Abstract
Objective
To examine the effects of an intensive 2-day course on physicians’ prescribing of opioids.
Design
Population-based retrospective observational study.
Setting
College of Physicians and Surgeons of Ontario (CPSO) in Toronto.
Participants
Ontario physicians who took the course between April 1, 2000, and May 30, 2008.
Intervention
A 2-day opioid-prescribing course with a maximum of 12 physician participants. Educational methods included didactic presentations, case discussions, and standardized patients. A detailed syllabus and office materials were provided.
Main outcome measures
Participants were matched with control physicians using specific variables. The primary outcome was the rate of opioid prescribing, expressed as milligrams of morphine equivalent per quarter.
Results
One hundred thirty-eight course participants (120 family physicians, 15 specialists, and 3 physicians whose status was uncertain) were eligible for analysis. Of these, 68.1% were self-referred and 31.9% were referred by the CPSO. Overall, among physicians referred by the CPSO, the rate of opioid prescribing decreased dramatically in the year before course participation compared with matched control physicians. The course had no added effect on the rate of physicians’ opioid prescribing in the subsequent 2 years. There was no statistically significant effect on the rate of opioid prescribing observed among the self-referred physicians. Among 15 of the self-referred physicians who, owing to the high quantities of opioids they prescribed, were not matched with control physicians, the rate of opioid prescribing decreased by 43.9% in the year following course completion.
Conclusion
Physicians markedly reduced the quantities of opioids they prescribed after medical regulators referred them to an opioid-prescribing course. The course itself did not lead to significant additional reductions; however, a subgroup of physicians who prescribed high quantities of opioids might have responded to what was taught in the course.
Résumé
Objectif
Évaluer l’effet d’un cours intensif de 2 heures sur la prescription d’opiacés par des médecins.
Type d’étude
Étude d’observation stratifiée et rétrospective.
Contexte
L’Ordre des médecins et chirurgiens de l’Ontario (OMCO).
Participants
Médecins ontariens ayant suivi le cours entre le 1er avril 2000 et le 30 mai 2008.
Intervention
Un cours de 2 jours sur la prescription d’opiacés, réunissant un maximum de 12 médecins. Les méthodes d’enseignement comprenaient des cours magistraux, des discussions de cas et des patients types. On fournissait aussi un plan de cours et du matériel de bureau.
Principaux paramètres à l’étude
Les participants étaient appariés à des médecins témoins selon des critères spécifiques. L’issue principale était le taux de prescription d’opiacés, exprimé en milligrammes de morphine par trimestre.
Résultats
Un total de 138 médecins ayant suivi le cours (120 médecins de famille, 15 spécialistes et 3 médecins au statut incertain) étaient admissibles pour l’analyse. De ceux-là, 68,1 % participaient de façon volontaire et 31,9 %, à la demande de l’OMCO. Par rapport aux médecins témoins appariés, le taux global de prescription d’opiacés chez les médecins désignés par l’OMCO avait diminué de façon dramatique dans l’année précédant leur participation au cours. Le cours n’a eu aucun effet additionnel sur le taux de prescription des médecins au cours des 2 années subséquentes. Chez les médecins participant de façon volontaire, on n’a observé aucun effet statistiquement significatif sur le taux de prescription d’opiacés. Toutefois, chez 15 de ces derniers qui, en raison de la grande quantité d’opiacés qu’ils prescrivaient, n’avaient pas été appariés à des médecins témoins, le taux de prescription d’opiacés a diminué de 43,9 % dans l’année suivant la fin du cours.
Conclusion
On a observé une diminution importante de la quantité d‘opiacés prescrits chez les médecins après qu’ils eurent été incités par les responsables de la réglementation à suivre un cours sur la prescription d’opiacés. Le cours en soi n’a entraîné aucune diminution additionnelle; toutefois, un sous-groupe de médecins qui prescrivaient de grandes quantités d’opiacés pourrait avoir répondu positivement aux notions enseignées dans le cours.
