Abstract
Background
The burden of chronic kidney disease (CKD) is a growing concern worldwide. The prevalence of hemodialysis in Taiwan is the highest in the world, and this may increase the prevalence of orthopedic fractures. The aim of this study was to explore the incidences of various orthopedic injuries and the related risk factors.
Methods
A nationwide prospective study based on the Taiwan National Health Insurance dataset was conducted during 2004–2008. A total of 82,491 CKD patients were selected as the fixed cohort population. The International Classification of Diseases 9-CM diagnosis codes and treatment codes were identified as the inclusion criteria for orthopedic injury.
Results
A total of 82,491 Taiwanese people with CKD were identified in 2004, and 4915 orthopedic injuries occurred during the 5-year follow-up period. The cumulative incidences of orthopedic injuries were 42.56‰ for lower limb fractures, and 12.93‰, 3.27‰, and 1.64‰ for upper limb fractures, vertebrae fractures, and joint dislocations, respectively. All three types of orthopedic fractures were more common in the oldest age stratum (≥65 years old). In the CKD patients, the risk ratio of osteoporosis was 3.47 (95% confidence interval, 3.10–3.89) for all orthopedic injuries. Patients of advanced age, the female gender, and those with high comorbidity were also at significant risk of sustaining orthopedic fractures.
Conclusion
The results from this Taiwanese CKD cohort support the strong influence of aging and osteoporosis on all kinds of orthopedic injuries. The postponing of osteoporosis may need to be taken into consideration for the prevention of orthopedic injury among CKD patients undergoing hemodialysis.
Keywords: chronic kidney disease, renal osteodystrophy, hemodialysis, orthopedic injuries
Introduction
The burden of chronic kidney disease (CKD) and end-stage renal disease (ESRD) are of increasing concern in the aging society worldwide.1,2 In the last decade, the incidence of ESRD in Taiwan increased rapidly and reached the highest in the world. The prevalence of hemodialysis in Taiwan is currently one of the highest of all countries.3 CKD patients undergoing long-term hemodialysis usually have a low bone mass and osteoporosis, so the outcome of CKD long-term hemodialysis is not only progression to ESRD, but also an increased risk of fracture.4–6 Previous studies have described that the amount of bone loss in the femoral neck affects the risk of femoral neck fracture in ESRD patients and the overall risk of fracture in the general population;4,7,8 however, little is known about the importance of relative risks with regards to several kinds of orthopedic fractures and injuries for early-stage CKD patients undergoing hemodialysis. The lengths of some investigation periods have not been sufficient, or some surveys have focused on osteoporosis and vertebra fracture using a cross-sectional study design. Hence, comprehensive information on the incidence at each site of orthopedic fractures and dislocations is still scarce.
The aims of this study are: (1) to explore the incidences of various orthopedic fractures or dislocations among the population undergoing hemodialysis in Taiwan; and (2) to analyze the risk factors for the occurrence of orthopedic injuries in the Taiwanese hemodialysis population. This information will inform hemodialysis patients on the plans for the prevention of orthopedic injuries.
Materials and methods
Data source, security, and quality control
Taiwan launched a single-payer National Health Insurance (NHI) program in 1995, and its coverage rate expanded to more than 98% of the Taiwanese population after the year 2004. All enrollees enjoy health care with a small copayment from most clinics and hospitals.9 The National Health Insurance Bureau (NHIB) established a nationwide research database, which includes nationwide population-based data with good quality control and representation. The NHI database contains registration files and original claims data, including patients’ demographics, diagnosis, treatment details related to in-hospital and outpatient claims for reimbursement, and access to the National Health Insurance Research Database (NHIRD).
The information of all subjects was encrypted with a double scrambling protocol for research purposes to protect the privacy of the patients. All researchers who wish to use the NHIRD and its data subsets are required to sign a written agreement declaring that they have no intention of attempting to obtain information that could potentially violate the privacy of patients or care providers. This study protocol was evaluated by the NHRI, who gave their agreement to the planned analysis of the NHIRD (Application and Agreement Number: 100047). This study was also approved by the Institutional Review Board of Taoyuan General Hospital, which has been certificated by the Department of Health, Taiwan (Institutional Review Board Approval Number: TYGH99037).
Inclusion and exclusion criteria of the study population and definition
Every claimant of the NHI program at any time during 2004–2008 was included in the studied population (22,134 × 103 people in 2004, increasing to 22,918 × 103 people in 2008).9 As a cohort study population, the registration and claims data of these individuals collected by the NHI program were traced, and two separate categories of expenditure were used as follows: (1) inpatient expenditure by admission (DD files); and (2) ambulatory care expenditure by visit (CD files). In order to investigate the incidence of orthopedic injuries in the population undergoing hemodialysis in this study, the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes were evaluated, and the major diagnosis codes for hemodialysis were defined as coded as 585 and 586 (chronic renal failure with hemodialysis), the 403 series (hypertensive heart and CKD with ESRD), and the 404 series (hypertensive heart with specific or nonspecific heart failure and ESRD). Also according to the coding rules of the Taiwan NHI database for identifying CKD and ESRD with hypertensive heart disease, when patients have both diseases, the diagnosis code would be coded first as one of the folowing codes: 403.01, 403.11, or 403.91, 404.02, 404.03, 404.12, 404.13, 404.92, 404.93 before coding as 585–586.
