Abstract
Background
The genes FTO and GNB3 are implicated in essential hypertension but their interaction remains to be explored. This study investigates the role of interaction between the two genes in the pathophysiology of essential hypertension.
Methods/Principal Findings
In a case-control study comprising 750 controls and 550 patients, interaction between the polymorphisms of FTO and GNB3 was examined using multifactor dimensionality reduction (MDR). The influence of interaction on clinical phenotypes like systolic and diastolic blood pressure, mean arterial pressure and body mass index was also investigated. The 3-locus MDR model comprising FTO rs8050136C/A and GNB3 rs1129649T/C and rs5443C/T emerged as the best disease conferring model. Moreover, the interacted-genotypes having either 1, 2, 3, 4 or 5 risk alleles correlated with linearly increasing odds ratios of 1.91 (P = 0.027); 3.93 (P = 2.08E–06); 4.51 (P = 7.63E–07); 7.44 (P = 3.66E–08) and 11.57 (P = 1.18E–05), respectively, when compared with interacted-genotypes devoid of risk alleles. Furthermore, interactions among haplotypes of FTO (H1−9) and GNB3 (Ha-d) differed by >1.5-fold for protective-haplotypes, CTGGC+TC [H2+Ha] and CTGAC+TC [H4+Ha] (OR = 0.39, P = 0.003; OR = 0.22, P = 6.86E–05, respectively) and risk-haplotypes, AAAGC+CT [H3+Hc] and AAAGC+TT [H3+Hd] (OR = 2.91, P = 9.98E–06; OR = 2.50, P = 0.004, respectively) compared to individual haplotypes. Moreover, the effectiveness of gene-gene interaction was further corroborated with a 1.29-, 1.25- and 1.38-fold higher SBP, MAP and BMI, respectively, in patients having risk interacted-haplotype H3+Hc and 2.48-fold higher SBP having risk interacted-haplotype H3+Hd compared to individual haplotypes.
Conclusion
Interactions between genetic variants of FTO and GNB3 influence clinical parameters to augment hypertension.
Introduction
Essential hypertension (EH) is a risk predictor of stroke and cardiovascular diseases and results in high mortality [1]. Studies in the last few decades have established the significance of various physiological pathways in EH [2], including the importance of the relative interactions between the autonomic nervous system (ANS) and G protein-coupled receptors (GPCRs) in the regulation of blood pressure (BP) [3]–[6]. Subsequent ongoing cohort studies have revealed that 40–60% of BP variability is genetically determined [7]–[9]. Among the various genes of these pathways, fat mass and obesity associated (FTO) and guanine nucleotide binding protein, β-polypeptide 3 (GNB3) appear relevant because the former is highly expressed in BP regulating centers of hypothalamus and the latter is involved in intracellular signaling pathways. Recent genome wide and meta-analysis reports have associated both individual genes with hypertension promoting risk factors e.g., BMI and adiposity especially in Asians [10]–[15].
FTO, originally identified in mice with fused toes, is highly expressed in paraventricular and dorsomedial nuclei of the hypothalamus [16]. Genome-wide linkage studies have identified linkages between FTO and BP [17], [18]. Similarly, GNB3, encoding the Gβ3 subunit of heterotrimeric signal transducing G proteins [19] has polymorphisms that have shown to be associated with susceptibility to EH [20]–[22].
Interestingly, the interactive role of FTO and GNB3 has not been studied despite the known role of both the genes in BP regulation. As EH is a multifactorial disease, the interaction between these two genes may be crucial. To address this question, we screened the potential single nucleotide polymorphisms (SNPs) of FTO and GNB3 in a case-control design and looked for their interactive effect in hypertension pathohysiology in correlation with clinical parameters including systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP) and body mass index (BMI).
Materials and Methods
Ethics Statement
The study protocol and consent form were approved by human ethics committee of both CSIR-IGIB and GB Pant hospital. Prior to written consent, subjects were informed of the objectives, study organization and implications of their participation.
Study Participants
Ethnically-matched consecutive unrelated 4000 North-Indian participants, over a period of 4 years, were screened in the hypertension and general outpatient clinic of GB Pant hospital, New Delhi. A significant number of subjects were excluded because they did not give consent for the study, were on medication, and to maintain both the age limit and the male to female ratio in the two groups. Moreover, physical examination and laboratory tests excluded individuals with coronary artery disease, vascular disease, stroke, secondary hypertension, diabetes mellitus and renal diseases. In the final analysis we included 1300 case-control participants comprising of 750 controls and 550 patients.
Inclusion Criteria and Clinical Evaluation
Controls recruitment criteria included: age 25–60 years, SBP<120 mmHg and DBP<80 mmHg, absence of family history of hypertension and any disease medication. Patients recruitment criteria was: age 25–60 years, SBP≥140 mmHg and/or DBP≥90 mmHg (JNC VII) and absence of antihypertensive medication. All the subjects were rested for 5 minutes prior to BP measurement. Three measurements of BP, in supine position, using a calibrated mercury sphygmomanometer with appropriate adult cuff size were recorded by the clinicians. The point at which the first of two or more Korotkoff sound was heard was recorded as SBP and the disappearance of Korotkoff sound as DBP. Blood was drawn in supine position after overnight fasting. Peripheral blood leucocytes were used for DNA extraction and plasma for the analysis of biochemical parameters; samples were stored at −40°C if not used immediately.
Routine Biochemical Assays
Total cholesterol, triglycerides, glucose and uric acid were estimated on a high-throughput autoanalyzer (Elecsys 2010, Roche, Germany) and SpectraMax384 spectrophotometer (Molecular Devices, Sunnyvale CA, USA). All the measurements were performed in duplicate. The intra- and inter-assay coefficient of variations were <5% for all the measurements.
Selection of FTO and GNB3 SNPs
Selection of SNPs was based on their location in respective genes, clinical and functional relevance, and their association with hypertension [13], [18], [21], [22]. Selection was also based on their tagging with other SNPs (www.hapmap.org) and association with BMI, obesity and diabetes that affect BP [10], [12], [23]–[26]. Among the FTO SNPs, rs8050136C/A, rs9939609T/A, rs9926289G/A, rs9930506A/G, rs9932754T/C, rs9933040A/T and rs62033414C/G were from intron 1; rs16952624C/T (Ala405Val) was from exon 9 and rs16953075C/T was from the 3' UTR. In case of GNB3 SNPs, rs1129649T/C (Ile685Thr) was from exon 1 and rs5443 (825C/T) was from exon 10. The selected SNPs cover around 16 kb and 4 kb of the FTO and GNB3 genes, respectively.
