Abstract
Breast cancer is one of the leading causes of cancer deaths among females. Many challenges exist in the current management of advanced stage breast cancer as there are fewer recognized therapeutic strategies, often due to therapy resistance. How breast cancer cells evade chemotherapy and the underlying mechanism remains unclear. We and others have observed that malignant cells that survive initial chemo-and radiation therapy express higher levels of CXCR2 ligands which might provide a survival benefit leading to therapy resistance. In this report, we test the hypothesis that CXCR2-dependent signaling in malignant cells might be critical for chemotherapy resistance and targeting this signaling axis may enhance the antitumor and antimetastatic activity of chemotherapeutic drugs and limit their toxicity. We used Cl66-wt, 4T1-wt, Cl66sh-CXCR2 and 4T1sh-CXCR2 cells expressing differential levels of the CXCR2 receptor to evaluate the role of targeting CXCR2 on chemotherapeutic responses. Knockdown of CXCR2 enhances paclitaxel and doxorubicin mediated toxicity at suboptimal doses. Moreover, we observed an increase in the expression of CXCL1, a CXCR2 ligand in paclitaxel and doxorubicin treated mammary tumor cells which were inhibited following CXCR2 knockdown. Knockdown of CXCR2 enhanced antitumor activity of paclitaxel in an in vivo mammary tumor model. We observed significant inhibition of spontaneous lung metastases in animals bearing CXCR2 knockdown tumors and treated with paclitaxel as compared to the control group. Our data suggest the novel role of CXCR2 and its ligands in maintaining chemotherapy resistance and provide evidence that targeting CXCR2-signaling in an adjuvant setting will help circumvent chemotherapy resistance.
Keywords: Chemokines, Chemotherapy, Therapy resistance, Angiogenesis
Introduction
Breast cancer is a heterogeneous disease which involves many dysregulated pathways (1;2). Metastasis and recurrence of disease after therapy further add to the complexity of this malignancy (3–6). Emerging resistance against conventional therapies has warranted developing alternative strategies to combat breast cancer.
Chronic inflammation can drive tumorigenesis, and tumors are inherently pro-inflammatory with infiltrating leukocytes thought to be critical for tumor maintenance and progression (7–11). Thus, molecules driving tumor-associated inflammation have considerable potential as therapeutic targets, yet this area remains relatively unexplored. Chemokines are secreted proteins that regulate cell behavior via G-protein coupled receptors, and subsets of CC and CXC chemokines, orchestrate tissue inflammation by recruiting and activating leukocytes, and by regulating endothelial and epithelial cells (12;13). Constitutive expression of pro-inflammatory chemokines, a hallmark of many human cancers, helps establish a supportive tumor stroma, and, in some cases, directly stimulates tumor proliferation and invasion via receptors on tumor cells (14).
CXCR2 is a G-protein coupled receptor which mediates its signaling after binding to CXC chemokines namely, CXCL1-3 and 5–8 (15). An increase in the transcription and secretion of CXCL8 and CXCL1 along with their receptor CXCR2 has been observed after oxaliplatin treatment in androgen-dependent prostate cancer (16). Increased expression levels of CXCR2 ligands have been implicated in the attenuation of chemotherapy induced apoptosis in prostate cancer, suggesting that CXCR2 and its ligands might be playing a role in therapy resistance (16). The Breast cancer cell line MCF-7 shows a dose dependent increase in CXCL8 expression following treatment with chemotherapeutic agents (17) implying that this signaling axis might be playing a role in therapy resistance. Our previous data and published reports suggest the involvement of chemokines in chemotherapy resistance in various cancers, however, the precise role and underlying mechanism(s) remains unclear.
In this report, we hypothesize that CXCR2-dependent signaling in malignant cells might be critical for chemotherapy resistance and that targeting CXCR2 receptor expression and/or activity may enhance the antitumor activity of chemotherapeutic agents along with reducing their toxicity. Our data indicate the novel role of CXCR2 and its ligands in maintaining chemotherapy resistance and suggest that targeting CXCR2 signaling in an adjuvant setting may help circumvent chemotherapy resistance.
Material and methods
Cell culture
Two murine mammary adenocarcinoma cell lines differing in their metastatic potential, 4T1 (highly metastatic) and Cl66 (moderately metastatic) (18;19) and five human breast cancer cell lines, MDA-MB-231, MDA-MB-468, MCF-7, SKBR3 and MCF-10A were used in this study. Murine cell lines were maintained in Dulbecco’s Modified Eagle Media (DMEM) (Mediatech, Hendon, VA) with 5% serum supreme (Biowhitaker, Walkersville, MD) or 5% foetal bovine serum (FBS), 1% vitamins, 1% L-glutamine and 0.08% gentamycin (Invitrogen, Carlsbad, CA). Human cell lines were maintained in DMEM supplemented with 10% FBS, 1% vitamins, 1% L-glutamine and 0.08% gentamycin (Invitrogen, Carlsbad, CA). Murine cells lines were obtained from Dr. F.R Miller’s laboratory18 and all the human cancer cell lines were obtained from ATCC (Manassas, VA). Cell lines were authenticated and tested for mycoplasma contamination using a kit, MycoAlert (Cambrex Bio Science Rockland. Inc).
mRNA expression analysis
Analysis of gene expression was performed using quantitative RT-PCR as described (20). Briefly, cDNA was synthesized from 5 μg total RNA using SuperScript™ II Reverse Transcriptase (Invitrogen) and oligo(dT) primer. Two micro liter of first strand cDNA (1:10 dilution) was amplified. The following primer sequences for murine chemokines and receptor were used: CXCR2, 5′-CAC CGA TGT CTA CCT GCT GA -3′ (forward) and 5′-CAC AGG GTT GAG CCA AAA GT -3′ (reverse); CXCR1, 5′-AAT CTG TTG TGG CTT CAC CCA -3′ (forward) and 5′-GCT ATC TTC CGC CAG GCA TAT -3′ (reverse); CXCL1, 5′-TCG CTT CTC TGT GCA GCG CT-3′ (forward) and 5′-GTG GTT GAC ACT TAG TGG TCT C-3′ (reverse); CXCL2, 5′-AGT GAA CTG CGC TGT CAA TG-3′ (forward) and 5′-TTC AGG GTC AAG GCA AAC TT-3′ (reverse); CXCL3, 5′-GCA AGT CCA GCT GAG CCG GGA-3′ (forward) and 5′-GAC ACC GTT GGG ATG GAT CGC TTT-3′ (reverse), CXCL7, 5′-TCG TCC TGC ACC AGG GCC TG-3′ (forward) and 5′-AAG GGG AGC CAG CGC AAC AA-3′ (reverse). Primers used for human chemokine and receptors were: CXCR1, 5′-GAG CCC CGA ATC TGA CAT TA-3′ (forward) and 5′-GCA GAC ACT GCA ACA CAC CT-3′ (reverse), CXCR2, 5′-ATT CTG GGC ATC CTT CAC AG-3′ (forward) and 5′-TGC ACT TAG GCA GGA GGT CT-3′ (reverse) and CXCL8, 5′-ACA TAC TCC AAA CCT TTC CAC CC-3′ (forward) and 5′-CAA CCC TCT GCA CCC AGT TTT C-3′ (reverse). For internal control, murine glyceraldehyde 3-phosphate dehydrogenase (GAPDH), 5′-AGC CTC GTC CCG TAG ACA AAA -3′ (forward), and 5′-GAT GAC AAG CTT CCC ATT CTC G -3′ (reverse) and human GAPDH 5′-CCA TCA CTG CCA CCC AGA AGA C-3′ (forward) and 5′-ATG ACC TTG CCC ACA GCC TTG-3′ (reverse) were used. Amplified products were resolved through a 1.5 % agarose gel containing ethidium bromide and analyzed using an Alpha Imager gel documentation system (AlphaInnotech, San Leandro, CA).