All subjects matching the inclusion criteria of these hemodialysis codes in the annual CD files or DD files during 2004–2008 were selected as the study cohort. In order to investigate the incidences of orthopedic fractures or dislocations in the study cohort population during 2004–2008, the concomitant ICD-9-CM diagnosis codes and treatment codes were evaluated and used as the inclusion criteria. The diagnosis codes of orthopedic injuries were defined as coded as four major categories. The first major orthopedic injuries category, fractures of upper limbs, was defined as injuries coded in the 810–811 series (fracture of the clavicle or scapula), 812 series (fracture of the humerus), and 813–817 series (fracture of the radius, ulna, or hand). The second major orthopedic injuries category, fractures of lower limbs, was coded as the 820–821 series (fracture of the femoral neck or shaft), 822–823 series (fracture of the patella, tibia, or fibula), and 824–826 series (fracture of the ankle or foot). The third category, fractures of the trunk, was coded as the 805–806 series (fracture of vertebrae) and 808 series (fracture of the pelvis). The fourth major orthopedic injuries category was orthopedic joints dislocation, which could be defined as upper limb dislocation (coded as the 831 series [shoulder dislocation], 832 series [elbow dislocation], 833 series [wrist dislocation], and 834 series [finger dislocation]) and lower limb dislocation (coded as the 835 series [hip dislocation], 836 series [knee dislocation], 837 series [ankle dislocation], and 838 series [foot dislocation]).10,11 The treatment codes for the orthopedic injuries mentioned above were classified as the 78 series (other operation on bones, except facial bones), 79 series (reduction of fracture and dislocation), and 81 series (repair and plastic operations on joint structures).11,12
We calculated the Charlson comorbidity index, developed by Charlson et al,13 which is based on medical diagnosis codes (ICD-9-CM). In order to focus on acute orthopedic injury cases, subsequent hospitalization for chronic care or other medical disorders was excluded. To avoid repeated calculation, the status of those who were admitted for other subsequent surgical procedures, such as removal of spinal implanted devices or debridement, was also excluded. Thus, we analyzed the period of 2004 to 2008, and a total of 82,491 subjects in this study.
Statistical analysis
Descriptive statistics are presented as numbers of cases, percentages, and means with standard deviation, cumulative incidences (I) of specific orthopedic injury types (per 1000 populations), and 95% confidence intervals for the estimated incidence were calculated based on the number of observed cases as per the following formula:
| (1) |
As a fixed cohort study, the risk ratios (RRs) of various accumulative orthopedic injuries in both genders were evaluated, and the 95% confidence intervals were calculated as per the following formula14,15:
| (2) |
(RR = (eci1, eci2); a: orthopedic injury and CKD male cases; a + c: CKD male subjects; b: orthopedic injury and CKD female cases; b + d: CKD female subjects).14
Pearson’s Chi-square (χ2) test was used for univariate analysis of categorical data; the Mantel–Haenszel Chi-square test was used to test for trends. Further categorical analysis by Poisson regression was performed to evaluate the factors related to the orthopedic injuries of the studied subjects. All analyses were conducted using SAS 9.2 (SAS Institute, Inc, Cary, NC, USA) and the Statistical Package for Social Sciences for Windows (SPSS for Windows 19.0; IBM Corporation, Armonk, NY, USA).14
Results
Incidence and characteristics of the Taiwanese hemodialysis patients in 2004
According to the ICD-9-CM coding of CKD with uremia (585–586), and coding of the first hypertensive heart with or without heart failure (403.00–403.91, 404.00–404.93) if diagnosed with both hypertensive heart and CKD, 125,094 Taiwanese patients received hemodialysis in 2004, and a total of 82,491 CKD patients underwent long-term hemodialysis regularly over 6 months. The largest age stratum of the studied population was over 65 years (49.6%), followed by 45–64 years (38.62%), and those aged below 18 years (0.48%). There was no gender difference in the age distribution of the CKD patients (male: 62.5 ± 14.9 versus female: 62.4 ± 14.2, P = 0.137). The majority of those patients undergoing long-term hemodialysis were diagnosed with CKD or chronic renal failure (94.81%), while the other 5.19% of the hemodialysis patients had both hypertensive heart disease and CKD (Table 1). Based on the above inclusion criteria, a total of 82,491 CKD patients undergoing long-term hemodialysis were initially enrolled as the fixed cohort group, and their medical records from 2004 to 2008 were examined.
