Abstract
Objective
There remains a need for biomarkers to guide therapy in antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis. Our objective was to determine whether measures of platelet activation or inflammation are associated with disease activity in Wegener’s granulomatosis (WG).
Methods
Study subjects were participants in a clinical trial. Soluble CD40 ligand (sCD40L), C-reactive protein, interleukin 6 (IL-6), IL-8, monocyte chemoattractant protein 1 (MCP-1), P-selectin, vascular endothelial growth factor, and proteinase 3 (PR3)-specific ANCA were measured by ELISA using plasma samples obtained at baseline (active disease), at remission, and prior to, during, and after first flares. Disease activity was assessed by the Birmingham Vasculitis Activity Score for WG (BVAS/WG). Association of biomarkers with disease activity was determined with conditional logistic and linear regression.
Results
Over a mean followup of 27 months, 180 subjects underwent 2044 visits; markers were measured in 563 samples. Longitudinally, all markers other than IL-6 were associated with disease activity. The strongest associations for active disease at baseline versus remission were observed for sCD40L (OR 4.72, 95% CI 2.47–9.03), P-selectin (OR 6.26, 95% CI 2.78–14.10), PR3-ANCA (OR 9.41, 4.03–21.99), and inversely for MCP-1 (OR 0.36, 95% CI 0.22–0.57). BVAS/WG increased by 0.80 (95% CI 0.44–1.16), 0.83 (95% CI 0.42–1.25), and 0.81 (95% CI 0.48–1.15) per unit-increase in PR3-ANCA, sCD40L, and P-selectin, respectively; and decreased by 1.54 (95% CI 0.96–2.12) per unit-increase in MCP-1.
Conclusion
Cytokines arising from within the circulation, including those of platelet activation, correlate with disease activity in WG.
Key Indexing Terms: VASCULITIS, BIOMARKERS, DISEASE ACTIVITY, WEGENER’S GRANULOMATOSIS
While most patients with antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis [AAV, Wegener’s granulomatosis (WG)] and microscopic polyangiitis achieve remission of disease, relapses are common and many patients sustain permanent damage from active disease or from toxic effects of treatment1,2,3. Associations between clinical and laboratory features of disease and subsequent relapse are weak4, and no reliable predictor for disease flares is available5,6. Useful biomarkers for prediction of relapse and response to therapy are needed and may provide insight into the pathophysiology of these challenging diseases.
Commonly used acute-phase reactants, such as C-reactive protein (CRP) and interleukin 6 (IL-6) are formed by the liver and activated tissue macrophages as a result of a systemic inflammatory response7. AAV is characterized by inflammation and necrosis occurring in the vascular endothelium8. Therefore, it is possible that cytokines and chemokines arising from within the circulation itself could identify disease activity in AAV better than markers originating from more distant sources. Further, there is increasing evidence for a biologic association between thrombosis and inflammation9,10, and the increased occurrence of venous thrombotic events among patients with AAV was recently described and confirmed11,12,13. We explored whether selected cytokines originating in the circulation, including markers related to platelet activation that might have a role in thrombosis, are associated with disease activity and/or predict disease flares in AAV.
MATERIALS AND METHODS
Study subjects were all participants in the Wegener’s Granulomatosis Etanercept Trial (WGET; Appendix)14, a multicenter, randomized, double-blind, placebo-controlled trial of standard therapy with the addition of etanercept or placebo for patients with active WG. Details of the study design and primary results of the WGET have been published14,15. One hundred eighty subjects were enrolled at a time of active vasculitis and followed for a median of 27 months. Disease activity and specific organ system involvement were assessed at study visits at baseline, 6 weeks, 3 months, and every 3 months thereafter.
Selection of biomarkers for study
Given the reported association between thrombosis and AAV11 and based on a literature review of the relationship between inflammation and thrombosis, 5 cytokines/chemokines arising from within the circulation with associations with platelet activation and inflammatory response were chosen for study: soluble CD40 ligand (sCD40L)16,17, IL-89,10, monocyte chemoattractant protein 1 (MCP-1)18,19, P-selectin20,21, and vascular endothelial growth factor (VEGF)22. Two commonly used markers of inflammation were also selected: C-reactive protein (CRP) and IL-6. These specific biomarkers were chosen because they were the most promising measures that could be tested on the stored plasma samples that had been originally collected with EDTA. Results from all markers measured are reported.
Measurement of biomarkers
Since collection during the WGET study, samples were stored at −80°C. All new measurements reported in our study were performed at a single laboratory: the High Throughput Gene Expression and Protein Analysis Laboratory at the Boston University Medical Campus. For each marker, all samples from individual patients were assayed on a single plate and all 7 markers were measured simultaneously from each subject. Commercial ELISA kits were used for measurements of all biomarkers. For sCD40L, human sCD40L BMS293 (Bender MedSystems, Vienna, Austria) was used. For other markers, the following ELISA kits from R&D Systems (Minneapolis, MN, USA) were used: Quantikine® HS Human C-Reactive Protein Enzyme-Immunoassay DCRP00, Quantikine® HS Human IL-6 Immunoassay HS600B, Quantikine® Human CXCL8/IL-8 Immunoassay D8000C, Quantikine® Human CCL2/MCP-1 Immunoassay DCP00, human soluble P-selectin immunoassay BBE6, and Quantikine® HS Human VEGF Immunoassay DVE00. All assays were used according to manufacturers’ specifications.