Genotyping
Genomic DNA was isolated from peripheral blood leukocytes using a standard protocol. All the nine SNPs of FTO and rs5443C/T SNP of GNB3 were analyzed by SNaPshot ddNTP primer extension PCR (Applied Biosystems, Foster City, USA). The GNB3 rs1129649T/C was genotyped by PCR-restriction fragment length polymorphism (RFLP). Two observers independently read and confirmed all the genotypes; discrepancies, if any, were resolved by repeating PCR-RFLP and SNaPshot. The primers, optimal conditions for amplification and restriction enzymes for digestion are presented in Tables S1 and S2.
Haplotypes and Linkage Disequilibrium
Haplotypes were estimated from genotypes using software PHASEv2.1.1 [27] and the best haplotypes were identified for protection and risk. Order of SNPs in inferred-haplotypes was based on their physical location, starting from SNPs at the upstream promoter region to downstream. Distribution of each haplotype was compared using multivariate logistic regression analysis. Haplotypes with <2% frequency were excluded. The extent of association, i.e., the Lewontin’s coefficient (D′) and squared correlation coefficient (r2) for pairwise linkage disequilibrium (LD), was calculated by Haploview-v4.0 [28].
Gene-Gene Interactions
Gene-gene interactions (epistasis), in same subjects, were analyzed in two ways, using (1) interacted-genotypes and (2) interacted-haplotypes.
(1) Interacted-genotypes
The interacted-genotypes between FTO and GNB3 were analyzed using multifactor dimensionality reduction (MDR-v.1.2.2) software [29]. The best disease predicting MDR model was identified on the basis of interacted-genotypes carrying different set of risk alleles using the gene counting method. The P value and odds ratio (OR) were calculated using multivariate logistic regression analysis after adjustment with seven confounders namely, age, gender, BMI, alcohol, smoking habit, triglyceride, cholesterol and also by Bonferroni’s correction test for multiple testing.
(2) Interacted-haplotypes
In this analysis, we first inferred risk and protective haplotypes of each gene on the basis of P value and OR at 95% confidence interval (CI). We then looked for haplotype-haplotype interactions through the interaction of risk or protective haplotypes between FTO and GNB3 using Haploview-v4.0 [28], Hap Evolution [30] and the gene counting method. Statistical significance was determined empirically using multivariate logistic-regression model after adjustment with seven confounders (the same as used for interacted-genotypes above) and Bonferroni’s correction test for multiple testing.
Correlation Analysis
To strengthen the genetic outcome, the investigated SNPs were analyzed for possible correlation with clinical characteristics. Genotypes and haplotypes were correlated with SBP, DBP, MAP and BMI. Likewise, both the interacted-genotypes and interacted-haplotypes of FTO and GNB3 were correlated with the same clinical parameters to determine the extent of gene-gene interaction.
Statistical Analysis
Unpaired Student’s t-test (two-tailed) was performed to compare the differences in baseline clinical and demographic characteristics between the two groups. A goodness-of-fit test was used for testing the Hardy-Weinberg Equilibrium (HWE) using DeFinetti program (http://ihg.gsf.de/cgi-bin/hw/hwa1.pl). Allele and genotype frequencies between the study subjects were estimated by χ2 test. The allelic distribution between our population and HapMap populations was compared after retrieving the data from www.hapmap.org. The risk of having hypertension was estimated as an odds ratio (OR) at 95% confidence interval (95% CI) using multivariate logistic regression analysis by SPSS-12 (SPSS Inc., Chicago, Illinois, USA). Haplotypes distribution was compared by multiple regression analysis based on the frequency of each haplotype individually versus all others combined between both the groups. The clinical parameters were expressed as mean ± SD. Further, P value and estimated difference at 95% CI for continuous variables e.g., SBP, DBP, MAP and BMI against categorical variables e.g., individual and interacted genotypes and haplotypes were analyzed using a general linear model (GLM) after adjustment for the seven confounding factors. The transcription factor binding site (TFBS) with respect to the studied SNPs was analyzed using TFSEARCH developed by Yutaka Akiyama ( http://www.rwcp.or.jp/papia ). The power of association at α = 0.05 was calculated using EPIINFO ver.6. A P value of <0.05 was considered statistically significant.
Results
Comparison of Demographic and Clinical Characteristics
Patients had significantly higher BMI (P = 0.003), clinical parameters e.g. SBP, DBP and MAP (P<0.0001, each) and the levels of routine biochemical parameters e.g., cholesterol and triglyceride (P<0.0001, each) when compared with controls (Table 1).
Table 1. Demographic and clinical characteristics of studied participants.
| Parameters | Patients | Controls | P |
| n = 550 | n = 750 | ||
| Gender | |||
| Male | 467(85%) | 649(87%) | – |
| Female | 83(15%) | 101(13%) | – |
| Clinical characteristics | |||
| Age, year | 49.8±11.0 | 48.5±13.0 | NS |
| BMI, kg/m2 | 25.0±3.7 | 24.0±7.4 | 0.003 |
| SBP, mmHg | 159.4±17.8 | 117.6±8.0 | <0.0001 |
| DBP, mmHg | 96.4±9.0 | 77.6±3.9 | <0.0001 |
| MAP, mmHg | 116.9±12.7 | 91.0±21.2 | <0.0001 |
| Biochemical parameters | |||
| Total cholesterol, mmol/L | 3.3±1.2 | 2.4±1.3 | <0.0001 |
| Triglycerides, mmol/L | 1.3±0.8 | 1.0±0.6 | <0.0001 |
| Uric acid, mg/dl | 4.7±1.6 | 4.6±1.4 | NS |
| Glucose, mg/dl | 101.0±22.0 | 98.1±32.0 | NS |
| Protein urea | Nil | Nil | – |
| Life style/history | |||
| Diet, non-veg | 68% | 30% | – |
| Family history, EH | 78% | None | – |
| Alcohol | 15% | 10% | – |
| Smoking history | 25% | 15% | – |
Data are presented as mean ± standard deviation; n, number of subjects; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; EH, essential hypertension. P-values were calculated using EPIINFO ver.6 (Center for Disease Control, Atlanta, Georgia, USA) software.
Single-locus Association Analyses
The allele and genotype frequencies of studied SNPs were in HWE ( P>0.05, Table S3). The allele frequency of the studied SNPs was comparable with HapMap Caucasian population (P>0.05, Table S4). The single-locus genotype distribution is shown in Table S5. The FTO alleles rs8050136A (P = 0.014), rs9939609A (P = 0.002), rs9926289A (P = 0.015) and the GNB3 alleles rs1129649C (P = 8.76E–06) and rs5443T (P = 9.45E–10) were associated with increased risk of hypertension.
