Abstract
Objective
To review the risk factors, management, and prevention of recreational water–related illness in family practice.
Sources of information
Original and review articles from January 1998 to February 2012 were identified using PubMed and the search terms water-related illness, recreational water illness, and swimmer illness.
Main message
There is a 3% to 8% risk of acute gastrointestinal illness (AGI) after swimming. The high-risk groups for AGI are children younger than 5 years, especially if they have not been vaccinated for rotavirus, and elderly and immunocompromised patients. Children are at higher risk because they swallow more water when swimming, stay in the water longer, and play in the shallow water and sand, which are more contaminated. Participants in sports with a lot of water contact like triathlon and kite surfing are also at high risk, and even activities involving partial water contact like boating and fishing carry a 40% to 50% increase in risk of AGI compared with nonwater recreational activities. Stool cultures should be done when a recreational water illness is suspected, and the clinical dehydration scale is a useful clinical tool for assessing the treatment needs of affected children.
Conclusion
Recreational water illness is the main attributable cause of AGI during swimming season. Recognition that swimming is a substantial source of illness can help prevent recurrent and secondary cases. Rotavirus vaccine is highly recommended for children who will swim frequently.
Case introduction
Emma, a usually healthy 3-year-old, is brought to your office by her father in August. For 3 days she has had severe diarrhea with up to 6 stools per day. There is no blood in the stool. She is not drinking well or eating any solid food. Her father is concerned about her becoming dehydrated and hopes to have her feeling better before the family heads back to the cottage for the weekend.
Sources of information
A PubMed search from January 1998 to February 2012 was completed using the search terms recreational water illness, water-related illness, and swimmer illness. Two systematic reviews of recreational water illness examined epidemiologic studies1,2; other sources of information included investigations of water-borne disease outbreaks3–5 and 1 experimental study of swimmer illness.6
Main message
Epidemiology
Acute gastrointestinal illness (AGI) is common, occurring in 8% to 9% of Canadians in any 4-week period.7,8 In one urban children’s emergency department (ED) it accounted for 9% of all visits.9
The greatest risk of bacterial, protozoal, and viral gastroenteritis during the swimming season is likely not from exposure through food consumption, drinking water, or at day care, but rather from exposure to recreational water.10,11
Illness from recreational water exposure is common. In field studies of swimmer illness, diarrhea rates of 3% to 8% are found in follow-up health surveys.2,6 The same organisms associated with illness from drinking water (ie, Campylobacter, Salmonella, and Giardia species, and enteric viruses) are found in swimming water but in much higher concentrations. The average water ingestion with swimming is estimated to be 10 to 150 mL per hour.12
Children younger than 10 years of age contract more illness from recreational water because they play in the shallow water and sand, which are most contaminated, have hand-to-mouth exposure, stay in the water longer, immerse their heads more often, and swallow more water while swimming.2,13,14
At public beaches, swimmer health is supposed to be protected by beach water sampling and beach closure when levels of indicator bacteria (ie, Escherichia coli and coliforms) exceed regulatory levels. However, high sampling and testing costs and long laboratory turnaround times (48 to 72 hours) make it difficult for many municipalities and health units to monitor beaches comprehensively or make real-time closure decisions. Current monitoring methods are not adequately protective against illness from swimming,15 and most swimming in Canada occurs in unmonitored water. Beach signage indicating that water will be unsafe after heavy rainfall, when waves are high, and when turbid is also used to prevent the public from swimming in unsafe water.
Important sources of human illness from beach water contamination include treated human waste water and agricultural run-off, especially from cattle manure.16 Crowded beaches have higher rates of swimmer illness, suggesting swimmer-to-swimmer illness transmission also plays a role.12,17 Modeling estimates suggest that norovirus and rotavirus account for 75% of human illness from swimming.12 As the enteroviruses are mainly species specific, this suggests that human contamination sources account for a high proportion of swimmer illness.