Table 1.
Characteristics of the enrolled subjects in 2004
| Male n = 43,014 |
Female n = 39,477 |
General n = 82,491 |
||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Cases | (%) | Cases | (%) | Cases | (%) | |
| Age stratum | ||||||
| Less than 18 years old | 247 | 0.6 | 150 | 0.4 | 397 | 0.5 |
| 18–44 years old | 5101 | 11.9 | 4224 | 10.7 | 9325 | 11.3 |
| 45–64 years old | 16,071 | 37.4 | 15,783 | 40.0 | 31,854 | 38.6 |
| 65+ years old | 21,595 | 50.2 | 19,320 | 48.9 | 40,915 | 49.6 |
| Types of CKD | ||||||
| CKD and chronic renal failurea | 40,744 | 94.7 | 37,430 | 94.8 | 78,174 | 94.8 |
| Hypertensive heart and end stage renal diseaseb | 2270 | 5.3 | 2047 | 5.2 | 4317 | 5.2 |
Notes:
ICD-9-CM diagnosis codes: 585,586;
ICD9_CM diagnosis codes: 403.01, 403.11, 403.91, 404.02, 404.03, 404.12, 404.13, 404.92, 404.93. Hypertensive heart and CKD, with or without heart failure, and with CKD stage 5 or end-stage renal disease.
Abbreviations: n, number; CKD, chronic kidney disease.
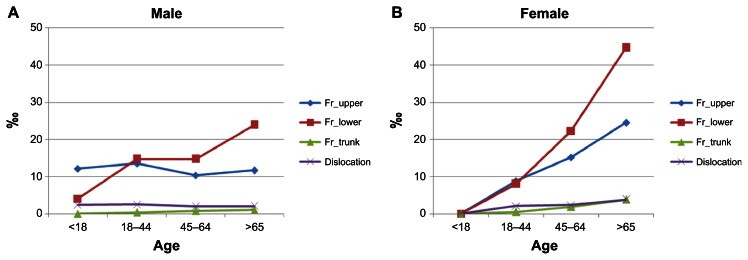
Table 2 details the orthopedic injury distributions of the fixed cohort during the follow-up period of 2004–2008. In general, 1028 cases of orthopedic injuries (including fractures and dislocations) occurred in 2004, and 1093, 969, 936, and 889 cases occurred in 2005, 2006, 2007, and 2008, respectively. The cumulative incidence of total orthopedic injuries for the CKD with hemodialysis population in Taiwan was 59.58‰, with a 95% CI of 57.97 to 61.20 per thousand persons in 5 years. The greatest incidence of orthopedic injuries among the CKD cohort was for lower limb fractures, which represented over 70% of various orthopedic injury cases annually. The cumulative incidence rate of lower limbs fractures, including the femoral neck/femur or tibia/fibula, was 42.56‰ in 5 years (95% CI: 41.18–43.94 per thousand persons). Figure 1 shows a gradual rate of increase in the four major categories of orthopedic injury with age in female genders. All three types of orthopedic fractures were more common in the oldest age stratum (≥65 years old). All incidences of fractures were higher than that of joint dislocations.
Table 2.
The incidences of various orthopedic injuries among CKD patients in Taiwan
| 2004 cases | 2005 cases | 2006 cases | 2007 cases | 2008 cases | 5-year cumulative incidence* | |
|---|---|---|---|---|---|---|
| Total incident orthopedic injuries with treatment | 1028 | 1093 | 969 | 936 | 889 | 59.58 (57.97–61.20) |
| Orthopedic fracture | 1006 | 1061 | 945 | 947 | 862 | 58.44 (56.84–60.04) |
| Upper limb | 204 | 216 | 214 | 221 | 212 | 12.93 (12.16–13.71) |
| Lower limb | 768 | 787 | 682 | 675 | 599 | 42.56 (41.18–43.94) |
| Trunka | 37 | 64 | 56 | 60 | 53 | 3.27 (2.88–3.66) |
| Orthopedic dislocation | 28 | 36 | 24 | 19 | 28 | 1.64 (1.36–1.91) |
| Upper limb | 12 | 20 | 16 | 10 | 13 | 0.86 (0.66–1.06) |
| Lower limb | 16 | 17 | 8 | 9 | 15 | 0.79 (0.60–0.98) |
Notes:
Per 1000 persons (‰);
Trunk: vertebrae and pelvis fracture.
Abbreviation: CKD, chronic kidney disease.
Figure 1.
The cumulative incidence of the four types of orthopedic injury by age stratum and gender. (A) The cumulative incidence of the different types of orthopedic injury by age for males. (B) The cumulative incidence of the different types of orthopedic injury by age for females.
Abbreviation: Fr, fractures.
