Abstract
The cornea is physiologically avascular. Following a corneal injury, wound healing often proceeds without neovascularization (NV); however, corneal NV may be induced during wound healing in certain inflammatory, infectious, degenerative, and traumatic states. Such states disrupt the physiologic balance between pro-angiogenic and anti-angiogenic mediators, favoring angiogenesis. Contributors to such states are matrix metalloproteinases (MMPs), which are key factors in both extracellular matrix remodeling and angiogenesis. Similarly, vascular endothelial growth factor A (VEGF-A) and basic fibroblast growth factor (bFGF) exert pro-angiogenic effects. Here, we elaborate on the facilitative role of MMPs—specifically Membrane Type 1 MMP (MT1-MMP, MMP14)—in corneal NV. Additionally, we provide new insight into the signaling relating to MT1-MMP, Ras, and ERK in the bFGF-induced VEGF-A expression pathways within the corneal fibroblasts.
Keywords: Basic FGF, corneal angiogenesis, ERK, MT1-MMP, Ras, VEGF-A
I. INTRODUCTION
Normal corneal physiology is characterized by a homeostatic maintenance of avascularity [1, 2], which has been termed angiogenic privilege [3–5]. This homeostasis may be disrupted pro-angiogenically by several mechanisms, including certain pathophysiologies, physical wounding, and chemical burning [1, 4]. Basic fibroblast growth factor (bFGF), also known as FGF-2, is a multifunctional peptide that exhibits a pro-angiogenic effect [6, 7]. Membrane type-1 matrix metalloproteinase (MT1-MMP) is a zinc-dependent enzyme, contributory to bFGF-induced corneal neovascularization (NV) [8], that mediates extracellular matrix (ECM) remodeling [1, 4, 9] in such physiological processes such as embryogenesis [10], wound-healing [11], and angiogenesis [6, 12]. MT1-MMP embeds a catalytic domain, a hemopexin domain that mediates binding to extracellular substrates and cell surface receptors [13], and a type I transmembrane domain with a carboxyl-terminal cytosolic tail that mediates the protease trafficking and signaling cascades [14, 15]. Vascular endothelial growth factor (VEGF, also referred to as VEGF-A), a heparin-binding glycoprotein [16], is a key mediator of angiogenesis, tumor growth, and tumor metastasis [17].
Our previous studies suggest that MT1-MMP potentiates corneal NV in part through mechanisms intertwining the bFGF signaling pathways and the VEGF-A expression pathways [4, 6]. This characterization agrees with published experimental models which demonstrate that: (i) cross-talk occurs between bFGF and VEGF-A during corneal NV [6, 18], (ii) the pro-angiogenic effect of MT1-MMP is mediated at least in part by the up-regulation of VEGF-A at both mRNA and protein levels [19], (iii) MT1-MMP and VEGF-A are functionally linked in tumor vasculogenesis [20], (iv) the hypoxia-induced up-regulation of MT1-MMP in bone marrow-derived stromal cells correlates with VEGF-A stimulation [21], and (v) FGF-1—a heparin-binding peptide regulatory to tissue growth, differentiation, and angiogenesis [4, 22]—induces MT1-MMP transcription in certain prostate carcinoma cell lines [23].
Our present study elaborates on the interactions between MT1-MMP and, respectively, fibroblast growth factor receptor 1(FGFR-1)—an endogenous bFGF receptor [4, 24] –, Ras, and ERK in the bFGF-induced VEGF-A expression pathways within the corneal fibroblasts. Toward the end, we cultured the MT1-MMP knock-out (KO) corneal fibroblasts and transfected them with MT1-MMP DNA to generate the knock-in (KI) corneal fibroblasts overexpressing MT1-MMP and assayed the interactions between the KI platform and Ras, and ERK. Furthermore, in order to more clearly situate such interactions within the VEGF-A expression pathways, we assayed the effects of the Ras and ERK inhibitor upon the VEGF-A mRNA and/or protein expression levels. We observed that (i) MT1-MMP modulated Ras-GTP binding and ERK phosphorylation in KI corneal fibroblasts, and (ii) the application of Ras or ERK inhibitor to corneal fibroblast model down-regulated VEGF-A mRNA expression. These results suggest an interposition of Ras and ERK activity within the MT1-MMP mediated VEGF-A expression pathway of the corneal fibroblasts.
