Abstract
CBA mice infected with the helminthSchistosomamansonidevelop severe CD4 T cell-mediated hepaticgranulomatous inflammation against parasite eggs associated with a robust Th17 cell response.We investigated the requisites for Th17 cell development using novel CD4 T cells expressing a transgenic (Tg) TCR specific for the major Sm-p40 egg Ag, which produce IL-17 when stimulated with live schistosome eggs. Neutralization of IL-23 or blockade of the IL-1 receptor, but not IL-6 neutralization, abrogated egg-induced IL-17 secretion by Tg T cells, whereas exogenous IL-23 or IL-1β reconstituted their ability to produce IL-17 stimulated by syngeneic IL-12p40-deficient DCs. Kinetic analysis demonstrated that IL-17 production was initiated by IL-23 and amplified by IL-1β. Significantly, schistosome-infected IL-12p40-deficientor IL-1R antagonist treated CBA mice developed markedly reduced hepatic immunopathologywith a dampened egg Ag-specific IL-17 response. These results demonstrate the IL-23-IL-1-IL-17 axis to play a central role in the development of severe schistosome egg-induced immunopathology.
Keywords: Rodent, T cells, Parasitic–Helminth, Cytokines, Transgenic/Knockout mice
Introduction
Schistosomes are blood dwelling trematodehelminths responsible for causing one of the most prevalent tropical parasitic diseases in the world, schistosomiasis. During infection with the species Schistosomamansoni, adult worm pairs within the mesenteric venous plexus produce an estimated 300 parasite eggs per day of which a fraction becomes lodged in the liver, precipitating CD4 T cell-mediated granulomatous inflammation and fibrosis (1). The majority of individuals develop a mild “intestinal” form of schistosomiasis, whereas in 5–10% of infected hosts with “hepatosplenic” schistosomiasis the disease is severe and life-threatening (1). A similar disease heterogeneity is observed in an experimental murine model of schistosomiasis, where C57BL/6 (BL/6) and CBA mice develop mild and severe periovalgranulomatous inflammation, respectively(2). In BL/6 mice, an initial proinflammatory Th1-type response to schistosome egg antigens (SEA) is gradually superseded by a Th2-dominated environment marked by the rise of IL-4, IL-5, IL-10 and IL-13, whereas in CBA mice, the Th1 response persists for the duration of the infection (2). An effective Th1 to Th2 switch is critical for the modulation of immunopathology and extended survival (3).
Concomitant immunization of typically low pathology wild type (WT) BL/6 mice with SEA emulsified in CFA (SEA/CFA) results in marked exacerbation of egg-induced granulomatous lesions bearing considerable resemblance to thosenaturally occurring in CBA mice (4). Significantly, infected SEA/CFA-immunized BL/6 mice deficient in the common IL-12/IL-23 subunit IL-12p40 produced low levels of IL-17 and IFN-γ, and were completely refractory to pathology exacerbation, whereas similarly treated mice deficient in the IL-12-specific subunit IL-12p35 developed severe lesions associated with high levels of IL-17, despite the absence of IFN-γ(5). Furthermore, infected SEA/CFA-immunized mice deficient for the IL-23-specific subunit, IL-23p19,developed reduced immunopathology and failed to express IL-17 in the granulomatous lesions (6), and in vivo administration of anti-IL-17 Ab significantly reduced immunopathology in both CBA and SEA/CFA-immunized BL/6 mice (5). Taken together, these data strongly implicate the IL-23/IL-17 axis, and thus Th17 cells, as major players in the development of severe forms of schistosomiasis.
In addition to the described proinflammatorycytokine milieu, we have found that high pathology in schistosome-infected CBA mice correlates with a significant expansion of CD4 T cells recognizing the immunodominantepitope234–246 of the major Sm-p40 schistosome egg antigen (Sm-p40234–246) (7). Sm-p40 is one of the most abundant components of the schistosome egg and was found to elicit a strong Th1 polarized response in CBA (H-2k) mice (2, 8).Analysis of the I-Ak-restricted Sm-p40234–246-specific CD4 T cells revealed a clonally restricted population with a TCR expressing Vα11.3Vβ8(7), which paved the way for the development of a novel CBA mouse expressing atransgenic (Tg) TCR specific for Sm-p40234–246.
In this report, we demonstrate that naïve schistosome egg antigen-specific Tg CD4 T cells produce IL-17 and IFN-γ when stimulated with peptide Sm-p40234–246 or with live schistosome eggs, which allowed us to investigate the requirements for egg Ag-specific Th17 cell development and function in the high pathology CBA strain. We found optimal IL-17 production by Sm-p40234–246-specific TCR Tg T cells stimulated by syngeneicdendritic cells (DCs) to be dependent on IL-23, which in turn, induces IL-1β secretion in the presence of live schistosome eggs.Importantly, the centralin vivo roles of IL-23 and IL-1β were demonstrated by a significant reduction in hepatic immunopathology and in IL-17 production in infected IL-12p40-deficient CBA mice, and CBA mice in which IL-1 signaling was blocked byIL-1Ra. These results identifyIL-23 and IL-1β as critical factors in the generation of egg Ag-specific Th17 cells involved in the pathogenesis of severe schistosomiasis.