Identification of Risk or Protection Associated Haplotypes
The pairwise LD was similar for both the groups (Figure S1). At >2% cutoff frequency, 9 haplotypes for FTO and 4 haplotypes for GNB3 were inferred (Figure S2). For convenience, the FTO haplotypes were marked as H1–9 and the GNB3 haplotypes as Ha-d. The haplotypes FTO H3: AAAGC and GNB3 Hc: CT and Hd: TT increased the risk of hypertension with ORs of 1.48 (P = 0.005), 1.74 (P = 4.36E–05) and 1.79 (P = 1.79E–04), respectively, while haplotypes FTO H4: CTGAC and GNB3 Ha: TC with ORs of 0.38 (P = 5.45E–05) and 0.49 (P = 2.12E–11), respectively, were protective. The omnibus global test for both FTO and GNB3 haplotypes showed significant association with hypertension (P<0.0001, each).
Gene-gene Interaction and Hypertension Risk
(A) Interacted-genotypes
The exhaustive data mining MDR analysis was used to evaluate the impact of interactions among the genotypes of the eleven SNPs of FTO and GNB3 on hypertension; Table 2 summarizes the results obtained for 2-locus to 7-locus models. The 3-locus model comprised of the polymorphisms FTO rs8050136C/A and GNB3 rs1129649T/C and rs5443C/T emerged as the best disease predicting model with the highest level of statistical significance (TA = 0.62, CVC = 9/10; OR = 3.9, P = 0.0005) and a prediction error of 0.38. Out of the seven expected interacted-genotypes, only six interacted-genotypes were obtained, with the number of risk alleles varying between 0 and 5. Of note, the five interacted-genotypes bearing 1, 2, 3, 4 and 5 risk alleles corresponded with linearly increasing ORs, which varied between 1.91 and 11.57 (P = 0.027−3.66E–08, Figure 1).
Table 2. Interaction between genotypes of FTO and GNB3 using MDR.
| FTO+GNB3 | Best models | TB | TA | CVC | P value | OR(95% CI) |
| 2L | rs1129649T/C rs5443C/T | 0.62 | 0.59 | 8/10 | 0.067 | 2.1(0.9–4.8) |
| 3L† | rs8050136C/A rs1129649T/C rs5443C/T | 0.63 | 0.62 | 9/10 | 0.0005 | 3.9(1.8–8.5) |
| 4L | rs9930506A/G rs9932754C/T rs1129649T/C rs5443C/T | 0.65 | 0.61 | 4/10 | 0.006 | 3.0(1.4–6.5) |
| 5L | rs9939609T/A rs9930506A/G rs9932754C/T rs1129649T/C rs5443C/T | 0.64 | 0.61 | 8/10 | 0.0002 | 4.9(2.0–11.7) |
| 6L | rs8050136C/A rs9939609T/A rs9926289G/A rs9932754C/T rs1129649T/C rs5443C/T | 0.63 | 0.59 | 6/10 | 0.0165 | 3.0(1.2–7.7) |
| 7L | rs8050136C/A rs9939609T/A rs9926289G/A rs9930506A/G rs9932754C/Trs1129649T/C rs5443C/T | 0.61 | 0.58 | 10/10 | 0.012 | 3.6(1.3–10.4) |
Overall best MDR model; TB, Testing balance accuracy; TA, Training accuracy; CVC, Cross validation consistency. 2L–7L, represents 2-locus to 7-locus MDR model carrying best interacted genotypes. P values were calculated by permuting the cases and controls 1000 times.
Figure 1. Interacted-genotypes carrying varied number of risk alleles of the 3-locus best model in hypertension susceptibility.
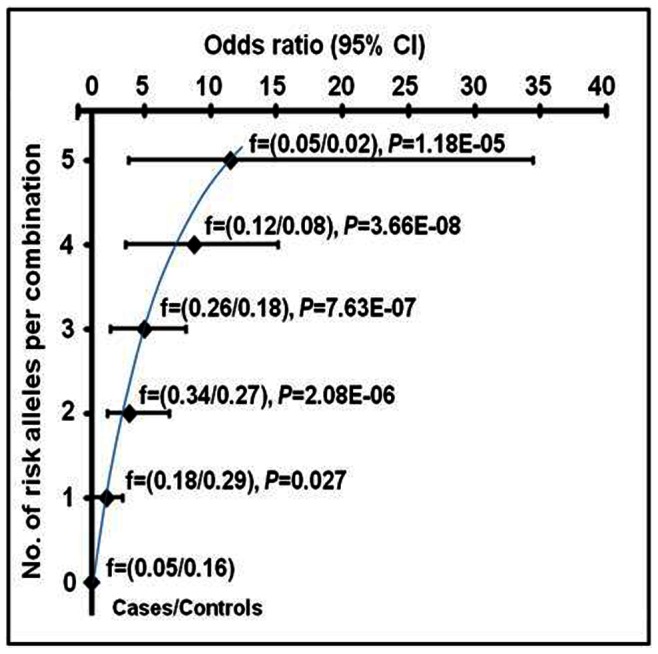
The MDR model consisted of the SNPs FTO, rs8050136C/A and GNB3, rs1129649T/C and rs5443C/T. Stratification was done on the basis of interacted-genotypes carrying risk alleles from 0 to 5. f, represents the frequency of the risk alleles in an interacted-genotypes. P-value and OR at 95% CI were calculated after adjustment for age, gender, BMI, alcohol, smoking, triglyceride and cholesterol using multivariate logistic regression analysis. The threshold P value was (0.05/6) = 0.008.
(B) Interacted-haplotypes
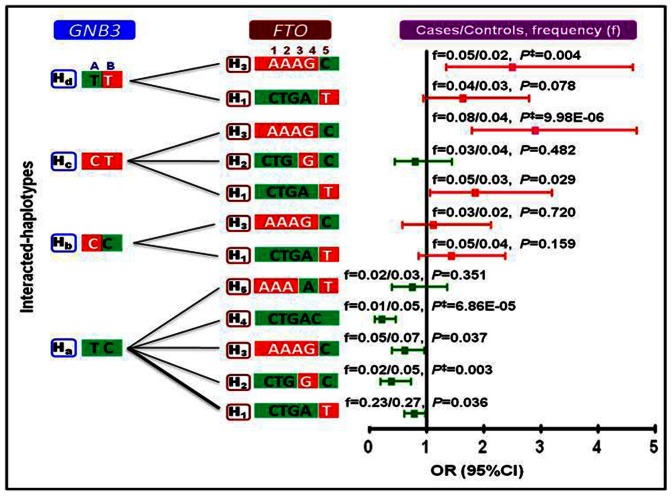
In this analysis, the 9 FTO and 4 GNB3 haplotypes were allowed to interact with each other and the haplotypes that showed significant interaction were selected. As shown in Figure 2, the FTO risk haplotype, H3: AAAGC interacted with GNB3 risk haplotypes, Hc: CT and Hd: TT. The interacted-haplotypes H3+Hc and H3+Hd contributed to 1.8- and 1.5-fold increase in hypertension susceptibility than the individual risk haplotypes of either gene alone (P = 9.98E–06; P = 0.004, respectively). FTO protective haplotypes H2: CTGGC and H4: CTGAC interacted with GNB3 protective haplotype Ha: TC to contribute to 1.5- and 2.0-fold lower hypertension susceptibility than the individual protective haplotypes of either gene alone (P = 0.003; P = 6.86E–05, respectively). Furthermore, the risk alleles FTO rs8050136A and rs9932754T and GNB3 rs5443T showed highest interaction ratio of 32%, 40% and 85%, respectively, and as a consequence all those haplotypes bearing these alleles associated with higher haplotype risk ratio (Figure S3).