II. MATERIALS AND METHODS
Generation of MT1-MMP KI Cell Lines
The immortalized mouse corneal fibroblast cell lines from the MT1-MMP KO eyes (provided courtesy of Dr. Zhongjun Zhou, University of Hong Kong) were generated as described previously [25]. Briefly, the entire MT1-MMP KO mouse corneal stroma was excised and incubated with Dulbecco’s modified Eagle’s medium (DMEM, HyClone Laboratories, Logan, UT) containing 3.3 mg/ml collagenase type II (Sigma-Aldrich, St. Louis, MO) at 37°C with shaking for 90 minutes. Isolated corneal fibroblasts were grown in DMEM supplemented with 10% fetal calf serum (FCS, HyClone Laboratories) at 37°C in a 5% CO2 humidified atmosphere. Subconfluent corneal fibroblasts were then immortalized with a pZIPTEX virus (containing SV40T antigen) containing polybrene (4 ng/ml) in order to generate the MT1-MMP KO cell line used in our studies.
A retroviral expression system was used to generate the stably expressing MT1-MMP KI cell line. Recombinant viruses were then generated by a co-transfection of 293T cells with either the recombinant enhanced green fluorescent protein- (EGFP) expressing pFB vector (Stratagene La Jolla, CA) encoding MT1-MMP, or an empty vector. Briefly, 2 μl of pFB-MT1-MMP DNA, 1 μg of pVPack Eco, and 1 μg of pVPack gag-pol (Stratagene) were mixed with 16 μl of Enhancer solution and 300 μl of transfection buffer (Qiagen, Valencia, CA). The samples were incubated for 5 minutes at room temperature, after which 60 μl of Effectene™ transfection reagent (Qiagen) was added and mixed for 10 sec. The final solution was incubated for 5–10 minutes at room temperature, then mixed with 3 ml growth medium (10% FBS, no antibiotics), and added drop wise to 7 ml fresh conditioned medium in wells containing the MT1-MMP KO corneal fibroblasts. After a 24 hr incubation period, the conditioned medium was replaced with fresh DMEM and incubated for another 24 hr. The medium was then collected from the cells at 2 and 3 days post-transfection and used to infect the MT1-MMP KO cells described above, resulting in the MT1-MMP KI cell line. The infected corneal fibroblasts were incubated at 37°C for 1–3 days and monitored daily for the presence EGFP expression, as described below.
Flow Cytometry and Cell Sorting
The MT1-MMP KI cells were sorted by flow cytometry based on EGFP expression. Single cell suspensions were prepared, washed with cold PBS, trypsinized, and centrifuged at 1000 rpm for 5 minutes. A flow cytometry analysis was performed using the Coulter EPICS XL-MCL flow cytometer (Coulter Electronics, Miami, FL). Using a Coulter ELITE cell sorter, the cells were sorted into EGFP-positive and EGFP-negative cells. The EGFP-positive cells were cultured and sorted again in order to facilitate the resolution of the KI from the KO fibroblasts.
Western Blots, Immunoassays, and Confocal Microscopy
The MT1-MMP KO, KI, and wild-type (WT) corneal fibroblasts were plated with a density of 5.0 × 106 cells in 75-cm2 flasks. After stimulation with bFGF (20 ng/ml), the cells were lysed with an ice-cold RIPA buffer containing a protease inhibitor and a phosphatase inhibitor (Roche, Indianapolis, IN). The total protein concentration was measured using a Bio-Rad protein assay (Bio-Rad, Hercules, CA), and each sample was adjusted to 1.0 mg/ml. Proteins were then subjected to a SDS-PAGE and transferred to Immobilon-P membranes (Millipore, Bedford, MA) that had been blocked with 3% BSA for 60 minutes. The membranes were incubated overnight with rabbit anti-ERK antibody (Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti-phospho-ERK antibody (Cell Signaling Technology, Danvers, MA), or rabbit anti-VEGF-A antibody (Millipore, Bedford, MA). Blots were then incubated with horseradish peroxidase-conjugated donkey anti-rabbit or anti-mouse IgG antibodies (Amersham Biosciences, UK) for 30 minutes. The membranes were washed with a TBST buffer (50mM Tris-HCl, pH 7.4, 150 mM NaCl and 0.05% Tween-20) and the antigen was detected by ECL (Pierce Biotechnology, Rockford, IL).
The MT1-MMP KO, KI, and WT corneal fibroblasts were seeded in chambers and mounted onto glass slides (Lab-Tek II Chamber Slide System). After culture media removal, the cells were treated with 4% paraformaldehyde for 10 minutes at room temperature. The cells were then blocked with 1% BSA and incubated with rabbit anti-VEGF-A antibody in 1% BSA for 1 hr at room temperature. Then, the cells were washed with PBS and incubated with C5-conjugated donkey anti-rabbit IgG. After three 10-minutes washes with PBS, the cells were mounted in Vectashield plus propidium iodide (Vector lab, Burlingame, CA) and digitally imaged using a Leica (Mannhein, Germany) TCS 4D confocal microscope.