Materials and Methods
Mice, infections and treatments
Schistosome egg Ag-specific TCR Tg mice were generated following the protocol described by Waldner et al (9). Briefly, the Vα11.3 and Vβ8 TCR chains were isolated from genomic DNA purified from aSm-p40234–246-specific T cell hybridoma and cloned, respectively, into pTαCass and pTβCass expression cassettes, kindly provided to us by Dr. Diane Mathis, Harvard Medical School, Boston, MA. LinearizedpTαCass and pTβCass vectors were introduced into BL/6 (H-2b) oocysts by microinjection to produce transgenic founders, which were subsequently backcrossed for >10 generations to CBA (H-2k) mice, resulting in a TCR Tg mouse on the high pathology-prone CBA background capable of presenting the I-Ak-restricted immunodominant peptide Sm-p40234–246. Mice were genotyped for the presence of the Vα11.3Vβ8 TCR gene rearrangement using standard PCR genotyping techniques on genomic DNA obtained from tail biopsies. The primer sequences used for these PCRs were:
Vα11.3 Forward: 5' GCCTCATGAATCTTTTTTACCTGG 3'
Vα11.3 Reverse: 5' TAGCCAGGTTGAGAACCATTGCCC 3'
Vβ8 Forward: 5' CACCCAAAGCCCAAGAAACAAGGT 3'
Vβ8 Reverse: 5'ATCCTTAGACCTGGGAAATGCTCC 3'
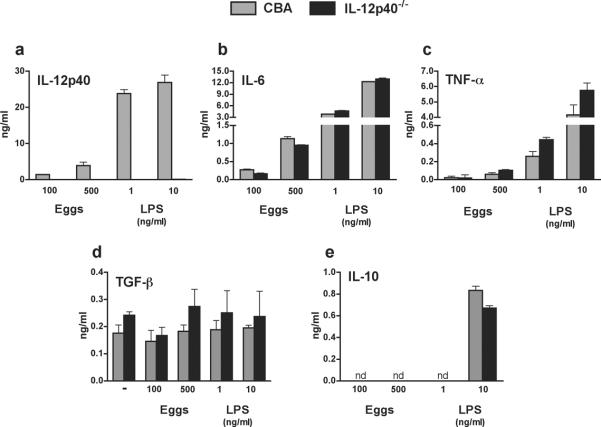
Female CBA/J (CBA), C57BL/6(BL/6) and IL-12p40-deficient B6.129S1-Il12btm1Jm/J mice were purchased from The Jackson Laboratory (Bar Harbor, ME), and Swiss Webster mice were obtained from Charles River (Wilmington, MA). To generate IL-12p40-deficient (IL-12p40−/−) mice on the CBA background, B6.129S1-Il12btm1Jm/J mice were backcrossed to CBA mice for>10 generations. Bone marrow-derived DCs (DCs) from the IL-12p40−/− mice failed to produce bioactive IL-12p40 protein following stimulation with schistosome eggs or LPS(Figure 3a).
Figure 3. Cytokine production profile of egg-stimulated WT CBA vs. CBA IL-12p40−/−DC.
DCs from WT CBA and CBA IL-12p40−/−mice (two mice per group) were cultured with eggs or control LPS for 24h, and culture supernatants were assayed for (a) IL-12p40, (b) IL-6 (c) TNF-α, (d) TGF-βand (e) IL-10 by ELISA. Cytokine levels are expressed as means of triplicate ELISA determinations ± SD; where appropriate, background cytokine levels from unstimulated controls were subtracted;nd, not detectable. Data are from one representative experiment of three.
In some experiments, age matched, female CBA, BL/6 and IL-12p40−/− CBA mice were infected by i.p. injection with 80 cercariae of S. mansoni(Puerto Rico strain), which were obtained from infected Biomphalariaglabrata snails provided to us by Dr. Fred Lewis from the Biomedical Research Institute (Rockville, MD), through NIH/NIAID contract N01AI-55270. Additionally, some mice were treated daily by i.p. injection with 300 μg of recombinant humanIL-1Ra for the last 10 days of a 7-week infection(10). Swiss Webster mice were infected in an identical fashion for the purpose of isolating schistosome eggs. All mice were maintained in the Animal Facility at Tufts University School of Medicine in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) guidelines.
Cell preparations
Bulk cell suspensions were prepared from the spleens of uninfected TCR Tg or non-Tg littermate control mice as described elsewhere (4). For some experiments, CD4 T cells were purified from the spleens of uninfected CBA, IL-12p40−/−, TCR Tgor non-Tg mice using CD4 MACS negative selection columns (MiltenyiBiotec, Auburn, CA) per manufacturer's instructions. CD4 T cell purity was >95% by FACS analysis.
DCs from uninfected CBA and IL-12p40−/− mice were derived from bone marrow using reagents and protocols described previously (11).Briefly, bone marrow cells from uninfected mice were cultured in medium containing 10% fetal calf serum (AlekenBiologicals, Nash, TX) and granulocyte-macrophage colony-stimulating factor (GM-CSF), produced by B cell hybridoma J558L. Three days after seeding, fresh GM-CSF-containing medium was added to the cultures. At day 10, non-adherent cells, which were >85% CD11c+, were harvested and used for experiments.
Characterization of the TCR Tg mice
To examine expression of the Vβ8 TCR chain, bulk splenocytes from TCR Tg and non-Tg mice were stained with FITC-conjugated anti-CD4 (GK1.5) and PE-conjugated anti-Vβ8 (MR5-2) mAbs (BD-Pharmingen, San Diego, CA) as described previously (4). Cells were acquired using a FACSCalibur flow cytometer using Cell Quest software version 3.2.1 (Becton Dickinson, Franklin Lakes, NJ) and the data were analyzed using FlowJo Flow Cytometry Analysis Software.
To verify their antigenic specificity, CD4 T cells were purified from bulk splenocyte suspensions from TCR Tg or non-Tg littermate control mice using CD4 MACS negative selection columns (MiltenyiBiotec). CD4 T cells (2 × 105) plus 2 × 105 irradiated (3000 rad) syngeneic non-Tgsplenocytes were cultured in the presence of Smp40 Ag, Sm-p40234–246 peptide, or irrelevant peptide Sm-p40304–333 for 72 h. Cultures were then pulsed with 0.5 μCi(3H) thymidine(New England Nuclear), and incubated for an additional 18h, after which incorporation was measured by scintillation spectroscopy.
Cytokine production by bulk splenocytes and CD4 T cells from TCR Tg mice was determined by ELISA. Bulk splenocytes (5 × 106) isolated from uninfected TCR Tg mice were cultured in the presence or absence of 10 μg/ml Sm-p40234–246 peptide for 72h. Additionally, CD4 T cells from TCR Tg mice (1 × 106 cells/ml) were co-cultured with syngeneic non-TgDCs (1.25 × 105 cells/ml) and stimulated with either Sm-p40 Ag or Smp40234–246 (both at 2 μg/ml) for 48 h. Culture supernatants were filtered and tested for IL-17, IFN-γ (R&D Systems, Minneapolis, MN) and IL-5 (BD Pharmingen) by ELISA.
Isolation of parasite eggs, preparation of SEA, preparation ofrSm-p40 Ag and synthesis of peptides
Schistosome eggs were isolated under sterile conditions from the livers of 7–8 week-infected Swiss Webster mice. Infected livers were blended and eggs were sifted from the tissue using a series of sieves and washes with saline solution (11). The absence of contaminating liver tissue was confirmed by microscopy. SEA was obtained as previously described (6).
