Abstract
Objective
An association between rheumatoid factor (RF) and increased mortality has been described in individuals with rheumatoid arthritis. The objective of this study was to determine the effect of RF on mortality and coronary heart disease (CHD) in the general population.
Methods
Subjects were participants in a population-based study focused on cardiovascular disease who attended for a study visit during the years 1974–84. RF was measured and information obtained on cardiovascular risk factors, joint symptoms and erythrocyte sedimentation rate (ESR). The subjects were followed with respect to mortality and incident CHD through 2005. Adjusted comparison of overall survival and CHD event-free survival in RF-positive versus RF-negative subjects was performed using Cox proportional hazards regression models.
Results
Of 11 872 subjects, 140 had positive RF. At baseline RF was associated with diabetes mellitus and smoking and inversely associated with serum cholesterol. RF-positive subjects had increased all-cause mortality (HR 1.47, 95% CI 1.19 to 1.80) and cardiovascular mortality (HR 1.57, 95% CI 1.15 to 2.14) after adjusting for age and sex. Further adjustment for cardiovascular risk factors and ESR only modestly attenuated this effect. An increase in CHD among the RF-positive subjects did not reach statistical significance (HR 1.32, 95% CI 0.96 to 1.81, adjusted for age and sex). Subjects with RF but without joint symptoms also had increased overall mortality and cardiovascular mortality (HR for overall mortality 1.33, 95% CI 1.01 to 1.74, after adjustment).
Conclusion
In a general population cohort, RF was associated with increased all-cause mortality and cardiovascular mortality after adjustment for cardiovascular risk factors, even in subjects without joint symptoms.
INTRODUCTION
There is increased mortality among individuals with rheumatoid arthritis (RA) which is mainly driven by cardiovascular disease. Epidemiological studies have found remarkably similar effects with about a twofold increase in mortality in people with RA,1–5 and an increase in cardiovascular events has also been found.6–8
In subjects with RA, rheumatoid factor (RF) has been associated with increased mortality.9–11 A study in people without chronic arthritis described an association between high-titre RF and cardiovascular mortality.12 Apart from that, it is not known whether RF unrelated to RA is associated with adverse health outcomes. In 1974–84 RF was measured in all subjects who came for a clinic visit to the Reykjavik Study regardless of musculoskeletal symptoms.
The aims of the present study are as follows:
To estimate the effect of RF on all-cause mortality, cardiovascular mortality and incident coronary heart disease (CHD) after adjusting for cardiovascular risk factors.
To compare this effect of RF in persons with and without inflammatory joint symptoms.
To explore whether the effect of RF is mediated by measures of inflammation.
METHODS
Study subjects
The Reykjavik Study is a population-based cohort study which has been described in detail elsewhere.13,14 Briefly, men born in 1907–34 and women born in 1908–35 living in Reykjavik or adjacent communities on 1 December 1966 were invited to participate. Study examinations took place from 1967 to 1996. For this analysis we used data from study visits made in 1974–84 when RF and variables pertaining to cardiovascular disease were measured (figure 1).
Figure 1.
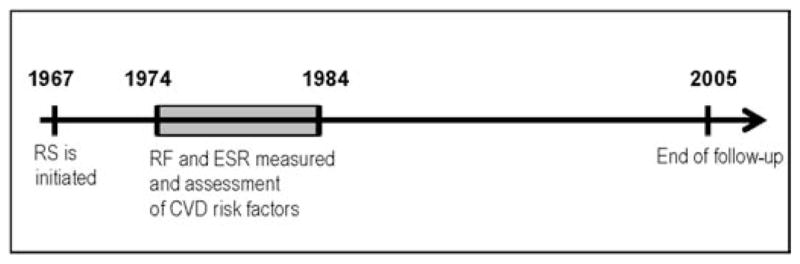
Study design. In 1967 the Reykjavik Study (RS) was initiated. During the period 1974–84 rheumatoid factor (RF) was measured in all study participants. All baseline data obtained for the purpose of this study were obtained during that period (shaded). All subjects were followed prospectively for incident coronary heart disease and death or until 31 December 2005. CVD, cardiovascular disease; ESR, erythrocyte sedimentation rate.