Figure 2. Interacted-haplotypes of FTO and GNB3 in cases and controls.
The red and green shaded areas in the haplotypes block represent the risk and non-risk alleles, respectively. The interactions were examined at a frequency of >2%. The thin and thick line represents the interaction frequency of >2% and 10%, respectively. H represents a haplotype, H with numeric and alphabet symbol represent the FTO and GNB3 haplotypes, respectively. The alleles in each block belonged to SNPs A = rs1129649T/C and B = 825C/T of GNB3 and 1 = rs8050136C/A, 2 = rs9939609T/A, 3 = rs9926289G/A, 4 = rs9930506A/G and 5 = rs9932754T/C of FTO. P ‡ value was statistically significant for risk and protective interacted-haplotypes. The P-value and OR were calculated after adjustment for potential confounding factors and Bonferroni’s correction. ‘f’, represents frequency of interacted-haplotypes. The threshold P-value was (0.05/7) = 0.007.
Correlation with Clinical Characteristics
(a) Genotypes/alleles versus clinical characteristics
The general linear model revealed a significant positive correlation of risk genotypes of FTO SNPs rs8050136C/A, rs9939609T/A and rs9926289G/A and GNB3 SNPs rs1129649T/C and rs5443C/T with SBP, MAP and BMI (P = 0.005−3.96E–07). As a consequence, FTO risk alleles rs8050136A and rs9939609A correlated with 3.51 and 2.47 mmHg higher SBP (P = 1.95E–05; P = 0.002, respectively); 1.95 and 1.53 mmHg higher MAP (P = 2.69E–04; P = 0.004, respectively) and 1.04 and 0.57 kg/m2 higher BMI (P = 1.01E–07; P = 0.003, respectively). FTO risk allele rs9926289A correlated with 2.37 and 1.68 mmHg higher SBP and MAP (P = 0.003; P = 0.001, respectively; Figure 3). The GNB3 risk allele rs1129649C correlated with 1.58 mmHg higher MAP (P = 0.003) and 0.62 kg/m2 higher BMI (P = 0.001); GNB3 risk allele rs5443T correlated with 2.32 mmHg higher SBP (P = 0.005), 2.08 mmHg higher MAP (P = 1.04E–04) and 0.97 kg/m2 higher BMI (P = 6.77E–07).
Figure 3. Difference in BP and BMI according to risk alleles of FTO and GNB3 SNPs.
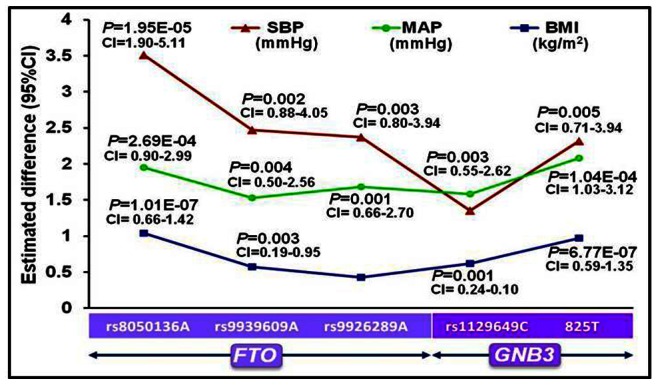
CI represents confidence interval. The general linear model was used to calculate P-value and estimated difference at 95% CI after adjustment for potential confounding factors and Bonferroni’s correction.
(b) Haplotypes versus clinical characteristics
As shown in Figure 4a, FTO risk haplotype H3 correlated with an increase of 3.59 mmHg SBP and 2.19 mmHg MAP(P = 0.002; P = 0.008, respectively). With respect to GNB3 risk haplotype Hc, the increase was 2.53 mmHg SBP (P = 0.04), 2.85 mmHg MAP (P = 2.15E–05) and 0.97 kg/m2 BMI (P = 1.10E–04). The protective haplotype Ha correlated with a decrease of 1.52 mmHg MAP (P = 0.02) and 0.85 kg/m2 BMI (P = 4.41E–05).
Figure 4. Correlation analyses for (a) individual haplotypes (b) interacted-haplotypes of FTO and GNB3 with clinical parameters.
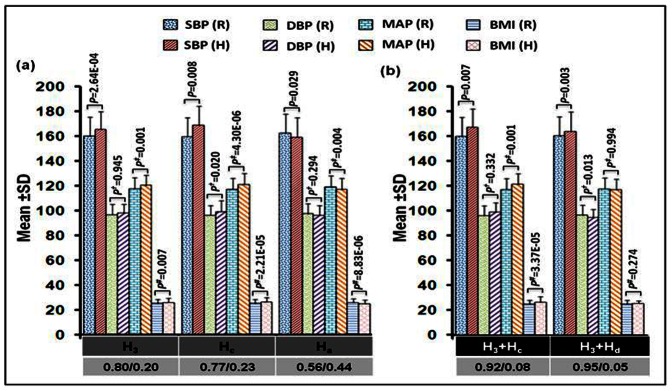
The general linear model was used to calculate significance level after adjustment with age, gender, smoking, alcohol, triglyceride, cholesterol and Bonferroni’s correction test. Besides, P was adjusted for DBP, BMI; P † for SBP, BMI and P # for SBP, DBP. (R), represents reference for remaining haplotypes against any studied individual haplotype (H). The X-axis represents individual haplotypes H3: AAAGC, Hc: CT and Ha: TC and interacted-haplotypes H3+Hc: AAAGC+CT and H3+Hd: AAAGC+TT. The numerator and denominator represent frequency of (R) and (H), respectively.
