Abstract
Various monoclonal antibodies (mAb) target immune system molecules to enhance immunity by costimulating T cells (i.e., CD137, OX40, CD40, GITR) or interfering in coinhibitory signals (i.e., CTLA-4, PD-1). These powerful agents can be guided by cancer vaccines to enhance immunity against tumor but not self tissues. Clinically powerful therapeutic synergies are at hand.
Malignancies actively shield themselves from immune attack. Soluble and membrane-attached molecules whose normal function is to regulate immunity and avoid self reactivity are cunningly perverted by tumors to permit immune escape. The PD-1 interaction with B7-H1 is important for peripheral T-cell tolerance as evidenced in gene-targeted mice (1). Interestingly, PD-1 marks anergic or exhausted T-lymphocytes in tumor-bearing and chronically virus-infected subjects. Indeed, targeting PD-1-B7-H1 binding with mAbs has been developed to augment tumor immunity and also has potential for chronic and latent viral infections (2, 3).
Conspicuously, B7-H1 is aberrantly overexpressed on the cell surface of many malignancies from a variety of tissue origins in humans (1). B7-H1 expression in mice provides an immune escape mechanism for the tumor cells and in humans correlates with poorer prognosis as an independent prediction factor. The suppressive mechanisms of PD-1-B7-H1 action involve not only coinhibitory signaling to T lymphocytes but also protective functions on the target tumor cells that are done by the cytoplasmic tail of B7-H1(4). This shielding function has been described to defend B7-H1 – expressing tumor cells from the tumoricidal effector activity of CTL and other apoptosis mechanisms (4).
Based on preclinical data, a fully human IgG4 mAb against PD-1 (MDX-1106) has completed a dose escalation phase I clinical trial in patients with a variety of solid tumors. Treatment was well tolerated and a few tumor partial responses (3 of 24) were reported (American Society of Clinical Oncology 2008 abstract 3006). Severe autoimmunity was not documented, although a few patients apparently developed manageable arthritis and mild thyroiditis. A clinical trial with this agent for chronic hepatitis C is now recruiting patients and its results will provide important information (NCT00703469).
An alternative would be to use blocking mAb directed toward B7-H1. The biological effects ought not to be fully equivalent. On the one hand the phenotype of B7-H1-/- mice indicates less risk for autoimmunity and on the other hand there is evidence for additional B7-H1 ligands (5). Additional ligands include one that remains to be identified and is involved in T-cell apoptosis and also a reportedly coinhibitory interaction with CD80, an interaction whose physiologic importance remains to be seen. A mAb directed to B7-H1 is approaching a first-to-human clinical trial.
One reason that makes immunotherapy of cancer an increasingly exciting field is that it can be added onto other treatments. Several lines of evidence indicate that immunotherapy is often a synergistic partner for efficacy in combinations without additive toxicity. Indeed, successful combinations with chemotherapy, surgery, and radiotherapy are being reported for many of the members of the immunostimulatory mAb family (6). Although these conventional therapies are believed to increase antigen exposure to host immune system, in addition to debulking cancer, an attractive approach will be the use of antigen-based vaccine to enforce the direction of tumor immune response.
Cancer vaccines (7) consist of strategies that intend to prime and boost specific immune responses in a tumor-bearing host. Immunogenic formulations of tumor antigens can be achieved in multiple ways that have battled over the years to induce more robust and lasting T-cell responses. It is difficult to say which is the best and none of them as a single agent has offered significant benefit for patients with advanced disease thus far. In our opinion, those vaccines that present the whole array of tumor antigens are more likely to be efficacious. The reason is that unique (not shared) tumor mutations can be the best targets and that focusing the immune response to a single antigen can result in antigen-lost escape variants. This type of vaccine relies on tumor cells, tumor cell lysates, apoptotic tumor cells, or total tumor mRNA. Artificial introduction of these antigens into the antigen presentation machinery of activated dendritic cells is a common theme in these strategies.