Risk factors of orthopedic injuries among the CKD cohort
Table 3 shows the results of univariate analysis to evaluate the risk factors of the four major orthopedic injuries in the study cohort. There was no statistical difference in the incidence of upper limb and trunk fractures among the CKD subgroups, even among the patients who were diagnosed with both hypertensive heart and CKD and were undergoing long-term hemodialysis. However, there was a statistical difference between younger and older patients for lower limb and trunk fractures. Since there were too few events in young age (<18 years old), young adulthood (age stratum 18–44 years of age) was used as a reference group to compare for vertebra/pelvis fractures. The RRs of the age strata of those aged 45–64 years and 65 years or above were 3.08 (95% CI: 1.10–8.58) and 5.84 (95% CI: 2.02–14.90), respectively. A similar aging effect was also seen for vertebra/trunk fractures. The CKD subjects with a history of osteoporosis also had a significantly higher risk of sustaining a trunk fracture (RR = 4.09; 95% CI = 3.63–4.55). Gender differences were seen for all four types of orthopedic injury (P < 0.05). CKD patients with more comorbidities (Charlson index ≥ 6) had a significantly greater tendency to sustain orthopedic fractures than patients with fewer comorbidities (P < 0.05).
Table 3.
Univariate analysis of orthopedic injuries among the CKD population in Taiwan
| Upper limb fractures | Lower limb fractures | Trunk fractures | Limb dislocations | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| RR | (95% CI) | RR | (95% CI) | RR | (95% CI) | RR | (95% CI) | |
| Age stratum | ||||||||
| Less than 18 years old | 1.00 | 1.00 | – | 1.00 | ||||
| 18–44 years | 1.51 | (0.48–4.78) | 4.68 | (0.65–33.64) | 1.00 | 0.94 | (0.13–6.97) | |
| 45–64 years | 1.70 | (0.54–5.30) | 7.45 | (1.04–53.09) | 3.08 | (1.10–8.58) | 0.86 | (0.12–6.21) |
| ≥65 years | 2.37 | (0.76–7.39) | 13.81 | (1.94–98.37) | 5.48 | (2.02–14.90) | 1.15 | (0.16–8.22) |
| Gender | ||||||||
| Female versus male | 1.67 | (1.49–1.87) | 1.64 | (1.51–1.79) | 3.09 | (2.13–4.50) | 1.48 | (1.13–1.95) |
| Hypertension | ||||||||
| Yes versus no | 1.04 | (0.93–1.17) | 0.99 | (0.91–1.08) | 0.73 | (0.52–1.25) | 1.40 | (1.07–1.84) |
| Osteoporosis | ||||||||
| Yes versus no | 3.86 | (3.56–3.88) | 4.91 | (4.80–5.02) | 4.09 | (3.63–4.55) | 2.48 | (2.02–2.94) |
| Osteoarthritis | ||||||||
| Yes versus no | 2.11 | (1.98–2.24) | 2.76 | (2.67–2.86) | 2.24 | (1.86–2.63) | 2.99 | (2.69–3.28) |
| Comorbidities | ||||||||
| Higher (≥6) versus lower | 1.77 | (1.62–1.91) | 2.26 | (2.16–2.36) | 1.54 | (1.10–1.98) | 1.90 | (1.56–3.32) |
Abbreviations: CKD, chronic kidney disease; RR, risk ratio; CI, confidence interval.
The effects of independent risk factors on orthopedic injuries were separately modeled by four subtypes, as shown in Table 4. The adjusted RRs and 95% CIs of potentially confounding factors for orthopedic injuries are also shown. Patients of advanced aged, those of the female gender, and those with osteoarthritis or osteoporosis were at significant risk of sustaining an orthopedic fracture. Among those patients undergoing long-term hemodialysis, high comorbidity was not related to joint dislocation (0.969, 0.637–1.476). Similarly, comorbidity and hypertensive renal failure were also not significantly associated with upper limb fracture (1.083, 0.915–1.281) or trunk fracture (0.803, 0.485–1.330).
Table 4.