(c) Interacted-genotypes versus clinical characteristics
As shown in Figure 5, the interacted-genotypes bearing 1, 2, 3, 4 and 5 risk alleles correlated with linear increase in SBP of 4.88–14.62 mmHg (P = 0.079−1.18E–04), MAP 4.29–11.29 mmHg (P = 0.016−3.73E–06) and 1.16–5.78 kg/m2 BMI (P = 0.067−1.40E–11) when compared against interacted-genotypes without risk alleles.
Figure 5. Variation in clinical characteristics according to number of risk-alleles in interacted-genotypes in 3-locus MDR model.
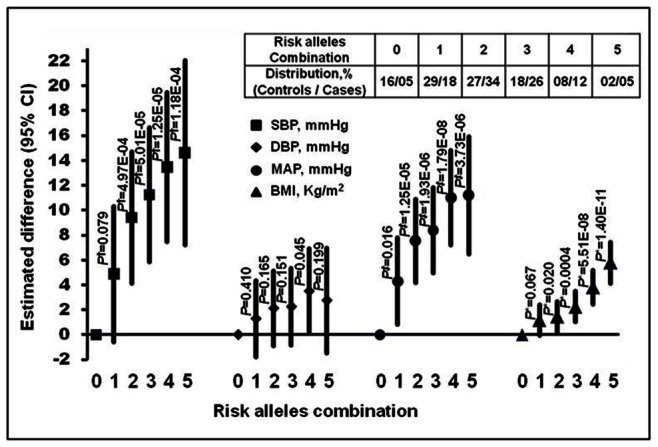
The general linear model was used to calculate significance level after adjustment with age, gender, smoking, alcohol, triglyceride, cholesterol and Bonferroni’s correction test. Besides, P † was adjustment with BMI and DBP; P ‡ with BMI; P* with SBP, DBP. The threshold P-value was (0.05/10) = 0.005.
(d) Interacted-haplotypes versus clinical characteristics
As shown in Figure 4b, an estimated increase of 3.96 mmHg SBP, 3.14 mmHg MAP and 1.11 kg/m2 BMI was observed in the presence of interacted-haplotype H3+Hc when compared against the remaining interacted-haplotypes from both the genes (P = 0.007; P = 0.001 and P = 3.37E–05, respectively). Of consequence, epistasis influence resulted in a 1.29-, 1.25- and 1.38-fold higher SBP, MAP and BMI, respectively, in the patients with interacted-haplotypes H3+Hc compared to individual risk haplotypes H3 and Hc. The second risk interacted-haplotype, H3+Hd significantly correlated with an estimated increase of 5.44 mmHg SBP (P = 0.003), with epistasis contributing a 2.48-fold higher SBP.
The SNPs and the Associated Transcription Factor
The transcription factor binding site (TFBS) in the presence of protective and risk alleles of both the genes changes the preference for the transcription factors (Figure S4). For example in the presence of rs8050136C allele, the transcription factors (TFs) were CDP-CR and cap; whereas in the presence of risk allele rs8050136A, the TFs were CdxA, Abd-B and Croc. Further, in the presence of FTO protective allele rs9930506A, the TFs were Dfd and MATalp and in the presence of risk allele rs9930506G, a single TF Dfd was noted. In the presence of FTO protective allele rs9932754T four TFs HNF-3b, Cap, Skn-1 and CdxA were observed; whereas, in the presence of risk allele rs9932754C a single TF HSF2 was observed. Likewise, in case of GNB3, the protective allele rs1129649T associated with three TFs NIT2, Cap and NF-1 whereas, in the presence of risk allele rs1129649C, only TF Cap was associated.
Discussion
In this study, the epistasis models of interacted-genotypes and interacted-haplotypes demonstrated increased hypertension susceptibility. Notably, stratification of the interacted-genotypes, as obtained in the best locus MDR model on the basis of presence of number of risk allele(s) in increasing order, correlated linearly with hypertension susceptibility. Furthermore, the interaction between FTO and GNB3 when analyzed through haplotype-haplotype interactions revealed substantial modifications in the ORs for risk and protection compared to individual haplotypes. Moreover, the general linear model showed substantial correlation of interacted-genotypes and interacted-haplotypes with clinical characteristics, e.g., SBP, DBP, MAP and BMI.
Our findings on individual genes were significant as it revealed higher OR for EH in the presence of risk alleles of FTO rs8050136C/A, rs9939609T/A and rs9926289G/A, and GNB3 rs1129649T/C and rs5443C/T SNPs, even after adjustment for potential confounders. Literature suggested an association of the FTO variants with hypertension [18], [23] or mediation through other hypertension risk factors like BMI or adiposity [10], [12], [31], [32]. Likewise, given the role of heterotrimeric G-proteins in intracellular signaling pathways, the GNB3 variants were studied in EH [20]–[22], [33]. The rs5443C/T of GNB3 was associated with enhanced activation of G protein-mediated signaling [20], noradrenaline-induced vasoconstriction [34], higher plasma sodium and lower potassium levels [33] and the C allele of rs1129649T/C was associated with salt sensitive BP [35].
Although our single locus results on FTO and GNB3 were encouraging, however, in a polygenic and multifactorial disease like hypertension, the magnitude of effect is bound to be missed if the genes are examined individually and without considering potential interactions [36]. The evaluation of gene-gene interactions not only increases the power to detect the effects but also helps in understanding the genetic influences on the biological and biochemical pathways that underpin the disease [37]. Two reasons encouraged us to look for interaction between these two genes. First, both the genes are involved in modulating sympathetic and parasympathetic activity [4], [6].Second, these genes are highly associated with phenotypes like adiposity and BMI [14], [15], [24]–[26], [38], which are major risk factors for hypertension [18], [23], [39]–[41]. Our study demonstrated that indeed there was a linear correlation between OR and interacted-genotypes that represented the risk convoking alleles in increasing order. The interaction between the two genes revealed higher OR for risk or lower OR for protection conferring interacted-haplotypes compared to individual respective haplotypes of each gene, thus, supporting the role of epistasis in the regulation of BP [42], [43]. Such interaction studies of FTO with other genes are not available. An interaction between GNB3 and ACE however, was documented in EH [44].
With regard to correlation analysis, our findings signified a major contribution of epistasis towards BP phenotypes. The GLM model revealed a significant linear correlation of interacted-genotypes and interacted-haplotypes with clinical parameters e.g., SBP, MAP and BMI. The latter two parameters were increased by >1-fold in the presence of the interacted-haplotypes H3+Hc and SBP was increased by 2.5-fold in the presence of H3+Hd, suggesting that the interactions of genetic variants played a significant role in determining the observed phenotype [37], [45].