For real time quantitative RT-PCR 2ul of the 1:10 diluted cDNA products were amplified per reaction in duplicate with SYBR green master mix (Roche, Indianapolis IN) and 10 mM primer mix for each gene in a Bio-Rad iCycler (Bio-Rad, Hercules, CA). The annealing temperatures used for murine primers: 60°C for CXCR2, CXCR1 and GAPDH, 55°C for CXCL1, 57°C for CXCL2 and 68°C for CXCL3 & 7. For human primers: 59°C for CXCR1, 2 and GAPDH, 55°C for CXCL8. Real time PCR products were quantitated using Gene Expression Macro™ Version 1.1 © 2004 Bio-Rad Laboratories.
Cytotoxicity assay
Tumor cells (5000 cells/well) were plated in triplicate in a 96 well plate and incubated for 24 hours at 37°C. Cells were treated with different concentrations of doxorubicin or paclitaxel (100, 50, 10, 5, 1, 0.5, 0.1, 0 nM for paclitaxel and 200, 100, 50, 10, 5, 1, 0.5, 0 nM for doxorubicin) for 72 hrs. After 72 hrs, supernatants were collected from each well and fresh media was added to the cells along with 30μL of 5mg/ml MTT (3-(4, 5-Dimethylthiazol-2yl)-2, 5-diphenyltetrazolium bromide) (MP Biomedicals) and incubated for 2–4 hours. After incubation media was aspirated and cells were lysed in 100 μL of DMSO (Fisher Scientific) with shaking. Absorbance was read at 450nm on ELx800 Bio-Tek plate reader. Percent inhibition was calculated using the following formula:
In vitro Apoptosis assay
Tumor cells (1000 cells/well) were plated in an 8-well micro-chamber slide and incubated for 24 hours at 37°C. Cells were treated with 10nM of paclitaxel for 30 mins. CaspACE™FITC-VAD-FMK in Situ marker (Promega, Madison WI) which is a fluoroisothiocyanate conjugate of the cell permeable caspase inhibitor VAD-FMK was used to conjugate the cells with active caspase-3. Apoptotic cells were quantitated by counting fluorescent cells in 5 different areas of the slide under a fluorescent microscope.
Enzyme-linked immunosorbant assay (ELISA)
Cell-free supernatants were collected from cells treated with varying concentrations of drugs at 72 hrs of treatment. ELISA plates were coated with 100μl per well of primary monoclonal antibody (2μg/ml rat anti-mouse CXCL1/KC monoclonal, R&D Systems Inc, 1μg/ml mouse anti-human CXCL1/GROα, R&D Systems Inc and 1μg/ml rabbit anti-human CXCL8 antibody, Endogen, Worburn, MA) diluted in PBS (pH=7.4) and incubated overnight at 4°C (CXCL1) or at room temperature (CXCL8). The next day plates were washed and blocked with 300μl of blocking buffer (as per manufacturer’s protocol) for 1 hr. Standards (recombinant proteins) and samples were added 100μl/well in duplicate. After incubation, plates were washed and then incubated with biotinylated secondary antibody 100μl/well (0.2μg/ml goat anti-mouse KC, R&D Systems Inc, 4μg/ml goat anti-human GROα/CXCL1, R&D Systems Inc and 0.1μg/ml mouse anti-human IL-8, Endogen, Worburn, MA). After washing strepavidin-horseradish peroxidase (1:20000) was added and 3,3′,5,5′-tetramethylbenzidine substrate (100μl/well)was used. Reactions were stopped and plates were read at 450nm using an ELx800 (Bio-Teck) plate reader. Concentrations were normalized to proliferation ODs from the MTT assay.
Tumor growth and metastasis
Female BALB/c mice (6–8 weeks old) were purchased from the National Cancer Institute and maintained under specific pathogen-free conditions. All procedures performed were in accordance with institutional guidelines and approved by the University of Nebraska Medical Center Institutional Animal Care and Use Committee. Cl66-wt or Cl66sh-CXCR2 cells (50,000 in 50 μl of HBSS) were injected orthotopically in mammary fat pad (MFP) to study tumor growth and spontaneous metastasis in response to chemotherapeutic treatment. Tumor growth was measured twice a week. Tumor volume was calculated using the formula π/6 X (smaller diameter) X (larger diameter)2. Tumors recovered from mice were fixed in zinc, embedded in paraffin and processed for histopathological evaluation and immunohistochemistry.
Tumor microvessel density
Immunohistochemical analysis was performed to determine micro-vessel density as previously described (21). In brief, 6-μm thick tumor sections were deparaffinized by xylenes and ethanol and blocked for 30 minutes. Tumor sections were incubated overnight in a humid chamber with mouse biotinylated anti-GS-IB4 (isolectin from Griffonia simplicifolia; 1:50; Vector Laboratories, Burlingame, CA) antibody. Immunoreactivity was detected using the ABC Elite kit and DAB substrate (Vector Laboratories) as per the manufacturer’s instructions. A reddish brown precipitate indicated a positive reaction. Negative controls had all reagents except antibody. The number of microvessels was quantitated microscopically with a 5×5 reticle grid (Klarmann Rulings, Litchfield, NH) using 400x objective (250 μm total area).