Baseline data
Information on smoking (never, former or current), education (no high school, some high school, some college or college graduate) and medication use was obtained from a questionnaire. Subjects were asked about three musculoskeletal symptoms during the preceding 12 months: joint pain, joint swelling and morning stiffness. Inflammatory joint symptoms were defined as answering ‘yes’ to all three questions. Those who answered ‘no’ to all three questions were considered to have no joint symptoms. Blood pressure was measured in a standardised fashion and hypertension defined as systolic blood pressure ≥140 mm Hg or use of medications to treat high blood pressure. Diabetes mellitus was defined as fasting blood glucose ≥6.05 mmol/l or the use of insulin or oral medications to treat diabetes. Height and weight were measured and body mass index (BMI = weight (kg)/height (m2)) calculated.
Blood was drawn (after overnight fasting) and total cholesterol, triglycerides and blood glucose measured. Erythrocyte sedimentation rate (ESR) was measured according to the Westergren method. RF was measured in two stages: first, by sheep red blood cell agglutination slide test (Rheumaton; Wampole Laboratories, Princeton, New Jersey, USA); agglutination was visually inspected and graded on an ordinal scale (0–3). Samples with agglutination of 2 or 3 were tested further using the Rose-Waaler technique15 and a titre of ≥1/10 (lowest titre measured) was considered positive. Those with a positive Rose-Waaler test were considered RF-positive in this study and all other subjects were RF-negative.
Study outcomes
The main study outcomes were all-cause mortality, cardiovascular mortality and incident CHD. The definition of cardiovascular mortality used for this study was adopted from the Systematic Coronary Risk Evaluation project16; CHD included myocardial infarction (MI), coronary artery bypass grafting, percutaneous coronary intervention or sudden cardiac death. Diagnostic criteria for MI included clinical symptoms, typical changes on an ECG, enzyme activity and signs of possible or definite MI on autopsy. The quality of the registry of MIs in the Reykjavik Study has been subject to external oversight and its accuracy has been found to be excellent.17 Those with a history of a CHD-defining event at baseline were excluded.
Statistical analysis
Baseline characteristics were compared between RF-positive and RF-negative subjects. A t test and Wilcoxon rank sum test were used for normally and non-normally continuously distributed variables, respectively. A χ2 test was used for dichotomous and ordinal variables.
Study subjects were followed from study entry to death or until 31 December 2005. Kaplan–Meier curves were constructed according to RF status for overall survival, survival until cardiovascular death and CHD-free survival. Crude differences between the groups were compared with the log rank test. To explore the effect of RF after adjusting for age, sex, cardiovascular risk factors and ESR, Cox proportional hazards regression models were constructed with all-cause mortality, cardiovascular mortality and CHD events as outcome measures. The effect of RF on outcomes was also assessed in the presence and absence of joint symptoms. In additional analysis the effect of RF on mortality using different thresholds for positive RF was explored. Effects of variables on time to outcomes are expressed as HRs with 95% CI.
The assumption of proportional hazards between RF-positive and RF-negative subjects was evaluated by a visual inspection of a plot of the minus log of the log survival against log time, and by testing an interaction term between time and RF status for statistical significance. Neither method suggested violation of the proportional hazards assumption. SAS Version 9.1 (SAS Institute, Cary, North Carolina, USA) was used for statistical analyses.