Of consequence, the interactions between genetic variants may modulate the FTO expression in metabolically relevant tissues such as hypothalamus, and this may influence subsequent translation of key signaling molecules like GNB3; however, this hypothesis needs to be further examined. The other important fact that cannot be ignored is that disease-associated SNPs detected in large-scale association studies are frequently located in noncoding regions, suggesting their involvement in gene regulation [46]; hence, we undertook the TF analysis and observed different sets of transcription factors associating with the risk and protective alleles of both the genes. It is known that the transcriptional regulatory system plays an essential role in controlling numerous biological processes and numerous diseases [46], [47]. Overall, our findings not only supported the available reports but also provided an insight into the interaction of risk variants of FTO and GNB3 in the susceptibility to EH.
Inconsistencies in genetic association studies may be due to, limited statistical power, population stratification and chance of false positive results. To minimize population stratification we recruited the patients and controls from the same region [48]. The likelihood of false positive results was decreased using the Bonferroni’s correction test for multiple testing. As already emphasized, our main aim was to investigate the role of FTO and GNB3 in EH; we adjusted all the results with BMI and other possible confounders to ascertain the direct effect of these genes on hypertension regulation. These adjustments provided evidence of FTO and GNB3 influencing BP. Additional prospective studies on gene-gene interaction are warranted to define the underlying mechanisms in the pathophysiology of EH. The present sample size has been adequate to provide statistically significant associations, but it needs to be tested in larger cohorts with different ethnicities.
In conclusion, the interaction between FTO and GNB3, through interacted-genotypes and interacted-haplotypes models, markedly has an epistatic effect and associated with altered clinical phenotypes and consequently with EH. The study has also suggested that gene-gene interaction holds robust information about the phenotype beyond analysis of individual SNPs, and thus including interaction between or among genes may improve the predictive accuracy of genetic-clinical correlations.
Supporting Information
Linkage disequilibrium (LD) among studied SNPs of FTO and GNB3. LD was calculated using Haploview-v4.0 in cases and controls. D’ box shading represents the strength of LD between SNPs. The light shade represents weak LD, whereas dark shade represents strong LD.
(TIF)
Individual haplotypes of FTO and GNB3 in cases and controls. Total 9 haplotypes of FTO were inferred from five SNPs (rs8050136C/A, rs9939609T/A, rs9926289G/A, rs9930506A/G and rs9932754T/C) and 4 haplotypes of GNB3 from 2 SNPs (rs1129649T/C and rs5443C/T) at overall cutoff frequency of >2%. The symbol *† represents statistically significant risk haplotypes, FTO H3, GNB3 Hc and Hd, (OR = 1.48, 95% CI = 1.13−1.94, P = 0.005; OR = 1.74, 95% CI = 1.33−2.26, P = 4.36E−05 and OR = 1.79, 95% CI = 1.32−2.43, P = 1.79E−04, respectively); whereas, symbol #† represents, statistically significant protective haplotypes, FTO H4 and GNB3 Ha (OR = 0.38, 95% CI = 0.23−0.61, P = 5.45E−05; OR = 0.49, 95% CI = 0.40−0.61, P = 2.12E−11, respectively). P-value and odds ratio (OR) were calculated after adjustment for age, gender, BMI, alcohol, smoking, triglyceride and cholesterol using multivariate logistic regression analysis and Bonferroni’s correction for multiple testing.
(TIF)
Gene-gene interaction between FTO and GNB3 . The gene-gene interaction was looked using Hap Evolution software in case-control haplotypes data. 1, represents major allele and 2, represents minor allele of each SNP. The SNPs GNB3 rs1129649T/C, rs5443 and FTO rs8050136C/T, rs9939609T/A, rs9926289G/A, rs9930506A/G and rs9932754T/C are arranged according to their position on chromosomes. Maximum interaction ratio was observed for minor allele GNB3 rs5443T and FTO rs9932754C. The P-value and haplotype risk ratio (HRR) were computed after permutation test.
(TIF)
Diagrammatic representation of the effects of FTO and GNB3 SNPs on transcription factors binding sites. The upper and lower TF, binding sites with BA represents the protective alleles (marked in black) and risk alleles (marked in red), respectively. The prediction of transcription factor, their binding sites and their binding affinity were performed by online software TFSEARCH: Searching Transcription Factor Binding Sites, http://www.rwcp.or.jp/papia/developed by Yutaka Akiyama. TF, transcription factor; BA, binding affinity (%). Flanking sequences in blue represent transcription factor binding sites (TFBS).
(TIF)
Primer sequences, normal and SNapShot PCR cycling conditions used for genotyping of FTO studied polymorphisms.
(DOC)
Primers, RFLP and SNapShot PCR cycling conditions for genotyping GNB3 polymorphisms.
(DOC)
Goodness-of-fit test for observed and expected genotypes distribution of FTO and GNB3 polymorphisms in patients and controls. Comparison between observed and expected frequencies was performed by an epidemiologic data management and analysis package (EPIINFO) ver.6
(DOC)
Comparison of the FTO and GNB3 studied alleles frequencies with Hapmap population. P-values were calculated using EPIINFO ver.6 (Center for Disease Control, Atlanta, Georgia, USA) software. Indian (IND) population was used as reference.
(DOC)
Genotypes and allele distribution of the FTO and GNB3 polymorphisms in controls and patients. P value, χ2 and odds ratio (OR) were calculated using multivariate logistic regression analysis after adjustment for age, gender, BMI, smoking, alcohol, triglycerides and cholesterol.
(DOC)
Acknowledgments
We highly appreciate the support and constant encouragement of the Director of CSIR-Institute of Genomics and Integrative Biology, Dr. Brian B. Graham, Program in Translational Lung Research, Division of Pulmonary Sciences and Critical Care Medicine, University of Colorado, Denver for editing and valuable suggestions and the staff at the Department of Cardiology, GB Pant Hospital.