Harms related to prescription opioids, including overdose and addiction, have increased substantially over the past 20 years.1–3 Evidence suggests that physicians’ opioid prescribing contributes to these harms. Among individuals in Ontario whose deaths were related to opioids and whose prescribing data were available, most were found to have received an opioid prescription within 4 weeks before death.2 Similarly, an Ontario study of patients attending a treatment facility for opioid addiction found that more than 80% of them acquired some or all of their opioids from physician prescriptions.4
Medical education has been suggested as one strategy to improve opioid prescribing among physicians.5,6 Randomized clinical trials and observational studies have demonstrated that educational interventions lead to modest and inconsistent improvements in prescribing of antibiotics, antidepressants, antipsychotics, and diabetes therapies.7–16 Educational interventions focused on opioid prescribing lead to positive improvement in physicians’ knowledge and self-reported practices17; however, few studies have used objective measures of opioid prescribing. In one such trial,18,19 46 physicians were randomized to receive a letter from the College of Physicians and Surgeons of British Columbia notifying them that they were prescribing high quantities of opioids and were either offered a 1-day workshop in the same letter or received only the notification letter with no intervention. At 6 months, the intervention groups reduced their prescribing by equivalent amounts, but both groups reverted to baseline within 1 year.
The College of Physicians and Surgeons of Ontario (CPSO) has offered an opioid-prescribing course several times per year since 1995. Participants are either self-referred or referred by the CPSO as a result of an investigation, a peer assessment, or a public complaint. We sought to examine the effect of the course on the rate of opioid prescribing by participating physicians.
METHODS
We conducted a population-based retrospective cohort study, comparing the opioid-prescribing practices of Ontario physicians who had taken the course, which is held in the offices of the CPSO in Toronto, Ont, with the practices of a matched control group. The study was approved by the research ethics board of Sunnybrook Health Sciences Centre in Toronto.
We matched physicians who took the course between April 1, 2000, and May 30, 2008, with a group of non-participating physicians on sex, age (within 5 years), specialty (family physician vs all other specialties), the number of opioid prescriptions written in the year before index date (within 50 prescriptions), and the number of patients eligible for Ontario Drug Benefits (ODB) aged 15 to 64 years and aged 65 years and older who had been treated by the physician in the year before the index date. Physicians who prescribed no opioids in the year before the index date were excluded. We designated the index date as the date of course completion for participating physicians. Control physicians were assigned the same index date as their matched pair. All participating physicians who could not be matched to at least 1 control physician were removed from the analyses and described separately.
The course is intended to improve physicians’ opioid prescribing for patients with chronic noncancer pain and to improve physicians’ skills in identifying and managing opioid misuse and addiction.20 The 2-day course involves didactic presentations, case discussions, standardized patients, a written test, and a telephone conference after the course is completed. A detailed syllabus, as well as didactic and office materials, is provided (Box 1).
Box 1. Description of the Appropriate Prescribing of Narcotic Analgesics course.
Faculty
Physiatrist with expertise in chronic pain management
2 family physicians with expertise in opioid addiction
Duration
2 days
Participants
Maximum of 12 participants
Syllabus
Mailed to participants before the course, with background notes, key articles, and office materials
Course components
4 didactic presentations on chronic pain, opioid prescribing, opioid addiction, and benzodiazepines (7 hours, including discussion time)
2 simulated clinical interviews with standardized patients; physicians receive feedback from standardized patients and have an individual review with faculty staff (2 hours)
Group discussion on cases from the physicians’ own practices (2 hours, in groups of 6)
Session on pros and cons of change, case vignettes on opioid and benzodiazepine prescribing, faculty demonstration of interview, and short-answer written test (2 hours)
Follow-up exercise
3 months after course completion, a 1-hour teleconference to review changes in practice since taking the course
Data sources
Identifiers for course participants, referral status, and course dates were obtained from the CPSO and linked to computerized records of the ODB Database. All Ontarians aged 65 years and older are eligible for ODB coverage, as are younger Ontarians who are receiving social assistance. We identified physician characteristics from the Institute for Clinical Evaluative Sciences Physician Database.
Outcome measurements
The primary outcome was the rate of opioid prescribing to ODB-eligible patients aged 15 years and older by each study physician in each calendar quarter. The total dose for each opioid prescription was calculated as the quantity dispensed multiplied by the strength, converted to morphine equivalents using ratios employed by the Canadian Guideline for Safe and Effective Use of Opioids for Chronic Non-Cancer Pain (http://nationalpaincentre.mcmaster.ca/opioid/).6 We included all prescriptions for codeine, morphine, oxycodone, hydromorphone, meperidine, or transdermal fentanyl in the analyses. Methadone was excluded because it is usually prescribed for treating addiction, not pain.