RESULTS
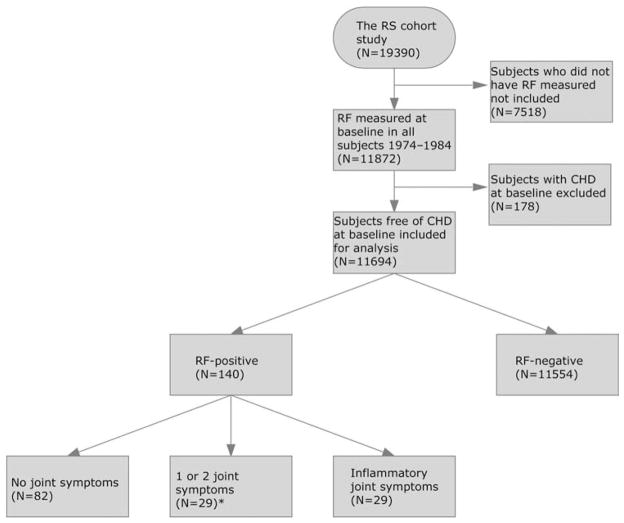
RF was measured in 11 872 subjects, of whom 178 subjects had prevalent CHD at baseline. There was no difference in RF in subjects with and without CHD at baseline. After exclusion of subjects with CHD at baseline, 11 694 subjects were included for analysis (figure 2).
Figure 2.
Exclusion process and groups included for analysis. Subgroups of RF-positive subjects with no joint symptoms and with inflammatory joint symptoms were compared with all RF-negative subjects. *No analysis was performed on this subgroup specifically. CHD, coronary heart disease; inflammatory joint symptoms, answering ‘yes’ to all three questions on musculoskeletal symptoms (joint pain, joint swelling and morning stiffness); RF, rheumatoid factor; RS, Reykjavik Study.
Baseline characteristics
At baseline, 1292 (10.8%) subjects screened positive for RF with the agglutination test (agglutination scored 2 or 3) and 140 of these (1.2% of the whole population) were positive for RF according to the Rose-Waaler test. RF-positive subjects were older than RF-negative subjects (58.2 years vs 55.4 years, p<0.001; table 1).
Table 1.
Baseline characteristics of RF-negative and RF-positive subjects
| RF-negative (n=11554) | RF-positive (n=140) | Significance (p value) | |
|---|---|---|---|
| Mean (SD) age (years) | 55.4 (7.33) | 58.2 (7.89) | <0.001 |
| Female sex (%) | 5559 (48.1) | 75 (53.6) | 0.19 |
| Smoking (%) | 0.03 | ||
| Never | 3922 (34.0) | 33 (23.6) | |
| Former | 2405 (20.8) | 30 (21.4) | |
| Current | 5527 (45.2) | 77 (55.0) | |
| Hypertension (%) | 4543 (39.3) | 49 (35.0) | 0.30 |
| Diabetes mellitus (%) | 401 (3.5) | 11 (7.9) | 0.005 |
| Mean (SD) cholesterol (mmol/l) | 6.44 (1.10) | 6.18 (1.22) | 0.01 |
| Triglycerides (mmol/l)* | 1.04 (0.8–1.4) | 1.03 (0.7–1.4) | 0.29 |
| Mean (SD) BMI (kg/m2) | 25.4 (3.85) | 25.3 (4.31) | 0.75 |
| Education (%) | 0.36 | ||
| No high school | 5011 (43.4) | 71 (50.7) | |
| Some high school | 4712 (40.8) | 51 (36.4) | |
| Some college | 1174 (10.2) | 11 (7.9) | |
| College graduate | 657 (5.7) | 7 (5.0) | |
| ESR* | 5 (2–10) | 6.5 (3–16) | 0.002 |
| Musculoskeletal symptoms | |||
| Joint pain | 3087 (26.7) | 50 (35.1) | 0.02 |
| Joint swelling | 1396 (12.1) | 33 (23.6) | <0.001 |
| Morning stiffness | 2718 (23.5) | 48 (34.2) | 0.003 |
| All joint symptoms (=inflammatory joint symptoms) | 947 (8.2) | 29 (20.7) | <0.001 |
| No joint symptoms | 7598 (65.8) | 82 (58.6) | 0.07 |
Presented as total counts with percentages in parentheses or mean (SD).
Not normally distributed and presented as median (IQR).
BMI, body mass index; ESR, erythrocyte sedimentation rate; RF, rheumatoid factor.
