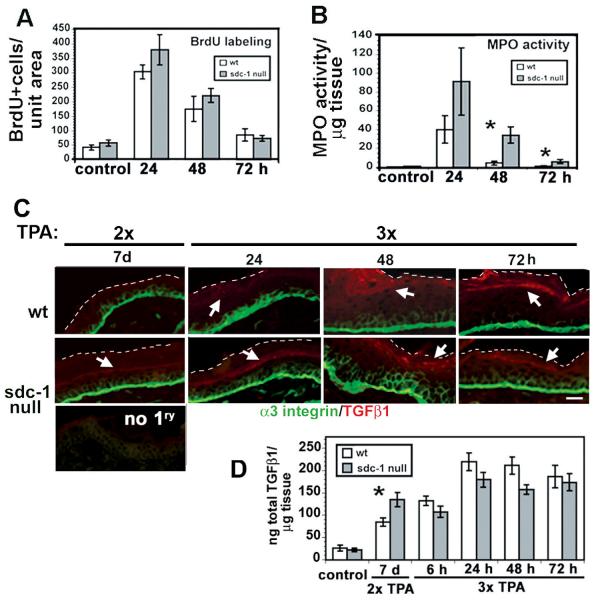
To determine the comparative response of sdc-1 null mouse skin to TPA treatment, we exposed age-matched wildtype and sdc-1 mice to a single or 3 weekly TPA treatments. Since data for cell proliferation and MPO-activity were similar after both single and multiple TPA treatments, results are presented in Figure 4 only for multiple TPA treatment studies. As shown in Figure 4A, BrdU incorporation into the interfollicular epidermis of wt and sdc-1 null skin was similar after multiple TPA treatments. To assess whether TPA induced inflammation in sdc-1 null skin, MPO activity was measured in extracts from skin biopsies taken from TPA-treated mice. MPO activity was higher in sdc-1 null mice at all time points assessed and was statistically significant at 48 hr and 72 hr after treatment (Figure 4B).
Figure 4. Response of wildtype and sdc-1 mice after 3 weekly treatments to topical TPA.
Shown are 3 timepoints after the last application of TPA. A. BrdU incorporation (2 hours IP), n=6. B. MPO activity in 8mm skin punch biopsies, n=6 C. Unfixed frozen sections were used to localize α3 integrin and TGFβ1. White dashed white lines demarcate the apical margin of the epidermis and arrows indicate TGFβ1 localization within the epidermis. D. Total epidermal and dermal TGFβ1 levels determined by ELISA assay at 3 timepoints after the third TPA treatment or 7 days after the second TPA treatment, n=6. Bar in C = 15 μm.
TGFβ1 reporter assays and pSmad2 immunoblot studies have shown that sdc-1 null keratinocytes produced more TGFβ1 in culture and that null cells had high endogenous levels of secreted TGFβ1 and elevated TGFβ1 signaling (3). Figure 4C shows enhanced suprabasal cell localization of α3 integrin in both wt and sdc-1 null skin 48 h after a 3rd TPA application; however, suprabasal α3 integrin is more persistent in sdc-1 null skin, remaining elevated for up to 72 h. TGFβ1 is increased in the more differentiated cell layers of sdc-1 null skin seven days after the second and prior to the third application of TPA and increased in both wt and sdc-1 null skin within 48 h of the 3rd TPA application and was detected in the dermis of null mouse skin at 72 h. While quantitation of TGFβ1 in total skin extracts failed to show a difference between the genotypes within 72 h of the last TPA exposure, the growth factor had persisted in the skin of sdc-1 null mice for a considerable time, here shown for 7 days after the second application of TPA (Figure 4D).