Multiple regression models of orthopedic injury among the CKD population in Taiwan
| Upper limb fractures | Lower limb fractures | Trunk fractures | Limb dislocations | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| RR | (95% CI) | RR | (95% CI) | RR | (95% CI) | RR | (95% CI) | |
| Age | ||||||||
| Less than 18 years old | 1.00 | 1.00 | – | – | 1.00 | |||
| 18–44 years | 1.42 | (0.45–4.50) | 4.36 | (0.61–31.36) | 1.00 | 0.82 | (0.11–6.11) | |
| 45–64 years | 1.46 | (0.47–4.56) | 6.17 | (0.87–44.02) | 2.80 | (1.00–7.82)** | 0.66 | (0.09–4.77) |
| ≥65 years | 1.80 | (0.58–5.64) | 9.66 | (1.36–68.87)* | 4.62 | (1.69–12.65)* | 0.73 | (0.10–5.26) |
| Gender | ||||||||
| Female versus male | 1.54 | (1.37–1.73)** | 1.48 | (1.35–1.62)** | 2.85 | (1.95– 4.16)** | 1.41 | (1.07–1.87)* |
| Hypertension | – | – | – | – | – | – | 1.33 | (0.99–1.79) |
| Osteoporosis | 2.79 | (2.33–3.35)** | 3.43 | (3.00–3.91)** | 2.68 | (1.62–4.45)** | 1.64 | (0.99–2.70) |
| Osteoarthritis | 1.63 | (1.40–1.90)** | 1.91 | (1.71–2.14)** | 1.64 | (1.07–2.52)* | 2.70 | (1.92–3.79)* |
| Comorbidities | ||||||||
| Higher versus lower | 1.08 | (0.92–1.28) | 1.17 | (1.03–1.33)* | 0.80 | (0.49–1.33) | 0.97 | (0.64–1.48) |
Notes:
P < 0.05;
P < 0.01.
Abbreviations: CKD, chronic kidney disease; RR, risk ratio; CI, confidence interval.
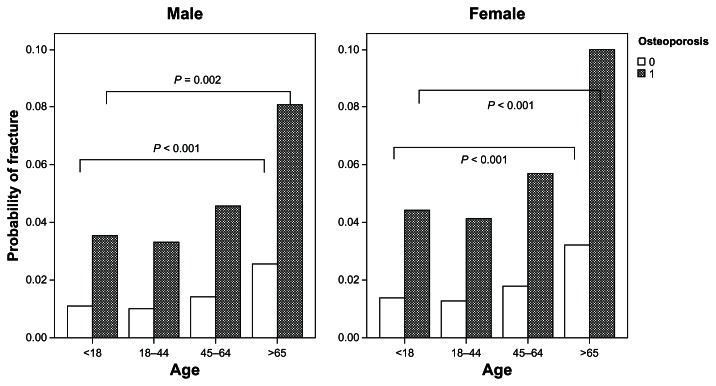
Table 5 reveals the results of this prospective study to examine the risk factors for the occurrence of orthopedic injuries. Among all the patients undergoing long-term hemodialysis, advanced age, presence of osteoporosis or osteoarthritis, and high comorbidity were the major risk factors for orthopedic injuries. Aging was a complex risk factor for orthopedic injuries, and female patients with older age (≥65 years) had a high risk (RR = 3.01, 95% CI, 2.35–3.85) of sustaining orthopedic injuries. In the male patients, we did not find a statistically significant age effect. Younger age may be protective against orthopedic injuries with a dose–response effect. After adjusting for age and medical history of osteoarthritis and comorbid conditions, patients with osteoporosis were still susceptible to getting orthopedic fractures. Using the prediction method of log linear regression analysis, the probabilities of any kind of orthopedic fracture by gender and by age strata could be compared (P < 0.01, age strata tested by the linear trend test). Of all the studied subjects, the CKD patients with osteoporosis were at a significantly greater risk of sustaining an orthopedic fracture than the patients without osteoporosis in both genders (Figure 2).
Table 5.
All kinds of orthopedic injury among the CKD population by gender
| Total kinds of orthopedic injury | Male | Female | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| RR | (95% CI) | RR | (95% CI) | RR | (95% CI) | |
| Age | ||||||
| Less than 18 years old | 1.00 | 1.00 | – | – | ||
| 18–44 years | 2.06 | (0.76–5.57) | 1.57 | (0.58–4.29) | 1.00 | |
| 45–64 years | 2.48 | (0.92–6.65) | 1.33 | (0.49–3.60) | 1.94 | (1.51–2.50)*** |
| ≥65 years | 3.48 | (1.30–9.34)* | 1.62 | (0.60–4.36) | 3.01 | (2.35–3.85)*** |
| Gender | ||||||
| Female | 1.48 | (1.37–1.60)*** | – | – | ||
| Male | 1.00 | – | – | |||
| Osteoporosis | 3.47 | (3.10–3.89)*** | 4.66 | (3.78–5.74)*** | 3.01 | (2.63–3.45)*** |
| Osteoarthritis | 1.79 | (1.63–1.97)*** | 2.08 | (1.78–2.43)*** | 1.63 | (1.44–1.84)*** |
| Comorbidities | ||||||
| Higher versus lower | 1.13 | (1.02–1.26)* | 0.92 | (0.77–1.09) | 1.28 | (1.12–1.46)*** |
Notes:
P < 0.05;
P < 0.001.
Abbeviations: CKD, chronic kidney disease; RR, risk ratio; CI, confidence interval.
Figure 2.
Probability of orthopedic fractures among patients with or without osteoporosis by gender and age group.