Measurement of ANCA titers
Titers of ANCA specific for proteinase-3 (PR3-ANCA) were previously measured in this cohort by immunofluorescence and capture ELISA using mature PR3 antigen as described5,23.
Clinical outcome assessments
Disease activity was assessed at all study visits using 2 instruments: (1) the Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis (BVAS/WG)24, which measures disease activity due to WG within the 4 weeks preceding a study visit by assessing 34 manifestations of disease weighted 1 or 3, resulting in a composite score ranging from 0 (remission) to 68; and (2) a physician’s global assessment (PGA), a visual analog scale scored continuously from 0 (remission) to 100. Remission was defined as BVAS/WG = 0 and flare of disease was defined as the first visit with BVAS > 0 after remission had been achieved. Venous thrombotic events were defined according to a prospective protocol, as described11.
A secondary analysis of the association of marker levels with “granulomatous” versus “vasculitic” manifestations of AAV was conducted. The following components of the BVAS/WG were defined as representing granulomatous disease: mouth ulcers, retroorbital mass/proptosis, bloody nasal discharge/nasal crusting/ulcer, sinus involvement, swollen salivary gland, subglottic inflammation, and conductive deafness. A granulomatous subscore of BVAS/WG was calculated by summing the above granulomatous components. The following components of the BVAS/WG were defined as representing vasculitic disease: purpura, skin ulcer, gangrene, scleritis, retinal exudates/hemorrhage, sensorineural deafness, mesenteric ischemia, alveolar hemorrhage, respiratory failure, hematuria, red blood cell casts, and rise in creatinine or fall in creatinine clearance. A vasculitic subscore of BVAS/WG was calculated by summing these vasculitic components11.
Selection of study samples
Plasma samples were collected at each trial visit. Biomarkers were measured on all subjects at baseline (a time of active disease) and at the 6-month visit (a time when most subjects were in remission). For those subjects who were not in remission at 6 months, a sample at the first visit during remission was chosen. For subjects who experienced a flare of disease, biomarkers were measured from samples from the visit prior to first flare, the visit of first flare, and the first visit after flare, regardless of whether the subject had achieved remission again.
Statistical analysis
At baseline, the cross-sectional association of disease activity (dependent variable) with marker levels (independent variable) was determined with linear regression.
The longitudinal association of biomarkers with disease activity was determined in 2 ways: (1) by comparing the difference in marker levels between baseline and remission with conditional logistic regression; standardized β coefficients were calculated to assess the relative strength of each marker’s association with disease activity; and (2) by multiple linear regression using all samples from all subjects with disease activity as the dependent variable and marker levels as independent variables. For both these sets of analyses, crude associations of markers with active disease versus remission were the primary analysis. In addition, to explore whether candidate markers could differentiate between active disease and remission beyond currently used markers, estimates adjusted for CRP and PR-3 ANCA were also obtained. In addition, a multivariate model with all markers included as predictors was constructed. General estimating equations were used to adjust for multiple samples from each subject.
Subgroup analysis
In a secondary analysis, crude associations of markers with vasculitic and granulomatous manifestations of disease were explored using linear regression, with marker levels as independent variables and the granulomatous-BVAS/WG and vasculitic-BVAS/WG as the outcome variables, respectively.
Although the WGET study did not demonstrate the clinical efficacy of etanercept, it is possible that tumor necrosis factor-α blockade could modify the relationship between marker levels and disease activity25. To assess whether the association between biomarkers and disease activity differed between the etanercept-treated and placebo-treated groups, regression models were run separately in the 2 treatment arms and then by including an interaction term between treatment and marker levels as an independent variable in the regression models.
Predictability of remission was determined by Cox proportional hazards regression models, with time to remission as the outcome variable and biomarker level at baseline as the predictor variable. An analogous approach was used to determine whether markers could predict flare of disease. Tests for proportionality of hazards were assessed by testing an interaction between time and marker levels for significance in the models.
To determine whether biomarkers were associated with thrombosis, logistic regression was done with thrombosis as the outcome measure and marker levels as the independent variable, using general estimating equations to adjust for multiple samples from each subject. Venous thrombotic events (VTE) were represented by a dichotomous variable: a value of 1 at the study visit immediately following a VTE and a value of zero for all other study visits.
All statistical analyses were performed on log-marker levels, except for PR3-ANCA. Results from linear regression are presented as β coefficients with 95% CI, from conditional logistic regression as OR with 95% CI, and from Cox proportional hazards regression as hazard ratio (HR) with 95% CI. A 2-sided α level of 0.05 was considered statistically significant. For the purpose of providing a graphical representation of change in marker levels at baseline, remission, and at times surrounding flares, a nonparametric smoothing curve was generated using the LOESS algorithm. All analyses were performed using SAS, version 9.1 (SAS Inc., Cary, NC, USA).
RESULTS
A total of 180 subjects were enrolled in the WGET study and were seen for a total of 2044 study visits. Samples from 563 of 592 selected visits (95%) were available for the bio-marker measurements.
Cross-sectional association of biomarkers with disease activity
From the time of active disease at baseline, 179 samples were available. IL-8 was significantly associated with disease activity according to BVAS/WG, with a 0.77 increase in BVAS/WG per 1-unit increase in log IL-8 (95% CI 0.23–1.32, p = 0.005), and P-selectin was significantly associated with disease activity according to PGA, with a 6.12 increase in PGA per 1-unit increase in log P-selectin (95% CI 1.13–11.11, p = 0.01).