Statistical analysis
For the in vitro studies the unpaired t-test was performed using Sigma plot 11 software. In vivo analysis was performed using the Mann-Whitney U-test and paired t-test. All the values are expressed as mean ± SEM. p ≤0.05 was considered statistically significant.
Results
Chemotherapy induced higher expression of CXCR2 ligands in aggressive breast cancer cells
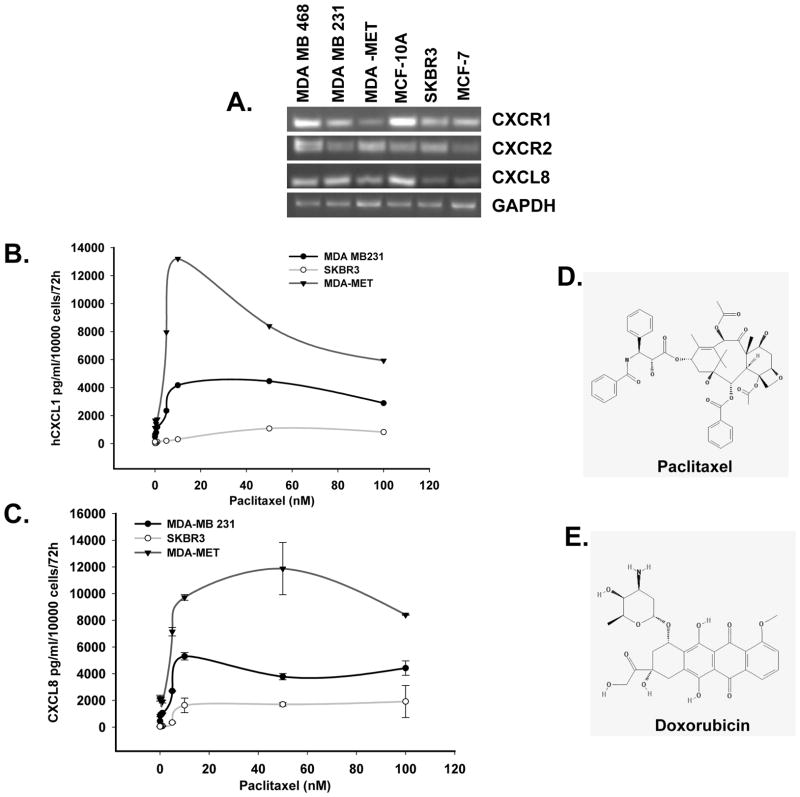
We screened human breast cancer cell lines, which differ in their metastatic potential and hormone receptor expression for CXCL8, CXCR1 and CXCR2 by semi-quantitative RT-PCR. We observed that the metastatic cell lines MDA-MB-231, MDA-MB-468 andMDA-MET have higher mRNA expression of CXCR2 and CXCL8 at mRNA level in comparison to the non-metastatic, MCF-7 and SKBR3 cell lines (Figure 1A). We examined the expression of CXCL1 and CXCL8 at the protein level in three of the human breast cancer cell lines MDA-MB-231, MDA-MET and SKBR3. We observed that aggressive MDA-MB-231 and MDA-MET cells express higher level of CXCL8 and CXCR2 at basal level in comparison to less aggressive SKBR3 cells (Figure 1B). When we treated these cells with paclitaxel and doxorubicin (data not shown for doxorubicin), we observed a significant increase in the expression of CXCL1 and -8 in these cells (Figure 1B and C).
Figure 1. Effect of chemotherapeutic agents on the expression of CXCR2 ligands.
A) PCR results showing expression of CXCR1, CXCR2 and CXCL8 in different breast cancer cell lines. B &C) ELISA results showing the expression of CXCL1 and CXCL8 in the supernatants of paclitaxel treated MDA-MB-231, MDA-MET or SKBR3 cells. D&E) Chemical structures of paclitaxel and doxorubicin drugs (adapted from NCBI PubChem Substance).
CXCR2 knockdown in mammary tumor cells enhance sensitivity to chemotherapy
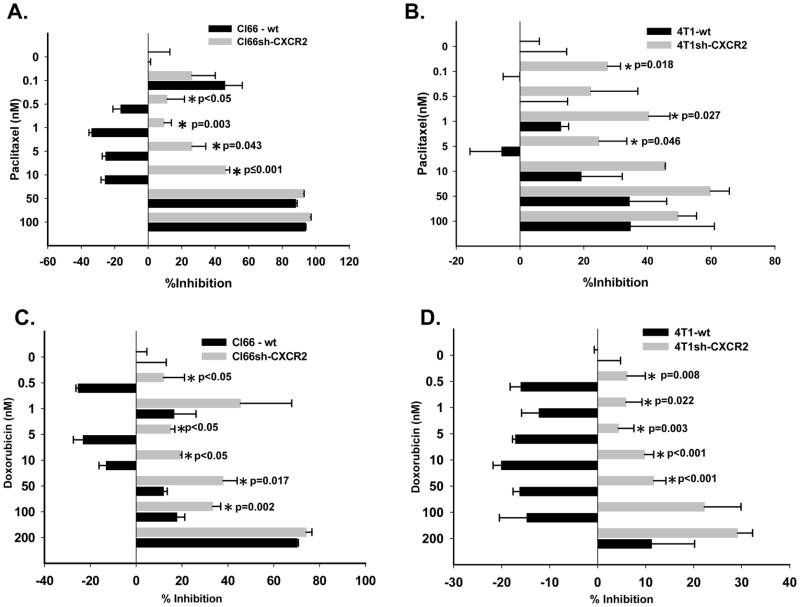
To evaluate the role of CXCR2 in modulating tumor cell sensitivity towards chemotherapy, we knocked down CXCR2 in Cl66 and 4T1 cells by shRNA (22). Cl66-wt and 4T1-wt, transfected with vector alone or Cl66-shCXCR2 and 4T1sh-CXCR2, transfected with shRNA directed against CXCR2 were used. We observed significantly enhanced sensitivity of Cl66sh-CXCR2 and 4T1sh-CXCR2 cells towards paclitaxel and doxorubicin as compared to Cl66-wt and 4T1-wt (Figure 2). We observed a significant difference in growth inhibition, with Cl66sh-CXCR2 and 4T1sh-CXCR2 cells more sensitive to chemotherapy in comparison to Cl66-wt and 4T1-wt cells at lower concentrations of 0.5, 1 and 5nM for both paclitaxel (Figure 2 A and B) and doxorubicin (p≤0.05) (Figure 2 C and D).
Figure 2. CXCR2 knockdown enhances sensitivity of mammary tumor cells toward chemotherapeutic agents.