Statistical analysis
We modeled the total amount of opioids dispensed using broken-line longitudinal regression.21,22 Break points were chosen at appropriate locations on the time axis (1 year before index date, index date, and 1 year after index date). We tested for differences in the rate of opioid prescribing (amount of opioids dispensed per quarter in milligrams of morphine equivalent [mg ME]) between segments of interest. From this model, we constructed several comparisons outlined in Figure 1. These comparisons included changes in opioid prescribing before the course (1 year before index date vs 2 to 5 years before index date), immediately after the course (1 year after index date vs 2 to 5 years before index date), and long-term changes after the course (2 years after index date vs 2 to 5 years before index date) (Figure 1). All comparisons excluded prescribing information in the 1 year before the index date to avoid potential contamination by the referral process among participating physicians. The model was further stratified by patient age group (patients 15 to 64 years of age vs patients 65 years of age and older) and physician referral status (physicians referred to the course vs those self-referred).
Figure 1.
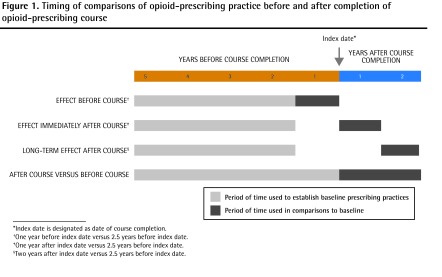
Timing of comparisons of opioid-prescribing practice before and after completion of opioid-prescribing course
*Index date is designated as date of course completion.
†One year before index date versus 2.5 years before index date.
‡One year after index date versus 2.5 years before index date.
§Two years after index date versus 2.5 years before index date.
We calculated descriptive statistics for baseline characteristics of physicians on their index date. Standardized differences were used to test for differences between groups. A standardized difference of greater than 0.10 is generally considered meaningful.23 All analyses used a type 1 error rate of .05 as the threshold for significance and were performed using SAS, version 9.2.24 Regression models were fit using Proc MIXED, and the correlation structure was chosen using the Akaike information criterion.
RESULTS
Over the 9-year study period, 138 physicians participated in the course (120 family physicians, 15 specialists, and 3 physicians whose status was uncertain). Of these, 44 (31.9%) were referred by the CPSO and 94 (68.1%) were self-referred. Among referred physicians, 29 (65.9%) were involved in a registrar’s investigation, 10 (22.7%) were the subject of a public complaint, and 5 (11.4%) were referred following peer assessment. The median time between the course referral and course completion was 1.7 years (interquartile range 0.9 to 2.9 years). Of all course participants, 105 (76.1%) were matched to non-participating physicians.
In general, course participants and their matched pairs were similar with respect to demographic characteristics, practice, and prescribing history; however, course participants were more likely to practise in an urban location and, on average, had practised medicine longer than their matched counterparts (Table 1). Thirty-three participants could not be matched to control physicians because of the high quantities of opioids they prescribed. These physicians were typically older (mean age 57 years), had a larger practice, and treated more ODB-eligible patients than physicians who were successfully matched to control physicians (Table 1). Eighteen (54.5%) of the unmatched physicians were referred to the course and 15 (45.5%) were self-referred.
Table 1.