Real-time PCR
Total RNA was purified from the MT1-MMP KO, KI, and WT corneal fibroblast using a Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. A reverse transcription was then performed using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). The following primer sets were used for amplification:
VEGF-A, sense 5′-CAAAAACGAAAGCGCAAGAAA-3′ and antisense 5′-CGCTCTGAACAAGGCTCACA-3′; GAPDH sense 5′-TTGCCATCAATGACCCCTTCA -3′ and antisense 5′-ATGGGCTTCCTGTTGATGACA-3′. A real-time PCR was performed by using the fluorescence detection method using the ABI Prism 7900HT Sequence Detection System with SYBR Green PCR Master Mix (Applied Biosystems). The cycling conditions were as follows: 50°C for 2 minutes, 95°C for 10 minutes, 40 cycles of 95°C for 15 seconds, and 60°C for 60 seconds. All experiments were performed in triplicate, and the data was analyzed using the ABI PRISM SDS 2.3 software (Foster City, CA). The relative amount of each mRNA was determined by the normalization to that of GAPDH in each sample.
Statistical Data Analysis
Statistical significance was determined using Sigmaplot (Systat Software, Inc., Chicago, IL) and a statistical difference was considered significant when the p value was 0.05 or less.
The procedures/methods followed in conducting this research are in accordance with the standards set forth in the Guide for the Care and Use of Laboratory Animals (published by the National Academy of Science (National Academy Press, Washington, D.C.).
III. RESULTS
Generation of MT1-MMP KO and KI Corneal Fibro-blast Cell Lines
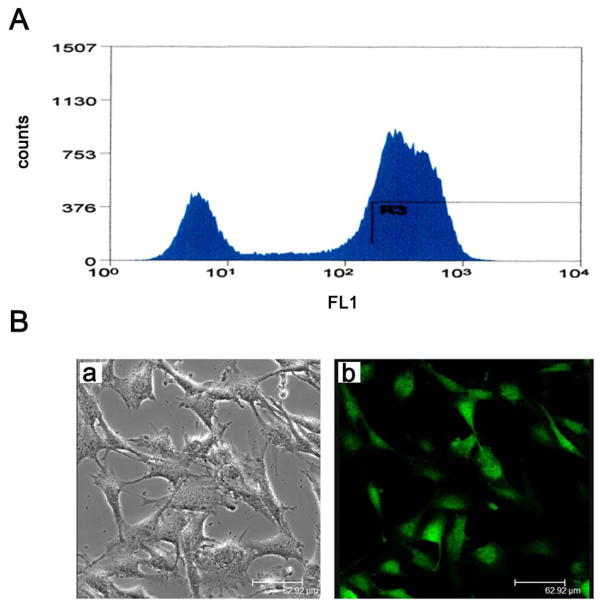
Our investigations began with the hypothesis that MT1-MMP expression would be apparent within the KI corneal fibroblasts and not within the KO variant. Thus, the MT1-MMP KI corneal fibroblasts were sorted by flow cytometry; visible reporting of EGFP in the KI corneal fibroblasts (Fig. 1A). EGFP expression in the MT1-MMP KI corneal fibro-blasts was also confirmed by visualization via the confocal microscopy (Fig. 1B).
Figure 1.
Generation of MT1-MMP KI cells. (A) MT1-MMP was reintroduced into KO cells, and KI cells were sorted for EGFP expression by flow cytometry. (B) EGFP expression in KI cells was visualized by confocal microscopy (a, bright field; b, confocal imaging).