Risk factors and musculoskeletal symptoms according to RF status
Diabetes mellitus and current smoking were more common in RF-positive subjects than in RF-negative subjects (7.9% vs 3.5%, p=0.005 and 55.0% vs 45.2%, p=0.03, respectively). However, the RF-positive subjects had lower cholesterol than the RF-negative subjects (6.18 mmol/l vs 6.43 mmol/l, p=0.01). All musculoskeletal symptoms were more common in RF-positive subjects although most of them (59%) had none (this compares with 65% in RF-negative subjects; table 1).
Survival analysis
Effect of RF
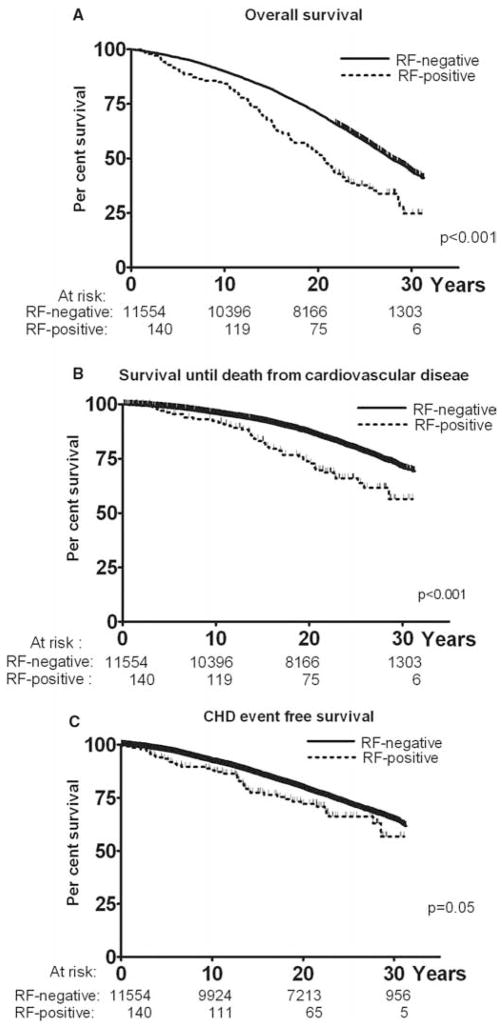
During the study period (median follow-up 23.1 years, range 8 days to 31.3 years) 5975 subjects (51.1%) died. RF was associated with increased all-cause mortality (p<0.001; figure 3). After adjustment for age and sex, subjects with RF had increased mortality (HR 1.47, 95% CI 1.19 to 1.80). Further adjustment for cardiovascular risk factors resulted in a small decrease in this estimate (HR 1.40, 95% CI 1.14 to 1.72). Yet further adjustment for ESR resulted in a modest further reduction in the effect estimate (HR 1.31, 95% CI 1.06 to 1.61; table 2).
Figure 3.
Kaplan–Meier curves according to rheumatoid factor (RF) status. (A) Overall survival. (B) Survival until death from cardiovascular disease. (C) Coronary heart disease (CHD) event-free survival. Vertical bars represent censoring.
Table 2.