Descriptive statistics for control physicians, course participants matched with control physicians, and course participants not matched with control physicians
| VARIABLE | CONTROL PHYSICIANS (N = 105), VALUE |
COURSE PARTICIPANTS MATCHED WITH CONTROL PHYSICIANS (N = 105)
|
COURSE PARTICIPANTS NOT MATCHED WITH CONTROL PHYSICIANS (N = 33)
|
||
|---|---|---|---|---|---|
| VALUE | STANDARDIZED DIFFERENCE | VALUE | STANDARDIZED DIFFERENCE | ||
| Mean (SD) age, y | 48.6 (10.4) | 48.5 (10.4) | 0.01 | 56.6 (9.8) | 0.78 |
| Male sex, N (%) | 84 (80.0) | 84 (80.0) | 0 | 25 (75.8) | 0.10 |
| Main specialty, N (%) | |||||
| • GPs and family physicians | 90 (85.7) | 90 (85.7) | 0 | 30 (90.9) | 0.15 |
| • Other | 15 (14.3) | 15 (14.3) | 0 | ≤ 5 (9.1) | 0.15 |
| Rural residence, N (%) | 8 (7.6) | 16 (15.2) | 0.24 | ≤ 5 (6.1) | 0.06 |
| Mean (SD) no. of patients | 5995 (3620) | 5074 (2990) | 0.28 | 8112 (4947) | 0.53 |
| Mean (SD) no. of years practising medicine | 23.3 (10.6) | 21.8 (11.6) | 0.13 | 31.5 (9.7) | 0.8 |
| Mean (SD) no. of ODB-eligible patients in year before course | 232.5 (189.3) | 230.2 (192.5) | 0.01 | 548.5 (333.6) | 1.36 |
| Mean (SD) no. of ODB-eligible patients aged 15–64 in year before course | 65.7 (53.2) | 66.5 (53.9) | 0.01 | 210.3 (157.0) | 1.61 |
| Mean (SD) no. of ODB-eligible patients aged ≥ 65 in year before course | 166.7 (152.4) | 163.6 (157.2) | 0.02 | 338.2 (216.5) | 1.01 |
| Median (IQR) no. of opioid prescriptions dispensed in year before course | 95 (22–278) | 96 (18–273) | 0 | 745 (383–1577) | 1.47 |
| Median (IQR) no. of opioid prescriptions dispensed to patients aged 15–64 in year before course | 31 (6–118) | 43 (9–130) | 0.1 | 558 (145–870) | 1.29 |
| Median (IQR) no. of opioid prescriptions dispensed to patients aged ≥ 65 in year before course | 42 (10–124) | 26 (6–99) | 0.08 | 278 (58–481) | 1.12 |
IQR—interquartile range, ODB—Ontario Drug Benefit.
Of the participants who were matched to control physicians, 26 (24.8%) were referred to the course and 79 (75.2%) were self-referred.
Effect of the opioid-prescribing course
Effect of the CPSO course on opioid-prescribing rates
In the primary analysis, there was neither an immediate (1 year) nor a long-term (2 years) reduction in the rate of opioid prescribing to younger ODB-eligible patients following course completion as compared with matched control physicians (reduction by 5103 mg ME per quarter [P = .22] and by 787 mg ME per quarter [P = .86], respectively) (Table 2). Similarly, the rate of opioid prescribing to older patients was unchanged in the first and second year following course completion (reduction by 243 mg ME per quarter [P = .74] and by 314 mg ME per quarter [P = .65], respectively).
Table 2.
Effect of the CPSO opioid-prescribing course on the rate of opioids prescribed (mg ME) per quarter by participating physicians
| COURSE PARTICIPANTS MATCHED WITH CONTROL PHYSICIANS |
EFFECT BEFORE COURSE*
|
EFFECT IMMEDIATELY AFTER COURSE†
|
LONG-TERM EFFECT AFTER COURSE‡
|
AFTER COURSE VS BEFORE COURSE
|
||||
|---|---|---|---|---|---|---|---|---|
| ESTIMATE, mg ME | P VALUE | ESTIMATE, mg ME | P VALUE | ESTIMATE, mg ME | P VALUE | ESTIMATE, mg ME | P VALUE | |
| All physicians | ||||||||
| • Patients aged 15–64 y | −7137 | .1022 | −5103 | .2215 | −787 | .8578 | 1338 | .6340 |
| • Patients aged ≥ 65 y | −465 | .5454 | −243 | .7410 | −314 | .6455 | −245 | .6275 |
| Referred physicians | ||||||||
| • Patients aged 15–64 y | −37 801 | < .001 | −26 857 | < .001 | −25 595 | .0002 | −1554 | .7216 |
| • Patients aged ≥ 65 y | −3599 | .0029 | −2099 | .0687 | −1183 | .2724 | −1296 | .1023 |
| Self-referred physicians | ||||||||
| • Patients aged 15–64 y | 3310 | .4824 | 2186 | .6275 | 7645 | .1044 | 2391 | .4355 |
| • Patients aged ≥ 65 y | 586 | .4805 | 366 | .6455 | −30 | .9674 | 77 | .8882 |
CPSO—College of Physicians and Surgeons of Ontario, mg ME—milligrams of morphine equivalent.
1 year before course versus 2–5 years before course.
1 year after course versus 2–5 years before course.
2 years after course versus 2–5 years before course.