MT1-MMP Modulates bFGF-induced ERK Phosphorylation
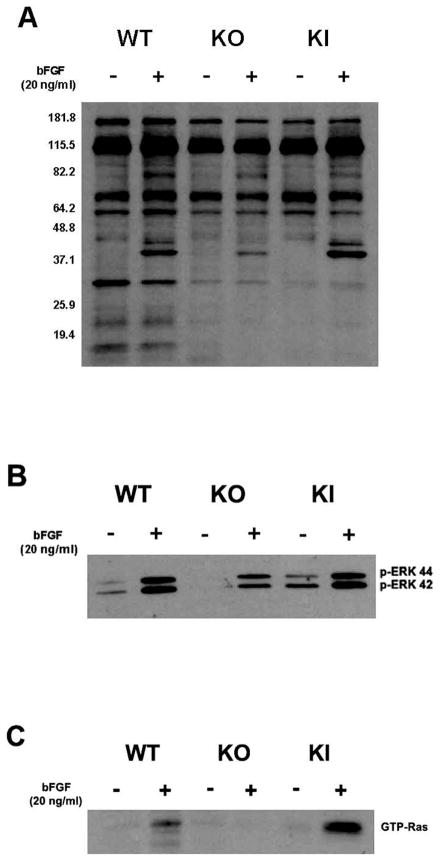
We next assayed cellular phosphorylation levels in the MT1-MMP WT, KO, and KI corneal fibroblasts under both naive and bFGF-induced conditions. The MT1-MMP WT, KO, and KI corneal fibroblasts were incubated in the presence or absence of bFGF, and cell lysates were prepared and subjected to a western blot analysis for the assay of the total cellular tyrosyl phosphorylation. We observed recovered tyrosyl phosphorylation in the KI corneal fibroblasts as opposed to the KO corneal fibroblasts when treated with bFGF (Fig. 2A). We tested the ERK phosphorylation, in order to determine a linkage between MT1-MMP activity and ERK phosphorylation, because ERK has been hypothesized as a regulator of VEGF-A expression in such cell types as osteoblasts [27] and placental tissue [28]. Our data demonstrated that the ERK tyrosyl phosphorylation was up-regulated in the WT and KI corneal fibroblasts as compared to corresponding phosphorylation levels in the KO corneal fibroblasts (Fig. 2B), irrespective of the bFGF treatment. Because phosphorylation is known to potentiate ERK activity [29], our results demonstrating MT1-MMP enhancement of ERK phosphorylation is suggestive of the interposition of ERK activity into the MT1-MMP modulated, bFGF induced VEGF-A pathway of corneal fibroblasts (discussed further below).
Figure 2.
Diminished bFGF-induced tyrosyl-phosphorylation substrates in KO cell lines. (A) WT, KO and KI cells were incubated in the presence or absence of bFGF for 5 minutes, and cell lysates were probed by Western blot for tyrosyl phosphorylation, and (B) in the presence of bFGF for 10 minutes before assessment of ERK phosphorylation. (C) Cell lysates from MT1-MMP WT, KO and KI fibroblasts were precipitated with GST-Raf beads, and samples were probed for GTP-Ras (after bFGF exposure for 30 minutes).
Enhanced Ras-GTP Binding in KI Corneal Fibroblasts
We assayed for Ras-GTP binding in the KO and KI corneal fibroblasts, in order to determine a linkage between the MT1-MMP expression and Ras activity, because Ras is an upstream regulator of certain ERK regulatory pathways governing proliferation, differentiation, and cell survival [27, 30]. We precipitated the bFGF-activated Ras protein from cell samples using GST-Raf beads, which specifically recognize and bind to active, GTP-bound forms of Ras. These samples were then subjected to a western immunoblot for GTP-Ras. We observed that the levels of GTP-bound Ras, which are indicative of ERK signal transduction [31], were substantially elevated in the WT and KI corneal fibroblasts over those detected in the KO corneal fibroblasts (Fig. 2C). Together, these results demonstrate a positive linkage between the MT1-MMP expression and the Ras activity within the corneal fibroblasts.
Enhanced VEGF-A Expression in MT1-MMP KI Corneal Fibroblasts; Inhibition of VEGF-A Expression by ERK and Ras Inhibitor in KI Corneal Fibroblasts
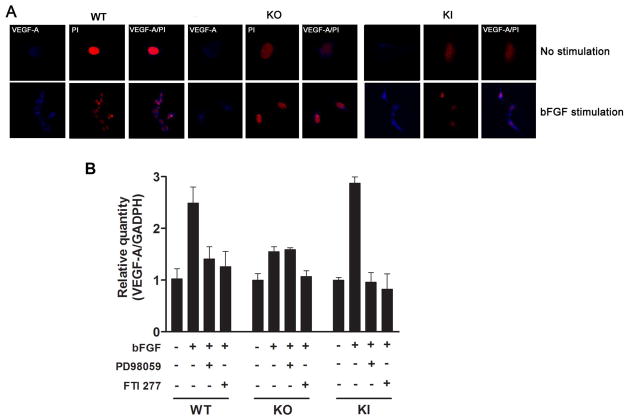
Because VEGF-A expression levels have been linked to MT1-MMP production [6], we assayed for VEGF-A expression by confocal immunohistochemistry in the bFGF-stimulated WT, KO, and KI corneal fibroblasts. We observed that the intensity of anti-VEGF-A immunostaining was greater in the bFGF-treated WT and KI corneal fibroblasts than in the KO corneal fibroblasts (Fig. 3A). Taken together, these data suggest a positive linkage between MT1-MMP activity and VEGF-A up regulation within the corneal fibro-blasts and are consistent with a model of ERK and Ras interposition within the VEGF-A expression pathways of the corneal fibroblasts.