Discussion
A growing amount of literature has reported that patients with CKD are also at an increased risk of fragility fracture.16,17 End-stage kidney disease decreases renal synthesis of 1,25(OH)2D3, which may adversely affect bone remodeling and accelerate the deterioration of the bone structure.1 The prevalence of total orthopedic fractures in 2005 was reported to be 6.1‰,11 and the annual incidence of joint dislocations among the Taiwanese general population is 42.1 per 100,000 people.12 In the present study, the cumulative incidence of orthopedic fracture was 59.6‰, and the annual incidence of orthopedic fracture among the CKD patients was higher than that in the general population by at least 1.72-fold.18 To our knowledge, a large sample prospective study with a long-term observation period has not previously been conducted in detail.19–21 One strength of our study was that we included study subjects from a nationwide cohort. We conducted a prospective cohort study of 82,491 hemodialysis patients and discovered the numbers of head-to-toe orthopedic injuries per 1000 persons annually. Osteoporosis remained the factor associated with the highest RR for orthopedic fractures.22,23
The present nationwide population study, consisting of CKD patients who underwent long-term hemodialysis and follow-up for 5 years, provided experimental evidence that CKD patients sustain many orthopedic injuries, even hemodialysis patients with less comorbidity. Theoretically, the pathogenesis of renal osteodystrophy exacerbated by CKD leads to fragility fractures.1 The results of Gabay et al’s7 study indicated that bone mass density (BMD) was decreased at all skeletal sites after hemodialysis, especially in the lumbar spine and femoral shaft, which were significantly negatively related to the duration of dialysis. This might be also the reason for which the highest incidence of fractures occurred in the lower limbs among CKD patients undergoing long-term hemodialysis.
The primary purpose of this study was to explore the incidences and risk factors of various orthopedic fractures or dislocations among the hemodialysis population in Taiwan. In the present prospective cohort study, the 5-year cumulative incidences of orthopedic injuries were 42.56‰ for lower limb fractures, and 12.93‰, 3.27‰, and 1.64‰ for upper limb fractures, vertebrae fractures, and joint dislocations. These high incidences were similar to those reported in the Rudser et al24 study, which focused on the long-term risks for fractures in America. Another similar result was also found in a European clinical study,25 which also reported the gender difference that vertebral cortical bone loss occurs significantly more in females than in males. Our study was consistent with previous studies.16,18Figure 2 demonstrates that osteoporosis in women increases the risk of orthopedic fracture in each age stratum.1
One retrospective study of the prevalence of spine and nonspine fractures in Japanese dialysis patients reported figures of 15% in men and 30% in women,22,26 which were much higher than our findings. That study also measured the bone mineral density at the lumbar spine and radius site, and reported significantly lower BMDs in women than in men (P < 0.001).26 Most of the investigations were conducted using a cross-sectional study design or a case-control study design, which did not enable the identification of the incidences of various fractures without recall bias. Table 2 clearly shows the incidences of various orthopedic injuries found in each year of our study. In addition, Figure 2 demonstrates the cumulative incidence rate of the four types of orthopedic trauma by gender and age strata, simultaneously. Our study provides detailed information regarding not only the gender differences, but also the age effect based on a nationwide database. In a Czech cohort study, time on dialysis was found to be independently correlated with a decrease in BMD (R = 0.35, P < 0.005) by multiple regression analysis, and a significantly greater decrease was seen in females than in males (P < 0.005).22
CKD is usually silent and may remain undetected until an orthopedic accident is sustained. At this point in the silent process, there are few opportunities to prevent malignant outcomes,19 such as further declines in bone strength and renal function necessitating hemodialysis. In a recent observational study of the Third National Health and Nutrition Examination Survey (NHANES III) in the United States, physical activity was found to be associated with health outcomes among end-stage kidney disease patients (estimated glomerular filtration rate < 60 mL/minute/1.73 m2). Insufficient activity was present in 13.5% of the non-CKD and 28.0% of the CKD groups (P < 0.001), with a higher mortality rate.27 Regarding the risk factors identified for fracture in previous studies,6,19,28,29 which included advanced age, the female gender, postmenopausal status, medical history of osteoarthritis, osteoporosis, and propensity to fall, our study was similar to those conducted in the general population.16,22,30,31 More comorbidities were associated with an increased fracture risk among CKD patients. Hemodialysis patients also sustain more orthopedic fractures with advancing age.
Age is a complex factor when exploring the relationships between gender and fracture risks. A significantly increased risk of fractures was observed in women who had CKD with long-term hemodialysis, especially in the age stratum of 65 years old and above, and the excess risk remained significant even after accounting for baseline medical diseases and related factors, including osteoarthritis, osteoporosis, and comorbid conditions.