Effect of RF, cardiovascular risk factors and inflammation on all-cause mortality
| Model 1: Adjustment for age and sex | Model 2: Further adjustment for smoking | Model 3: Further adjustment for other cardiovascular risk factors | Model 4: Further adjustment for ESR | |
|---|---|---|---|---|
| RF | 1.47 (1.19 to 1.80) | 1.38 (1.12 to 1.70) | 1.40 (1.14 to 1.72) | 1.31 (1.06 to 1.61) |
| Age (years) | 1.12 (1.11 to 1.12) | 1.12 (1.12 to 1.13) | 1.11 (1.11 to 1.12) | 1.11 (1.11 to 1.11) |
| Male sex | 1.72 (1.63 to 1.81) | 1.60 (1.51 to 1.69) | 1.58 (1.49 to 1.68) | 1.73 (1.63 to 1.84) |
| Tobacco use | ||||
| Never smoked | Referent | Referent | Referent | |
| Former smoker | 1.14 (1.05 to 1.23) | 1.12 (1.04 to 1.21) | 1.12 (1.03 to 1.21) | |
| Current smoker | 1.75 (1.65 to 1.87) | 1.75 (1.65 to 1.87) | 1.72 (1.62 to 1.84) | |
| Hypertension | 1.26 (1.19 to 1.33) | 1.24 (1.17 to 1.31) | ||
| Cholesterol (mmol/l) | 1.00 (0.98 to 1.03) | 0.99 (0.97 to 1.02) | ||
| Diabetes mellitus | 1.73 (1.54 to 1.94) | 1.68 (1.50 to 1.88) | ||
| Triglycerides | 1.13 (1.08 to 1.17) | 1.11 (1.07 to 1.16) | ||
| BMI | 1.00 (0.99 to 1.01) | 1.00 (0.99 to 1.00) | ||
| Education | ||||
| No high school | Referent | Referent | ||
| Some high school | 0.83 (0.78 to 0.88) | 0.84 (0.79 to 0.89) | ||
| Some college | 0.78 (0.71 to 0.86) | 0.79 (0.72 to 0.87) | ||
| College graduate | 0.77 (0.68 to 0.88) | 0.78 (0.68 to 0.88) | ||
| ESR (10 mm/h) | 1.19 (1.15 to 1.22) | |||
Results from four Cox proportional hazards models are expressed. Data presented as HRs with 95% CI.
BMI, body mass index; ESR, erythrocyte sedimentation rate; RF, rheumatoid factor.
A total of 2385 subjects (20.4%) died from cardiovascular disease and this rate was higher among RF-positive subjects than RF-negative subjects (p<0.001; figure 3). After adjustment for age, sex and cardiovascular risk factors, this difference remained significant (HR 1.57, 95% CI 1.15 to 2.14). Further adjustment for ESR resulted in minimal change in this estimate (HR 1.45, 95% CI 1.07 to 1.98).
During the follow-up period 3335 subjects (28.5%) died from causes other than cardiovascular disease. This non-cardiovascular mortality was also associated with positive RF status (HR 1.40, 95% CI 1.06 to 1.85) adjusted for age and sex (table 3).
Table 3.
Effect of RF with and without joint symptoms on mortality, cardiovascular mortality, non-cardiovascular mortality and incident CHD
| Model 1: Adjustment for age and sex | Model 2: Further adjustment for smoking | Model 3: Further adjustment for cardiovascular risk factors | Model 4: Further adjustment for ESR | |
|---|---|---|---|---|
| Effects of RF (140 subjects with positive RF) | ||||
| All-cause mortality | 1.47 (1.19 to 1.80) | 1.38 (1.12 to 1.70) | 1.40 (1.14 to 1.72) | 1.31 (1.06 to 1.61) |
| Cardiovascular mortality | 1.57 (1.15 to 2.13) | 1.47 (1.08 to 2.00) | 1.57 (1.15 to 2.14) | 1.45 (1.07 to 1.98) |
| Non-cardiovascular mortality | 1.40 (1.06 to 1.85) | 1.32 (1.00 to 1.74) | 1.29 (0.97 to 1.70) | 1.20 (0.91 to 1.59) |
| Incident CHD | 1.32 (0.96 to 1.81) | 1.25 (0.91 to 1.72) | 1.33 (0.97 to 1.83) | 1.24 (0.90 to 1.70) |
| Effect of positive RF and no joint symptoms (82 subjects with positive RF and no joint symptoms) | ||||
| All-cause mortality | 1.29 (0.98 to 1.70) | 1.24 (0.95 to 1.63) | 1.30 (0.99 to 1.71) | 1.33 (1.01 to 1.74) |
| Cardiovascular mortality | 1.45 (0.98 to 2.15) | 1.40 (0.94 to 2.07) | 1.57 (1.05 to 2.32) | 1.60 (1.08 to 2.37) |
| Non-cardiovascular mortality | 1.17 (0.80 to 1.72) | 1.13 (0.77 to 1.65) | 1.12 (0.77 to 1.64) | 1.14 (0.78 to 1.67) |