Marker levels during active disease at baseline, remission, and first flares
One hundred sixty-one of 180 subjects achieved remission at the 6-month visit or later; samples were available for 151 subjects (94%). Five of the 7 markers were positively associated with active disease versus remission (Table 1). Based on the standardized β estimates, sCD40L and P-selectin appeared to have the strongest association with active disease. For sCD40L, the OR for active disease was 4.72 (95% CI 2.47–9.03) per unit-increase in marker level. For P-selectin, the OR for active disease was 6.26 (95% CI 2.78–14.1) per unit-increase in marker level. MCP-1 was inversely associated with disease activity (OR 0.36, 95% CI 0.22–0.57). There was no difference in IL-6 during active disease versus remission (OR 1.04, 95% CI 0.63–1.71). PR3-ANCA was highly significantly associated with active disease versus remission (OR 9.41, 95% CI 4.03–21.99). All markers remained significantly associated with active disease after adjustment for CRP and PR3-ANCA, respectively, with only modest changes in the effect estimates. In a multivariate model with adjustments with all other markers, sCD40L, P-selectin, MCP-1, or PR3-ANCA each remained significantly associated with active disease.
Table 1.
Conditional logistic regression of log-marker levels with active disease vs remission (n = 151). Results expressed as OR for active disease per unit-increase in log-marker level.
| Marker | Crude Association | Adjusted for CRP | Adjusted for PR3-ANCA | Adjusted for All Markers | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | Std β | OR (95% CI) | Std β | OR (95% CI) | Std β | OR (95% CI) | Std β | |
| sCD40L | 4.72 (2.47, 9.03) | 0.75 | 4.26 (2.22, 8.17) | 0.70 | 3.08 (1.60, 5.96) | 0.54 | 3.66 (1.38, 9.72) | 0.62 |
| CRP | 1.54 (1.16, 2.04) | 0.33 | — | 1.47 (1.04, 2.08) | 0.30 | 1.06 (0.59, 1.90) | 0.05 | |
| IL-6 | 1.04 (0.63, 1.71) | 0.01 | 0.79 (0.45, 1.40) | −0.07 | 0.64 (0.34, 1.19) | −0.14 | 0.92 (0.23, 3.65) | −0.02 |
| IL-8 | 1.93 (1.33, 2.80) | 0.33 | 1.68 (1.15, 2.47) | 0.26 | 1.89 (1.22, 2.92) | 0.32 | 3.19 (1.23, 8.28) | 0.59 |
| MCP-1 | 0.36 (0.22, 0.57) | −0.46 | 0.23 (0.13, 0.42) | −0.58 | 0.45 (0.27, 0.76) | −0.35 | 0.13 (0.04, 0.51) | −0.78 |
| P-selectin | 6.26 (2.78, 14.10) | 0.82 | 5.26 (2.27, 12.19) | 0.70 | 3.57 (1.52, 8.41) | 0.57 | 2.54 (0.82, 7.84) | 0.40 |
| VEGF | 2.13 (1.38, 3.29) | 0.44 | 1.77 (1.13, 2.77) | 0.33 | 2.05 (1.21, 3.46) | 0.41 | 1.28 (0.46, 3.61) | 0.14 |
| PR3-ANCA | 9.41 (4.03, 21.99) | 1.01 | 11.86 (4.40, 31.95) | 1.10 | — | 5.56 (1.49, 20.74) | 0.75 | |
Std β: standardized β estimate; sCD40L: soluble CD40 ligand; CRP: C-reactive protein; IL: interleukin; MCP-1: monocyte chemoattractant protein 1; VEGF: vascular endothelial growth factor; PR3-ANCA: antiproteinase 3 antineutrophil cytoplasmic antibody.
The associations of each marker with active versus inactive disease were similar among subjects randomized to etanercept and those randomized to placebo, both according to the analyses conducted separately for each treatment strata and when using an interaction term between marker level and treatment strata in the regression models (data not shown).
Of the 161 subjects who had achieved remission at the 6-month visit or later, 67 had a subsequent flare of disease; samples were available for 50 subjects both at remission and during subsequent flare. Only CRP was significantly associated with active disease during flare versus remission (OR 1.75, 95% CI 1.02–3.01).
Longitudinal association of biomarkers with disease activity
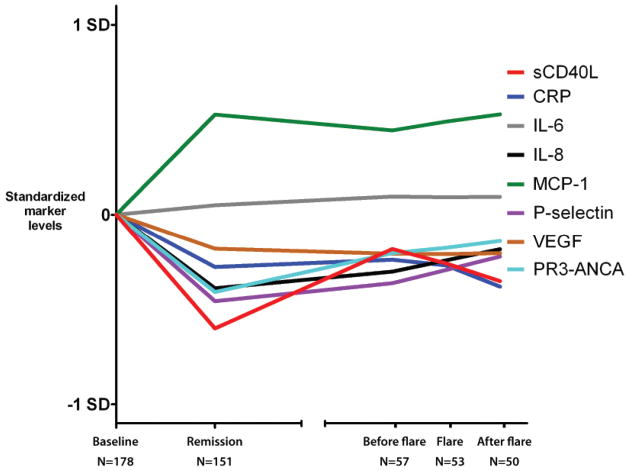
When analyzing all 563 samples from the 180 subjects, there was statistically significant association between disease activity (BVAS/WG score) with all markers except IL-6 (Table 2). P-selectin and sCD40L had the strongest associations with disease activity, with a 0.83 increase in log-sCD40L per unit-increase in BVAS/WG and a 0.81 increase in log P-selectin per unit-increase in BVAS/WG. MCP-1 was inversely associated with disease activity, with a 0.66 decrease in log MCP-1 per 1-unit increase in BVAS/WG. Results did not vary by treatment arm. In models adjusted for CRP or PR3-ANCA, respectively, all markers remained significantly associated with disease activity. In a multiple linear regression model adjusting for other markers, sCD40L, P-selectin, and IL-8 remained positively associated with disease activity, and MCP-1 was inversely associated with disease activity. Analysis using PGA for disease activity did not result in different conclusions about the estimates of association or their statistical significance (data not shown). Figure 1 shows changes in levels of markers at baseline, remission, and time of flare.