In vivo grafts of rasHa transduced sdc-1 null keratinocytes convert to SCC with high frequency
To confirm and extend the skin painting data, wt and sdc-1 null keratinocytes were transduced with an oncogenic rasHa retrovirus and grafted onto the backs of nude mice along with primary wt BALB/c fibroblasts. By this method approximately 75–85% of engraftments developed into papillomas. Data from three separate grafting experiments are summarized in Table 1 and reveal that 36% of tumors derived from sdc-1 null keratinocytes converted to SCC compared to 19% of wildtype tumors, a difference which was not quite statistically significant. Immunostaining revealed that tumors generated using sdc-1 null cells displayed extended suprabasal expression of both α3 and β4 integrins and an expanded basement membrane zone (BMZ) compared to papillomas generated from wt cells (Figure 5A); localization of LM332 confirmed that the BMZ in the sdc-1 null papillomas was thicker (Figure 5B). Using IHC, we confirmed that sdc-1 was lost as papillomas formed from grafted wt ras-activated keratinocytes progressed to SCCs (Figure 5C). SCCs derived from both wt and sdc-1 null ras-transduced keratinocytes showed downregulation of epithelial integrins within tumor cells (Figure 5D) but staining was retained in blood vessels.
Table 1.
Tumorigenicity of sdc-1 deficient keratinocytes expressing oncogenic ras in nude mouse skin grafts.
| Experiment | Genotype rasHA-keratinocytes | Genotype dermal fibroblasts | # of mice | Tumor incidence | % papilloma* | % carcinoma* |
|---|---|---|---|---|---|---|
| 1 | wt | wt | 6 | 5 | 80% | 20% |
|
|
||||||
| sdc-1 null | wt | 7 | 6 | 83% | 17% | |
|
| ||||||
| 2 | wt | wt | 7 | 6 | 83% | 17% |
|
|
||||||
| sdc-1 null | wt | 10 | 9 | 56% | 44% | |
|
| ||||||
| 3 | wt | wt | 7 | 6 | 83% | 17% |
|
|
||||||
| sdc-1 null | wt | 14 | 10 | 60% | 40% | |
|
| ||||||
| total: | wt | wt | 20 | 17 | 82% | 18% |
|
|
||||||
| sdc-1 null | wt | 34 | 25 | 64% | 36% | |
Data were not significant by the Chi-squared or Fisher's Exact tests (p=0.3).
Figure 5. Integrin and laminin localization in tumors developing from grafts of oncogenic ras transduced sdc-1 null and wildtype keratinocytes.
Images from serial frozen sections of representative tumors from nude mice grafted with wt or sdc-1 null rasHa-transduced keratinocytes. Indirect immunofluorescence for α3 and β4 integrins (A) and LN332 (B) are presented. Asterisks indicate areas shown below after 3-fold magnification and arrows indicate suprabasal localization in sdc-1 null tumors. C. IHC showing the loss of sdc-1 in tumors from grafted rasHa transduced wildtype keratinocytes as they progress from benign to malignant D. Typical wt and sdc-1 null SCCs from nude mouse grafts stained for α3 and β4 integrins or LM332 and β4 integrin Bar in A and B = 60 μm, Bar in C = 100 μm and the Bar in D = 15 μm.
rasHa-transduced primary sdc-1 null keratinocytes differ from their wildtype counterparts in vitro
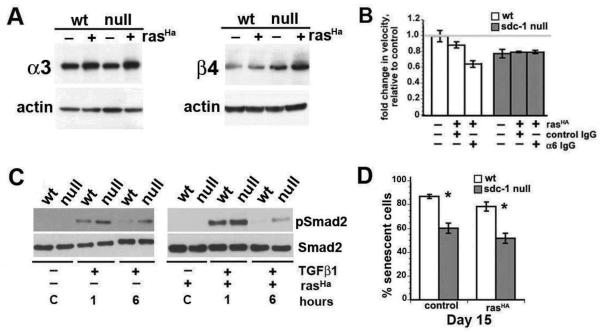
To examine the impact of rasHa activation in vitro, we assessed integrin expression by immunoblots in normal and ras-transduced keratinocytes. As detected in tumors, total α3 and β4 integrin expression are elevated in wt and sdc-1 null keratinocytes by ras transformation with higher expression detected in sdc-1null keratinocytes (Figure 6A). Previously, we reported in time lapse studies that sdc-1 null cells were more adhesive, less migratory, and increased their migration to wt levels upon treatment with GoH3 (α6IgG), an α6 integrin function blocker (3). Figure 6B indicates that ras-activated sdc-1 null cells are less migratory compared to wt cells and ras activation renders them resistant to the effect of the α6 integrin function blocker. In contrast, the α6 integrin function blocker reduces migration of ras-transduced wt cells. Thus, ras-transduction of wildtype keratinocytes converts α6β4 from an integrin that reduces cell migration into one that enhances cell migration whereas α6β4 functions are not altered in ras transduced sdc-1 null keratinocytes.