A & B) Cell toxicity determined by MTT assay in CXCR2 knockdown Cl66 and 4T1 cells (Cl66sh-CXCR2, 4T1sh-CXCR2) and wild type cells transfected with vector alone (Cl66-wt, 4T1-wt) at lower doses of paclitaxel (p ≤0.05) and (C & D) doxorubicin (p ≤0.05).
CXCR2 knockdown along with chemotherapeutic drugs induces apoptosis in murine mammary tumor cells
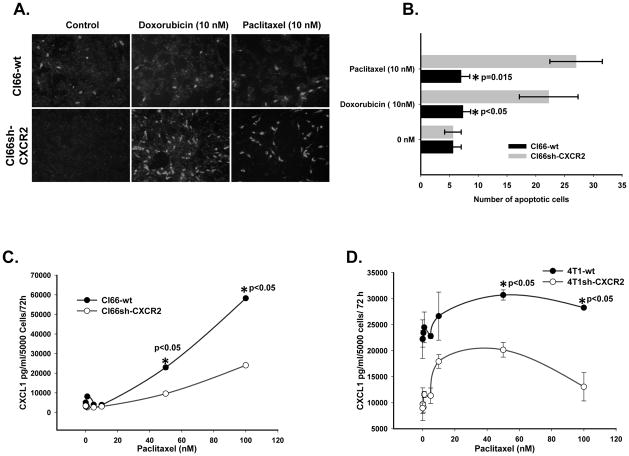
To determine the effect of CXCR2 knockdown in mediating chemotherapy induced apoptosis on mammary tumor cells, we treated Cl66-wt and Cl66-shCXCR2 cells with 10nM of paclitaxel or doxorubicin. We observed a significant increase (p≤0.05 and 0.015) in the number of apoptotic cells in Cl66sh-CXCR2 cells as compared to Cl66-wt cells after chemotherapy (Figure 3 A and B).
Figure 3. Effect of chemotherapeutic agents on the expression of CXCR2 ligands.
A) Apoptosis in CXCR2 knockdown cells after treatment with 10 nM dose of paclitaxel or doxorubicin and vector transfected Cl66 cells evaluated using CaspACE™ FITC-VAD-FMK in Situ marker. B) Quantification of apoptosis after treatment with paclitaxel or doxorubicin of Cl66-wt and Cl66 sh-CXCR2 cells (p ≤0.05). C & D) Expression of CXCL1 in the supernatants of Cl66-wt, Cl66sh-CXCR2, 4T1-wt and 4T1sh-CXCR2 cells after treatment with paclitaxel demonstrated by ELISA (p ≤0.05).
Chemotherapy-induced decrease in the expression of CXCR2 ligands in CXCR2 knockdown cells
We investigated whether knockdown of CXCR2 regulates chemotherapy-induced expression of CXCR2 ligands in murine mammary tumor cells. Supernatants from Cl66-wt, 4t1-wt, Cl66sh-CXCR2 and 4T1sh-CXCR2 cells following paclitaxel or doxorubicin treatment were examined for CXCL1. We observed an increase in the expression of CXCL1 in both wild-type and CXCR2 knockdown cells after chemotherapy in a concentration-dependent manner (p<0.05). However, the increase was significantly lower in CXCR2 knockdown cells in comparison to wild type cells (Figure 3 C and D). We also evaluated the mRNA expression of other known CXCR2 ligands in Cl66-wt and Cl66sh-CXCR2 cells by RT-qPCR and found a similar decrease in the CXCR2 knockdown cells when compared to wild-type cells (data not shown) suggesting CXCR2 knockdown downregulated chemotherapy-induced CXCR2 ligand expression.
CXCR2 knockdown enhances anti-tumor response and inhibits mammary tumors growth with paclitaxel treatment
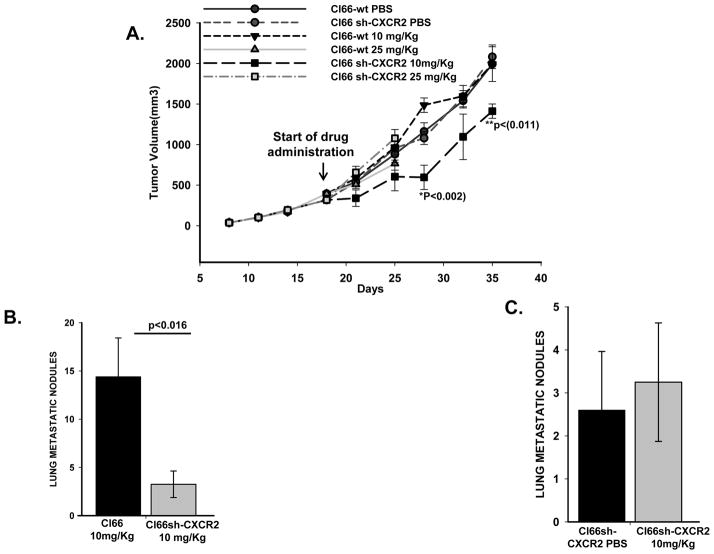
We injected Cl66-wt and Cl66sh-CXCR2 cells in the mammary fat pad of BALB/c mice. The mice were treated with two different doses of paclitaxel, 10mg/Kg or 25mg/Kg body weight biweekly once the tumors were 8–9 mm in diameter. We observed mice injected with the 25mg/Kg dose of paclitaxel showed signs of sickness after only 2–3 doses. Because of the side effects, we discontinued this dose. However, we continued using the sub-optimal 10mg/Kg dose of paclitaxel. Tumor size was measured twice a week. Control groups were treated with PBS alone. We observed a significant (p=0.011 and 0.002) decrease in tumor growth after paclitaxel treatment in mice with Cl66sh-CXCR2 tumors as compared to Cl66-wt tumors after 2 weeks of drug administration (Figure 4A). These results suggest that CXCR2 knockdown enhances the anti-tumor activity of paclitaxel.
Figure 4. Paclitaxel treatment along with CXCR2 knockdown reduces tumor growth and metastasis.
A) Growth of tumors formed by Cl66-wt and Cl66sh-CXCR2 cells injected in the mammary fat pad of Balb/c mice. Mice were treated with two different doses of paclitaxel, 10mg/Kg or 25mg/Kg body weight. Control group tumors were treated with PBS. Tumor size was measured twice a week and treatment with drugs was started 2 weeks after injection of tumor cells. p≤0.05 was considered significant. B) Lungs from mice harboring Cl66-wt tumor or Cl66sh-CXCR2 tumor treated with 10mg/Kg paclitaxel showing metastatic nodules which were quantitated and represented as a bar graph (p<0.05). C) Quantification of the metastatic nodules from mice bearing Cl66sh-CXCR2 tumors treated with PBS or 10 mg/Kg of paclitaxel.