Figure 3.
Enhanced bFGF-induced VEGF-A expression in KI cell lines. (A) MT1-MMP WT, KO and KI fibroblast cells were unstimulated or stimulated with bFGF for 24 hours. Cells were immunostained with anti-VEGF-A (blue) and PI (red). Merged images are indicated in the right panels. Bar, 24 um. (B) WT, KO and KI cells were incubated in the presence or absence of bFGF (6 hr bFGF exposure) and/or the ERK kinase inhibitor (PD98059) and Ras inhibitor (FTI-277) inhibited bFGF-induced VEGF-A as indicated, and total RNA was extracted and subjected to real-time PCR for VEGF-A. Data were normalized to that of GAPDH.
Inhibition of VEGF-A mRNA Expression by ERK Inhibitor in KI Corneal Fibroblasts
To further elucidate a linkage between ERK and VEGF-A expression in the corneal fibroblasts, we next assayed the VEGF-A mRNA expression in the bFGF-treated WT, KO, and KI corneal fibroblasts via real-time PCR. The bFGF-induced VEGF-A mRNA expression was substantially attenuated in the KO corneal fibroblasts, as compared to the WT and KI fibroblasts (Fig. 3B). In addition, the treatment of cells with the ERK kinase inhibitor PD98059 and Ras inhibitor FTI-277 inhibited the bFGF-induced VEGF-A expression in the WT and KI corneal fibroblasts (Fig. 3B). These results suggest a linkage between ERK activation (phosphorylation) levels and VEGF-A mRNA expression levels within the corneal fibroblasts.
DISCUSSION/CONCLUSION
The VEGF-A expression is tightly controlled, and an elevation in the VEGF-A levels are contributory to corneal NV [4] and tumorigenesis [32]. Notably, (i) an enhancement of the MT1-MMP expression levels correlates with an increased bFGF-induced VEGF-A up-regulation and corneal NV in mice [1, 6] and (ii) the expression of VEGF-A and its receptors (VEGFR-1 and VEGFR-2) is implicated in vascular endothelial cell proliferation and NV [17, 33–35]. Published studies suggest bFGF and MT1-MMP complicity [4, 6] in the regulation of the VEGF-A expression precedent to aberrant corneal vascularization patterns. In the present study, we undertook the task of more fully characterizing the MT1-MMP modulation of a repertoire of key molecules implicated in the bFGF-induced VEGF-A expression pathways in the corneal fibroblasts.
Our investigations began with the ligand-receptor coupling of FGFR-1, a tyrosine kinase receptor, and bFGF [4, 36, 37]. Our published data demonstrated both a positive correlation of MT1-MMP overexpression and FGFR-1 up-regulation in the KI corneal fibroblasts and heterogeneity of the FGFR expression across the MT1-MMP KO and KI fibroblast platforms, suggesting a differential sensitivity to the MT1-MMP activity [4]. While the FGFR-2 expression levels were not statistically significantly different across the KO and KI platforms, the FGFR-1 expression levels demonstrated a statistically significant down-regulation in the MT1-MMP KO corneal fibroblasts, as compared with corresponding expression levels in the KI corneal fibroblasts [4]. This finding accords with published models of interaction linking MT1-MMP and bFGF signaling pathways, as bFGF has been previously shown to up-regulate the FGFR-1 expression [7, 38]. Furthermore, our previous studies have demonstrated that MT1-MMP potentiates bFGF-induced corneal NV in vivo [4, 6].
We characterized the VEGF-A expression pathway in the corneal fibroblasts downstream of the FGFR-1 expression, utilizing immunohistochemically characterized cell lines. Our data demonstrated that Ras-GTP binding was down-regulated in the MT1-MMP KO cells as compared to the KI corneal fibroblasts. Because Ras is an upstream regulator of certain ERK regulatory pathways governing proliferation, differentiation, and cell survival [27, 30], our data supports a model of regulatory interaction linking Ras-GTP binding to the extracellular catalysis of neovascularization processes via MT1-MMP activity in the corneal fibroblasts. This model aligns with our findings that ERK phosphorylation is reduced in the KO corneal fibroblasts, because, in addition to Ras, MT1-MMP is also an upstream regulator of ERK [39, 40], a MAP kinase homologue important for MT1-MMP-dependent cell migration [40, 41].