Figure 6. Comparative analysis of cultured rasHa-transduced primary sdc-1 null and wildtype keratinocytes.
Isolated primary keratinocytes from both genotypes were cultured in 0.05 mM Ca2+-medium to select for basal cells, mock or rasHa transduced on day 3, and analyzed 5 days later. A. Immunoblots of integrin expression before and after rasHa transduction. B. Time lapse studies to quantify relative migration in the presence or absence of functional α6 integrin using a blocking antibody. C. Immunoblot for pSmad2 and total Smad2 in the presence or absence of TGFβ1 for the indicated time. D. Senescence associated β-galactosidase positive cells were counted as a function of time. Shown are results for wt and sdc-1 null mock and rasHa-transduced primary keratinocytes at day 15 in culture.
Since TGFβ1 upregulates integrin expression in keratinocytes (3), we assessed TGFβ1 activity by looking at pSmad2 expression in serum starved, TGFβ1-treated, ras-transduced primary wt and sdc-1 null keratinocytes. Data presented in Figure 6C show that pSmad2 is increased 1 and 6 h after TGFβ1 treatment in keratinocytes from both genotypes but the levels are higher and sustained longer in sdc-1 null keratinocytes whether or not they are expressing oncogenic ras.
Elevated TGFβ1 expression and activation in keratinocytes are known to have a cytostatic impact reducing cell proliferation and inducing senescence; yet, the trend was for sdc-1 null ras-transduced cells to convert more readily to SCC. To assess whether the ablation of sdc-1 altered senescence of keratinocytes, we measured the numbers of senescence associated β-galactosidase positive cells. Both constitutive and ras-transduced sdc-1 null keratinocytes are significantly more resistant to senescence than wt cells (Figure 6D).
DISCUSSION
Although sdc-1 null mice do not demonstrate a constitutive skin phenotype, the importance of sdc-1 in cutaneous homeostasis has been clearly shown by experimental studies in mice. In vivo, skin wound healing is substantially delayed by the absence of sdc-1 (17). Further, cultured sdc-1 null keratinocytes have reduced migration rates, enhanced matrix adhesion, altered expression of cell surface integrins and elevated TGFβ1 expression and signaling (3) when compared to wildtype keratinocytes of the same genetic background. We now show that sdc-1 contributes to the development and progression of skin tumors both in mice and man. Previous reports of sdc-1 expression in human skin tumors have suggested a correlation between loss of the proteoglycan with increasing invasiveness (23–25). Remarkably, the loss is detected in both BCC and SCC, tumor types with a markedly different molecular pathogenesis, suggesting that sdc-1 plays a general role in suppressing cancer development. This is not likely to simply represent a relation to proliferation rate, as sdc-1 is not lost in hyperproliferative epidermis after TPA treatment of wildtype mouse skin (not shown). Although more study is needed, particularly of premalignant skin lesions, sdc-1 could serve as a marker for malignant progression in human skin cancer.