CXCR2 knock-down enhanced anti-metastatic activity of paclitaxel
Previous studies from our laboratory showed a significant reduction in the number of lung metastatic nodules formed in mice harboring Cl66sh-CXCR2 tumors when compared to Cl66-wt tumor bearing mice treated (22). We observed similar results in this study with PBS treatment (data not shown), suggesting a role for CXCR2 in the lung metastasis of mammary tumor cells. Although we did not find any difference in the number of lung nodules for Cl66sh-CXCR2 and Cl66sh-CXCR2 treated 10mg/kg paclitaxel, the numbers of lung metastatic nodules were few for statistical comparison (Figure 4 C). Similar comparison between the lungs of Cl66-wt and Cl66sh-CXCR2 groups treated with 10mg/kg dose of paclitaxel showed an enhanced reduction of lung metastatic nodules in mice harboring CXCR2 knock down tumors (Figure 4B).
Differential expression of CXCR2 ligands in mammary tumors
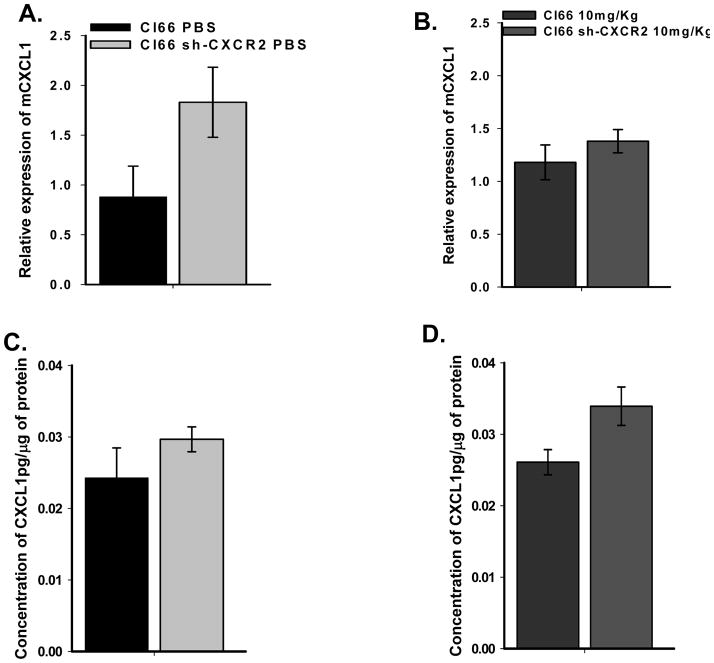
Next we investigated mRNA expression of CXCR2 ligands in wild-type and CXCR2 knock down tumors with or without paclitaxel treatment. We observed a decrease in the expression of CXCL2 and CXCL7 in knockdown tumors and no change after paclitaxel treatment (data not shown). In contrast to CXCL2 and 7, we observed an increase in the expression of CXCL1 in mammary tumors formed by Cl66-wt and which treated with10mg/kg paclitaxel (Figure 5). Expression of CXCL1 was also slightly higher in tumors formed by Cl66sh-CXCR2 cells and treated with PBS. This expression was higher in Cl66sh-CXCR2 tumors after paclitaxel treatment (Figure 5). These observations were similar both at the RNA level (RT-qPCR) as well as at the protein level (ELISA) suggesting that receptor knock down has an inverse effect on CXCL1 expression and that treatment with chemotherapeutic agents enhances CXCL1 expression in vivo.
Figure 5. Expression of CXCL1 in tumors.
A & B) qRT-PCR of CXCL1 in primary tumors formed by Cl66-wt or Cl66sh-CXCR2 cells treated with PBS or 10mg/Kg paclitaxel. C & D) Expression of CXCL1 in tumors evaluated by ELISA.
CXCR2 knockdown in tumor cells along with paclitaxel treatment reduces neovascularization in mammary tumors
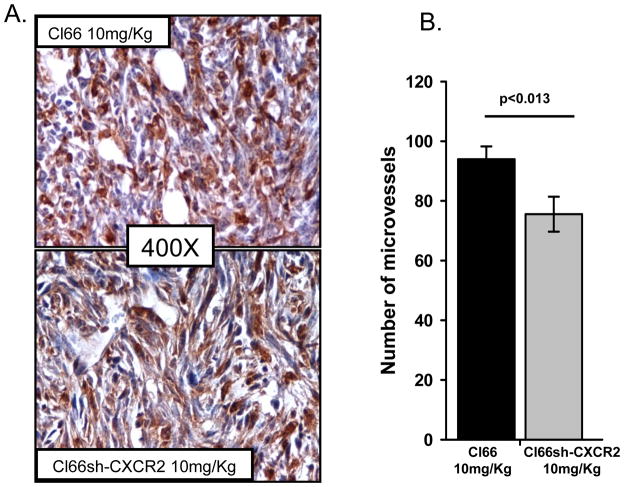
Previous reports from our laboratory demonstrated an increase in apoptosis, a decrease in proliferation and microvessel density in tumors formed by Cl66sh-CXCR2 (22). We observed similar results in tumors formed by Cl66sh-CXCR2 cells undergoing PBS treatment. However, we observed that Cl66sh-CXCR2 tumors treated with a suboptimal 10mg/Kg dose of paclitaxel had a significant reduction in microvessel density as compared to Cl66-wt tumors treated with the same dose of paclitaxel (Figure 6). These results suggest that CXCR2 knockdown in combination with paclitaxel treatment decreases microvessel density in mammary tumors.
Figure 6. Angiogenesis in tumors formed by cells varying the expression of CXCR2.
A) Immunohistochemical analysis of tumor sections for Isolectin-B4 to evaluate micro-vessel density in tumor formed by CXCR2 knockdown Cl66 cells (Cl66sh-CXCR2) treated with PBS or 10mg/Kg paclitaxel. B) Quantification of micro-vessels in CXCR2 knockdown tumors after paclitaxel treatment (p<0.05).