Although AGI is the most common recreational water–related illness, respiratory pathogens such as adenovirus are also found in swimming water and might cause summer respiratory illness.4 There are 2 other groups of water-borne illness at opposite ends of the disease spectrum. Minor conditions such as swimmer’s itch rash, conjunctivitis, and otitis externa might be caused by water-borne pathogens, both bacterial and viral. Swimmer’s itch is a schistosome, found across the entire border region between Canada and the United States, that burrows under the skin and causes papular summer rashes. At the extreme end of the spectrum are rare cases of diseases caused by unusual organisms such as leptospirosis,18 the duck-borne Naegleria fowleri (primary amebic encephalitis), or liver dysfunction from toxic chemicals such as microcystin-LR released by dying algal blooms in warm inland waters.4
Individuals at high risk of illness from recreational water include children younger than 10 years of age, the elderly, and the immunocompromised.14,19 Children younger than 5 years of age are at higher risk because they have not yet acquired immunity to rotavirus (unless they have been vaccinated). Beach sand is an important reservoir of bacteria and a source of illness for young children; play that includes being buried in sand poses an especially high risk.13
In one experimental study,6 swimmers (the exposure group) were required to swim for at least 10 minutes with at least 3 head immersions. This exposure, although apparently small, resulted in much higher 1-week AGI rates than in the nonswimming group (8.6% vs 1.3%, P < .001). There was a dose-response relationship: swimming in more contaminated water and swallowing more water both resulted in higher AGI rates. Sports with high water immersion or high water contact that carry high risks of water-borne infection include triathlon,18 surfing, windsurfing, kite surfing,20,21 and diving.22 Even limited-water-contact recreation, such as boating and fishing, increases the risk of AGI by 40% to 50% relative to nonwater recreational activities.23
Table 1 outlines common recreational water illness pathogens and their management.
Table 1.
Recreational water illness pathogens and their management
| PATHOGEN | SYMPTOMS AND UNIQUE FEATURES | TREATMENT AND PREVENTION | SPECIAL RISK GROUPS |
|---|---|---|---|
| Bacteria | |||
|
| |||
| • Campylobacter | Diarrhea, fever; 50% of children have blood in stools Incubation 3 d (range 1–7 d) |
Self-limited; rare use of fluoroquinolones or azithromycin | Pregnant women, children, the elderly, immunocompromised patients Users of PPIs Those handling raw meat, especially poultry Users of private wells |
| • Salmonella | Fever more common; nausea, diarrhea, cramps Incubation 8–72 h (longer if water-borne than if food-borne) |
Rehydration Occasional antibiotics, especially in children younger than 1 y or in severe illness |
Corticosteroid users Patients with AIDS or cancer Transplant recipients |
| • Shigella | Highly infective Abdominal pain, frequent but low-volume stools Affects lower GI tract, so dehydration is less common |
Oral rehydration Antibiotic use more common Azithromycin or ceftriaxone in those < 18 y Fluoroquinolone in those 18 y or older Avoid antimotility agents |
Young children Patients with poor nutrition International travelers |
| • Enteropathogenic Escherichia coli | Highly infective, short incubation Enterotoxin-producing strains cause bloody diarrhea and hemolytic uremic syndrome (higher risk in children) |
Monitor creatinine Avoid antibiotics |
Children, the elderly Commonly, those in the same household as the infected patient |
| Protozoan parasites | |||
|
| |||
| • Giardia | Incubation 7–14 d Loose, foul-smelling stools; flatulence; fatigue Induces lactose intolerance |
Oral metronidazole if symptomatic Lactose avoidance Avoid swimming until asymptomatic for 14 d |
Those with immune deficiencies Children < 5 y Wilderness water users |
| • Cryptosporidium | Incubation 5–7 d The organism is chlorine-resistant |
Self-limiting in immunocompetent patients Nitazoxanide for young children (some trials have also used it in adults with HIV) |
Those with immune deficiencies Solid organ transplant recipients after surgery |
| Viruses | |||
|
| |||
| • Rotavirus | More severe and prolonged diarrhea | Rehydration Vaccine 50% effective for prevention |
Children < 5 y Unimmunized patients |
| • Norovirus (Norwalk-like virus) | Short incubation | Rehydration Probiotics (limited evidence) Zinc if malnourished |
Young children, the elderly Those with immune deficiencies |
| • Adenovirus | Respiratory and GI symptoms Fever, pneumonia, and diarrhea in children Keratoconjunctivitis |
Symptomatic treatment | Young children Those with immune deficiencies |
| Harmful algal bloom toxins | |||
|
| |||
| • Microcystin-LR and many others; released by some algal blooms | Exacerbations of asthma; hepatic and neurotoxins; probable carcinogens | Supportive treatment Avoid swimming in water with visible algal blooms |
Toxic to all age groups |
GI—gastrointestinal, PPI—proton pump inhibitors.