The results presented here show that sdc-1 loss has a biphasic influence on experimental skin cancer development, reducing the frequency of benign tumors but enhancing malignant conversion of the benign tumors that erupt after initiation and promotion. Enhanced malignant conversion was also seen after grafting of rasHatransduced keratinocytes onto nude mice. Similar results from experimental models involving distinct mechanisms of initiation suggest that sdc-1 does not act on the earliest mutagenic events in cancer induction. This is consistent with previous work indicating that the metabolism of DMBA by mouse liver from sdc-1 null and wildtype mice are equivalent (26). The limited number of studies on tumor induction in sdc-1 null mice have yielded mixed results (26, 27), likely due to differences in the background mouse strain or agex of tumor induction. For example, DMBA exposure of perinatal sdc-1 null BALB/c mice resulted in a reduced tumor incidence in several organ sites that was not reproduced with DMBA exposure to adult mice (26). This was interpreted to suggest that genetic ablation of sdc-1 in a multipotential tumor precursor cell type may have an influence on tumor outcome. Such an idea could also underlie the common influence of sdc-1 loss in disparate tumor types such as BCC and SCC.
α6 integrin can form heterodimers with either β1 or β4 integrin. In epithelial tissues and carcinomas, α6 has been shown to complex with β4 integrin (28, 29); no convincing evidence has been presented for the existence of α6β1 integrin heterodimers in skin carcinomas (29) although α6β1 has been convincingly shown to be present in prostate (30) and breast (31) cancers. A study by Witkowski and colleagues used mouse cell lines derived from papillomas and SCCs to look for the presence of α6β1 heterodimers in cells derived from skin tumors using surface biotinylation and found only α6β4 heterodimers (32). Whether α6β1 is expressed in vivo in advanced skin SCCs remains to be determined.
As far as we can tell, our study is the first to test the contribution of sdc-1 in tumor formation in a multistep model involving initiation and promotion. Given equivalent initiation, the reduced papilloma yield would suggest that sdc-1 contributes to tumor promotion. Sdc-1 is known to enhance tumor angiogenesis (10) and our studies suggest that in the absence of sdc-1, neutrophilic infiltrate and cutaneous TGFβ increase relative to wildtype skin after TPA application. Together these changes would produce an unfavorable promoting environment and contribute to the reduced papilloma yield.
Examination of sdc-1 null papillomas and rasHa transduced keratinocytes in vitro has given some insight concerning potential mechanisms underlying their greater propensity to undergo malignant conversion. In vitro, transformed sdc-1 null keratinocytes are more resistant to senescence than their transformed wildtype counterparts and this property is linked to malignant progression in multiple cell types (33). Both in vitro and in vivo, ras-transduced sdc-1 null keratinocytes or carcinogen-induced or grafted papillomas display increased expression and suprabasal localization of β4 integrin. This has been linked previously with high risk of premalignant progression and malignant conversion in mouse skin studies (5, 6, 34). Direct evidence of this linkage was provided by Owens and colleagues (34) who transgenically targeted α6β4 integrin to suprabasal epidermis, thereby increasing malignant conversion of initiation/promotion induced skin tumors. Furthermore these studies indicate that disruption of TGFβ signaling contributes to these biological results. Earlier studies had indicated that increased expression of TGFβ1 targeted to the epidermis of transgenic mice subjected to chemical carcinogenesis reduced the yield of papillomas but enhanced malignant conversion (35). The similarity of this result to our study in sdc-1 null mice suggests that the increase in TGFβ signaling observed after oncogenic ras transduction in sdc-1 null keratinocytes could also contribute to the higher conversion frequency. Together these results suggest several pathways associated with sdc-1 loss that require further exploration. Even in the absence of full understanding of the mechanisms involved, loss of sdc-1 now serves as a marker to monitor progression in the major non-melanoma human skin cancers.
Acknowledgements
We want to thank Adam Glick, Mariam Malik, Jonathan Jones, and Luowei Li for advice and assistance at various stages of this project as well as to Aleksandra Michalowski for help with statistics. This work was initiated during a sabbatical from GWU Medical School for MAS and was supported in part by the intramural research program of the NIH, National Cancer Institute, Center for Cancer Research. In addition, this work was funded by NIH RO1-EY08512 and EY13559 (MAS).