Expression of granulocyte macrophage colony-stimulating factor in tumor cells (autologous or allogenic of the same histology type) is a potent way to enhance immunogenicity (8). The vaccination activity of the granulocyte macrophage colony-stimulating factor transfectants relies largely in the processing and presentation of tumor antigens by endogenous dendritic cells attracted and differentiated by the transgene product, a process termed antigen cross-presentation. This strategy has been tested in the clinic for various indications using either autologous tumor cells or allogenic cell lines. Plenty of evidence indicates biological activity in terms of raising cellular immune responses. However, recently two phase III clinical trials testing this type of vaccine for hormone refractory prostate cancer patients were terminated because of lack of evidence for clinical benefit.4
In this issue of CCR, Li et al. report (9) that PD-1 blockade and vaccination with granulocyte macrophage colony-stimulating factor – transfected tumor cells synergize for antitumor activity against transplanted mouse tumors, accompanied with stronger tumor-specific immune responses in mice. Autologous granulocyte macrophage colony-stimulating factor tumor transfectants shows synergistic effects in mice when combined with anti–CTLA-4 mAb (10), anti-OX40 mAb (11), or anti-CD137 mAb (12). One wonders what would be the potency of triple or even quadruple combinations of these agents. Recently, retrospective observations in human trials provide circumstantial evidence for a role of granulocyte macrophage colony-stimulating factor transfectants vaccine at sensitizing patients for a favorable outcome after a subsequent treatment with anti-CTLA4 blocking mAb given several months later (13).
As a result of all these reports, it is clear that tumor vaccines are to be combined with immunostimulatory mAb in clinical trials. Not every combination ought to work but the rationale is simple: The vaccine evokes antigen specific immunity, whereas the antibody acting as an immunostimulatory tool fosters costimulation or unleashes immunoregulatory constraints (Fig. 1). Which immunizing procedure is best is a question that deserves much attention from future comparative research. It must be kept in mind that turning a tumor lesion into a vaccine (autovaccination) can still be an important option. Clinically developing more than two agents together, however, is very challenging for industry and clashes with conventional wisdom for clinical drug development. Regulatory agencies demand safety studies of each combination at the preclinical and clinical level and combinations can be tested only at much later stages. The stress on caution clearly slows down progress of combinational therapies in conjunction with other hurdles posed by need for arrangements of business interest and intellectual property on the compounds.
Fig. 1.
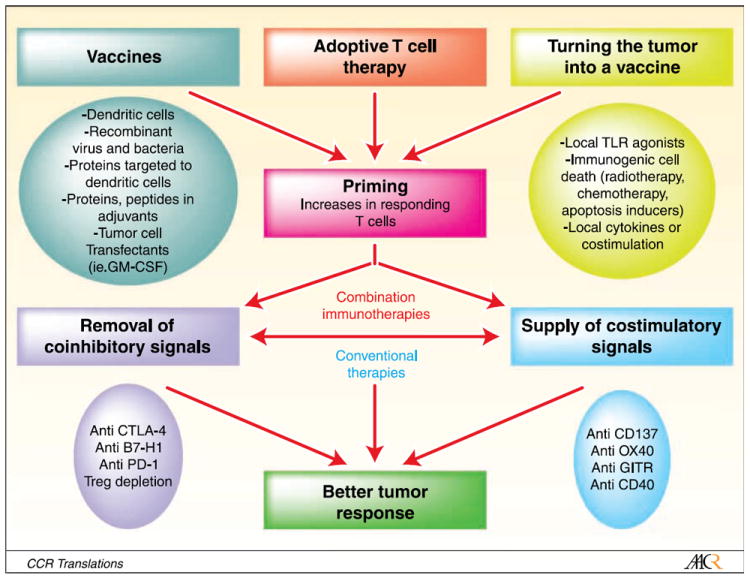
Strategies for immunotherapy combinations. Stepwise rationale approaches for combinations of immunostimulatory mAb and procedures of immunization against malignant antigens. Mouse experimentation provides a recipe for clinicians: First, use procedures that enhance the number of primed nonanergic T cells in the organism by means of vaccines, adoptive transfer, or procedures to enhance the immunogenicity of a tumor lesion. Then apply strategies to de-repress or stimulate artificially the ongoing immune response, for the most part considering immunostimulatory mAb. The optimal interval between immunization procedures and the further immunostimulatory treatment may vary. Therapies involving various elements have made a difference in AIDS, tuberculosis, and chemotherapy for certain malignancies. In cancer immunotherapy, no single action is expected to reach dramatic efficacy in human patients but it is likely that L’union fait la force.