Opioid prescribing among self-referred physicians
On average, self-referred physicians prescribed 37 777 mg ME of opioids per quarter to patients aged 15 to 64 years, and 12 673 mg ME per quarter to patients aged 65 years and older during the 7-year observation window. The opioid-prescribing rates for both younger and older patients did not change significantly in the first and second year following course completion compared with matched controls (younger patients: rate increase by 2186 mg ME per quarter [P = .63] and by 7645 mg ME per quarter [P = .10], respectively [Table 2, Figure 2]; older patients: rate increase by 366 mg ME per quarter [P = .65] and rate reduction by 30 mg ME per quarter [P = .97] in the first and second years, respectively [Table 2, Figure 3]).
Figure 2.
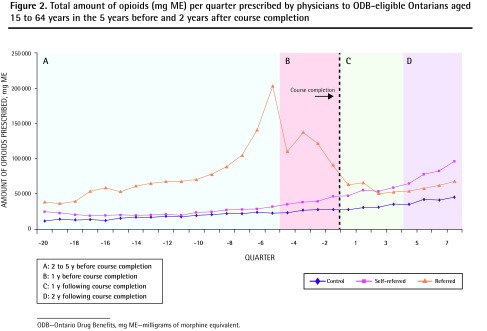
Total amount of opioids (mg ME) per quarter prescribed by physicians to ODB-eligible Ontarians aged 15 to 64 years in the 5 years before and 2 years after course completion
ODB—Ontario Drug Benefits, mg ME—milligrams of morphine equivalent.
Figure 3.
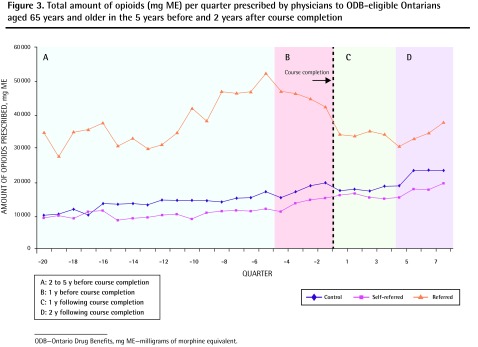
Total amount of opioids (mg ME) per quarter prescribed by physicians to ODB-eligible Ontarians aged 65 years and older in the 5 years before and 2 years after course completion
ODB—Ontario Drug Benefits, mg ME—milligrams of morphine equivalent.
Opioid prescribing among physicians referred by the CPSO
On average, physicians who were referred to the course prescribed 77 338 mg ME per quarter to patients aged 15 to 64 years, and 37 918 mg ME per quarter to patients aged 65 years and older during the study period. Among these physicians, the rate of opioid prescribing to both younger and older ODB-eligible patients was reduced significantly in the 1 year before the course (reduction by 37 801 mg ME per quarter [P < .001] and by 3599 mg ME per quarter [P = .003], respectively [Table 2]). Among younger patients, this decreased prescribing rate was sustained over the subsequent 2 years (reduction by 25 595 mg ME per quarter in the second year after the course [P < .001]) (Figure 2). Among older patients, this reduction was not sustained in the first year after course completion (reduction by 2099 mg ME per quarter; [P = .07]) (Figure 3).
Opioid prescribing among unmatched physicians
The prescribing trends of physicians who participated in the course but could not be matched to control physicians are presented in Figure 4. Prescribing of opioids to older patients did not change significantly following course completion. However, unmatched physicians who were self-referred reduced their rate of opioid prescribing to younger patients by 32.4% in the first year after the course (from 669 754 mg ME per quarter to 452 721 mg ME per quarter). By the end of the 2-year follow-up period, prescribing in this group had risen again to 641 531 mg ME per quarter. In contrast, the rate of opioid prescribing to younger patients by unmatched physicians referred to the course was reduced in the year before course completion (from 573 311 mg ME per quarter to 380 379 mg ME per quarter, 33.7% reduction) and remained relatively stable thereafter.
Figure 4.
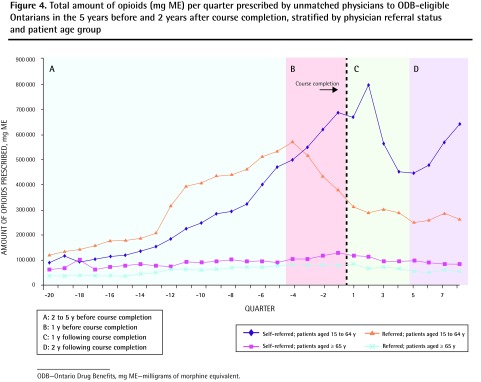
Total amount of opioids (mg ME) per quarter prescribed by unmatched physicians to ODB-eligible Ontarians in the 5 years before and 2 years after course completion, stratified by physician referral status and patient age group
ODB—Ontario Drug Benefits, mg ME—milligrams of morphine equivalent.