Limitations of the present study exist. Complete information regarding clinical symptoms was not available from our database because the national health registry records have ICD-9-CM diagnosis codes only. The BMD, a traditional fracture risk factor, was not recorded in the national health registry. Therefore, the ICD-9-CM code for osteoporosis, which has appeared over three times, was used to define bone mass loss in our study. The general population study with a long-term prospective observation period would need to be conducted in the future.
Previous investigations have focused on a single type of injury, such as fractures. It is the strength of our study that more is now known about the various characteristics of orthopedic injuries and their relationships among CKD patients undergoing hemodialysis. This study also confirms the strong influence of osteoarthritis and osteoporosis on all kinds of orthopedic injuries. We hope to present the risk factors as a reference point through which to inform hemodialysis patients to prevent orthopedic injuries; the deterioration of osteoporosis should be postponed and the symptoms of osteoarthritis should be lessened. We suggest that these high-risk patients should be taken care of intensively in order to maintain safety in their daily lives.
Conclusion
Hemodialysis for CKD may carry increased fracture risks in older women. The results from this Taiwanese CKD cohort support the conclusion that aging, the female gender, and suffering from osteoporosis and osteoarthritis are high risk factors for orthopedic injuries. The impact of osteoporosis may need to be taken into consideration for the prevention of orthopedic injuries among CKD patients undergoing hemodialysis.
Acknowledgments
This study was funded by the Department of Health, Executive Yuan, Taiwan.
Footnotes
Disclosure
The authors report no conflicts of interest in this work. All authors declare that they have no conflicts of interest including directorships, stock holding, or contracts.
References
- 1.Nickolas TL, Leonard MB, Shane E. Chronic kidney disease and bone fracture: a growing concern. Kidney Int. 2008;74(6):721–731. doi: 10.1038/ki.2008.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu X, Cheng MH, Shi CG, et al. Variability of glomerular filtration rate estimation equations in elderly Chinese patients with chronic kidney disease. Clin Interv Aging. 2012;7:409–415. doi: 10.2147/CIA.S36152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins AJ, Kasiske B, Herzog C, et al. Excerpts from the United States Renal Data System 2006 Annual Data Report. Am J Kidney Dis. 2007;49(1 Suppl 1):A6–A7. S1–S296. doi: 10.1053/j.ajkd.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Alem AM, Sherrard DJ, Gillen DL, et al. Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int. 2000;58(1):396–399. doi: 10.1046/j.1523-1755.2000.00178.x. [DOI] [PubMed] [Google Scholar]
- 5.Stehman-Breen CO, Sherrard DJ, Alem AM, et al. Risk factors for hip fracture among patients with end-stage renal disease. Kidney Int. 2000;58(5):2200–2205. doi: 10.1111/j.1523-1755.2000.00394.x. [DOI] [PubMed] [Google Scholar]
- 6.Huang GS, Chu TS, Lou MF, Hwang SL, Yang RS. Factors associated with low bone mass in the hemodialysis patients – a cross-sectional correlation study. BMC Musculoskelet Disord. 2009;10:60. doi: 10.1186/1471-2474-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gabay C, Ruedin P, Slosman D, Bonjour JP, Leski M, Rizzoli R. Bone mineral density in patients with end-stage renal failure. Am J Nephrol. 1993;13(2):115–123. doi: 10.1159/000168600. [DOI] [PubMed] [Google Scholar]
- 8.Nickolas TL, McMahon DJ, Shane E. Relationship between moderate to severe kidney disease and hip fracture in the United States. J Am Soc Nephrol. 2006;17(11):3223–3232. doi: 10.1681/ASN.2005111194. [DOI] [PubMed] [Google Scholar]
- 9.Wen CP, Tsai SP, Chung WS. A 10-year experience with universal health insurance in Taiwan: measuring changes in health and health disparity. Ann Intern Med. 2008;148(4):258–267. doi: 10.7326/0003-4819-148-4-200802190-00004. [DOI] [PubMed] [Google Scholar]
- 10.Bureau of National Health Insurance, Department of Health, Executive Yuan. Taipei: Taiwan NHI Information for the public: essential data of ensured affair [homepage on the Internet] Taiwan: Bureau of National Health Insurance Department of Health, Executive Yuan; [Accessed November 15, 2012]. Available from: http://www.nhi.gov.tw/webdata/webdata.aspx?menu=17&menu_id=661&WD_ID=689&webdata_id=805. [Google Scholar]