For instance, if two new coming agents exist, such as a vaccine and a mAb, they are more likely to be combined with standard treatments than with each other. The reasons are not scientific, such as the expected efficacy, but instead, the simplicity and feasibility in terms of regulatory affairs. To make things even more challenging, the best possible immunotherapy combinations may need more than two agents, and then feasibility is more entangled. For instance in the paper by Li et al. (9), the combined therapy does not have an effect on the regulatory T-cell compartment, thereby clearly offering another step for improvement. Companies that rapidly make alliances or already own a portfolio of these immunotherapy agents will have a lot of advantage.
Regulatory contraints must be urgently reconsidered because development of single immunotherapy agents are takes a long time and many agents that are only marginally efficacious as monotherapies are expected to be very active in combinations. Immunostimulatory mAbs combined with strong tumor immunogens (even if still just investigational products) should undergo early clinical testing. If the synergies observed in mouse models are sustained, these combined strategies hold a lot of hope.
Acknowledgments
Grant support: IM recieves financial support form the European Union 7th framework program (Encite), UTE Project Centro de Investigación Médica Aplicada, MEC (SAF2005-03131 and SAF2008-03294), Departamento de Educación del Gobierno de Navarra, Departamento de Salud del Gobierno de Navarra (Beca Ortiz de Landázuri), RTICC, Fondo de investigación sanitaria (PI060932), and US NIH grant CA 106861 and AI72592.
Footnotes
Disclosure of Potential Conflicts of Interest
The authors have served as consultants for Bristol Myers Squibb, Pfizer, and Medarex.
References
- 1.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–77. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 2.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–4. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 3.Hirano F, Kaneko K, Tamura H, et al. Blockade of B7 – 1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–96. [PubMed] [Google Scholar]
- 4.Azuma T, Yao S, Zhu G, Flies AS, Flies SJ, Chen L. B7-1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood. 2008;111:3635–43. doi: 10.1182/blood-2007-11-123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melero I, Hervas-Stubbs S, Glennie M, Pardoll DM, Chen L. Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev Cancer. 2007;7:95–106. doi: 10.1038/nrc2051. [DOI] [PubMed] [Google Scholar]
- 7.Gilboa E. The promise of cancer vaccines. Nat Rev Cancer. 2004;4:401–11. doi: 10.1038/nrc1359. [DOI] [PubMed] [Google Scholar]
- 8.Dranoff G, Jaffee E, Lazenby A, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993;90:3539–43. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li B, VanRoey M, Wang C, Chen TT, Korman A, Jooss K. Anti-Programmed Death-1 Synergizes with Granulocyte Macrophage Colony-Stimulating Factor – Secreting Tumor Cell Immunotherapy Providing Therapeutic Benefit to Mice with Established Tumors. Clin Cancer Res. 2009;15:1623–34. doi: 10.1158/1078-0432.CCR-08-1825. [DOI] [PubMed] [Google Scholar]
- 10.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–66. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murata S, Ladle BH, Kim PS, et al. OX40 costimulation synergizes with GM-CSF whole-cell vaccination to overcome established CD8+ T cell tolerance to an endogenous tumor antigen. J Immunol. 2006;176:974–83. doi: 10.4049/jimmunol.176.2.974. [DOI] [PubMed] [Google Scholar]
- 12.Li B, Lin J, Vanroey M, Jure-Kunkel M, Jooss K. Established B16 tumors are rejected following treatment with GM-CSF-secreting tumor cell immunotherapy in combination with anti-4-1BB mAb. Clin Immunol. 2007;125:76–87. doi: 10.1016/j.clim.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Hodi FS, Butler M, Oble DA, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci U S A. 2008;105:3005–10. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]