Table 2.
Longitudinal association of marker levels with disease activity using the Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis (BVAS/WG). Results expressed as increase in disease activity score on the BVAS/WG per unit-increase in log-marker level.
| Marker | Crude Association | Adjusted for CRP | Adjusted for PR3-ANCA | Adjusted for All Markers | ||||
|---|---|---|---|---|---|---|---|---|
| β Estimate (95% CI) | Std β | β Estimate (95% CI) | Std β | β Estimate (95% CI) | Std β | β Estimate (95% CI) | Std β | |
| sCD40L | 0.83 (0.42, 1.25) | 0.19 | 0.81 (0.39, 1.24) | 0.19 | 0.83 (0.44, 1.22) | 0.20 | 0.66 (0.24, 1.07) | 0.16 |
| CRP | 0.28 (0.09, 0.47) | 0.10 | — | 0.28 (0.09, 0.47) | 0.11 | −0.02 (−0.26, 0.21) | −0.01 | |
| IL-6 | 0.16 (−0.40, 0.72) | 0.02 | 0.01 (−0.61, 0.62) | 0.00 | 0.07 (−0.49, 0.64) | 0.01 | 0.69 (−0.06, 1.44) | 0.11 |
| IL-8 | 0.79 (0.45, 1.13) | 0.19 | 0.69 (0.35, 1.03) | 0.17 | 0.86 (0.51, 1.21) | 0.21 | 0.93 (0.46, 1.41) | 0.23 |
| MCP-1 | −0.66 (−1.09, −0.24) | −0.13 | −0.86 (−1.28, −0.44) | −0.17 | −0.62 (−1.05, −0.19) | −0.13 | −1.49 (−2.05, −0.93) | −0.31 |
| P-selectin | 0.81 (0.48, 1.15) | 0.17 | 0.67 (0.32, 1.02) | 0.14 | 0.82 (0.47, 1.17) | 0.17 | 0.46 (0.06, 0.86) | 0.10 |
| VEGF | 0.33 (0.09, 0.58) | 0.09 | 0.30 (0.04, 0.55) | 0.08 | 0.35 (0.09, 0.61) | 0.10 | −0.08 (−0.45, 0.29) | −0.02 |
| PR3-ANCA | 0.80 (0.44, 1.16) | 0.17 | 0.73 (0.35, 1.12) | 0.16 | — | 0.71 (0.26, 1.16) | 0.16 | |
sCD40L: soluble CD40 ligand; CRP: C-reactive protein; IL: interleukin; MCP-1: monocyte chemoattractant protein 1; VEGF: vascular endothelial growth factor; PR3-ANCA: antiproteinase 3 antineutrophil cytoplasmic antibody Std β: standardized β estimate.
Figure 1.
Longitudinal changes of marker levels. Nonparametric smoothing of marker levels at several timepoints in the study. X axis represents different timepoints, and the Y axis standardized marker levels after subtraction of the baseline value (all baseline values set to zero). Broken X axis represents that flares did not occur at the same times for all study subjects.
Predictability of marker levels on future remission and disease flare
Out of 180 subjects at baseline, 167 achieved remission during the followup period. The median time to remission was 3 months. Subjects with higher baseline CRP values had decreased rates of remission: HR 0.82 (95% CI 0.73–0.93) per 1-unit increase in log CRP levels. Other markers were not associated with time to remission. Additionally, at the time of remission none of the markers was associated with time to flare of disease.
Association of marker levels with granulomatous vs vasculitic manifestations of WG
Secondary analysis of the association of marker levels with granulomatous-BVAS/WG and vasculitic-BVAS/WG found that the association of sCD40L and CRP and the inverse association of MCP-1 was mainly driven by association with the granulomatous components of the BVAS/WG, while the associations of VEGF and PR3-ANCA were stronger with the vasculitic components of the BVAS/WG (Table 3).
Table 3.