Abbreviations
- BCC
basal cell carcinoma
- BMZ
basement membrane zone
- BrdU
bromodexyuridine
- DMBA
7,12-Dimethylbenz(a)anthracene
- LM332
laminin molecule composed of the Lm-α2, Lm-β3, and Lm-γ2 chains; previously called laminin 5
- MMP
Matrix metaloproteinases
- SCC
squamous cell carcinoma
- Sdc-1
Syndecan-1
- Smad2
a regulatory protein that controls TGFβ signaling; SMAD proteins are homologs of both the Caenorhabditis elegans protein SMA protein and the drosophila protein, mothers against decapentaplegic (MAD)
- TBST
Tris-buffered saline-Tween-20 buffer
- TGFβ
Transforming growth factor β
- TPA
Phorbol-12-myristate-13-acetate
- v-rasHa
transforming gene originally isolated from Harvey murine sarcoma virus
- wt
wildtype
REFERENCES
- 1).Fears CY, Woods A. The role of syndecans in disease and wound healing. Matrix Biol. 2006;25:443–456. doi: 10.1016/j.matbio.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 2).Marinkovich MP. Tumour microenvironment: laminin 332 in squamous-cell carcinoma. Nat Rev Cancer. 2007;7:370–380. doi: 10.1038/nrc2089. [DOI] [PubMed] [Google Scholar]
- 3).Stepp MA, Liu Y, Pal-Ghosh S, Jurjus RA, Tadvalkar G, Sekaran A, Losicco K, Jiang L, Larsen M, Li L, Yuspa SH. Reduced migration, altered matrix and enhanced TGFβ1 signaling are signatures of mouse keratinocytes lacking Sdc1. J Cell Sci. 2007;120:2851–2863. doi: 10.1242/jcs.03480. [DOI] [PubMed] [Google Scholar]
- 4).Jurjus RA, Liu Y, Pal-Ghosh S, Tadvalkar G, Stepp MA. Primary dermal fibroblasts derived from sdc-1 deficient mice migrate faster and have altered αv integrin function. Wound Repair Regen. 2008;16:649–660. doi: 10.1111/j.1524-475X.2008.00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Tennenbaum T, Yuspa SH, Grover A, Castronovo V, Sobel ME, Yamada Y, De Luca LM. Extracellular matrix receptors and mouse skin carcinogenesis: altered expression linked to appearance of early markers of tumor progression. Cancer Res. 1992;52:2966–2976. [PubMed] [Google Scholar]
- 6).Tennenbaum T, Belanger AJ, Quaranta V, Yuspa SH. Differential regulation of integrins and extracellular matrix binding in epidermal differentiation and squamous tumor progression. J Investig Dermatol Symp Proc. 1996;1:157–161. [PubMed] [Google Scholar]
- 7).Ortiz-Urda S, Garcia J, Green CL, Chen L, Lin Q, Veitch DP, Sakai LY, Lee H, Marinkovich MP, Khavari PA. Type VII collagen is required for Ras-driven human epidermal tumorigenesis. Science. 2005;307:1773–1776. doi: 10.1126/science.1106209. [DOI] [PubMed] [Google Scholar]
- 8).Bachy S, Letourneur F, Rousselle P. Syndecan-1 interaction with the LG4/5 domain in laminin-332 is essential for keratinocyte migration. J Cell Physiol. 2008;214:238–249. doi: 10.1002/jcp.21184. [DOI] [PubMed] [Google Scholar]