Role of climate change
Climate change might play an important role in water-related illness patterns. The 2 highest-probability climate-change events are more frequent heavy rainfall occurrences and more frequent and intense heat waves,24 both with probability greater than 90%. Both these climate-change effects can impair water quality. Heavy rain washes pathogens into surface water, and higher temperatures promote the growth of algae and bacteria. A US study3 demonstrated that during the previous 46 years, 68% of water-borne disease outbreaks were preceded by heavy rainfall above the 80th percentile (P < .001). At a Milwaukee pediatric ED, any rainfall in the previous 4 days increased the rate of visits for AGI by 11%.25
Chronic sequelae
Acute gastrointestinal illness might be viewed as minor, but its economic effects and long-term sequelae are substantial. An Ontario study estimated a cost per case for AGI of $1089 when over-the-counter medications, lost patient or parental work time, and costs to the health care system were included.26 The Walkerton Health Study followed up with patients who became infected with E coli or Campylobacter species from drinking water and provided surprising new information about the serious chronic sequelae of AGI. Adults who were most severely affected (measured by seeking health care) had a 38% rate of diarrhea-predominant irritable bowel syndrome (IBS), diagnosed by the Rome criteria.27 The IBS symptoms were mostly resolved by 6 years after the AGI. Children from the same outbreak also developed postinfectious IBS at higher rates (odds ratio 4.6, 95% CI 1.6 to 13.3) than those who were not exposed. Risk factors for development of IBS in the children were increased illness severity and use of antibiotics during the outbreak.28 Other chronic health sequelae of acute bacterial gastroenteritis included hypertension, proteinuria, and glomerular filtration rate below 60 mL/min.29 The latter 2 complications might occur with or without hemolytic uremic syndrome, and affect children and adults.
Case discussion
Emma is assessed in the office. Using the clinical dehydration scale, a useful triaging tool for children younger than 6 years of age (Table 2),30 she scores 5 out of a possible 8, indicating moderate to severe dehydration. She is referred to the ED for further treatment. She receives ultrarapid intravenous rehydration,31 consisting of 50 mL/kg of normal saline over 1 hour. Stool cultures are collected and she is discharged. Results of stool culture are reported 4 days later; cultures were positive for rotavirus.
Table 2.
Clinical dehydration scale: A score of 0 represents no dehydration, 1–4 represents some dehydration, and 5–8 represents moderate to severe dehydration; higher score is predictive of need for IV fluids and longer ED stay after assessment by physician (P < .01).
| ASSESSED FEATURE | POSSIBLE SCORE |
|---|---|
| General appearance | 0–2 |
| Eyes | 0–2 |
| Mucous membranes | 0–2 |
| Tears | 0–2 |
ED—emergency department, IV—intravenous.
Data from Bailey et al.30
Studies of AGI in pediatric settings have consistently found that rotavirus causes 20% to 30% of all AGI.9,32 Approximately 29% of outpatient visits for diarrhea and 25% of diarrhea-associated ED visits in pediatric settings are due to rotavirus.31 With increasing use of the 2-dose oral rotavirus vaccine before age 1, the rate of ED visits and hospitalizations of children for rotavirus has declined by 70% to 100%.33,34 After 5 years of age, there is a high rate of naturally acquired rotavirus immunity in unvaccinated children.