DISCUSSION
We found that physicians who were referred to the course by medical regulators had a marked and sustained decline in their opioid prescribing. This decline cannot be attributed to the course itself or to external events because prescribing did not change among matched control physicians or among physicians who took the course voluntarily. This suggests that regulation might have a substantially greater effect than education on physicians’ opioid prescribing.
In a descriptive analysis, self-referred physicians who prescribed high quantities of opioids (and thus were not matched to a control physician) reduced their opioid prescribing to younger patients in the first year after the course; however, these reductions were not sustained in the second year. Further research is needed to confirm this finding. Interventions targeted at physicians who prescribe opioids more frequently are an important public health priority because overdose deaths are concentrated in patients of high prescribers of opioids.25
Limitations
Some limitations merit emphasis. We measured only the quantity of opioids prescribed, not the quality of opioid prescribing. Hence, we were unable to assess the effect of the CPSO course on prescribing practices such as patient selection, titration, and tapering. Also, as the primary outcome measure was the total amount of opioids prescribed per physician, our analysis might have missed changes to physician practice involving small numbers of high-risk patients. Finally, we only had access to prescribing data for patients who were eligible for public coverage.
Studies in other jurisdictions have confirmed the effect of regulatory interventions. For example, in 2007 Washington State introduced a policy requiring physicians to obtain a formal or informal consultation for patients receiving opioids in doses above 120 mg ME per day. The policy was associated with a 35% reduction in doses greater than 120 mg ME daily and a 50% reduction in opioid-related deaths among patients receiving workers’ compensation benefits.26
While our course had a limited effect on prescribing patterns, educational interventions that were multifaceted and sustained have had promising results. Utah’s Prescription Safety Program was associated with a 14% reduction in opioid-overdose deaths.27,28 The program used widespread provider detailing, a public education campaign, dissemination of prescribing guidelines, and a prescription database.
Conclusion
A brief but intensive educational program was not independently associated with reduced opioid prescribing among physicians. However, referral to the opioid-prescribing course by a medical regulator was associated with a significant (P < .001) and sustained reduction in opioid prescribing to younger patients.
EDITOR’S KEY POINTS
While conferences and workshops about opioids are among the main sources of information for family physicians about these drugs, there is limited evidence that they are effective in improving physicians’ opioid prescribing.
Among the physicians who were referred to the opioid-prescribing course, opioid-prescribing rates declined dramatically after they were notified by a medical regulator of an impending complaint or investigation. The decline occurred well before they actually took the course. Among the self-referred physicians, there was no change in opioid prescribing before the course or after course completion. This suggests that the course had little effect on opioid prescribing (except perhaps among a subgroup of physicians who prescribed high quantities of opioids), whereas notification from a medical regulator had a considerable effect.
The study demonstrates that regulatory interventions are far more effective at changing physician behaviour than educational interventions.
POINTS DE REPÈRE DU RÉDACTEUR
Les conférences et ateliers traitant d’opiacés constituent la principale source d’information pour les médecins de famille, mais il y a peu de preuves que ces interventions soient efficaces pour améliorer la prescription d’opiacés par les médecins.
Chez les médecins qui avaient été incités à suivre le cours sur la prescription d’opiacés par l’OMCO, le taux de prescription d’opiacés a diminué de façon dramatique après que les responsables de la réglementation les eurent avertis qu’ils pourraient faire l’objet d’une plainte ou d’une enquête. Cette diminution est survenue bien avant qu’ils aient suivi le cours. Chez les médecins qui participaient de façon volontaire, il n’y a pas eu de changement dans la prescription d’opiacés avant et après le cours. Cela suggère que le cours a eu peu d’effet sur la prescription d’opiacés (sauf, peut-être, pour un sousgroupe de médecins qui prescrivaient déjà de fortes quantités d’opiacés); par contre, les interventions de type réglementaire ont eu un effet considérable.