| Incident CHD | 1.20 (0.80 to 1.81) | 1.16 (0.77 to 1.75) | 1.28 (0.85 to 1.93) | 1.30 (0.86 to 1.97) |
| Effect of positive RF and inflammatory joint symptoms (29 subjects with positive RF and inflammatory joint symptoms) | ||||
| All-cause mortality | 2.25 (1.45 to 3.50) | 2.04 (1.31 to 3.17) | 2.24 (1.44 to 3.48) | 1.56 (1.00 to 2.43) |
| Cardiovascular mortality | 1.41 (0.58 to 3.38) | 1.26 (0.52 to 3.03) | 1.55 (0.64 to 3.72) | 1.06 (0.44 to 2.56) |
| Non-cardiovascular mortality | 2.84 (1.71 to 4.71) | 2.59 (1.56 to 4.30) | 2.63 (1.58 to 4.36) | 1.87 (1.11 to 3.14) |
| Incident CHD | 2.34 (1.22 to 4.51) | 2.14 (1.11 to 4.12) | 2.54 (1.32 to 4.88) | 1.76 (0.91 to 3.42) |
Results from four Cox proportional hazards models are expressed. Data presented as HRs with 95% CI.
CHD, coronary heart disease; ESR, erythrocyte sedimentation rate; RF, rheumatoid factor.
Incident CHD occurred in 3049 subjects (26.1%) (median follow-up for CHD 22.8 years, range 3 days to 31.3 years). Positive RF was associated with a borderline decrease in event-free survival (p=0.05; figure 3) which was not significant after adjustment for covariates (table 3).
RF-positive subjects with no joint symptoms
Of 140 subjects with positive RF, 82 had no joint symptoms. In comparison with all RF-negative subjects, this group had modestly increased mortality (HR 1.29, 0.98 to 1.70). Adjustment for cardiovascular risk factors and ESR had a small effect on this estimate which reached statistical significance (HR 1.33, 95% CI 1.01 to 1.74). In this group there was also an increase in cardiovascular mortality (HR 1.45, 95% CI 0.98 to 2.15) which was statistically significant after adjustment for cardiovascular risk factors and ESR (HR 1.60, 95% CI 1.08 to 2.37). There was no increase in incident CHD in this group (HR 1.20, 95% CI 0.80 to 1.81; table 3). Comparison of this subgroup with RF-negative subjects who were also free of joint symptoms gave similar results.
RF-positive subjects with inflammatory joint symptoms
Twenty-nine RF-positive subjects had inflammatory joint symptoms and had markedly increased mortality compared with all RF-negative subjects after adjusting for age, sex and cardiovascular risk factors (HR 2.24, 95% CI 1.44 to 3.48). Further adjustment for ESR resulted in a substantial decrease in this HR estimate (HR 1.56, 95% CI 1.00 to 2.43). Cardiovascular mortality was not significantly increased in this subgroup but both mortality from other causes and incident CHD were (table 3). Comparison of this subgroup with RF-negative subjects who also had inflammatory joint symptoms gave similar results.
Effect of different thresholds for positive RF
Three thresholds for positive RF were tested according to the agglutination screening test (1, 2 and 3). With these definitions of RF, all effects of RF on mortality and CHD were minimal and solely driven by subjects who also had positive RF according to the confirmatory Rose-Waaler test.
DISCUSSION
We have shown that RF is independently associated with increased all-cause mortality and cardiovascular mortality. The increased mortality risks were present even in those without any joint symptoms. RF was not significantly associated with incident CHD but a modestly increased risk cannot be excluded.
RF was measured in a large population cohort regardless of joint symptoms. Other strengths of our approach include a comparison group drawn from the same population, detailed information on cardiovascular risk factors and a measure of inflammation obtained in an identical fashion in all subjects without respect to RF status.