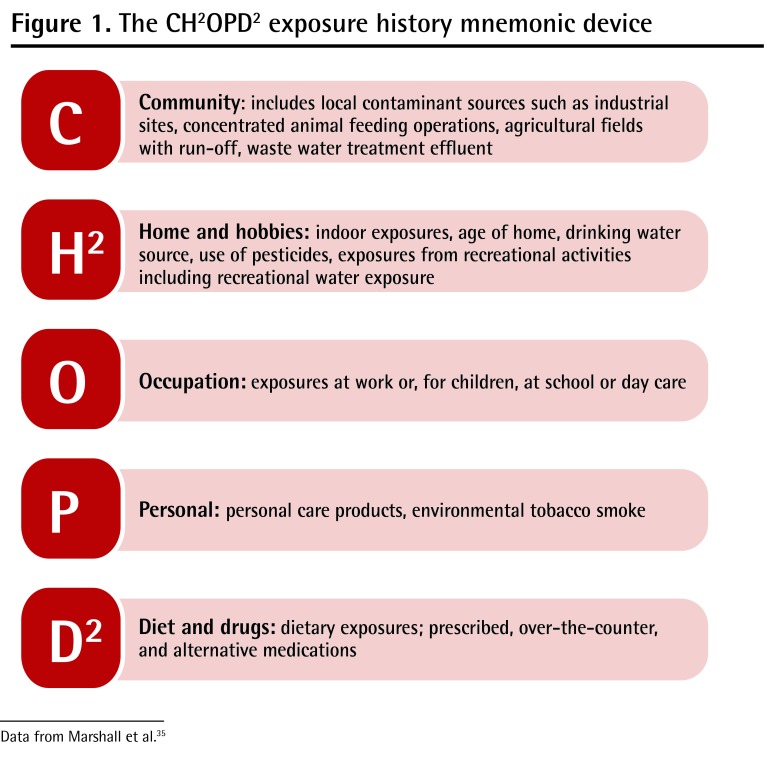
Exposure history
The most important element of the office management of Emma’s case, after acute care, is trying to determine a possible source of the gastroenteritis. This begins with a food- and water-exposure history. The exposure history can be taken using a standardized approach (Figure 1),35 which should include questions to determine the child’s drinking water sources and whether there has been recreational water exposure. High-risk foods include not only undercooked beef, poultry, and fish, but also raw fruits, nuts, and vegetables.36
Emma had been swimming the previous weekend and had spent 3 to 4 hours a day playing in the shallow water and beach sand at the cottage. This is a potentially large exposure that increases the risk of recreational water–related illness.
The family uses a prefilter and ultraviolet light to treat the lake water for cottage use. Emma’s parents have not tested the drinking water at the cottage for 2 years. At home they use municipally treated water for drinking.
Figure 1.
The CH2OPD2 exposure history mnemonic device
Data from Marshall et al.35
Two important clues in this case are the season and the father’s comment about returning to the cottage on the weekend. Cottages often use treated surface water with uncomplicated treatment systems. Lakes and rivers can become contaminated in the summer by agricultural run-off following heavy rainfall, faulty septic systems near lakes and streams, and waste water effluent from treatment plants. The frequency of beach closures due to microbial contamination and algal blooms, which might harbour toxin-producing microorganisms, increases in late summer and early fall, and after heavy rainfall events. Private swimming areas at cottages are seldom monitored for water quality.
In this case, the moderate to severe dehydration (clinical dehydration scale score of 5 out of 8) made ED treatment appropriate, and stool culture results, exposure history, and timing of onset pointed to a probable recreational water source.
Stool cultures, although ordered for only 33% of patients who present to doctors with diarrhea,37 are important for diagnosing the occasional cause (such as Giardia) that is treatable with antibiotics.
Inadequately treated surface water used for drinking at the cottage was another possible source of this child’s infection. Increased risk of gastrointestinal illness in populations using private wells is well documented, but even municipal systems that rely on treated surface water might confer a higher risk than purely groundwater sources.38
As well as recognizing recreational water illness, family physicians have an important role in reminding patients who use private water systems to test 2 or 3 times yearly, including after spring run-off and after heavy rainfall events. Children younger than age 5 who will be swimming frequently have a higher risk of rotavirus infection, so immunization should be strongly recommended.
EDITOR’S KEY POINTS
Acute gastrointestinal illness is common, occurring in 8% to 9% of Canadians in any 4-week period. The greatest risk of bacterial, protozoal, and viral gastroenteritis during the swimming season is likely not from exposure through food consumption, drinking water, or at day care, but rather from exposure to recreational water.