We next characterized correlations between the Ras and ERK activity and the VEGF-A mRNA and protein expression levels in the corneal fibroblasts via immunohistochemical techniques and a quantitative real-time PCR of the VEGF-A mRNA levels in an ERK inhibitor context. We observed that (i) the ERK inhibitor PD98059 and Ras inhibitor FTI-277 inhibited the bFGF-induced VEGF-A protein expression in the KI but not the KO corneal fibroblasts and (ii) that the bFGF-induced VEGF-A mRNA expression was substantially attenuated in the KO corneal fibroblasts, as compared to the KI fibroblasts; these findings establish supplementary support for the interposition of ERK and Ras signaling activity within the VEGF-A expression pathway of the corneal fibroblasts.
Taken together, our findings thus characterize MT1-MMP modulation of Ras, and ERK, a repertoire of key molecules interposed within the bFGF-induced VEGF-A expression pathway in the corneal fibroblasts. Additional experimental studies are required in order to comprehensively model the bFGF-induced, MT1-MMP-mediated signaling dynamics downstream of ERK phosphorylation and upstream of VEGF-A expression within the corneal fibro-blasts. Our findings suggest important insights into the physiological expression pathways implicated in tumor proliferation, malignant cellular transformation, and aberrant corneal vascularization patterns.
Acknowledgments
This work was partially supported by National Institutes of Health grants EY10101, EY001792 (DTA), EY021886 (JHC) and an unrestricted grant from Research to Prevent Blindness, New York, NY.
Footnotes
Send Orders of Reprints at reprints@benthamscience.org
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
References
- 1.Azar DT. Corneal angiogenic privilege: angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2006;104:264–302. [PMC free article] [PubMed] [Google Scholar]
- 2.Chang JH, Han KY, Azar DT. Wound healing fibroblasts modulate corneal angiogenic privilege: interplay of basic fibroblast growth factor and matrix metalloproteinases in corneal angiogenesis. Jpn J Ophthalmol. 2010;54:199–205. doi: 10.1007/s10384-010-0801-5. [DOI] [PubMed] [Google Scholar]
- 3.Chang JH, Gabison EE, Kato T, Azar DT. Corneal neovascularization. Curr Opin Ophthalmol. 2001;12:242–9. doi: 10.1097/00055735-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Ellenberg D, Azar DT, Hallak JA, Tobaigy F, Han KY, Jain S, Zhou Z, Chang JH. Novel aspects of corneal angiogenic and lymphangiogenic privilege. Prog Retin Eye Res. 2010;29:208–48. doi: 10.1016/j.preteyeres.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cursiefen C. Immune privilege and angiogenic privilege of the cornea. Chem Immunol Allergy. 2007;92:50–7. doi: 10.1159/000099253. [DOI] [PubMed] [Google Scholar]
- 6.Onguchi T, Han KY, Chang JH, Azar DT. Membrane type-1 matrix metalloproteinase potentiates basic fibroblast growth factor-induced corneal neovascularization. Am J Pathol. 2009;174:1564–71. doi: 10.2353/ajpath.2009.080452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang H, Xia Y, Lu SQ, Soong TW, Feng ZW. Basic fibroblast growth factor-induced neuronal differentiation of mouse bone marrow stromal cells requires FGFR-1, MAPK/ERK, and transcription factor AP-1. J Biol Chem. 2008;283:5287–95. doi: 10.1074/jbc.M706917200. [DOI] [PubMed] [Google Scholar]
- 8.Zhou Z, Apte SS, Soininen R, Cao R, Baaklini GY, Rauser RW, Wang J, Cao Y, Tryggvason K. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc Natl Acad Sci USA. 2000;97:4052–7. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mimura T, Han KY, Onguchi T, Chang JH, Kim TI, Kojima T, Zhou Z, Azar DT. MT1-MMP-mediated cleavage of decorin in corneal angiogenesis. J Vasc Res. 2009;46:541–50. doi: 10.1159/000226222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanwar YS, Ota K, Yang Q, Wada J, Kashihara N, Tian Y, Wallner EI. Role of membrane-type matrix metalloproteinase 1 (MT-1-MMP), MMP-2: and its inhibitor in nephrogenesis. Am J physiol. 1999;277:F934–47. doi: 10.1152/ajprenal.1999.277.6.F934. [DOI] [PubMed] [Google Scholar]