Our study also has some important limitations. RF was measured by the Rose-Waaler method which is now rarely used in clinical practice. To isolate the effect of RF, restricted analysis to RF-positive subjects with no joint symptoms was performed. We expect that by doing so we excluded most subjects with RA. However, it is possible that some subjects who indeed had RA or who developed it after the survey was administered were still included. It is therefore possible that some of our observed effects of RF, even among those with no joint symptoms, are in fact driven by RA.
Our findings add to previous knowledge on the effect of RF on mortality and cardiovascular disease. First, RF was associated with cardiovascular risk factors. RF was positively associated with smoking, as previously reported from this cohort18 and found in other population cohorts and in subjects with RA.19,20 In addition, RF was associated with DM and inversely associated with serum cholesterol levels. Second, there was increased mortality in subjects with RF that was both driven by increased cardiovascular mortality and mortality from other causes. This is in agreement with previous findings reported from this cohort, where positive RF of the IgA isotype was found to be associated with incident malignant disease.21 Third, while isolated positive RF without joint symptoms was associated with mortality, the constellation of RF and inflammatory joint symptoms has a stronger association with mortality and incident CHD than positive RF alone.
How RF leads to vascular disease and mortality remains unclear. Adjustment for risk factors, including separate adjustment for smoking, only minimally attenuated the effect of RF. The effect of RF cannot therefore be explained by smoking being a confounder or as a factor causing positive RF. Inflammation has been found to predict cardiovascular events and mortality13,22,23 and might explain the effects of RF. It is also possible that, in RF-positive subjects, immunological factors have a role that is independent of inflammation: circulating immune complexes have been associated with incident MI24 and it is possible that RF has direct pathological effects on the endothelium.25 Our study suggests that the effect of RF is mediated only to a limited extent by inflammation, at least as it is captured by ESR, but we recognise that ESR might not reflect all inflammatory elements that contribute to cardiovascular risk.
In conclusion, in a general population cohort RF was independently associated with all-cause and cardiovascular mortality, even among subjects without joint symptoms. The mechanism by which RF leads to vascular disease remains to be determined.
Acknowledgments
Funding NIH AR47785.
Footnotes
Ethics approval This study was conducted with the approval of the Boston Medical Center IRB. The conduct of the Reykjavik Study was approved by the National Bioethics Committee of Iceland.
Competing interests None.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Goodson N, Marks J, Lunt M, et al. Cardiovascular admissions and mortality in an inception cohort of patients with rheumatoid arthritis with onset in the 1980s and 1990s. Ann Rheum Dis. 2005;64:1595–601. doi: 10.1136/ard.2004.034777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Symmons DP, Jones MA, Scott DL, et al. Longterm mortality outcome in patients with rheumatoid arthritis: early presenters continue to do well. J Rheumatol. 1998;25:1072–7. [PubMed] [Google Scholar]
- 3.Wallberg-Jonsson S, Ohman ML, Dahlqvist SR. Cardiovascular morbidity and mortality in patients with seropositive rheumatoid arthritis in Northern Sweden. J Rheumatol. 1997;24:445–51. [PubMed] [Google Scholar]
- 4.Wolfe F, Mitchell DM, Sibley JT, et al. The mortality of rheumatoid arthritis. Arthritis Rheum. 1994;37:481–94. doi: 10.1002/art.1780370408. [DOI] [PubMed] [Google Scholar]
- 5.Young A, Koduri G, Batley M, et al. Mortality in rheumatoid arthritis. Increased in the early course of disease, in ischaemic heart disease and in pulmonary fibrosis. Rheumatology (Oxford) 2007;46:350–7. doi: 10.1093/rheumatology/kel253. [DOI] [PubMed] [Google Scholar]