Funding Statement
URL of funder’s website: http://csirhrdg.res.in/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, et al. (2005) Global burden of hypertension: analysis of worldwide data. Lancet 365: 217–223. [DOI] [PubMed] [Google Scholar]
- 2. Joyner MJ, Charkoudian N, Wallin BG (2008) A sympathetic view of the sympathetic nervous system and human blood pressure regulation. Exp Physiol 93: 715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xia H, Suda S, Bindom S, Feng Y, Gurley SB, et al. (2011) ACE2-Mediated reduction of oxidative stress in the central nervous system is associated with improvement of autonomic function. PLoS One 6: e22682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guyenet PG (2006) The sympathetic control of blood pressure. Nat Rev Neurosci 7: 335–346. [DOI] [PubMed] [Google Scholar]
- 5. Bunnett NW, Cottrell GS (2010) Trafficking and signaling of G protein-coupled receptors in the nervous system: implications for disease and therapy. CNS Neurol Disord Drug Targets 9: 539–556. [DOI] [PubMed] [Google Scholar]
- 6. Zolk O, Kouchi I, Schnabel P, Bohm M (2000) Heterotrimeric G proteins in heart disease. Can J Physiol Pharmacol 78: 187–198. [PubMed] [Google Scholar]
- 7. Mitchell BD, Kammerer CM, Blangero J, Mahaney MC, Rainwater DL, et al. (1996) Genetic and environmental contributions to cardiovascular risk factors in Mexican Americans. The San Antonio Family Heart Study. Circulation 94: 2159–2170. [DOI] [PubMed] [Google Scholar]
- 8. Hong Y, de FU, Heller DA, McClearn GE, Pedersen N (1994) Genetic and environmental influences on blood pressure in elderly twins. Hypertension 24: 663–670. [DOI] [PubMed] [Google Scholar]
- 9. Harrap SB, Stebbing M, Hopper JL, Hoang HN, Giles GG (2000) Familial patterns of covariation for cardiovascular risk factors in adults: The victorian family heart study. Am J Epidemiol 152: 704–715. [DOI] [PubMed] [Google Scholar]
- 10. Yang J, Loos RJ, Powell JE, Medland SE, Speliotes EK, et al. (2012) FTO genotype is associated with phenotypic variability of body mass index. Nature 490: 267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fall T, Ingelsson E (2012) Genome-wide association studies of obesity and metabolic syndrome. Mol Cell Endocrinol. S0303-7207(12)00413-3. [DOI] [PubMed]
- 12. Peng S, Zhu Y, Xu F, Ren X, Li X, et al. (2011) FTO gene polymorphisms and obesity risk: a meta-analysis. BMC Med 9: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Niu W, Qi Y (2011) Association of alpha-adducin and G-protein beta3 genetic polymorphisms with hypertension: a meta-analysis of Chinese populations. PLoS One 6: e17052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wen W, Cho YS, Zheng W, Dorajoo R, Kato N, et al. (2012) Meta-analysis identifies common variants associated with body mass index in east Asians. Nat Genet 44: 307–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pemberton TJ, Mehta NU, Witonsky D, Di RA, Allayee H, et al. (2008) Prevalence of common disease-associated variants in Asian Indians. BMC Genet 9: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gerken T, Girard CA, Tung YC, Webby CJ, Saudek V, et al. (2007) The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science 318: 1469–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hamet P, Merlo E, Seda O, Broeckel U, Tremblay J, et al. (2005) Quantitative founder-effect analysis of French Canadian families identifies specific loci contributing to metabolic phenotypes of hypertension. Am J Hum Genet 76: 815–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pausova Z, Syme C, Abrahamowicz M, Xiao Y, Leonard GT, et al. (2009) A common variant of the FTO gene is associated with not only increased adiposity but also elevated blood pressure in French Canadians. Circ Cardiovasc Genet 2: 260–269. [DOI] [PubMed] [Google Scholar]
- 19. Neves SR, Ram PT, Iyengar R (2002) G protein pathways. Science 296: 1636–1639. [DOI] [PubMed] [Google Scholar]
- 20. Siffert W, Rosskopf D, Siffert G, Busch S, Moritz A, et al. (1998) Association of a human G-protein beta3 subunit variant with hypertension. Nat Genet 18: 45–48. [DOI] [PubMed] [Google Scholar]
- 21. Siffert W (2003) G-protein beta3 subunit 825T allele and hypertension. Curr Hypertens Rep 5: 47–53. [DOI] [PubMed] [Google Scholar]
- 22. Bagos PG, Elefsinioti AL, Nikolopoulos GK, Hamodrakas SJ (2007) The GNB3 C825T polymorphism and essential hypertension: a meta-analysis of 34 studies including 14,094 cases and 17,760 controls. J Hypertens 25: 487–500. [DOI] [PubMed] [Google Scholar]
- 23. Timpson NJ, Harbord R, Davey SG, Zacho J, Tybjaerg-Hansen A, et al. (2009) Does greater adiposity increase blood pressure and hypertension risk?: Mendelian randomization using the FTO/MC4R genotype. Hypertension 54: 84–90. [DOI] [PubMed] [Google Scholar]
- 24. Chang YC, Liu PH, Lee WJ, Chang TJ, Jiang YD, et al. (2008) Common variation in the fat mass and obesity-associated (FTO) gene confers risk of obesity and modulates BMI in the Chinese population. Diabetes 57: 2245–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Souza RP, De LV, Muscettola G, Rosa DV, de BA, et al. (2008) Association of antipsychotic induced weight gain and body mass index with GNB3 gene: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 32: 1848–1853. [DOI] [PubMed] [Google Scholar]
- 26. Klenke S, Kussmann M, Siffert W (2011) The GNB3 C825T polymorphism as a pharmacogenetic marker in the treatment of hypertension, obesity, and depression. Pharmacogenet Genomics 21: 594–606. [DOI] [PubMed] [Google Scholar]
- 27. Stephens M, Smith NJ, Donnelly P (2001) A new statistical method for haplotype reconstruction from population data. Am J Hum Genet 68: 978–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Barrett JC, Fry B, Maller J, Daly MJ (2005) Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21: 263–265. [DOI] [PubMed] [Google Scholar]
- 29. Hahn LW, Ritchie MD, Moore JH (2003) Multifactor dimensionality reduction software for detecting gene-gene and gene-environment interactions. Bioinformatics 19: 376–382. [DOI] [PubMed] [Google Scholar]
- 30. Potter MA, De Jong KA (2000) Cooperative coevolution: an architecture for evolving coadapted subcomponents. Evol Comput 8: 1–29. [DOI] [PubMed] [Google Scholar]
- 31. Ahmad T, Chasman DI, Mora S, Pare G, Cook NR, et al. (2010) The fat-mass and obesity-associated (FTO) gene, physical activity, and risk of incident cardiovascular events in white women. Am Heart J 160: 1163–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sentinelli F, Incani M, Coccia F, Capoccia D, Cambuli VM, et al. (2012) Association of FTO polymorphisms with early age of obesity in obese Italian subjects. Exp Diabetes Res. 2012: 872176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nejatizadeh A, Kumar R, Stobdan T, Qadar Pasha MA (2011) Association of GNB3 C825T polymorphism with plasma electrolyte balance and susceptibility to hypertension. Genet Mol Biol 34: 553–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wenzel RR, Siffert W, Bruck H, Philipp T, Schafers RF (2002) Enhanced vasoconstriction to endothelin-1, angiotensin II and noradrenaline in carriers of the GNB3 825T allele in the skin microcirculation. Pharmacogenetics 12: 489–495. [DOI] [PubMed] [Google Scholar]