Lakes and rivers can become contaminated, particularly in the summer, by agricultural run-off following heavy rainfall, faulty septic systems near lakes and streams, and waste water effluent from treatment plants. The frequency of beach closures due to microbial contamination and algal blooms, which might harbour toxin-producing microorganisms, increases in late summer and early fall, and after heavy rainfall.
Family physicians also have an important role in reminding patients who use private water systems to test the water 2 or 3 times yearly, including after spring run-off and after heavy rainfall. Children younger than age 5 who will be swimming frequently have a higher risk of rotavirus infection, so immunization should be strongly recommended.
Footnotes
This article has been peer reviewed.
This article is eligible for Mainpro-M1 credits. To earn credits, go to www.cfp.ca and click on the Mainpro link.
La traduction en français de cet article se trouve à www.cfp.ca dans la table des matières du numéro de mai 2013 à la page e225.
Contributors
Drs Sanborn and Takaro contributed to the literature review and writing the article.
Competing interests
None declared
References
- 1.Prüss A. Review of epidemiological studies on health effects from exposure to recreational water. Int J Epidemiol. 1998;27(1):1–9. doi: 10.1093/ije/27.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Wade TJ, Pai N, Eisenberg J, Colford JM., Jr Do US Environmental Protection Agency water quality guidelines for recreational waters prevent gastrointestinal illness? A systematic review and meta-analysis. Environ Health Perspect. 2003;111(8):1102–9. doi: 10.1289/ehp.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curriero FC, Patz JA, Rose JB, Lele S. The association between extreme precipitation and waterborne disease outbreaks in the United States, 1948–1994. Am J Public Health. 2001;91(8):1194–9. doi: 10.2105/ajph.91.8.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoder JS, Hlavsa MC, Craun GF, Hill V, Roberts V, Yu PA, et al. Surveillance for waterborne diseases and outbreaks associated with recreational water use and other aquatic facility-associated health events—United States, 2005–2006. MMWR Surveill Summ. 2008;57(9):1–29. [PubMed] [Google Scholar]
- 5.Hlavsa MC, Roberts VA, Anderson AR, Hill VR, Kahler AM, Orr M. Surveillance for waterborne disease outbreaks and other health events associated with recreational water—United States, 2007–2008. MMWR Surveill Summ. 2011;60(12):1–32. [PubMed] [Google Scholar]
- 6.Wiedenmann A, Krüger P, Dietz K, López-Pila JM, Szewzyk R, Botzenharts K. A randomized controlled trial assessing infectious disease risks from bathing in fresh recreational waters in relation to the concentration of Escherichia coli, intestinal enterococci, Clostridium perfringens, and somatic coliphages. Environ Health Perspect. 2006;114(2):228–36. doi: 10.1289/ehp.8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sargeant JM, Majowicz SE, Snelgrove J. The burden of acute gastrointestinal illness in Ontario, Canada, 2005–2006. Epidemiol Infect. 2008;136(4):451–60. doi: 10.1017/S0950268807008837. Epub 2007 Jun 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas MK, Majowicz SE, MacDougall L, Sockett PN, Kovacs SJ, Fyfe M, et al. Population distribution and burden of acute gastrointestinal illness in British Columbia, Canada. BMC Public Health. 2006;6:307. doi: 10.1186/1471-2458-6-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yee EL, Staat MA, Azimi P, Bernstein DI, Ward RL, Schubert C, et al. Burden of rotavirus disease among children visiting pediatric emergency departments in Cincinnati, Ohio, and Oakland, California, in 1999–2000. Pediatrics. 2008;122(5):971–7. doi: 10.1542/peds.2007-1609. [DOI] [PubMed] [Google Scholar]