Longitudinal association of marker levels with granulomatous and vasculitic components of disease activity. Results expressed as increase in disease activity score on the Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis (BVAS/WG) per unit-increase in log-marker level.
| Marker | Crude Association with BVAS/WG | Crude Association with Granulomatous BVAS | Crude Association with Vasculitic BVAS | |||
|---|---|---|---|---|---|---|
| β Estimate (95% CI) | Std β | β Estimate (95% CI) | Std β | β Estimate (95% CI) | Std β | |
| sCD40L | 0.83 (0.42, 1.25) | 0.19 | 0.36 (0.23, 0.49) | 0.26 | 0.20 (−0.03, 0.44) | 0.08 |
| CRP | 0.28 (0.09, 0.47) | 0.10 | 0.18 (0.12, 0.24) | 0.21 | −0.02 (−0.13, 0.10) | −0.01 |
| IL-6 | 0.16 (−0.40, 0.72) | 0.02 | −0.01 (−0.21, 0.18) | 0.00 | 0.13 (−0.22, 0.47) | 0.04 |
| IL-8 | 0.79 (0.45, 1.13) | 0.19 | 0.16 (0.05, 0.27) | 0.12 | 0.47 (0.26, 0.69) | 0.20 |
| MCP-1 | −0.66 (−1.09, −0.24) | −0.13 | −0.42 (−0.59, −0.25) | −0.26 | −0.03 (−0.27, 0.22) | −0.01 |
| P-selectin | 0.81 (0.48, 1.15) | 0.17 | 0.26 (0.12, 0.40) | 0.16 | 0.32 (0.13, 0.51) | 0.12 |
| VEGF | 0.33 (0.09, 0.58) | 0.09 | 0.03 (−0.06, 0.12) | 0.03 | 0.16 (−0.01, 0.33) | 0.08 |
| PR3-ANCA | 0.80 (0.44, 1.16) | 0.17 | 0.12 (−0.02, 0.27) | 0.08 | 0.38 (0.17, 0.59) | 0.15 |
sCD40L: soluble CD40 ligand; CRP: C-reactive protein; IL: interleukin; MCP-1: monocyte chemoattractant protein 1; VEGF: vascular endothelial growth factor; PR3-ANCA: antiproteinase 3 antineutrophil cytoplasmic antibody Std β: standardized β estimate.
Association of markers with venous thromboembolic events
During the followup period, 16 subjects had a first VTE; in most of these subjects the thrombosis occurred during a period of active disease. IL-6 was associated with VTE (OR 3.17, 95% CI 1.33–7.55). Adjusting for disease activity (BVAS/WG) did not change this estimate (OR 3.63, 95% CI 1.37–9.66). No other markers were associated with VTE.
DISCUSSION
This large cohort study identified several cytokines as candidate biomarkers for measuring disease activity in WG. P-selectin, sCD40L, IL-8, and VEGF were positively, and MCP-1 inversely, associated with vasculitis disease activity. The candidate markers in our study remained significantly associated with active disease even after adjustment for CRP, a standard measure of inflammation, or PR-3-ANCA, the most studied biomarker for AAV5,6,26, indicating that the new markers might identify different elements of disease activity and offer additive value beyond that of these traditional biomarkers. Further, in a multivariate analysis, sCD40L, MCP-1, IL-8, and P-selectin remained independently associated with disease activity after adjustment for other markers. While the cytokines/chemokines measured in our study likely have several cellular sources, there is evidence that they originate from cells in close spatial proximity to the endothelium where inflammation and destruction occurs in AAV, specifically: sCD40L and P-selectin from platelets27,28, IL-8 from endothelial cells29 and circulating monocytes30, VEGF from endothelial cells31 and platelets32, and MCP-1 from endothelial cells and circulating monocytes30. In contrast, widely accepted acute-phase reactants such as CRP and IL-6 have more distant sources: CRP is synthesized in the liver and IL-6 in activated tissue macrophages7. Cytokines that operate at the endothelial level may more directly measure the key biologic processes in small-vessel vasculitis.
The inverse association of MCP-1 with disease activity was unexpected and contrasts with previous studies. Monocyte activation has been found to correlate with disease activity in AAV19. One small study found elevated MCP-1 in serum of patients with WG compared to healthy controls33, and elevated urinary levels of MCP-1 have been described in subjects with active renal vasculitis34. Studies describing the upregulation of MCP-1 at the site of vascular injury35 but the effective immobilization of MCP-1 by activated platelets36 might offer a biologic explanation for the decreased concentration of MCP-1 in plasma samples at times of active versus inactive disease. The absence of association between IL-6 and disease activity was unexpected19,37,38. In our study, there was significant positive correlation of IL-6 with both IL-8, a marker positively associated with disease activity, and with MCP-1, a marker strongly inversely associated with disease activity (data not shown). These results suggest that IL-6 has relationships to distinct biologic processes that have different associations with disease activity in AAV.
Our findings of positive associations of sCD40L, CRP, and an inverse association of MCP-1 with granulomatous disease activity and positive associations between PR3-ANCA and VEGF with vasculitic disease activity are interesting and may provide insight into the biology of the disease. However, given the difficulties in differentiating granulomatous versus vasculitic disease manifestations, the results of this secondary analysis should be considered exploratory.
The finding of an association between IL-6 and clinical thrombotic events is consistent with previous reports9,10. However, given a total of only 16 new VTE in the cohort, statistically significant associations between thromboses and marker levels were not expected.
Our study has important strengths. Plasma samples were collected prospectively from a well defined longitudinal cohort of patients with WG. Disease activity from all subjects was scored according to a predefined protocol. All experimental measurements were performed in an identical fashion at a single laboratory with samples from each subject run in single batches. This report includes the results of all measured tests, both individually and in combination, as per the a priori analytic plan. The analysis was planned to study not only bivariate associations between individual markers and disease activity and relapse, but also the additive value of the markers when studied in combination.