- 35. Kelly TN, Rice TK, Gu D, Hixson JE, Chen J, et al. (2009) Novel genetic variants in the alpha-adducin and guanine nucleotide binding protein beta-polypeptide 3 genes and salt sensitivity of blood pressure. Am J Hypertens 22: 985–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cordell HJ (2009) Detecting gene-gene interactions that underlie human diseases. Nat Rev Genet 10: 392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moore JH (2003) The ubiquitous nature of epistasis in determining susceptibility to common human diseases. Hum Hered 56: 73–82. [DOI] [PubMed] [Google Scholar]
- 38. Dina C, Meyre D, Gallina S, Durand E, Korner A, et al. (2007) Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet 39: 724–726. [DOI] [PubMed] [Google Scholar]
- 39. Mamun AA, Lawlor DA, O’Callaghan MJ, Williams GM, Najman JM (2005) Effect of body mass index changes between ages 5 and 14 on blood pressure at age 14: findings from a birth cohort study. Hypertension 45: 1083–1087. [DOI] [PubMed] [Google Scholar]
- 40. Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM (2003) Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension 42: 878–884. [DOI] [PubMed] [Google Scholar]
- 41. Jones DW (1996) Body weight and blood pressure. Effects of weight reduction on hypertension. Am J Hypertens 9: 50s–54s. [DOI] [PubMed] [Google Scholar]
- 42. Rana BK, Insel PA, Payne SH, Abel K, Beutler E, et al. (2007) Population-based sample reveals gene-gender interactions in blood pressure in White Americans. Hypertension 49: 96–106. [DOI] [PubMed] [Google Scholar]
- 43. Zhao Q, Wang L, Yang W, Chen S, Huang J, et al. (2008) Interactions among genetic variants from contractile pathway of vascular smooth muscle cell in essential hypertension susceptibility of Chinese Han population. Pharmacogenet Genomics 18: 459–466. [DOI] [PubMed] [Google Scholar]
- 44. Bae Y, Park C, Han J, Hong YJ, Song HH, et al. (2007) Interaction between GNB3 C825T and ACE I/D polymorphisms in essential hypertension in Koreans. J Hum Hypertens 21: 159–166. [DOI] [PubMed] [Google Scholar]
- 45. Newton-Cheh C, Hirschhorn JN (2005) Genetic association studies of complex traits: design and analysis issues. Mutat Res 573: 54–69. [DOI] [PubMed] [Google Scholar]
- 46. Ameur A, Rada-Iglesias A, Komorowski J, Wadelius C (2009) Identification of candidate regulatory SNPs by combination of transcription-factor-binding site prediction, SNP genotyping and haploChIP. Nucleic Acids Res 37: e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vaquerizas JM, Kummerfeld SK, Teichmann SA, Luscombe NM (2009) A census of human transcription factors: function, expression and evolution. Nat Rev Genet 10: 252–263. [DOI] [PubMed] [Google Scholar]
- 48. Indian Genome Variation Consortium (2008) Genetic landscape of the people of India: a canvas for disease gene exploration. J Genet 87: 3–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Linkage disequilibrium (LD) among studied SNPs of FTO and GNB3. LD was calculated using Haploview-v4.0 in cases and controls. D’ box shading represents the strength of LD between SNPs. The light shade represents weak LD, whereas dark shade represents strong LD.
(TIF)
Individual haplotypes of FTO and GNB3 in cases and controls. Total 9 haplotypes of FTO were inferred from five SNPs (rs8050136C/A, rs9939609T/A, rs9926289G/A, rs9930506A/G and rs9932754T/C) and 4 haplotypes of GNB3 from 2 SNPs (rs1129649T/C and rs5443C/T) at overall cutoff frequency of >2%. The symbol *† represents statistically significant risk haplotypes, FTO H3, GNB3 Hc and Hd, (OR = 1.48, 95% CI = 1.13−1.94, P = 0.005; OR = 1.74, 95% CI = 1.33−2.26, P = 4.36E−05 and OR = 1.79, 95% CI = 1.32−2.43, P = 1.79E−04, respectively); whereas, symbol #† represents, statistically significant protective haplotypes, FTO H4 and GNB3 Ha (OR = 0.38, 95% CI = 0.23−0.61, P = 5.45E−05; OR = 0.49, 95% CI = 0.40−0.61, P = 2.12E−11, respectively). P-value and odds ratio (OR) were calculated after adjustment for age, gender, BMI, alcohol, smoking, triglyceride and cholesterol using multivariate logistic regression analysis and Bonferroni’s correction for multiple testing.
(TIF)
Gene-gene interaction between FTO and GNB3 . The gene-gene interaction was looked using Hap Evolution software in case-control haplotypes data. 1, represents major allele and 2, represents minor allele of each SNP. The SNPs GNB3 rs1129649T/C, rs5443 and FTO rs8050136C/T, rs9939609T/A, rs9926289G/A, rs9930506A/G and rs9932754T/C are arranged according to their position on chromosomes. Maximum interaction ratio was observed for minor allele GNB3 rs5443T and FTO rs9932754C. The P-value and haplotype risk ratio (HRR) were computed after permutation test.
(TIF)
Diagrammatic representation of the effects of FTO and GNB3 SNPs on transcription factors binding sites. The upper and lower TF, binding sites with BA represents the protective alleles (marked in black) and risk alleles (marked in red), respectively. The prediction of transcription factor, their binding sites and their binding affinity were performed by online software TFSEARCH: Searching Transcription Factor Binding Sites, http://www.rwcp.or.jp/papia/developed by Yutaka Akiyama. TF, transcription factor; BA, binding affinity (%). Flanking sequences in blue represent transcription factor binding sites (TFBS).
(TIF)
Primer sequences, normal and SNapShot PCR cycling conditions used for genotyping of FTO studied polymorphisms.
(DOC)
Primers, RFLP and SNapShot PCR cycling conditions for genotyping GNB3 polymorphisms.
(DOC)
Goodness-of-fit test for observed and expected genotypes distribution of FTO and GNB3 polymorphisms in patients and controls. Comparison between observed and expected frequencies was performed by an epidemiologic data management and analysis package (EPIINFO) ver.6
(DOC)
Comparison of the FTO and GNB3 studied alleles frequencies with Hapmap population. P-values were calculated using EPIINFO ver.6 (Center for Disease Control, Atlanta, Georgia, USA) software. Indian (IND) population was used as reference.
(DOC)
Genotypes and allele distribution of the FTO and GNB3 polymorphisms in controls and patients. P value, χ2 and odds ratio (OR) were calculated using multivariate logistic regression analysis after adjustment for age, gender, BMI, smoking, alcohol, triglycerides and cholesterol.
(DOC)