The coding sequence for the Sm-p40 egg Ag was amplified from egg cDNAand subsequently cloned into a pET30 vector that contained a poly-His tag (Novagen, Darmstadt, Germany). To overexpress the Sm-p40 protein, competent E.coli Rosetta DE3 bacteria (Pierce, Rockford, IL) were transformed with the recombined vector. Cells expressing the Sm-p40 egg antigen-coding vector were inoculated into Luria Broth (LB) at a 1:50 dilution, grown for 1 h and stimulated with 1 mM IPTG for an additional 4 h. The bacterial cells were pelleted by centrifugation and solubilized protein was extractedusing B-Per reagent according to the manufacturer's instructions (Pierce, Rockford, IL). Recombinant Sm-p40 was purified by affinity chromatography using the 6X His Fusion Protein Purification Kit (Pierce). To eliminate any potential contaminating endotoxin, the Sm-p40 preparation was treated using the Endotoxin Removal Maxi Kit (NorgenBiotek, Thorold, ON, Canada) and purification was verified using the Limulus AmebocyteLysate assay (LAL, Cambrex, East Rutherford, NJ). Protein concentrations were assessed with the Bradford Protein Assay Kit (Pierce).
The 30-mer peptide (QVAVRPKSDNQIKAVPASQALVAKGVHGLS), which harbors the immunodominantepitope Sm-p40234–246 (in bold), as well as peptide Sm-p40304–333were synthesized and purified by HPLC at the Tufts University Core Facility.
In vitro co-cultures and cytokine analysis
DCs derived from CBA or IL-12p40−/− mice (1 × 106 cells/ml) were stimulated with 100 or 500 live eggs or LPS (Sigma, St. Louis, MO). After 24 h, culture supernatants were collected, sterile filtered and assayed by ELISA to measure the levels of IL-12p40, IL-10 and TGF-β using mAb, standards and protocols from BD-Pharmingen, and for IL-6 and TNF-α, using mAb, standards and protocols from R&D Systems.
DCs (1.25 × 105) plus CD4 T cells (1 ×106) purified from the spleens of uninfected TCR Tg mice were cultured in 1 ml of medium together with Sm-p40 egg Ag, Sm-p40234–246(both 2μg/ml), or 50 or 200 eggs. After 4 days, culture supernatants were removed, sterile filtered and assayed by ELISA for IL-17, IL-6 and IFN-γ using mAb, standards and protocols from R&D Systems. In some experiments, neutralizing mAb against IL-12p40, IL-6 (both 2 μg/ml, BD-Pharmingen), and IL-23p19 (10 μg/ml, eBioscience, San Diego, CA), IL-1Ra, at indicated concentrations, IL-6 (1 ng/ml), IL-23, and IL-1β (both at 0.05–0.2ng/ml, all R&D Systems) were added either individually or in combinations to the DC-CD4 T cell-egg co-cultures, as indicated.
Cell preparations and cytokine analysis from infected mice
After a 7 week infection, MLNC were isolated fromCBA, BL/6, IL-12p40−/− and CBA IL-1Ra treated mice, as previously described (4). Cell preparations were >95% viable by trypan blue exclusion. Bulk MLNC suspensions (5 × 106) were cultured in the presence or absence of 15 μg/ml of SEA for 48h, after which culture supernatants were tested for the presence of IL-17 and IFN-γ by ELISA (R&D Systems).
Real-Time Quantitative RT-PCR
Total RNA was isolated from DC-T cells co-cultures in the presence or absence of schistosome eggs using the Trizol method per manufacturer's instructions (Invitrogen, Carlsbad, CA). RNA (1–5 μg) was subjected to DNase I treatment (Roche Molecular Biochemicals, Indianapolis, IN) and reverse-transcribed using RT2 first strand kit (C-03). Real-time quantitative RT-PCR on 10 ng of cDNA from each sample was performed by Taqman analysis. All reactions were performed using an ABI 7300 instrument. GAPDH levels were measured in a separate reaction and used to normalize the data. Reagents and protocols forTaqman real-time quantitative PCR were obtained from Applied Biosystems (Foster City, CA). Using the average mean cycle threshold (Ct) value for GAPDH and the gene of interest for each sample, the equation 1.8 e (Ct GAPDH minus Ct gene of interest) × 104 was used to obtain normalized values (11).
Hepatic immunopathology
Liver tissue obtained from infected CBA, BL/6, IL-12p40−/−, and CBA IL-1Ra-treatedmice were fixed in 10% buffered formalin, processed and sectioned by routine histopathologic technique, stained with hematoxylin and eosin, and examined by optic microscopy. The size of the granulomatous lesions was measured by computer-assisted morphometric analysis (Image-ProPlus Software by Media Cybernetics, Bethesda, MD), as described elsewhere (4). For each liver, 8 to 15 granulomas with single eggs were analyzed.
Statistical Analysis
ANOVA and Student's t tests were used to determine thestatistical analysis of the differences between groups. P valuesof <0.05 were considered significant and were calculatedwith GraphPad Prism.
Results
Characterization of the Sm-p40 egg Ag-specific TCR Tg CD4 T cell response
The discovery of an expanded and remarkably restricted CD4 T cell response against the major Sm-p40 egg Ag in schistosome-infected highpathology CBA mice (7) enabled us to develop a mouse transgenicallyexpressing a Vα11.3Vβ8 TCR specific for the immunodominant peptide Sm-p40234–246. Indeed, 96% of splenic CD4 T cells from Tg mice expressed Vβ8 compared to only 9% in T cells from WT controls (Fig. 1a,b). Naïve Tgsplenocytes exhibited strong proliferative responses to Sm-p40 and peptide Smp40234–246,but not to irrelevant peptide Sm-p40304–333; neither Ag stimulated non-Tg cells (Fig. 1c,d). Strikingly, stimulation with peptide Sm-p40234–246 induced robust production of IL-17 and IFN-γ(Fig. 1e,f), but only elicited a milder IL-5 and IL-10 response (Fig. 1g,h).Sm-p40, Sm-p40234–246, or live schistosome eggs serving as a natural source of Sm-p40, elicited robust IL-17 production by TCR Tg CD4 T cells in cultures with syngeneicDCs(Fig. 1i). TCR Tg CD4 T cells also secreted IFN-γ, but no detectable IL-5 or IL-10 (data not shown), suggesting that they are biased toward a proinflammatory cytokine response. These findings demonstrate the successful development of the first mouse TCR Tg T cell system specific for a schistosome egg-or any other helminth-derived Ag.