Cette étude montre que les interventions de type réglementaire sont beaucoup plus efficaces que les interventions formatrices pour modifier le comportement des médecins.
Footnotes
This article has been peer reviewed.
Cet article a fait l’objet d’une révision par des pairs.
Contributors
All authors contributed to the concept and design of the study; data analysis and interpretation; and preparing the manuscript for submission.
Competing interests
None declared
References
- 1.Maxwell JC. The prescription drug epidemic in the United States: a perfect storm. Drug Alcohol Rev. 2011;30(3):264–70. doi: 10.1111/j.1465-3362.2011.00291.x. [DOI] [PubMed] [Google Scholar]
- 2.Dhalla IA, Mamdani MM, Sivilotti ML, Kopp A, Qureshi O, Juurlink DN. Prescribing of opioid analgesics and related mortality before and after the introduction of long-acting oxycodone. CMAJ. 2009;181(12):891–6. doi: 10.1503/cmaj.090784. Epub 2009 Dec 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fischer B, Nakamura N, Rush B, Rehm J, Urbanoski K. Changes in and characteristics of admissions to treatment related to problematic prescription opioid use in Ontario, 2004–2009. Drug Alcohol Depend. 2010;109(1–3):257–60. doi: 10.1016/j.drugalcdep.2010.02.001. Epub 2010 Mar 1. [DOI] [PubMed] [Google Scholar]
- 4.Sproule B, Brands B, Li S, Catz-Biro L. Changing patterns in opioid addiction. Characterizing users of oxycodone and other opioids. Can Fam Physician. 2009;55:68-9.e1–5. Available from: www.cfp.ca/content/55/1/68.full.pdf+html. Accessed 2013 Apr 11. [PMC free article] [PubMed] [Google Scholar]
- 5.College of Physicians and Surgeons of Ontario . Avoiding abuse, achieving a balance: tackling the opioid public health crisis. Toronto, ON: College of Physicians and Surgeons of Ontario; 2010. Available from: www.cpso.on.ca/uploadedFiles/policies/positions/Opioid%20report%20final.pdf. Accessed 2013 Apr 11. [Google Scholar]
- 6.National Opioid Use Guideline Group . Canadian guideline for safe and effective use of opioids for chronic non-cancer pain. Hamilton, ON: National Opioid Use Guideline Group; 2010. [PMC free article] [PubMed] [Google Scholar]
- 7.Bregnhøj L, Thirstrup S, Kristensen MB, Bjerrum L, Sonne J. Combined intervention programme reduces inappropriate prescribing in elderly patients exposed to polypharmacy in primary care. Eur J Clin Pharmacol. 2009;65(2):199–207. doi: 10.1007/s00228-008-0558-7. Epub 2008 Sep 21. [DOI] [PubMed] [Google Scholar]
- 8.Gören JL, Beck SE, Mills BJ, Shtasel DL, Dufresne RL. Development and delivery of a quality improvement program to reduce antipsychotic polytherapy. J Manag Care Pharm. 2010;16(6):393–401. doi: 10.18553/jmcp.2010.16.6.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henriksson S, Isacsson G. Increased antidepressant use and fewer suicides in Jämtland county, Sweden, after a primary care educational programme on the treatment of depression. Acta Psychiatr Scand. 2006;114(3):159–67. doi: 10.1111/j.1600-0447.2006.00822.x. [DOI] [PubMed] [Google Scholar]
- 10.Kasje WN, Denig P, Stewart RE, de Graeff PA, Haaijer-Ruskamp FM. An educational programme for peer review groups to improve treatment of chronic heart failure and diabetes mellitus type 2 in general practice. J Eval Clin Pract. 2006;12(6):613–21. doi: 10.1111/j.1365-2753.2005.00625.x. [DOI] [PubMed] [Google Scholar]