Discussion
In this study, we report that the CXCR2-ligands axis plays an important role in breast cancer therapy resistance. We observed that aggressive breast cancer cell lines expressed higher basal level of CXCR2 and its ligands. The level of CXCR2 ligands increased following chemotherapeutic treatment in aggressive breast cancer cells suggesting an important role of these CXC chemokines in cancer cell survival. Our CXCR2 knockdown studies in murine mammary tumor cells showed enhanced anti-tumor and anti-metastatic activity of chemotherapeutic agents. We observed that both CXCR2 and paclitaxel have their independent effects and when used in an adjuvant setting reduced tumor growth and decreased angiogenesis in mammary tumors. We also analyzed the affect of CXCR2 over-expression in breast cancer cells using SKBR3 cells (which expressed lower CXCR2 ligands). Our data showed that over-expression of CXCR2 makes SKBR3 cells resistant to drugs (Supplemental Figure 1), suggesting an important role of CXCR2 in therapy response. In our over-expression studies with SBKR3, we observed that drugs increases expression of CXCL8 (Supplemental Figure 2) but the increase was not significant when compared to vector-transfected control cells. However, SKBR3 cells inherently expressed low levels of CXCL8 and permanent transfections instead of transient transfections might be able to provide significant differences.
The pro-tumorigenic role of CXC chemokines and CXCR2 has been documented in various cancers (23;24). The serum of patients with advanced stages of breast cancer has been reported to have higher levels of various CXC chemokines (25;26). Elevated levels of CXCL8 have been linked to poor prognosis, metastasis and angiogenesis in breast cancer (27–29). It has also been shown that the level of CXCL8, a CXC chemokine, increases in a dose dependent manner in breast cancer cells in response to chemotherapeutic agents suggesting that it might be crucial for providing resistance to these cells (30). However their role in therapy-resistance remains unclear. Recent reports showed that levels of CXCL1, CXCL6 and CXCL8 increase after chemotherapy (17;30–32) and this has been implicated to be responsible for therapy resistance in breast cancer cells. We and others have shown that the level of CXCL8 increase in breast cancer and melanoma cells after chemotherapeutic treatment (17). We also found that CXCL1 increases in a similar manner. This suggests the importance of CXCR2-mediated signaling in advance stages of breast cancer and therapy resistance. Moreover, CXCR2 has been shown to be expressed in various cancers including breast cancer (33–37), where it enhances malignant cell proliferation and survival.
CXCL1 and CXCL5, both CXCR2 ligand have recently been shown to promote the migration of PyMT mammary cancer cell lines when they were treated with conditioned media (containing CXCL1 and CXCL5) derived from mesenchymal stem cells, suggesting that CXCR2 and its ligands are important in breast cancer (38). Based on published reports and our preliminary observations, we have targeted CXCR2 receptor, considering blocking this receptor will prevent chemotherapy-induced CXCR2 ligands-dependent cellular responses in breast cancer cells. NFκB, one of the downstream targets of CXCR2 mediated signaling has been shown to translocate to the nucleus upon chemotherapeutic treatment (39;40). NFκB has been reported in various cancers to help cancer cells escape apoptosis and enhance proliferation. Increased NFκB activity along with the promotion of anti-apoptotic gene transcription has been observed after treatment of prostate cancer cells with chemotherapeutic agents (41). Studies also indicate that a complex cooperation exists between NFκB and high expression of CXCL8 in invasive breast cancer cells (42–44). While many pathways may mediate resistance to chemotherapy, on the basis of published data and our own observations we propose NFκB to be a potential candidate for driving anti-apoptotic gene transcription. The role of CXCR2-mediated signaling and NFκB in regulating apoptosis, suggests that they might be interconnected in inducing chemotherapy resistance in cancers. We analyzed the effect of blocking CXCR2 signaling in murine mammary tumor cell lines on their sensitivity towards paclitaxel and doxorubicin. Our results implicate that CXCR2 knockdown in murine mammary tumor cells enhances their sensitivity toward these drugs and increases apoptosis in CXCR2 knockdown cells suggesting an essential role of CXCR2 in therapy resistance. These results also indicate that using CXCR2 targeting agents in combination with chemotherapeutic drugs may provide a promising strategy to combat drug resistance in breast cancer. The finding that mCXCL1 similar to human CXCL8, expression increases after CXCR2 knockdown provides evidence for a feedback loop operating between the ligand and the receptor. It has been reported earlier that at the protein level the expression of CXCR1 and CXCR2 are co-regulated as well as trans-regulated (45;46). CXCR1 similar to CXCR2 binds to CXCL6 and CXCL8. In prostate cancer depletion of CXCR1 was found to down-regulate CXCR2 suggesting that in prostate cancer CXCR1 regulates CXCR2 expression (47). We also observed a lower expression of CXCR1 in the tumors formed by CXCR2 knockdown cells (data not shown) suggesting that in mammary tumor cells CXCR2 expression influences CXCR1 expression. Although we didn’t test whether our oligos were targeting both CXCR1 and CXCR2 in the cells and hence the mechanism responsible for the regulation of CXCR1 and CXCR2 expression in mammary tumor cells still needs to be further investigated. Furthermore, it has been reported that treatment of MCF-7 cells with chemotherapeutic agents like floxuridine results in the release of CXCL8 in a dose dependent manner (17). We have observed that paclitaxel and doxorubicin also elicit a similar response releasing CXCL1 and 8 in other breast cancer cells. The exact mechanism for this response is not known, however, it has been proposed that persistent DNA damage signaling might trigger secretion of inflammatory cytokines like CXCL8 and IL6 in cancer cells (48).
Paclitaxel treatment alone played a marked role in reducing the proliferation of mammary tumor cells in vivo both in mice harboring wild-type as well as knockdown tumors (data not shown). CXCR2 knockdown alone decreases proliferation of mammary tumor cells in vivo and the reduction in tumor growth in tumors formed by CXCR2 knockdown cells when treated with paclitaxel suggest a joint effect of CXCR2 knockdown and paclitaxel treatment. Our laboratory has reported that CXCR2 knockdown in mammary tumor cells increases apoptosis and decreases angiogenesis in mammary tumors (22). We found similar results in this model system with a further reduction in angiogenesis after paclitaxel treatment when combine with CXCR2 knockdown. We observed that paclitaxel itself does not have any role in controlling angiogenesis in our model system. These observations suggest that both CXCR2 and paclitaxel have separate effects in decreasing mammary tumor growth and when combined together paclitaxel increases the effect of CXCR2 knockdown in reducing microvessel density in mammary tumors.
To conclude, our data implicate that blocking CXCR2 signaling in mammary tumor cells makes them sensitive toward chemotherapeutic agents, by decreasing their survival and increasing apoptosis. Moreover, we report that blocking CXCR2 in combination with paclitaxel decreases mammary tumor growth, metastatic lung nodule formation and reduces angiogenesis in mammary tumors. This study also proposes that targeting CXCR2 in an adjuvant setting in mammary tumors may provide an effective strategy to reduce therapy resistance.