Figure 1. TCR Tg CD4 T cells strongly respond to Sm-p40 with a proinflammatory cytokine profile.
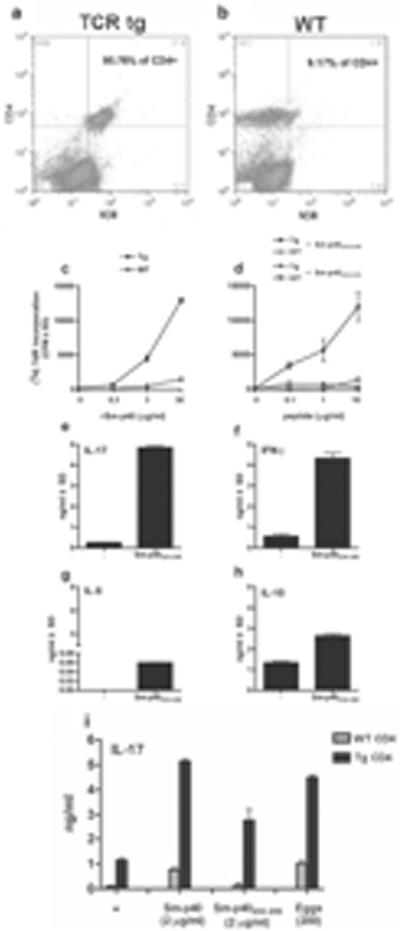
Bulk spleen cells from three to five (a) TCR Tg CBA or (b) WT mice were stained with anti-CD4 and anti-Vβ8 mAbs and analyzed by flow cytometry. SplenicCD4 T cells from TCR Tg and WT CBA were stimulated with (c) Sm-p40 egg Ag, or (d) peptides thereof, in the presence of irradiated syngeneic WT splenocytes for 72h. Cells were pulsed with (3H) thymidine for the last 18h of culture and incorporation was measured by scintillation spectroscopy. Bulk spleen cells from TCR Tg or WT CBA were cultured for 72h in the presence or absence of 2μg/ml of peptide Sm-p40234–246, and (e) IL-17, (f) IFN-γ,(g) IL-5and (h) IL-10levels in culture supernatants were measured by ELISA. (i) DCs from WT CBA mice plus TCR Tg or WT CBA CD4 T cells were stimulated with Sm-p40 Ag, peptide Sm-p40234–246, or 200 liveschistosome eggs for 48h, and IL-17 levels in culture supernatants were measured by ELISA. Cytokine levels are expressed as means of triplicate ELISA determinations ± SD. Data are from one representative experiment of three.
IL-23 and IL-1β are required for IL-17 production by schistosome egg Ag-specific TCR Tg CD4 T cells
Although initially shown to depend on IL-23, the differentiation of the Th17 lineage was subsequently demonstrated in various experimental systems to depend on IL-6 and TGF-β, as well as IL-1β and IL-21 (12). We previously demonstrated that schistosome egg-stimulated CBA, but not BL/6 DCs, induce T cell IL-17 production following non-specific TCR engagement via IL-23 and IL-1β(11). The development of Sm-p40-specific TCR Tg mice enabled us to analyze the differentiation and effector functions of schistosome egg antigen-specific T cells. When these TCR Tg CD4 T cells were co-cultured with DCs and live schistosome eggs, they produced significant amounts of IL-17. Abs against IL-12-p40 and IL-23p19 significantly inhibited IL-17 production; IL-1Ra was equally effective (Fig. 2).In contrast, anti-IL-6 Ab, used at a concentration capable of neutralizing 10ngof recombinant IL-6, which far exceeds the amount naturally produced by egg-stimulated CBA DCs(Fig. 3b and data not shown), failed to significantly affect IL-17 production (Fig 2).
Figure 2. Neutralization of IL-23 or IL-1receptor blockade inhibits egg-induced IL-17 production by TCR Tg CD4 T cells.
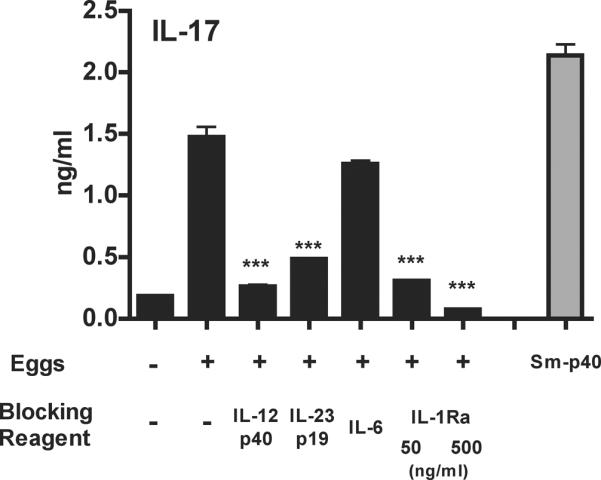
WT CBADCs (from two mice) and TCR Tg CD4 T cells (from five mice) were cultured in the presence or absence of 200 eggs together with the indicated blocking reagents used at the following concentrations: anti-IL-12p40 (2 μg/ml), anti-IL-23p19 (10 μg/ml), anti-IL-6 (2 μg/ml) and IL-1Ra (50 or 500 ng/ml). IL-17 levels in 48h supernatants were measured by ELISA. Cytokine levels are expressed as means of triplicate ELISA determinations ± SD. ***, p<0.001 compared to egg-stimulated cultures without blocking reagent. Data are from one representative experiment of three. Results from a culture stimulated with 2 μg/ml Sm-p40 are shown for comparison.
To further examine the cytokine requirements for Th17 cell differentiation, we made use of DCs from novel IL-12p40-deficient (IL-12p40−/−) mice, which we developed by backcrossing IL-12p40−/− BL/6 mice to the CBA background for >10 generations. DCs from the IL-12p40−/− CBA mice failed to produce detectable levels of IL-12p40 upon stimulation with either eggs or LPS (Fig. 3a), while their IL-6 (Fig. 3b) and TNF-α(Fig. 3c) responses were similar to those of WT controls. Production of theimmunomodulatory cytokine TGF-β was similar between unstimulatedWT CBA and IL-12p40−/−DCs, but was not further enhanced upon stimulation with eggs or LPS (Fig. 3d), and both DC populations demonstrated similar IL-10 responses following stimulation with LPS, but not in the presence of liveeggs (Fig. 3e).Egg-stimulated DCs from IL-12p40−/− mice induced significantly less IL-17 secretion by TCR Tg CD4 T cells (Fig. 4a) and also elicited a reduced IFN-γ response(Fig. 4b). However, egg-stimulated IL-6 production wassimilar in IL-12p40−/− and WT DC-CD4 T cell co-cultures(Fig. 4c).Taken together,these findings demonstratethat IL-23 and IL-1β are crucial for inducing robust egg Ag-specific Th17 responses.