- 11.Yang NP, Chan CL, Yu IL, Lee CY, Chou P. Estimated prevalence of orthopaedic fractures in Taiwan – A cross-sectional study based on nationwide insurance data. Injury. 2010;41(12):1266–1272. doi: 10.1016/j.injury.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 12.Yang NP, Chen HC, Phan DV, et al. Epidemiological survey of orthopedic joint dislocations based on nationwide insurance data in Taiwan, 2000–2005. BMC Musculoskelet Disord. 2011;12:253. doi: 10.1186/1471-2474-12-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 14.Yang NP, Lee YH, Chang NT, et al. Treatment incidence of orthopedic injuries among HIV-infected subjects in Taiwan: a dynamic cohort survey, 2005–2008. HealthMED. 2012;6(8):2700–2708. [Google Scholar]
- 15.Agresti A. Categorical Data Analysis. 2nd ed. Hoboken: John Wiley & Sons, Inc; 2002. [Google Scholar]
- 16.Jamal SA, Hayden JA, Beyene J. Low bone mineral density and fractures in long-term hemodialysis patients: a meta-analysis. Am J Kidney Dis. 2007;49(5):674–681. doi: 10.1053/j.ajkd.2007.02.264. [DOI] [PubMed] [Google Scholar]
- 17.Rosansky S, Glassock RJ, Clark WF. Early start of dialysis: a critical review. Clin J Am Soc Nephrol. 2011;6(5):1222–1228. doi: 10.2215/CJN.09301010. [DOI] [PubMed] [Google Scholar]
- 18.Hung HC, Yang RS, Tsauo JY. The epidemiology of hip fracture in Taiwan. Formosan Journal of Medicine. 2005;9(1):29–38. Japanese. [Google Scholar]
- 19.St Peter WL. Introduction: chronic kidney disease: a burgeoning health epidemic. J Manag Care Pharm. 2007;13(9 Suppl D):S2–S5. doi: 10.18553/jmcp.2007.13.9-d.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361(16):1539–1547. doi: 10.1056/NEJMoa0904655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joly D, Anglicheau D, Alberti C, et al. Octogenarians reaching end-stage renal disease: cohort study of decision-making and clinical outcomes. J Am Soc Nephrol. 2003;14(4):1012–1021. doi: 10.1097/01.asn.0000054493.04151.80. [DOI] [PubMed] [Google Scholar]
- 22.Toussaint ND, Elder GJ, Kerr PG. A rational guide to reducing fracture risk in dialysis patients. Semin Dial. 2010;23(1):43–54. doi: 10.1111/j.1525-139X.2009.00650.x. [DOI] [PubMed] [Google Scholar]
- 23.Perico N, Remuzzi G. Chronic kidney disease: a research and public health priority. Nephrol Dial Transplant. 2012;27(Suppl 3):iii19–iii26. doi: 10.1093/ndt/gfs284. [DOI] [PubMed] [Google Scholar]
- 24.Rudser KD, de Boer IH, Dooley A, Young B, Kestenbaum B. Fracture risk after parathyroidectomy among chronic hemodialysis patients. J Am Soc Nephrol. 2007;18(8):2401–2407. doi: 10.1681/ASN.2007010022. [DOI] [PubMed] [Google Scholar]
- 25.Mares J, Ohlidalova K, Opatrna S, Ferda J. Determinants of prevalent vertebral fractures and progressive bone loss in long-term hemodialysis patients. J Bone Miner Metab. 2009;27(2):217–223. doi: 10.1007/s00774-008-0030-x. [DOI] [PubMed] [Google Scholar]
- 26.Yamaguchi T, Kanno E, Tsubota J, Shiomi T, Nakai M, Hattori S. Retrospective study on the usefulness of radius and lumbar bone density in the separation of hemodialysis patients with fractures from those without fractures. Bone. 1996;19(5):549–555. doi: 10.1016/s8756-3282(96)00246-3. [DOI] [PubMed] [Google Scholar]
- 27.Beddhu S, Baird BC, Zitterkoph J, Neilson J, Greene T. Physical activity and mortality in chronic kidney disease (NHANES III) Clin J Am Soc Nephrol. 2009;4(12):1901–1906. doi: 10.2215/CJN.01970309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Danese MD, Kim J, Doan QV, Dylan M, Griffiths R, Chertow GM. PTH and the risks for hip, vertebral, and pelvic fractures among patients on dialysis. Am J Kidney Dis. 2006;47(1):149–156. doi: 10.1053/j.ajkd.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 29.Rajapurkar M, Dabhi M. Burden of disease – prevalence and incidence of renal disease in India. Clin Nephrol. 2010;74( Suppl 1):S9–S12. doi: 10.5414/cnp74s009. [DOI] [PubMed] [Google Scholar]
- 30.Chang NT, Yang NP, Chou P. Incidence, risk factors and consequences of falling injuries among the community-dwelling elderly in Shihpai, Taiwan. Aging Clin Exp Res. 2010;22(1):70–77. doi: 10.1007/BF03324818. [DOI] [PubMed] [Google Scholar]
- 31.Ensrud KE, Lui LY, Taylor BC for Osteoporotic Fractures Research Group. Renal function and risk of hip and vertebral fractures in older women. Arch Intern Med. 2007;167(2):133–139. doi: 10.1001/archinte.167.2.133. [DOI] [PubMed] [Google Scholar]