Our study also has limitations. The standardized β coefficients, provided to offer greater data interpretability, were calculated using the standard deviation of markers and were thus affected by the patient population, quality of samples, and reliability of the ELISA kits. Therefore, relative strengths of associations of individual markers with disease activity should be interpreted with caution. The association between PR3-ANCA and disease activity is reported here for comparison to the candidate markers but a much fuller description and analysis has been published5.
Our study demonstrates that several biomarkers arising from within the circulation are associated with disease activity in WG. Future studies of these and related biomarkers in different patient cohorts may lead to a greater understanding of the pathophysiology of vasculitis and to the development of novel measurements of disease activity. It is possible that if several biomarkers are identified, each identifying different domains of disease activity, that a composite measure could be developed to help guide therapy.
Acknowledgments
Supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) Multidisciplinary Clinical Research Center grant P60 AR047785. The Wegener’s Granulomatosis Etanercept Trial (WGET) was supported by the NIAMS, US National Institutes of Health (NIH) N01-AR92240, and the Office of Orphan Products, US Food and Drug Administration (grant FD-R-001652), General Clinical Research Center grants M01-RRO-00533 (Boston University), M01-RRO-0042 (The University of Michigan), MO1-RR-30 (Duke University), and M01-RRO-2719 (Johns Hopkins University School of Medicine), from the National Center for Research Resources/NIH. Drs. Stone, Merkel, and St. Clair were supported by NIAMS grants K24 AR049185-01, K24 AR2224-01A1, and K24 AR02126-04. Dr. Freedman’s laboratory is supported by US National Heart, Lung, and Blood Institute grants R01 HL 083801 and R01 HL087201.
The authors thank Monica V. Talor, Sharleen Johnson, and Kristine Morin for their expert technical assistance.
APPENDIX
List of study collaborators. The Wegener’s Granulomatosis Etanercept Trial (WGET) Research Group. Janet T. Holbrook, PhD, MPH, Director; Curtis L. Meinert, PhD, Associate Director; John Dodge, Systems Analyst; Jessica Donithan, Research Coordinator; Nancy Min, PhD, Biostatistician; Laurel Murrow, MSc, Trial Coordinator (former); Jacki Smith, Research Data Assistant; Andrea K. Tibbs, BS, Trial Coordinator; Mark Van Natta, MHS, Biostatistician, Coordinating Center, The Johns Hopkins University Center for Clinical Trials; Rosanne Berman, MPH; Sandy Enuha, MPH, Clinical Center; The Beth Israel Medical Center, New York; Rondi Gelbard, BS; Melynn Nuite, RN; Aileen Schiller, MS, Boston University: David Blumenthal, MD; Debora Bork, MFA; Tiffany Clark, CNP; Sonya L. Crook, RN; Leonard H. Calabrese, DO; Sharon Farkas; Sudhakar Sridharan, MD; Kimberly Strom, CNP; William Wilke, MD, The Cleveland Clinic Foundation; Nancy B. Allen, MD; Karen Rodin, RN; Edna Scarlett, Duke University; David B. Hellmann, MD; Amanda M. Moore, BS; Lourdes Pinachos, RN, BSN; Michael J. Regan, MD, MRCP; Misty L. Uhlfelder, MPH, Johns Hopkins University; Kristin Bradt; Kimberly Carlson; Susan Fisher, RN; Boleyn Hammel; Kathy Mieras; Steven Ytterberg, MD, The Mayo Clinic; Maureen Fitzpatrick, MPH; Ken Fye, MD; Steve Lund, MSN, NP, University of California, San Francisco; Billie Jo Coomer, BS; Barbara Gilson, RN; Hilary Haftel, MD; Ana Morrel-Samuels, BA; Sandra Neckel, RN, University of Michigan; Noel R. Rose, MD, PhD; C. Lynne Burek, PhD; Jobert Barin, BS; Monica Talor, MS, Resource Centers, The Johns Hopkins University Immune Diseases Laboratory; Paul L. Canner, PhD, Data and Safety Monitoring Board, Maryland Medical Research Institute; Doyt L. Conn, MD, Emory University (Safety Officer); Jack H. Klippel, MD, Arthritis Foundation (Chair); J. Richard Landis, PhD, University of Pennsylvania.