Figure 4. Egg-stimulated IL-12p40−/−DCs fail to induce significant IL-17 secretion by TCR Tg CD4 T cells.
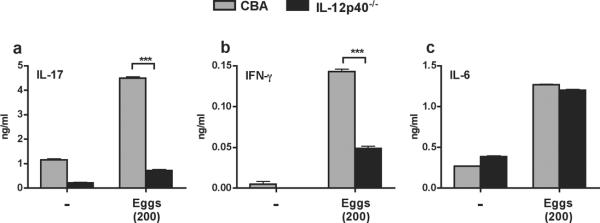
DCs from WT CBA and CBA IL-12p40−/− mice plus TCR Tg CD4 T cells were cultured in the presence or absence of eggs for 48 h, and culture supernatants were assayed for (a) IL-17, (b) IFN-γ and (c) IL-6 by ELISA. Each DC population was obtained from two mice; CD4 T cells from 3–5 mice. Cytokine levels are expressed as means of triplicate ELISA determinations ± SD. *** p<0.001. Data are from one representative experiment of three.
Exogenous IL-23 and IL-1βsequentially reconstitute the ability of IL-12p40−/−DCs to stimulate egg-induced IL-17 production by TCR Tg CD4 T cell
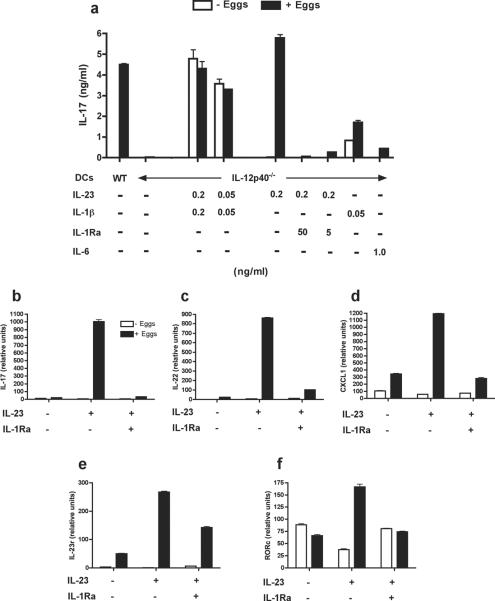
Wefurther investigated the role of IL-23 and IL-1βin egg Ag-specific Th17 cell development using egg-stimulated DC-Tg T cell co-cultures in vitro. In this system, live eggs stimulate WT CBA DCs to induce robust IL-17 production by TCR Tg CD4 T cells, whereas IL-12p40−/−DCs, which cannot make IL-23, are completely unable to do so (Fig. 5a). The addition of IL-23 and IL-1β to IL-12p40−/− DC-T cell cultures leads to expected IL-17 production, even in the absence of eggs, suggesting that these two cytokines per se are sufficient to induce IL-17 without any further stimulation from the schistosome eggs (Fig. 5a). Accordingly, the addition of exogenous IL-23 fully reconstitutes egg-stimulated IL-17 expression and secretion by Tg CD4 T cellsin cultures withIL-12-p40−/− DCs(Fig. 5a and b),implying that IL-1 is contributed by the egg-stimulated DCs. This was demonstrated to be the case as IL-17 levels were significantly reduced in egg-stimulated co-cultures treated with IL-1Ra (Fig. 5a and b). Interestingly, the addition of IL-1β alone inducedsome IL-17 production, which was enhanced in the presence of eggs. However, by comparison, the addition of IL-6 failed to elicit significant levels of IL-17 in co-cultures with DCs from IL-12p40−/− mice (Fig. 5a). Furthermore, exogenous IL-23 in egg-stimulated cultures augmented the expression of the Th17-associated effector cytokine and chemokine, Il22 and Cxcl1, respectively, as well as Il23r and the lineage specific transcription factor Rorγt (Rorc)(12)(Fig. 5 c–f). The enhanced expression of these factors was also strongly dependent upon intact IL-1 signaling, as the addition of IL-1Ra completely abrogated the IL-23-induced effects. Taken together, these findings suggest that the TCR Tg Th17 phenotype can be attained by cognate interaction via specific Ag, or an appropriate cytokine combination independent of antigenic stimulation.
Figure 5. IL-23 and IL-1β reconstitute the ability of egg-stimulated IL-12p40−/−DCs to induce IL-17 production by TCR Tg CD4 T cells.
DCsfrom WT CBA or CBA IL-12p40−/− mice plus TCR Tg CD4 T cells were cultured in the presence of eggs and recombinant cytokines or IL-1Raat indicated concentrations for 48h. (a) Culture supernatants were assayed for IL-17 by ELISA. (b) Il17, (c) Il22, (d) Cxcl1, (e) Il23r and (f) Rorcexpressionwas determined by real-time quantitative RT-PCR. Each DC population was obtained from two mice; CD4 T cells from 3–5 mice. Cytokine levels are expressed as means of triplicate ELISA determinations ± SD. For RT-PCR data, each bar represents the mean mRNA level from duplicate determinations ± SD. Data are from one representative experiment of two to three.
A kinetic analysis of IL-23 and IL-1β secretion involved in the differentiation of TCR Tg Th17 cells in co-cultures with cytokine-sufficient WT DCs revealed that stimulation with 50 schistosome eggs first elicited IL-23 production, which reached peak levels at 12 hours and then declined, but was followed by a steady increase in IL-1β(Fig. 6a). Most importantly, only with increasing levels of IL-1β, was there effective IL-17 production (Fig. 6a).A similar profile was observed when 200 eggs were used for stimulation except that IL-23 levels were slightly higher at 36 hours and absolute cytokine levels were correspondingly increased (Fig. 6b).
Figure 6. Kinetic analysis of IL-23, IL-1β and IL-17 production in WT CBA DCs and TCR Tg CD4 T cell co-cultures following stimulation with eggs.