- 10.Denno DM, Keene WE, Hutter CM, Koepsell JK, Patnode M, Flodin-Hursh, et al. Tri-county comprehensive assessment of risk factors for sporadic reportable bacterial enteric infection in children. J Infect Dis. 2009;199(4):467–76. doi: 10.1086/596555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valderrama AL, Hlavsa MC, Cronquist A, Cosgrove S, Johnston SP, Roberts JM, et al. Multiple risk factors associated with a large statewide increase in cryptosporidiosis. Epidemiol Infect. 2009;137(12):1781–8. doi: 10.1017/S0950268809002842. Epub 2009 May 27. [DOI] [PubMed] [Google Scholar]
- 12.Soller JA, Schoen ME, Bartrand T, Ravenscroft JE, Ashbolt NJ. Estimated human health risks from exposure to recreational waters impacted by human and non-human sources of faecal contamination. Water Res. 2010;44(16):4674–91. doi: 10.1016/j.watres.2010.06.049. Epub 2010 Jun 25. [DOI] [PubMed] [Google Scholar]
- 13.Heaney CD, Sams E, Wing S, Marshall S, Brenner K, Dufour AP, et al. Contact with beach sand among beachgoers and risk of illness. Am J Epidemiol. 2009;170(2):164–72. doi: 10.1093/aje/kwp152. Epub 2009 Jun 18. [DOI] [PubMed] [Google Scholar]
- 14.Wade TJ, Calderon RL, Brenner KP, Sams E, Beach M, Haugland R, et al. High sensitivity of children to swimming-associated gastrointestinal illness: results using a rapid assay of recreational water quality. Epidemiology. 2008;19(3):375–83. doi: 10.1097/EDE.0b013e318169cc87. [DOI] [PubMed] [Google Scholar]
- 15.Wu J, Long C, Das D, Dorner SM. Are microbial indicators and pathogens correlated? A statistical analysis of 40 years of research. J Water Health. 2011;9(2):265–78. doi: 10.2166/wh.2011.117. [DOI] [PubMed] [Google Scholar]
- 16.Soller JA, Bartrand T, Ashbolt NJ, Ravenscroft J, Wade TJ. Estimating the primary etiologic agents in recreational freshwaters impacted by human sources of faecal contamination. Water Res. 2010;44(16):4736–47. doi: 10.1016/j.watres.2010.07.064. Epub 2010 Jul 29. [DOI] [PubMed] [Google Scholar]
- 17.Papastergiou P, Mouchtouri VA, Rachiotis G, Pinaka O, Katsiaflaka A, Hadjichristodoulou C. Bather density as a predominant factor for health effects related to recreational bathing: results from the Greek bathers cohort study. Mar Pollut Bull. 2011;62(3):590–5. doi: 10.1016/j.marpolbul.2010.11.023. Epub 2010 Dec 22. [DOI] [PubMed] [Google Scholar]
- 18.Morgan J, Bornstein SL, Karpati AM, Bruce M, Bolin CA, Austin CC, et al. Outbreak of leptospirosis among triathlon participants and community residents in Springfield, Illinois, 1998. Clin Infect Dis. 2002;34(12):1593–9. doi: 10.1086/340615. Epub 2002 May 24. [DOI] [PubMed] [Google Scholar]
- 19.Graczyk TK, Sunderland D, Tamang L, Shields TM, Lucy FE, Breysse PN. Quantitative evaluation of the impact of bather density on levels of human-virulent microsporidian spores in recreational water. Appl Environ Microbiol. 2007;73(13):4095–9. doi: 10.1128/AEM.00365-07. Epub 2007 May 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dwight RH, Baker DB, Semenza JC, Olson BH. Health effects associated with recreational coastal water use: urban versus rural California. Am J Public Health. 2004;94(4):565–7. doi: 10.2105/ajph.94.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turbow DJ, Kent EE, Jiang SC. Web-based investigation of water associated illness in marine bathers. Environ Res. 2008;106(1):101–9. doi: 10.1016/j.envres.2007.06.006. Epub 2007 Aug 1. [DOI] [PubMed] [Google Scholar]
- 22.Schijven J, de Roda Husman AM. A survey of diving behaviour and accidental water ingestion among Dutch occupational and sport divers to assess the risk of infection with waterborne pathogenic microorganisms. Environ Health Perspect. 2006;114(5):712–7. doi: 10.1289/ehp.8523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dorevitch S, Pratap P, Wroblewski M, Hryhorczuk DO, Li H, Liu LC, et al. Health risks of limited-contact water recreation. Environ Health Perspect. 2012;120(2):192–7. doi: 10.1289/ehp.1103934. Epub 2011 Oct 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luber G, Prudent N. Climate change and human health. Trans Am Clin Climatol Assoc. 2009;120:113–7. [PMC free article] [PubMed] [Google Scholar]