Supplementary Material
Acknowledgments
This work was supported in part by Susan G. Komen for the Cure grant KG090860 (R.K.Singh), COBRE grant RR021937 (Nebraska Center for Nanomedicine)(R.K.Singh), and Cancer Center Support Grant (P30CA036727) from National Cancer Institute, and National Institutes of Health. Bhawna Sharma is supported through University of Nebraska Medical Center Graduate Student Fellowship/Assistantship.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
Reference List
- 1.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000 Aug 17;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 2.Sotiriou C, Neo SY, McShane LM, Korn EL, Long PM, Jazaeri A, et al. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci U S A. 2003 Sep 2;100:10393–8. doi: 10.1073/pnas.1732912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gatenby RA, Silva AS, Gillies RJ, Frieden BR. Adaptive therapy. Cancer Res. 2009 Jun 1;69:4894–903. doi: 10.1158/0008-5472.CAN-08-3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007;608:1–22. doi: 10.1007/978-0-387-74039-3_1. [DOI] [PubMed] [Google Scholar]
- 5.Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008 May 7;100:672–9. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 6.Zielske SP, Spalding AC, Wicha MS, Lawrence TS. Ablation of breast cancer stem cells with radiation. Transl Oncol. 2011 Aug;4:227–33. doi: 10.1593/tlo.10247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cordon-Cardo C, Prives C. At the crossroads of inflammation and tumorigenesis. J Exp Med. 1999 Nov 15;190:1367–70. doi: 10.1084/jem.190.10.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez-Novoa JM, Nieto MA. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med. 2009 Sep;1:303–14. doi: 10.1002/emmm.200900043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hudson JD, Shoaibi MA, Maestro R, Carnero A, Hannon GJ, Beach DH. A proinflammatory cytokine inhibits p53 tumor suppressor activity. J Exp Med. 1999 Nov 15;190:1375–82. doi: 10.1084/jem.190.10.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang JM, Deng X, Gong W, Su S. Chemokines and their role in tumor growth and metastasis. J Immunol Methods. 1998 Nov 1;220:1–17. doi: 10.1016/s0022-1759(98)00128-8. [DOI] [PubMed] [Google Scholar]
- 11.Frommel TO, Zarling EJ. Chronic inflammation and cancer: potential role of Bcl-2 gene family members as regulators of cellular antioxidant status. Med Hypotheses. 1999 Jan;52:27–30. doi: 10.1054/mehy.1997.0621. [DOI] [PubMed] [Google Scholar]
- 12.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 13.Wang JM, Deng X, Gong W, Su S. Chemokines and their role in tumor growth and metastasis. J Immunol Methods. 1998 Nov 1;220:1–17. doi: 10.1016/s0022-1759(98)00128-8. [DOI] [PubMed] [Google Scholar]
- 14.Vandercappellen J, Van Damme J, Struyf S. The role of CXC chemokines and their receptors in cancer. Cancer Letters. 2008 Aug 28;267:226–44. doi: 10.1016/j.canlet.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 15.Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- 16.Waugh DJJ, Wilson C. The Interleukin-8 Pathway in Cancer. Clinical Cancer Research. 2008 Nov 1;14:6735–41. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 17.Lev DC, Ruiz M, Mills L, McGary EC, Price JE, Bar-Eli M. Dacarbazine causes transcriptional up-regulation of interleukin 8 and vascular endothelial growth factor in melanoma cells: a possible escape mechanism from chemotherapy. Mol Cancer Ther. 2003 Aug;2:753–63. [PubMed] [Google Scholar]
- 18.Aslakson CJ, Miller FR. Selective Events in the Metastatic Process Defined by Analysis of the Sequential Dissemination of Subpopulations of a Mouse Mammary Tumor. Cancer Res. 1992 Mar 15;52:1399–405. [PubMed] [Google Scholar]
- 19.Wilson TJ, Nannuru KC, Futakuchi M, Sadanandam A, Singh RK. Cathepsin G Enhances Mammary Tumor-Induced Osteolysis by Generating Soluble Receptor Activator of Nuclear Factor-{kappa}B Ligand. Cancer Res. 2008 Jul 15;68:5803–11. doi: 10.1158/0008-5472.CAN-07-5889. [DOI] [PubMed] [Google Scholar]
- 20.Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003 Mar 15;170:3369–76. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- 21.Varney ML, Johansson SL, Singh RK. Distinct expression of CXCL8 and its receptors CXCR1 and CXCR2 and their association with vessel density and aggressiveness in malignant melanoma. Am J Clin Pathol. 2006 Feb;125:209–16. doi: 10.1309/VPL5-R3JR-7F1D-6V03. [DOI] [PubMed] [Google Scholar]
- 22.Nannuru KC, Sharma B, Varney ML, Singh RK. Role of chemokine receptor CXCR2 expression in mammary tumor growth, angiogenesis and metastasis. J Carcinog. 2011;10:40. doi: 10.4103/1477-3163.92308. Epub@2011 Dec 31.:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vicari AP, Caux C. Chemokines in cancer. Cytokine Growth Factor Rev. 2002 Apr;13:143–54. doi: 10.1016/s1359-6101(01)00033-8. [DOI] [PubMed] [Google Scholar]
- 24.Zlotnik A. Chemokines in neoplastic progression. Semin Cancer Biol. 2004 Jun;14:181–5. doi: 10.1016/j.semcancer.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Benoy IH, Salgado R, Van DP, Geboers K, Van ME, Scharpe S, et al. Increased serum interleukin-8 in patients with early and metastatic breast cancer correlates with early dissemination and survival. Clin Cancer Res. 2004 Nov 1;10:7157–62. doi: 10.1158/1078-0432.CCR-04-0812. [DOI] [PubMed] [Google Scholar]
- 26.Kozlowski L, Zakrzewska I, Tokajuk P, Wojtukiewicz MZ. Concentration of interleukin-6 (IL-6), interleukin-8 (IL-8) and interleukin-10 (IL-10) in blood serum of breast cancer patients. Rocz Akad Med Bialymst. 2003;48:82–4. [PubMed] [Google Scholar]