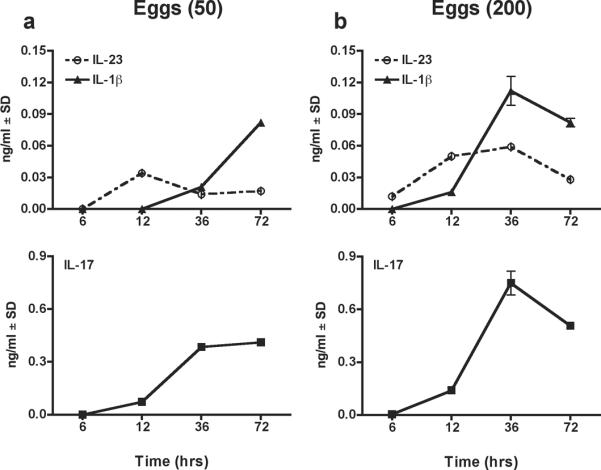
WT CBA DCs plus TCR Tg CD4 T cells were stimulated with (a) 50 or (b) 200 eggs. At the indicated time points, culture supernatants were tested for IL-23, IL-1β and IL-17 by ELISA. DCs were obtained from two mice; CD4 T cells from 3–5 mice. Cytokine levels are expressed as means of triplicate ELISA determinations ± SD. Data are from one representative experiment of two.
Schistosome-infectedIL-12p40−/−CBA mice, or CBA mice treated with IL-1Ra, develop milder hepatic immunopathology and produce reduced levels of IL-17
Thus far, we demonstrated the importance of IL-23 and IL-1βin the development of Sm-p40-specific Th17 cells in vitro. To lend in vivo relevance to these observations, we examined the egg-induced hepatic immunopathology and Th17 responses in infected IL-12p40−/− mice and WT CBA mice treated in vivo with IL-1Ra during the last 10 days of a 7-week infection. Schistosome-infected IL-12p40−/− mice exhibited a significant reduction of granulomatous inflammation in comparison with WT CBA mice (Fig. 7a), which was paralleled by a significant drop in serum levels of AST, an enzyme that reflects liver cell damage (not shown). SEA-stimulated MLNC from infected IL-12p40−/− mice produced significantly lower amounts of IL-17 than the CBA controls (Fig. 7b). Moreover, not unexpectedly, IFN-γlevels were also markedly reduced (Fig. 7c), as the production of this cytokine is largely dependent on IL-12p70, of which IL-12p40 is a subunit (13). Additionally, there also was significantly less granulomatous inflammation (Fig. 7d), as well aslower IL-17(Fig. 7e) and IFN-γ production(Fig. 7f) in IL-1Ra-treated mice than in untreated controls.These findings imply that the loss of IL-23 or IL-1 causes a significant reduction of immunopathology and in the levels of IL-17, thus supporting their role in mediating disease severity in vivo.
Figure 7. Reduced hepatic immunopathology and proinflammatorycytokine production in schistosome-infected CBA IL-12p40−/− mice and WT CBA mice treated with IL-1Ra.
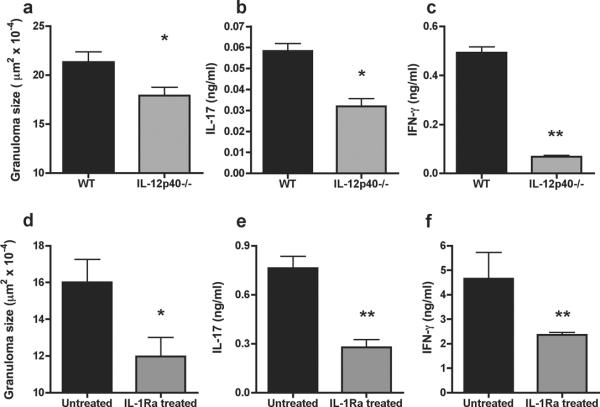
(a–c) WT CBA and IL-12p40−/−mice, or (d–f) WT CBA mice treated or not with IL-1Ra, were infected with 85 cercariae of S. mansoni. After 7 weeks, (a,d) granulomatous lesions were measured on H&E-stained liver sections by computer assisted morphometric analysis, and MLNC were stimulated with 15 μg/ml of SEA for 48h and (b,e) IL-17 and (c,f) IFN-γ in culture supernatants were measured by ELISA. Cytokine levels are expressed as means of triplicate determinations ± SD; background levels from unstimulatedMLNC were subtracted. *, p<0.05; **, p<0.01. Each group consisted of 3–5 mice. Data are from one experiment representative of five for (a–c) and two for (d–f).
Discussion
The development of a novel CBA mouse transgenically expressing a TCR recognizing the immunodominant peptide Sm-p40234–246 allowed us to directly assess the cytokine requirements for the development of schistosome egg Ag-specific Th17 cells. The naïve TCR Tg CD4 T cells are highly sensitive to stimulation with Sm-p40, either in recombinant form, or naturally delivered by schistosome eggs, the pathogenic form of the S. mansoni life cycle. In the CBA mice, these I-Ak-restricted TCR Tg T cells develop into IL-17- and IFN-γ-producing cells, representing the Th17 and Th1 subsets, but secrete exceedingly low levels of the Th2 cytokine, IL-5. Although both Th17 and Th1 cells are proinflammatory and pathogenic in a variety of infectious and autoimmune conditions (14), only the Th17 cells are clearly associated with exacerbated immunopathology in experimental schistosomiasis(5, 6). Instead, Th1 cells normally restrain Th17-mediated pathology as judged by the increase in egg-induced inflammation observed in infected SEA/CFA-immunized mice deficient in the lineage-specific transcription factor T-bet, in which Th1 cells fail to develop (15).
Our results demonstrate that IL-23 and IL-1β are necessary for optimal development of schistosome egg Ag-specific TCR Tg T cells into IL-17 producers in vitro. A kinetic analysis of cytokine production by egg-stimulated DC-TCR Tg CD4 cell co-cultures revealed an initial temporary IL-23 peak, followed by IL-1β, with ensuing IL-17 levels closely following those of IL-1β, suggesting that IL-23 precipitates Th17 cell differentiation, which is amplified by IL-1β. The importance of both IL-23 and IL-1β was further highlighted by the lack of IL-17 production in cultures using IL-12p40−/−DCs, or in IL-23 sufficient cultures in which IL-1β function was blocked by IL-1Ra. Significantly, both IL-23 and IL-1β were also effective in vivo as mice lacking the IL-12p40 subunit or treated with IL-1Ra produced lower levels of IL-17 and exhibited milder egg-induced hepatic immunopathology. Several other pathogen-based and autoimmune models such as Candida albicans, Klebsiellapneumoniae, experimental autoimmune encephalomyelitis (EAE), collagen induced arthritis (CIA) and inflammatory bowel disease (IBD), similarly emphasize the importance of IL-23 and/or IL-1β as critical Th17 cell inducers (16–19). Of importance is that IL-1β has been widely implicated as an essential factor in human Th17 cell development, either independently, or in cooperation with IL-23, IL-6 and TGF-β(20).