- 11.Ye HQ, Maeda M, Yu FS, Azar DT. Differential expression of MT1-MMP (MMP-14) and collagenase III (MMP-13) genes in normal wounded rat corneas. Invest Ophthalmol Vis Sci. 2000;41:2894–9. [PubMed] [Google Scholar]
- 12.Przewratil P, Sitkiewicz A, Andrzejewska E. Soluble receptors for vascular endothelial growth factor (sVEGFR1/sVEGFR2) in infantile hemangioma. Growth Factors. 2010;28:417–25. doi: 10.3109/08977194.2010.505566. [DOI] [PubMed] [Google Scholar]
- 13.Mori H, Gjorevski N, Inman JL, Bissell MJ, Nelson CM. Self-organization of engineered epithelial tubules by differential cellular motility. Proc Natl Acad Sci USA. 2009;106:14890–5. doi: 10.1073/pnas.0901269106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li XY, Ota I, Yana I, Sabeh F, Weiss SJ. Molecular dissection of the structural machinery underlying the tissue-invasive activity of membrane type-1 matrix metalloproteinase. Mol Biol Cell. 2008;19:3221–33. doi: 10.1091/mbc.E08-01-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seiki M. The cell surface: the stage for matrix metalloproteinase regulation of migration. CurrOpin Cell Biol. 2002;14:624–32. doi: 10.1016/s0955-0674(02)00363-0. [DOI] [PubMed] [Google Scholar]
- 16.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nature Medicine. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 17.Penn JS, Madan A, Caldwell RB, Bartoli M, Caldwell RW, Hartnett ME. Vascular endothelial growth factor in eye disease. Prog Retin Eye Res. 2008;27:331–71. doi: 10.1016/j.preteyeres.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Komarova Y, Malik AB. FGF signaling preserves the integrity of endothelial adherens junctions. Dev Cell. 2008;15:335–6. doi: 10.1016/j.devcel.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sounni NE, Devy L, Hajitou A, Frankenne F, Munaut C, Gilles C, Deroanne C, Thompson EW, Foidart JM, Noel A. MT1-MMP expression promotes tumor growth and angiogenesis through an up-regulation of vascular endothelial growth factor expression. FASEB J. 2002;16:555–64. doi: 10.1096/fj.01-0790com. [DOI] [PubMed] [Google Scholar]
- 20.Sounni NE, Janssen M, Foidart JM, Noel A. Membrane type-1 matrix metalloproteinase and TIMP-2 in tumor angiogenesis. Matrix Biol. 2003;22:55–61. doi: 10.1016/s0945-053x(03)00003-9. [DOI] [PubMed] [Google Scholar]
- 21.Annabi B, Lee YT, Turcotte S, Naud E, Desrosiers RR, Champagne M, Eliopoulos N, Galipeau J, Beliveau R. Hypoxia promotes murine bone-marrow-derived stromal cell migration and tube formation. Stem Cells. 2003;21:337–47. doi: 10.1634/stemcells.21-3-337. [DOI] [PubMed] [Google Scholar]
- 22.Yamaji T, Tsuboi H, Murata N, Uchida M, Kohno T, Sugino E, Hibino S, Shimamura M, Oikawa T. Anti-angiogenic activity of a novel synthetic agent, 9alpha-fluorome-droxyprogesterone acetate. Cancer Lett. 1999;145:107–14. doi: 10.1016/s0304-3835(99)00239-6. [DOI] [PubMed] [Google Scholar]
- 23.Udayakumar TS, Nagle RB, Bowden GT. Fibroblast growth factor-1 transcriptionally induces membrane type-1 matrix metalloproteinase expression in prostate carcinoma cell line. Prostate. 2004;58:66–75. doi: 10.1002/pros.10293. [DOI] [PubMed] [Google Scholar]
- 24.Giri D, Ropiquet F, Ittmann M. Alterations in expression of basic fibroblast growth factor (FGF) 2 and its receptor FGFR-1 in human prostate cancer. Clin Cancer Res. 1999;5:1063–71. [PubMed] [Google Scholar]
- 25.Azar DT, Casanova FH, Mimura T, Jain S, Chang JH. Effect of MT1-MMP deficiency and overexpression in corneal keratocytes on vascular endothelial cell migration and proliferation. Curr Eye Res. 2008;33:954–62. doi: 10.1080/02713680802461106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seghezzi G, Patel S, Ren CJ, Gualandris A, Pintucci G, Robbins ES, Shapiro RL, Galloway AC, Rifkin DB, Mignatti P. Fibroblast growth factor-2 (FGF-2) induces vascular endothelial growth factor (VEGF) expression in the endothelial cells of forming capillaries: an autocrine mechanism contributing to angiogenesis. J Cell Biol. 1998;141:1659–73. doi: 10.1083/jcb.141.7.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang FS, Wang CJ, Chen YJ, Chang PR, Huang YT, Sun YC, Huang HC, Yang YJ, Yang KD. Ras induction of superoxide activates ERK-dependent angiogenic transcription factor HIF-1alpha and VEGF-A expression in shock wave-stimulated osteoblasts. J Biol Chem. 2004;279:10331–7. doi: 10.1074/jbc.M308013200. [DOI] [PubMed] [Google Scholar]