References
- 1.Hoffman GS, Kerr GS, Leavitt RY, Hallahan CW, Lebovics RS, Travis WD, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med. 1992;116:488–98. doi: 10.7326/0003-4819-116-6-488. [DOI] [PubMed] [Google Scholar]
- 2.De Groot K, Rasmussen N, Bacon PA, Tervaert JW, Feighery C, Gregorini G, et al. Randomized trial of cyclophosphamide versus methotrexate for induction of remission in early systemic antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2005;52:2461–9. doi: 10.1002/art.21142. [DOI] [PubMed] [Google Scholar]
- 3.Seo P, Min YI, Holbrook JT, Hoffman GS, Merkel PA, Spiera R, et al. Damage caused by Wegener’s granulomatosis and its treatment: prospective data from the Wegener’s Granulomatosis Etanercept Trial (WGET) Arthritis Rheum. 2005;52:2168–78. doi: 10.1002/art.21117. [DOI] [PubMed] [Google Scholar]
- 4.Pagnoux C, Hogan SL, Chin H, Jennette JC, Falk RJ, Guillevin L, et al. Predictors of treatment resistance and relapse in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis: Comparison of two independent cohorts. Arthritis Rheum. 2008;58:2908–18. doi: 10.1002/art.23800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finkielman JD, Merkel PA, Schroeder D, Hoffman GS, Spiera R, St Clair EW, et al. Antiproteinase 3 antineutrophil cytoplasmic antibodies and disease activity in Wegener granulomatosis. Ann Intern Med. 2007;147:611–9. doi: 10.7326/0003-4819-147-9-200711060-00005. [DOI] [PubMed] [Google Scholar]
- 6.Girard T, Mahr A, Noel LH, Cordier JF, Lesavre P, Andre MH, et al. Are antineutrophil cytoplasmic antibodies a marker predictive of relapse in Wegener’s granulomatosis? A prospective study. Rheumatology. 2001;40:147–51. doi: 10.1093/rheumatology/40.2.147. [DOI] [PubMed] [Google Scholar]
- 7.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 8.Jennette JC, Falk RJ, Andrassy K, Bacon PA, Churg J, Gross WL, et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37:187–92. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 9.Roumen-Klappe EM, den Heijer M, van Uum SH, van der Ven-Jongekrijg J, van der Graaf F, Wollersheim H. Inflammatory response in the acute phase of deep vein thrombosis. J Vasc Surg. 2002;35:701–6. doi: 10.1067/mva.2002.121746. [DOI] [PubMed] [Google Scholar]
- 10.van Aken BE, den Heijer M, Bos GM, van Deventer SJ, Reitsma PH. Recurrent venous thrombosis and markers of inflammation. Thromb Haemost. 2000;83:536–9. [PubMed] [Google Scholar]
- 11.Merkel PA, Lo GH, Holbrook JT, Tibbs AK, Allen NB, Davis JC, Jr, et al. Brief communication: high incidence of venous thrombotic events among patients with Wegener granulomatosis: the Wegener’s Clinical Occurrence of Thrombosis (WeCLOT) Study. Ann Intern Med. 2005;142:620–6. doi: 10.7326/0003-4819-142-8-200505030-00011. [DOI] [PubMed] [Google Scholar]
- 12.Weidner S, Hafezi-Rachti S, Rupprecht HD. Thromboembolic events as a complication of antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2006;55:146–9. doi: 10.1002/art.21704. [DOI] [PubMed] [Google Scholar]
- 13.Stassen PM, Derks RPH, Kallenberg CGM, Stegeman CA. Venous thromboembolism in ANCA-associated vasculitis — incidence and risk factors. Rheumatology. 2008;47:530–4. doi: 10.1093/rheumatology/ken035. [DOI] [PubMed] [Google Scholar]
- 14.Wegener’s Granulomatosis Etanercept Trial (WGET) Research Group. Etanercept plus standard therapy for Wegener’s granulomatosis. N Engl J Med. 2005;352:351–61. doi: 10.1056/NEJMoa041884. [DOI] [PubMed] [Google Scholar]
- 15.WGET Research Group. Design of the Wegener’s Granulomatosis Etanercept Trial (WGET) Control Clin Trials. 2002;23:450–68. doi: 10.1016/s0197-2456(02)00209-x. [DOI] [PubMed] [Google Scholar]
- 16.Henn V, Slupsky JR, Grafe M, Anagnostopoulos I, Forster R, Muller-Berghaus G, et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–4. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 17.Slupsky JR, Kalbas M, Willuweit A, Henn V, Kroczek RA, Muller-Berghaus G. Activated platelets induce tissue factor expression on human umbilical vein endothelial cells by ligation of CD40. Thromb Haemost. 1998;80:1008–14. [PubMed] [Google Scholar]
- 18.Jiang Y, Beller DI, Frendl G, Graves DT. Monocyte chemoattractant protein-1 regulates adhesion molecule expression and cytokine production in human monocytes. J Immunol. 1992;148:2423–8. [PubMed] [Google Scholar]
- 19.Muller Kobold AC, Kallenberg CG, Tervaert JW. Monocyte activation in patients with Wegener’s granulomatosis. Ann Rheum Dis. 1999;58:237–45. doi: 10.1136/ard.58.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindmark E, Tenno T, Siegbahn A. Role of platelet P-selectin and CD40 ligand in the induction of monocytic tissue factor expression. Arterioscler Thromb Vasc Biol. 2000;20:2322–8. doi: 10.1161/01.atv.20.10.2322. [DOI] [PubMed] [Google Scholar]
- 21.Palabrica T, Lobb R, Furie BC, Aronovitz M, Benjamin C, Hsu YM, et al. Leukocyte accumulation promoting fibrin deposition is mediated in vivo by P-selectin on adherent platelets. Nature. 1992;359:848–51. doi: 10.1038/359848a0. [DOI] [PubMed] [Google Scholar]