- 27.Raman D, Baugher PJ, Thu YM, Richmond A. Role of chemokines in tumor growth. Cancer Lett. 2007 Oct 28;256:137–65. doi: 10.1016/j.canlet.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Addison CL, Daniel TO, Burdick MD, Liu H, Ehlert JE, Xue YY, et al. The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokine-induced angiogenic activity. J Immunol. 2000 Nov 1;165:5269–77. doi: 10.4049/jimmunol.165.9.5269. [DOI] [PubMed] [Google Scholar]
- 29.Heidemann J, Ogawa H, Dwinell MB, Rafiee P, Maaser C, Gockel HR, et al. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem. 2003 Mar 7;278:8508–15. doi: 10.1074/jbc.M208231200. [DOI] [PubMed] [Google Scholar]
- 30.De Larco JE, Wuertz BR, Manivel JC, Furcht LT. Progression and enhancement of metastatic potential after exposure of tumor cells to chemotherapeutic agents. Cancer Res. 2001 Apr 1;61:2857–61. [PubMed] [Google Scholar]
- 31.De Larco JE, Wuertz BR, Rosner KA, Erickson SA, Gamache DE, Manivel JC, et al. A potential role for interleukin-8 in the metastatic phenotype of breast carcinoma cells. Am J Pathol. 2001 Feb;158:639–46. doi: 10.1016/S0002-9440(10)64005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maxwell PJ, Gallagher R, Seaton A, Wilson C, Scullin P, Pettigrew J, et al. HIF-1 and NF-kappaB-mediated upregulation of CXCR1 and CXCR2 expression promotes cell survival in hypoxic prostate cancer cells. Oncogene. 2007 Nov 15;26:7333–45. doi: 10.1038/sj.onc.1210536. [DOI] [PubMed] [Google Scholar]
- 33.Miller LJ, Kurtzman SH, Wang Y, Anderson KH, Lindquist RR, Kreutzer DL. Expression of interleukin-8 receptors on tumor cells and vascular endothelial cells in human breast cancer tissue. Anticancer Res. 1998 Jan;18:77–81. [PubMed] [Google Scholar]
- 34.Richards BL, Eisma RJ, Spiro JD, Lindquist RL, Kreutzer DL. Coexpression of interleukin-8 receptors in head and neck squamous cell carcinoma. Am J Surg. 1997 Nov;174:507–12. doi: 10.1016/s0002-9610(97)00165-7. [DOI] [PubMed] [Google Scholar]
- 35.Norgauer J, Metzner B, Schraufstatter I. Expression and growth-promoting function of the IL-8 receptor beta in human melanoma cells. J Immunol. 1996 Feb 1;156:1132–7. [PubMed] [Google Scholar]
- 36.Takamori H, Oades ZG, Hoch OC, Burger M, Schraufstatter IU. Autocrine growth effect of IL-8 and GROalpha on a human pancreatic cancer cell line, Capan-1. Pancreas. 2000 Jul;21:52–6. doi: 10.1097/00006676-200007000-00051. [DOI] [PubMed] [Google Scholar]
- 37.Venkatakrishnan G, Salgia R, Groopman JE. Chemokine receptors CXCR-1/2 activate mitogen-activated protein kinase via the epidermal growth factor receptor in ovarian cancer cells. J Biol Chem. 2000 Mar 10;275:6868–75. doi: 10.1074/jbc.275.10.6868. [DOI] [PubMed] [Google Scholar]
- 38.Halpern JL, Kilbarger A, Lynch CC. Mesenchymal stem cells promote mammary cancer cell migration in vitro via the CXCR2 receptor. Cancer Lett. 2011 Sep 1;308:91–9. doi: 10.1016/j.canlet.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang TT, Wuerzberger-Davis SM, Seufzer BJ, Shumway SD, Kurama T, Boothman DA, et al. NF-kappaB activation by camptothecin. A linkage between nuclear DNA damage and cytoplasmic signaling events. J Biol Chem. 2000 Mar 31;275:9501–9. doi: 10.1074/jbc.275.13.9501. [DOI] [PubMed] [Google Scholar]
- 40.Brea-Calvo G, Siendones E, Sanchez-Alcazar JA, de CR, Navas P. Cell survival from chemotherapy depends on NF-kappaB transcriptional up-regulation of coenzyme Q biosynthesis. PLoS One. 2009;4:e5301. doi: 10.1371/journal.pone.0005301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson C, Purcell C, Seaton A, Oladipo O, Maxwell PJ, O’Sullivan JM, et al. Chemotherapy-Induced CXC-Chemokine/CXC-Chemokine Receptor Signaling in Metastatic Prostate Cancer Cells Confers Resistance to Oxaliplatin through Potentiation of Nuclear Factor-{kappa}B Transcription and Evasion of Apoptosis. Journal of Pharmacology And Experimental Therapeutics. 2008 Dec 1;327:746–59. doi: 10.1124/jpet.108.143826. [DOI] [PubMed] [Google Scholar]
- 42.Kawada K, Sonoshita M, Sakashita H, Takabayashi A, Yamaoka Y, Manabe T, et al. Pivotal role of CXCR3 in melanoma cell metastasis to lymph nodes. Cancer Res. 2004 Jun 1;64:4010–7. doi: 10.1158/0008-5472.CAN-03-1757. [DOI] [PubMed] [Google Scholar]
- 43.De Larco JE, Wuertz BR, Rosner KA, Erickson SA, Gamache DE, Manivel JC, et al. A potential role for interleukin-8 in the metastatic phenotype of breast carcinoma cells. Am J Pathol. 2001 Feb;158:639–46. doi: 10.1016/S0002-9440(10)64005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie JH, Nomura N, Lu M, Chen SL, Koch GE, Weng Y, et al. Antibody-mediated blockade of the CXCR3 chemokine receptor results in diminished recruitment of T helper 1 cells into sites of inflammation. J Leukoc Biol. 2003 Jun;73:771–80. doi: 10.1189/jlb.1102573. [DOI] [PubMed] [Google Scholar]
- 45.Richardson RM, Pridgen BC, Haribabu B, Ali H, Snyderman R. Differential cross-regulation of the human chemokine receptors CXCR1 and CXCR2. Evidence for time-dependent signal generation. J Biol Chem. 1998 Sep 11;273:23830–6. doi: 10.1074/jbc.273.37.23830. [DOI] [PubMed] [Google Scholar]
- 46.Attal H, Cohen-Hillel E, Meshel T, Wang JM, Gong W, Ben-Baruch A. Intracellular cross-talk between the GPCR CXCR1 and CXCR2: role of carboxyl terminus phosphorylation sites. Exp Cell Res. 2008 Jan 15;314:352–65. doi: 10.1016/j.yexcr.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 47.Shamaladevi N, Lyn DA, Escudero DO, Lokeshwar BL. CXC receptor-1 silencing inhibits androgen-independent prostate cancer. Cancer Res. 2009 Nov 1;69:8265–74. doi: 10.1158/0008-5472.CAN-09-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Munoz DP, Raza SR, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009 Aug;11:973–9. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.