- 9).Ogawa T, Tsubota Y, Hashimoto J, Kariya Y, Miyazaki K. The short arm of laminin γ2 chain of laminin-5 (laminin-332) binds syndecan-1 and regulates cellular adhesion and migration by suppressing phosphorylation of integrin β4 chain. Mol Biol Cell. 2007;18:1621–1633. doi: 10.1091/mbc.E06-09-0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Beauvais DM, Ell BJ, McWhorter AR, Rapraeger AC. Syndecan-1 regulates αvβ3 and αvβ5 integrin activation during angiogenesis and is blocked by synstatin, a novel peptide inhibitor. J Exp Med. 2009;206:691–705. doi: 10.1084/jem.20081278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. The integrin αvβ8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-β1. J Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Glick AB. TGFβ1, back to the future: revisiting its role as a transforming growth factor. Cancer Biol Ther. 2004;3:276–283. doi: 10.4161/cbt.3.3.849. [DOI] [PubMed] [Google Scholar]
- 13).Wakefield LM, Stuelten C. Keeping order in the neighborhood: new roles for TGFβ in maintaining epithelial homeostasis. Cancer Cell. 2007;12:293–295. doi: 10.1016/j.ccr.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 14).Bae DS, Blazanin N, Licata M, Lee J, Glick AB. Tumor suppressor and oncogene actions of TGFβ1 occur early in skin carcinogenesis and are mediated by Smad3. Mol Carcinog. 2009;48:441–453. doi: 10.1002/mc.20482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Padua D, Massagué J. Roles of TGFβ in metastasis. Cell Res. 2009;19:89–102. doi: 10.1038/cr.2008.316. [DOI] [PubMed] [Google Scholar]
- 16).Hayashida K, Johnston DR, Goldberger O, Park PW. Syndecan-1 expression in epithelial cells is induced by transforming growth factor beta through a PKA-dependent pathway. J Biol Chem. 2006;281:24365–24374. doi: 10.1074/jbc.M509320200. [DOI] [PubMed] [Google Scholar]
- 17).Stepp MA, Gibson HE, Gala PH, Iglesia DD, Pajoohesh-Ganji A, Pal-Ghosh S, Brown M, Aquino C, Schwartz AM, Goldberger O, Hinkes MT, Bernfield M. Defects in keratinocyte activation during wound healing in the syndecan-1-deficient mouse. J Cell Sci. 2002;115:4517–4531. doi: 10.1242/jcs.00128. [DOI] [PubMed] [Google Scholar]
- 18).Sta. Iglesia DD, Gala PH, Qiu T, Stepp MA. Integrin expression during epithelial migration and restratification in the tenascin-C-deficient mouse cornea. J Histochem Cytochem. 2000;48:363–376. doi: 10.1177/002215540004800306. [DOI] [PubMed] [Google Scholar]
- 19).Lichti U, Anders J, Yuspa SH. Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nat Protoc. 2008;3:799–810. doi: 10.1038/nprot.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Cataisson C, Pearson AJ, Torgerson S, Nedospasov SA, Yuspa SH. Protein kinase C α-mediated chemotaxis of neutrophils requires NF-kappa B activity but is independent of TNF alpha signaling in mouse skin in vivo. J Immunol. 2005;174:1686–1692. doi: 10.4049/jimmunol.174.3.1686. [DOI] [PubMed] [Google Scholar]
- 21).Shukla A, Malik M, Cataisson C, Ho Y, Friesen T, Suh KS, Yuspa SH. TGF-β signalling is regulated by Schnurri-2-dependent nuclear translocation of CLIC4 and consequent stabilization of phospho-Smad2 and 3. Nat Cell Biol. 2009;11:777–784. doi: 10.1038/ncb1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Woodworth CD, Michael E, Smith L, Vijayachandra K, Glick A, Hennings H, Yuspa SH. Strain-dependent differences in malignant conversion of mouse skin tumors is an inherent property of the epidermal keratinocyte. Carcinogenesis. 2004;25:1771–1778. doi: 10.1093/carcin/bgh170. [DOI] [PubMed] [Google Scholar]