- 25.Drayna P, McLellan S, Simpson P, Li SH, Gorelick MH. Association between rainfall and pediatric emergency visits for acute gastrointestinal illness. Environ Health Perspect. 2010;118(10):1439–43. doi: 10.1289/ehp.0901671. Epub 2010 May 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Majowicz SE, McNab WB, Sockett P, Henson TS, Doré K, Edge VL, et al. Burden and cost of gastroenteritis in a Canadian community. J Food Prot. 2006;69(3):651–9. doi: 10.4315/0362-028x-69.3.651. [DOI] [PubMed] [Google Scholar]
- 27.Marshall JK, Thabane M, Garg AX, Clark WF, Salvadori M, Collins SM. Incidence and epidemiology of irritable bowel syndrome after a large waterborne disease outbreak of bacterial dysentery. Gastroenterology. 2006;131(2):445–50. doi: 10.1053/j.gastro.2006.05.053. [DOI] [PubMed] [Google Scholar]
- 28.Thabane M, Simunovic M, Akhtar-Danesh N, Garg AX, Clark WF, Collins SM, et al. An outbreak of acute bacterial gastroenteritis is associated with an increased incidence of irritable bowel syndrome in children. Am J Gastroenterol. 2010;105(4):933–9. doi: 10.1038/ajg.2010.74. Epub 2010 Feb 23. [DOI] [PubMed] [Google Scholar]
- 29.Garg AX, Moist L, Matsell D, Thiessen-Philbrook HR, Haynes RB, Suri RS, et al. Risk of hypertension and reduced kidney function after acute gastroenteritis from bacteria-contaminated drinking water. CMAJ. 2005;173(3):261–8. doi: 10.1503/cmaj.050581. Epub 2005 May 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bailey B, Gravel J, Goldman RD, Friedman JN, Parkin PC. External validation of the clinical dehydration scale for children with acute gastroenteritis. Acad Emerg Med. 2010;17(6):583–8. doi: 10.1111/j.1553-2712.2010.00767.x. [DOI] [PubMed] [Google Scholar]
- 31.Nager AL, Wang VJ. Comparison of ultrarapid and rapid intravenous hydration in pediatric patients with dehydration. Am J Emerg Med. 2010;28(2):123–9. doi: 10.1016/j.ajem.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 32.Klein EJ, Boster DR, Stapp JR, Wells JG, Qin X, Clausen CR, et al. Diarrhea etiology in a children’s hospital emergency department: a prospective cohort study. Clin Infect Dis. 2006;43(7):807–13. doi: 10.1086/507335. [DOI] [PubMed] [Google Scholar]
- 33.Dennehy PH. Effects of vaccine on rotavirus disease in the pediatric population. Curr Opin Pediatr. 2012;24(1):76–84. doi: 10.1097/MOP.0b013e32834ee594. [DOI] [PubMed] [Google Scholar]
- 34.Goldman RD. Effectiveness of rotavirus vaccine in preventing severe acute gastroenteritis in children. Can Fam Physician. 2012;58:270–1. [PMC free article] [PubMed] [Google Scholar]
- 35.Marshall L, Weir E, Abelsohn A, Sanborn M. Identifying and managing adverse environmental health effects: 1. Taking an exposure history. CMAJ. 2002;166(8):1049–55. [PMC free article] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention Surveillance for foodborne disease outbreaks—United States, 2008. MMWR Morb Mortal Wkly Rep. 2011;60(35):1197–202. [PubMed] [Google Scholar]
- 37.Majowicz SE, Edge VL, Fazil A, McNabb WB, Dore KA, Sockett PN, et al. Estimating the under-reporting rate for infectious gastrointestinal illness in Ontario. Can J Public Health. 2005;96(3):178–81. doi: 10.1007/BF03403685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uhlmann S, Galanis E, Takaro TK, Mak S, Gustafson L, Embree G, et al. Where’s the pump? Associating sporadic enteric disease with drinking water using a geographic information system, in British Columbia, Canada, 1996–2005. J Water Health. 2009;7(4):692–8. doi: 10.2166/wh.2009.108. [DOI] [PubMed] [Google Scholar]