- 11.Lanas A, Esplugues JV, Zapardiel J, Sobreviela E. Education-based approach to addressing non-evidence-based practice in preventing NSAID-associated gastrointestinal complications. World J Gastroenterol. 2009;15(47):5953–9. doi: 10.3748/wjg.15.5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Connor PJ, Sperl-Hillen JM, Johnson PE, Rush WA, Asche SE, Dutta P, et al. Simulated physician learning intervention to improve safety and quality of diabetes care: a randomized trial. Diabetes Care. 2009;32(4):585–90. doi: 10.2337/dc08-0944. Epub 2009 Jan 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peters-Klimm F, Campbell S, Müller-Tasch T, Schellberg D, Gelbrich G, Herzog W, et al. Primary care-based multifaceted, interdisciplinary medical educational intervention for patients with systolic heart failure: lessons learned from a cluster randomised controlled trial. Trials. 2009;10:68. doi: 10.1186/1745-6215-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinman MA, Ranji SR, Shojania KG, Gonzales R. Improving antibiotic selection: a systematic review and quantitative analysis of quality improvement strategies. Med Care. 2006;44(7):617–28. doi: 10.1097/01.mlr.0000215846.25591.22. [DOI] [PubMed] [Google Scholar]
- 15.O’Brien MA, Rogers S, Jamtvedt G, Oxman AD, Odgaard-Jensen J, Kristoffersen DT, et al. Educational outreach visits: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2007;4:CD000409. doi: 10.1002/14651858.CD000409.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zillich AJ, Ackermann RT, Stump TE, Ambuehl RJ, Downs SM, Holmes AM, et al. An evaluation of educational outreach to improve evidence-based prescribing in Medicaid: a cautionary tale. J Eval Clin Pract. 2008;14(5):854–60. doi: 10.1111/j.1365-2753.2008.01035.x. [DOI] [PubMed] [Google Scholar]
- 17.Midmer D, Kahan M, Marlow B. Effects of a distance learning program on physicians’ opioid- and benzodiazepine-prescribing skills. J Contin Educ Health Prof. 2006;26(4):294–301. doi: 10.1002/chp.82. [DOI] [PubMed] [Google Scholar]
- 18.Anderson JF, McEwan KL, Hrudey WP. Effectiveness of notification and group education in modifying prescribing of regulated analgesics. CMAJ. 1996;154(1):31–9. [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson JF, McEwan KL. Modifying prescribing of regulated analgesics. CMAJ. 1997;156(5):636. [PMC free article] [PubMed] [Google Scholar]
- 20.Furlan AD, Reardon R, Weppler C, National Opioid Use Guideline Group Opioids for chronic noncancer pain: a new Canadian practice guideline. CMAJ. 2010;182(9):923–30. doi: 10.1503/cmaj.100187. Epub 2010 May 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toms JD, Lesperance ML. Piecewise regression: a tool for identifying ecological thresholds. Ecology. 2003;84(8):2034–41. [Google Scholar]
- 22.Verbeke G, Molenberghs G. Linear mixed models for longitudinal data. New York, NY: Springer-Verlag; 2000. p. 569. [Google Scholar]
- 23.Mamdani M, Sykora K, Li P, Normand SL, Streiner DL, Austin PC, et al. Reader’s guide to critical appraisal of cohort studies: 2. Assessing potential for confounding. BMJ. 2005;330(7497):960–2. doi: 10.1136/bmj.330.7497.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Statistical Analysis System [software]. Version 9.2 . Cary, NC: SAS Institute; 2010. [Google Scholar]
- 25.Dhalla IA, Mamdani MM, Gomes T, Juurlink DN. Clustering of opioid prescribing and opioid-related mortality among family physicians in Ontario. Can Fam Physician. 2011;57:e92–6. Available from: www.cfp.ca/content/57/3/e92.full.pdf+html. Accessed 2013 Apr 11. [PMC free article] [PubMed] [Google Scholar]
- 26.Franklin GM, Mai J, Turner J, Sullivan M, Wickizer T, Fulton-Kehoe D. Bending the prescription opioid dosing and mortality curves: impact of the Washington State opioid dosing guideline. Am J Ind Med. 2012;55(4):325–31. doi: 10.1002/ajim.21998. Epub 2011 Dec 27. [DOI] [PubMed] [Google Scholar]
- 27.Cochella S, Bateman K. Provider detailing: an intervention to decrease prescription opioid deaths in Utah. Pain Med. 2011;12(Suppl 2):S73–6. doi: 10.1111/j.1526-4637.2011.01125.x. [DOI] [PubMed] [Google Scholar]
- 28.Johnson EM, Porucznik CA, Anderson JW, Rolfs RT. State-level strategies for reducing prescription drug overdose deaths: Utah’s prescription safety program. Pain Med. 2011;12(Suppl 2):S66–72. doi: 10.1111/j.1526-4637.2011.01126.x. [DOI] [PubMed] [Google Scholar]