- 23).Bayer-Garner IB, Sanderson RD, Smoller BR. Syndecan-1 expression is diminished in acantholytic cutaneous squamous cell carcinoma. J Cutan Pathol. 1999;26:386–390. doi: 10.1111/j.1600-0560.1999.tb01862.x. [DOI] [PubMed] [Google Scholar]
- 24).Bayer-Garner I, Dilday B, Sanderson R, Smoller B. Acantholysis and spongiosis are associated with loss of syndecan-1 expression. J Cutan Pathol. 2001;28:135–139. doi: 10.1034/j.1600-0560.2001.028003135.x. [DOI] [PubMed] [Google Scholar]
- 25).Bayer-Garner IB, Dilday B, Sanderson RD, Smoller BR. Syndecan-1 expression is decreased with increasing aggressiveness of basal cell carcinoma. Am J Dermatopathol. 2000;22:119–122. doi: 10.1097/00000372-200004000-00005. [DOI] [PubMed] [Google Scholar]
- 26).McDermott SP, Ranheim EA, Leatherberry VS, Khwaja SS, Klos KS, Alexander CM. Juvenile syndecan-1 null mice are protected from carcinogen-induced tumor development. Oncogene. 2007;26:1407–1416. doi: 10.1038/sj.onc.1209930. [DOI] [PubMed] [Google Scholar]
- 27).Alexander CM, Reichsman F, Hinkes MT, Lincecum J, Becker KA, Cumberledge S, Bernfield M. Syndecan-1 is required for Wnt-1-induced mammary tumorigenesis in mice. Nat Genet. 2000;25:329–332. doi: 10.1038/77108. [DOI] [PubMed] [Google Scholar]
- 28).Hemler ME, Crouse C, Sonnenberg A. Association of the VLA α6 subunit with a novel protein. A possible alternative to the common VLA β1 subunit on certain cell lines. J Biol Chem. 1989;264:6529–6535. [PubMed] [Google Scholar]
- 29).Tennenbaum T, Weiner AK, Belanger AJ, Glick AB, Hennings H, Yuspa SH. The suprabasal expression of α6β4 integrin is associated with a high risk for malignant progression in mouse skin carcinogenesis. Cancer Res. 1993;53:4803–4810. [PubMed] [Google Scholar]
- 30).Cress AE, Rabinovitz I, Zhu W, Nagle RB. The α6β1 and α6β4 integrins in human prostate cancer progression. Cancer Metastasis Rev. 1995;14:219–228. doi: 10.1007/BF00690293. [DOI] [PubMed] [Google Scholar]
- 31).Maschler S, Wirl G, Spring H, Bredow DV, Sordat I, Beug H, Reichmann E. Tumor cell invasiveness correlates with changes in integrin expression and localization. Oncogene. 2005;24:2032–2041. doi: 10.1038/sj.onc.1208423. [DOI] [PubMed] [Google Scholar]
- 32).Witkowski CM, Bowden GT, Nagle RB, Cress AE. Altered surface expression and increased turnover of the α6β4 integrin in an undifferentiated carcinoma. Carcinogenesis. 2000;21(2):325–30. doi: 10.1093/carcin/21.2.325. [DOI] [PubMed] [Google Scholar]
- 33).Lowe SW, Cepero E, Evan G. Intrinsic tumour suppression. Nature. 2004;432:307–315. doi: 10.1038/nature03098. [DOI] [PubMed] [Google Scholar]
- 34).Owens DM, Romero MR, Gardner C, Watt FM. Suprabasal α6β4 integrin expression in epidermis results in enhanced tumourigenesis and disruption of TGFβ signalling. J Cell Sci. 2003;116:3783–3791. doi: 10.1242/jcs.00725. [DOI] [PubMed] [Google Scholar]
- 35).Cui W, Fowlis DJ, Bryson S, Duffie E, Ireland H, Balmain A, Akhurst RJ. TGFβ1 inhibits the formation of benign skin tumors, but enhances progression to invasive spindle carcinomas in transgenic mice. Cell. 1996;86:531–542. doi: 10.1016/s0092-8674(00)80127-0. [DOI] [PubMed] [Google Scholar]