The molecular mechanisms leading to IL-23 and IL-1 production for Th17 cell development vary greatly according to the structure of pathogen-derived ligands, the type of host innate receptors that recognize them, and the ensuing downstream signaling circuits resulting in cytokine-specific gene activation (20, 21). For example, the yeast form of C. albicansengages TLR2, TLR4 and dectin-1, resulting in the secretion of both IL-12p70 and IL-23, while the hyphal form is recognized by TLR2 and dectin-2 leading to the sole secretion of IL-23 (20). Moreover, a Syk-CARD9-dependent signaling cascade initiated following β-glucan binding by dectin-1 or dectin-2 is essential for the development of Th17 responses (19). Although DCs stimulated with the β-glucan-containing molecule, zymosan, elicited the production of TNF-α, IL-6 and IL-23, the differentiation of Th17 cells was shown to be completely dependent upon IL-23 (19). In human T cells, co-recognition of bacterial peptidoglycan by TLR2 and nucleotide-binding oligomerization domain 2 (NOD2) elicited the production of IL-23 and IL-1, resulting in the differentiation of Th17 cells (22). An additional layer of complexity concerns the transcription factors that control the expression of cytokines critical for Th17 cell development, as well as those expressed by Th17 cells themselves. Thus, mice deficient for the NF-κB family member, c-Rel, exhibited a significant decrease in Il23p19 expression (23), a defect in the differentiation of Th17 cells, and consequent resistance to immunization-induced EAE (24).STAT3 was also demonstrated to influence IL-23-induced IL-17 production (25). Specifically, IL-23, in tandem with IL-1β, augmented the expression of Il23r and modified the Il17 and Rorcloci, emphasizing the importance of IL-23 in the differentiation of Th17 cells (26). In addition to the original master regulator of the Th17 lineage, RoRγt, other transcription factors such as RORα and interferon regulatory factor 4 (IRF4) were also shown to contribute to the development of Th17 cells (12).
We previously demonstrated that stimulation with schistosome eggs is under genetic control resulting in a Th17 response in high pathology-prone CBA mice, but not in low-pathology BL/6 mice (11). However, it is still unclear if this is a consequence of differential recognition of egg-derived ligands by a distinct display of innate receptors or if the divergence occurs at the level of intracellular signaling. As egg components are heavily glycosylated(27), glycans and glycoconjugates are some of the more intensively investigated schistosome-derived ligands. For example, glycansterminating in Lewis (Le) Lexor pseudo-Leywere shown to interact with TLR4 or the CLR DC-SIGN (CD209), respectively, while LacdiNAc was identified as a putative ligand for the S-type lectin, galectin-3 (27). On the other hand, the egg glycoproteinOmega-1 was shown to induce Th2-biased immune responses in a MyD88/TRIF-independent fashion, suggesting that Toll-like receptors (TLRs) were not the primary PRRs involved in its recognition (28). Thus, the precise ligands, receptors and signaling pathways involved in the development of immunopathogenic responses in schistosomiasis still need to be elucidated.
A somewhat controversial issue concerns the role of IL-6 in the generation of Th17 cells. In our hands, IL-6 played no apparent role in inducing IL-17 responses by schistosome egg Ag-specific CD4 T cells, a finding that contrasts with the notion that IL-6, in combination with TGF-β, is required for the differentiation of the Th17 cell subset (29, 30). This difference may be explained by the nature of the T cells used in each experimental system. The requirement for IL-6 and TGF-β for inducing Th17 cell differentiation was demonstrated using FACS-sorted CD4+CD44lo or CD4+CD62Lhi naïve lymphocytes (12). In contrast, the CD4 T cells used in our study were purified from a normal, unselected spleen cell population. As such, these T cells had been previously exposed to IL-6, leading to enhanced IL-23R expression (31) and consequent increased susceptibility to stimulation with DC-derived IL-23 and IL-1β for Th17 cell differentiation without the need for additional exogenous IL-6. This scenario is more compatible with one encountered by a normal mature immune system following exposure to a new Ag. Further evidence of shifting paradigms is a recent study demonstrating that TGF-β is dispensable for Th17 cell development and, in agreement with our findings, that Th17 cells with most pathogenic potential are dependent on IL-23 and amplified by IL-1β(26). Nevertheless, it is possible that IL-6 could play a role in Th17 responses to certain microbial PRR ligands, which in contrast to schistosome egg Ags result in greater DC activation(32, 33). Taken together, our observations provide novel insight into the cytokine circuits involved in the development of egg Ag-specific Th17 cells that mediate high-pathology schistosomiasis and identify possible targets for intervention to curtail severe disease.
Acknowledgments
Supported by US Public Health Service grant RO1-18919 (to MJS) and AI5614 (to CAD). The authors thank Dr. HanspeterWaldner for helpful suggestions during the development of the Tg mouse, and Dr. Lina Du for expert technical assistance.