- 22.Bozoglu E, Dinc A, Erdem H, Pay S, Simsek I, Kocar IH. Vascular endothelial growth factor and monocyte chemoattractant protein-1 in Behcet’s patients with venous thrombosis. Clin Exp Rheumatol. 2005;4 (Suppl 38):S42–8. [PubMed] [Google Scholar]
- 23.Russell KA, Wiegert E, Schroeder DR, Homburger HA, Specks U. Detection of anti-neutrophil cytoplasmic antibodies under actual clinical testing conditions. Clin Immunol. 2002;103:196–203. doi: 10.1006/clim.2001.5200. [DOI] [PubMed] [Google Scholar]
- 24.Stone JH, Hoffman GS, Merkel PA, Min YI, Uhlfelder ML, Hellmann DB, et al. A disease-specific activity index for Wegener’s granulomatosis: modification of the Birmingham Vasculitis Activity Score. International Network for the Study of the Systemic Vasculitides (INSSYS) Arthritis Rheum. 2001;44:912–20. doi: 10.1002/1529-0131(200104)44:4<912::AID-ANR148>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 25.Booth AD, Jayne DR, Kharbanda RK, McEniery CM, Mackenzie IS, Brown J, et al. Infliximab improves endothelial dysfunction in systemic vasculitis: a model of vascular inflammation. Circulation. 2004;109:1718–23. doi: 10.1161/01.CIR.0000124720.18538.DD. [DOI] [PubMed] [Google Scholar]
- 26.Slot MC, Tervaert JW, Boomsma MM, Stegeman CA. Positive classic antineutrophil cytoplasmic antibody (C-ANCA) titer at switch to azathioprine therapy associated with relapse in proteinase 3-related vasculitis. Arthritis Rheum. 2004;51:269–73. doi: 10.1002/art.20234. [DOI] [PubMed] [Google Scholar]
- 27.Henn V, Steinbach S, Buchner K, Presek P, Kroczek RA. The inflammatory action of CD40 ligand (CD154) expressed on activated human platelets is temporally limited by coexpressed CD40. Blood. 2001;98:1047–54. doi: 10.1182/blood.v98.4.1047. [DOI] [PubMed] [Google Scholar]
- 28.Blann AD, Lip GY. Hypothesis: is soluble P-selectin a new marker of platelet activation? Atherosclerosis. 1997;128:135–8. doi: 10.1016/s0021-9150(96)05980-1. [DOI] [PubMed] [Google Scholar]
- 29.Lee TH, Avraham H, Lee SH, Avraham S. Vascular endothelial growth factor modulates neutrophil transendothelial migration via up-regulation of interleukin-8 in human brain microvascular endothelial cells. J Biol Chem. 2002;277:10445–51. doi: 10.1074/jbc.M107348200. [DOI] [PubMed] [Google Scholar]
- 30.Rezaie-Majd A, Maca T, Bucek RA, Valent P, Muller MR, Husslein P, et al. Simvastatin reduces expression of cytokines interleukin-6, interleukin-8, and monocyte chemoattractant protein-1 in circulating monocytes from hypercholesterolemic patients. Arterioscler Thromb Vasc Biol. 2002;22:1194–9. doi: 10.1161/01.atv.0000022694.16328.cc. [DOI] [PubMed] [Google Scholar]
- 31.Kourembanas S, Morita T, Liu Y, Christou H. Mechanisms by which oxygen regulates gene expression and cell-cell interaction in the vasculature. Kidney Int. 1997;51:438–43. doi: 10.1038/ki.1997.58. [DOI] [PubMed] [Google Scholar]
- 32.Arisato T, Hashiguchi T, Sarker KP, Arimura K, Asano M, Matsuo K, et al. Highly accumulated platelet vascular endothelial growth factor in coagulant thrombotic region. J Thromb Haemost. 2003;1:2589–93. doi: 10.1046/j.1538-7836.2003.00475.x. [DOI] [PubMed] [Google Scholar]
- 33.Torheim EA, Yndestad A, Bjerkeli V, Halvorsen B, Aukrust P, Froland SS. Increased expression of chemokines in patients with Wegener’s granulomatosis — modulating effects of methylprednisolone in vitro. Clin Exp Immunol. 2005;140:376–83. doi: 10.1111/j.1365-2249.2005.02770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tam FW, Sanders JS, George A, Hammad T, Miller C, Dougan T, et al. Urinary monocyte chemoattractant protein-1 (MCP-1) is a marker of active renal vasculitis. Nephrol Dial Transplant. 2004;19:2761–8. doi: 10.1093/ndt/gfh487. [DOI] [PubMed] [Google Scholar]
- 35.Furukawa Y, Matsumori A, Ohashi N, Shioi T, Ono K, Harada A, et al. Anti-monocyte chemoattractant protein-1/monocyte chemotactic and activating factor antibody inhibits neointimal hyperplasia in injured rat carotid arteries. Circ Res. 1999;84:306–14. doi: 10.1161/01.res.84.3.306. [DOI] [PubMed] [Google Scholar]
- 36.Schober A, Zernecke A, Liehn EA, von Hundelshausen P, Knarren S, Kuziel WA, et al. Crucial role of the CCL2/CCR2 axis in neointimal hyperplasia after arterial injury in hyperlipidemic mice involves early monocyte recruitment and CCL2 presentation on platelets. Circ Res. 2004;95:1125–33. doi: 10.1161/01.RES.0000149518.86865.3e. [DOI] [PubMed] [Google Scholar]
- 37.Ohlsson S, Wieslander J, Segelmark M. Circulating cytokine profile in anti-neutrophilic cytoplasmatic autoantibody-associated vasculitis: prediction of outcome? Mediators Inflamm. 2004;13:275–83. doi: 10.1080/09629350400003100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohta N, Fukase S, Aoyagi M. Serum levels of soluble adhesion molecules ICAM-1, VCAM-1 and E-selectin in patients with Wegener’s granulomatosis. Auris Nasus Larynx. 2001;28:311–4. doi: 10.1016/s0385-8146(01)00097-9. [DOI] [PubMed] [Google Scholar]