- 6.Solomon DH, Karlson EW, Rimm EB, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107:1303–7. doi: 10.1161/01.cir.0000054612.26458.b2. [DOI] [PubMed] [Google Scholar]
- 7.Maradit-Kremers H, Crowson CS, Nicola PJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 2005;52:402–11. doi: 10.1002/art.20853. [DOI] [PubMed] [Google Scholar]
- 8.del Rincón ID, Williams K, Stern MP, et al. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44:2737–45. doi: 10.1002/1529-0131(200112)44:12<2737::AID-ART460>3.0.CO;2-%23. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez A, Icen M, Kremers HM, et al. Mortality trends in rheumatoid arthritis: the role of rheumatoid factor. J Rheumatol. 2008;35:1009–14. [PMC free article] [PubMed] [Google Scholar]
- 10.Goodson NJ, Wiles NJ, Lunt M, et al. Mortality in early inflammatory polyarthritis: cardiovascular mortality is increased in seropositive patients. Arthritis Rheum. 2002;46:2010–19. doi: 10.1002/art.10419. [DOI] [PubMed] [Google Scholar]
- 11.van Schaardenburg D, Hazes JM, de Boer A, et al. Outcome of rheumatoid arthritis in relation to age and rheumatoid factor at diagnosis. J Rheumatol. 1993;20:45–52. [PubMed] [Google Scholar]
- 12.Heliövaara M, Aho K, Knekt P, et al. Rheumatoid factor, chronic arthritis and mortality. Ann Rheum Dis. 1995;54:811–14. doi: 10.1136/ard.54.10.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andresdottir MB, Sigfusson N, Sigvaldason H, et al. Erythrocyte sedimentation rate, an independent predictor of coronary heart disease in men and women: the Reykjavik Study. Am J Epidemiol. 2003;158:844–51. doi: 10.1093/aje/kwg222. [DOI] [PubMed] [Google Scholar]
- 14.Harris TB, Launer LJ, Eiriksdottir G, et al. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165:1076–87. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waaler E. On the occurence of a factor in human serum activating specifiagglutination of sheep blood corpuscles. Acta Pathol Micribiol Scand. 1940;17:172–88. doi: 10.1111/j.1600-0463.2007.apm_682a.x. [DOI] [PubMed] [Google Scholar]
- 16.Conroy RM, Pyörälä K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. doi: 10.1016/s0195-668x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 17.Tunstall-Pedoe H, Kuulasmaa K, Amouyel P, et al. Myocardial infarction and coronary deaths in the World Health Organization MONICA Project. Registration procedures, event rates, and case-fatality rates in 38 populations from 21 countries in four continents. Circulation. 1994;90:583–612. doi: 10.1161/01.cir.90.1.583. [DOI] [PubMed] [Google Scholar]
- 18.Jónsson T, Thorsteinsson J, Valdimarsson H. Does smoking stimulate rheumatoid factor production in non-rheumatic individuals? APMIS. 1998;106:970–4. doi: 10.1111/j.1699-0463.1998.tb00247.x. [DOI] [PubMed] [Google Scholar]
- 19.Mathews JD, Whittingham S, Hooper BM, et al. Association of autoantibodies with smoking, cardiovascular morbidity, and death in the Busselton population. Lancet. 1973;2:754–8. doi: 10.1016/s0140-6736(73)91037-4. [DOI] [PubMed] [Google Scholar]
- 20.Wolfe F. The effect of smoking on clinical, laboratory, and radiographic status in rheumatoid arthritis. J Rheumatol. 2000;27:630–7. [PubMed] [Google Scholar]
- 21.Jónsson T, Thorsteinsson J, Valdimarsson H. Rheumatoid factor isotypes and cancer prognosis. Cancer. 1992;69:2160–5. doi: 10.1002/1097-0142(19920415)69:8<2160::aid-cncr2820690824>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 22.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–97. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 23.Ridker PM, Hennekens CH, Buring JE, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 24.Mustafa A, Nityanand S, Berglund L, et al. Circulating immune complexes in 50-year-old men as a strong and independent risk factor for myocardial infarction. Circulation. 2000;102:2576–81. doi: 10.1161/01.cir.102.21.2576. [DOI] [PubMed] [Google Scholar]
- 25.Kato H, Yamakawa M, Ogino T. Complement mediated vascular endothelial injury in rheumatoid nodules: a histopathological and immunohistochemical study. J Rheumatol. 2000;27:1839–47. [PubMed] [Google Scholar]