References
- 1.Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- 2.Stadecker MJ, Asahi H, Finger E, Hernandez HJ, Rutitzky LI, Sun J. The immunobiology of Th1 polarization in high-pathology schistosomiasis. Immunol Rev. 2004;201:168–179. doi: 10.1111/j.0105-2896.2004.00197.x. [DOI] [PubMed] [Google Scholar]
- 3.Herbert DR, Holscher C, Mohrs M, Arendse B, Schwegmann A, Radwanska M, Leeto M, Kirsch R, Hall P, Mossmann H, Claussen B, Forster I, Brombacher F. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity. 2004;20:623–635. doi: 10.1016/s1074-7613(04)00107-4. [DOI] [PubMed] [Google Scholar]
- 4.Rutitzky LI, Hernandez HJ, Stadecker MJ. Th1-polarizing immunization with egg antigens correlates with severe exacerbation of immunopathology and death in schistosome infection. Proc. Natl. Acad. Sci. USA. 2001;98:13243–13248. doi: 10.1073/pnas.231258498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rutitzky LI, Lopes da Rosa JR, Stadecker MJ. Severe CD4 T cell-mediated immunopathology in murine schistosomiasis is dependent on IL-12p40 and correlates with high levels of IL-17. J Immunol. 2005;175:3920–3926. doi: 10.4049/jimmunol.175.6.3920. [DOI] [PubMed] [Google Scholar]
- 6.Rutitzky LI, Bazzone L, Shainheit MG, Joyce-Shaikh B, Cua DJ, Stadecker MJ. IL-23 is required for the development of severe egg-induced immunopathology in schistosomiasis and for lesional expression of IL-17. J Immunol. 2008;180:2486–2495. doi: 10.4049/jimmunol.180.4.2486. [DOI] [PubMed] [Google Scholar]
- 7.Finger E, Brodeur PH, Hernandez HJ, Stadecker MJ. Expansion of CD4 T cells expressing a highly restricted TCR structure specific for a single parasite epitope correlates with high pathology in murine schistosomiasis. Eur J Immunol. 2005;35:2659–2669. doi: 10.1002/eji.200526344. [DOI] [PubMed] [Google Scholar]
- 8.Cass CL, Johnson JR, Califf LL, Xu T, Hernandez HJ, Stadecker MJ, Yates JR, 3rd, Williams DL. Proteomic analysis of Schistosoma mansoni egg secretions. Mol Biochem Parasitol. 2007;155:84–93. doi: 10.1016/j.molbiopara.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waldner H, Whitters MJ, Sobel RA, Collins M, Kuchroo VK. Fulminant spontaneous autoimmunity of the central nervous system in mice transgenic for the myelin proteolipid protein-specific T cell receptor. Proc Natl Acad Sci U S A. 2000;97:3412–3417. doi: 10.1073/pnas.97.7.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shainheit MG, Smith PM, Bazzone LE, Wang AC, Rutitzky LI, Stadecker MJ. Dendritic cell IL-23 and IL-1 production in response to schistosome eggs induces Th17 cells in a mouse strain prone to severe immunopathology. J Immunol. 2008;181:8559–8567. doi: 10.4049/jimmunol.181.12.8559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 13.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 14.Harrington LE, Mangan PR, Weaver CT. Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr Opin Immunol. 2006;18:349–356. doi: 10.1016/j.coi.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 15.Rutitzky LI, Smith PM, Stadecker MJ. T-bet protects against exacerbation of schistosome egg-induced immunopathology by regulating Th17-mediated inflammation. Eur J Immunol. 2009;39:2470–2481. doi: 10.1002/eji.200939325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGeachy MJ, Cua DJ. The link between IL-23 and Th17 cell-mediated immune pathologies. Semin Immunol. 2007;19:372–376. doi: 10.1016/j.smim.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 18.Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol. 2005;5:521–531. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 19.LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, Schweighoffer E, Tybulewicz V, Brown GD, Ruland J, Reis e Sousa C. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 20.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 21.Goriely S, Neurath MF, Goldman M. How microorganisms tip the balance between interleukin-12 family members. Nat Rev Immunol. 2008;8:81–86. doi: 10.1038/nri2225. [DOI] [PubMed] [Google Scholar]
- 22.van Beelen AJ, Zelinkova Z, Taanman-Kueter EW, Muller FJ, Hommes DW, Zaat SA, Kapsenberg ML, de Jong EC. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity. 2007;27:660–669. doi: 10.1016/j.immuni.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 23.Carmody RJ, Ruan Q, Liou HC, Chen YH. Essential roles of c-Rel in TLR-induced IL-23 p19 gene expression in dendritic cells. J Immunol. 2007;178:186–191. doi: 10.4049/jimmunol.178.1.186. [DOI] [PubMed] [Google Scholar]
- 24.Hilliard BA, Mason N, Xu L, Sun J, Lamhamedi-Cherradi SE, Liou HC, Hunter C, Chen YH. Critical roles of c-Rel in autoimmune inflammation and helper T cell differentiation. J Clin Invest. 2002;110:843–850. doi: 10.1172/JCI15254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho ML, Kang JW, Moon YM, Nam HJ, Jhun JY, Heo SB, Jin HT, Min SY, Ju JH, Park KS, Cho YG, Yoon CH, Park SH, Sung YC, Kim HY. STAT3 and NF-kappaB signal pathway is required for IL-23-mediated IL-17 production in spontaneous arthritis animal model IL-1 receptor antagonist-deficient mice. J Immunol. 2006;176:5652–5661. doi: 10.4049/jimmunol.176.9.5652. [DOI] [PubMed] [Google Scholar]
- 26.Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, Ramos HL, Wei L, Davidson TS, Bouladoux N, Grainger JR, Chen Q, Kanno Y, Watford WT, Sun HW, Eberl G, Shevach EM, Belkaid Y, Cua DJ, Chen W, O'Shea JJ. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hokke CH, Yazdanbakhsh M. Schistosome glycans and innate immunity. Parasite Immunol. 2005;27:257–264. doi: 10.1111/j.1365-3024.2005.00781.x. [DOI] [PubMed] [Google Scholar]
- 28.Steinfelder S, Andersen JF, Cannons JL, Feng CG, Joshi M, Dwyer D, Caspar P, Schwartzberg PL, Sher A, Jankovic D. The major component in schistosome eggs responsible for conditioning dendritic cells for Th2 polarization is a T2 ribonuclease (omega-1) J Exp Med. 2009;206:1681–1690. doi: 10.1084/jem.20082462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 30.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 32.Iezzi G, Sonderegger I, Ampenberger F, Schmitz N, Marsland BJ, Kopf M. CD40-CD40L cross-talk integrates strong antigenic signals and microbial stimuli to induce development of IL-17-producing CD4+ T cells. Proc Natl Acad Sci U S A. 2009;106:876–881. doi: 10.1073/pnas.0810769106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perona-Wright G, Jenkins SJ, O'Connor RA, Zienkiewicz D, McSorley HJ, Maizels RM, Anderton SM, MacDonald AS. A pivotal role for CD40-mediated IL-6 production by dendritic cells during IL-17 induction in vivo. J Immunol. 2009;182:2808–2815. doi: 10.4049/jimmunol.0803553. [DOI] [PubMed] [Google Scholar]