- 28.Galabova-Kovacs G, Matzen D, Piazzolla D, Meissl K, Plyushch T, Chen AP, Silva A, Baccarini M. Essential role of B-Raf in ERK activation during extraembryonic development. Proc Natl Acad Sci USA. 2006;103:1325–30. doi: 10.1073/pnas.0507399103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boudreau NJ, Jones PL. Extracellular matrix and integrin signalling: the shape of things to come. Biochem J. 1999;339(Pt 3):481–8. [PMC free article] [PubMed] [Google Scholar]
- 30.Orton RJ, Sturm OE, Vyshemirsky V, Calder M, Gilbert DR, Kolch W. Computational modelling of the receptor-tyrosine-kinase-activated MAPK pathway. Biochem J. 2005;392:249–61. doi: 10.1042/BJ20050908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan EY, Stang SL, Bottorff DA, Stone JC. Hypothermic stress leads to activation of Ras-Erk signaling. J Clin Invest. 1999;103:1337–44. doi: 10.1172/JCI5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langlois S, Gingras D, Beliveau R. Membrane type 1-matrix metalloproteinase (MT1-MMP) cooperates with sphingosine 1-phosphate to induce endothelial cell migration and morphogenic differentiation. Blood. 2004;103:3020–8. doi: 10.1182/blood-2003-08-2968. [DOI] [PubMed] [Google Scholar]
- 33.Cursiefen C, Cao J, Chen L, Liu Y, Maruyama K, Jackson D, Kruse FE, Wiegand SJ, Dana MR, Streilein JW. Inhibition of hemangiogenesis and lymphangiogenesis after normal-risk corneal transplantation by neutralizing VEGF promotes graft survival. Invest Ophthalmol Vis Sci. 2004;45:2666–73. doi: 10.1167/iovs.03-1380. [DOI] [PubMed] [Google Scholar]
- 34.Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C, D’Amore PA, Dana MR, Wiegand SJ, Streilein JW. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–50. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Zhang MC, Hu YZ, Yu CT. Inhibition of rat corneal neovascularization by inhibitor of nuclear factor-kappaB. Zhonghua Yan Ke Za Zhi. 2005;41:1124–8. [PubMed] [Google Scholar]
- 36.Myoken Y, Okamoto T, Sato JD, Kan M, McKeehan WL, Nakahara M, Takada K. Immunohistochemical study of overexpression of fibroblast growth factor-1 (FGF-1), FGF-2: and FGF receptor-1 in human malignant salivary gland tumours. J Pathol. 1996;178:429–36. doi: 10.1002/(SICI)1096-9896(199604)178:4<429::AID-PATH495>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 37.Ornitz DM, Xu J, Colvin JS, McEwen DG, MacArthur CA, Coulier F, Gao G, Goldfarb M. Receptor specificity of the fibroblast growth factor family. J Biol Chem. 1996;271:15292–7. doi: 10.1074/jbc.271.25.15292. [DOI] [PubMed] [Google Scholar]
- 38.Shiang JC, Cheng CJ, Tsai MK, Hung YJ, Hsu YJ, Yang SS, Chu SJ, Lin SH. Therapeutic analysis in Chinese patients with thyrotoxic periodic paralysis over 6 years. Eur J Endocrinol. 2009;161:911–6. doi: 10.1530/EJE-09-0553. [DOI] [PubMed] [Google Scholar]
- 39.Kolch W. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J. 2000;351(Pt 2):289–305. [PMC free article] [PubMed] [Google Scholar]
- 40.Takino T, Miyamori H, Watanabe Y, Yoshioka K, Seiki M, Sato H. Membrane type 1 matrix metalloproteinase regulates collagen-dependent mitogen-activated protein/extracellular signal-related kinase activation and cell migration. Cancer Res. 2004;64:1044–9. doi: 10.1158/0008-5472.can-03-1843. [DOI] [PubMed] [Google Scholar]
- 41.Gingras D, Bousquet-Gagnon N, Langlois S, Lachambre MP, Annabi B, Beliveau R. Activation of the extracellular signal-regulated protein kinase (ERK) cascade by membrane-type-1 matrix metalloproteinase (MT1-MMP) FEBS Lett. 2001;507:231–6. doi: 10.1016/s0014-5793(01)02985-4. [DOI] [PubMed] [Google Scholar]