Abstract
The bean bug Riptortus pedestris possesses a specialized symbiotic organ in a posterior region of the midgut, where numerous crypts harbor extracellular betaproteobacterial symbionts of the genus Burkholderia. Second instar nymphs orally acquire the symbiont from the environment, and the symbiont infection benefits the host by facilitating growth and by occasionally conferring insecticide resistance. Here we performed comparative transcriptomic analyses of insect genes expressed in symbiotic and non-symbiotic regions of the midgut dissected from Burkholderia-infected and uninfected R. pedestris. Expression sequence tag analysis of cDNA libraries and quantitative reverse transcription PCR identified a number of insect genes expressed in symbiosis- or aposymbiosis-associated patterns. For example, genes up-regulated in symbiotic relative to aposymbiotic individuals, including many cysteine-rich secreted protein genes and many cathepsin protease genes, are likely to play a role in regulating the symbiosis. Conversely, genes up-regulated in aposymbiotic relative to symbiotic individuals, including a chicken-type lysozyme gene and a defensin-like protein gene, are possibly involved in regulation of non-symbiotic bacterial infections. Our study presents the first transcriptomic data on gut symbiotic organ of a stinkbug, which provides initial clues to understanding of molecular mechanisms underlying the insect-bacterium gut symbiosis and sheds light on several intriguing commonalities between endocellular and extracellular symbiotic associations.
Introduction
The majority of insects are associated with microbial symbionts within their alimentary tract, body cavity and/or cells, and they are often benefited from the symbiosis for their growth, viability and fecundity. Hence, understanding of the mechanisms of establishment, maintenance and fitness consequences of such host-symbiont associations is of fundamental importance [1]–[6].
Transcriptomic analyses of the bacteriomes, which are specialized insect organs consisting of bacteriocytes for harboring microbial symbionts, have been conducted for aphid-Buchnera, weevil-Sodalis and bedbug-Wolbachia endosymbiotic associations of obligate nature [7]–[12]. Comparative transcriptomics of symbiont-infected and uninfected individuals have been applied to diverse arthropods and their facultative endosymbionts like Wolbachia, Cardinium and Serratia [13]–[18]. These studies show that the expression of immune-related genes such as lysozyme genes and antimicrobial peptide genes is often affected by endosymbiont infection in a tissue-specific manner. Notably, a number of cysteine-rich secreted proteins are highly expressed in the aphid bacteriocytes [7], which has been also known in plant symbioses such as legume-Rhizobium associations [19].
Within the insect suborder Heteroptera, more than 12,500 species of true bugs or stinkbugs constitute the infraorder Pentatomomorpha [20]. Besides relatively few predacious and mycophagous species, most of the phytophagous stinkbugs possess a specialized symbiotic region in the posterior midgut. The gut symbiotic organ is equipped with a number of sac- or tube-like crypts, whose lumen harbors specific extracellular symbiotic bacteria. In general, these gut symbionts significantly benefit their host stinkbugs: symbiont-deprived insects suffer retarded growth, increased nymphal mortality and/or adult sterility [3], [21], [22]. To our knowledge, no transcriptomic studies have been conducted on such insect symbiotic organs associated with specific extracellular symbionts.
The bean bug Riptortus pedestris (Hemiptera: Heteroptera: Alydidae) possesses the midgut symbiotic organ with numerous crypts, whose lumen is full of betaproteobacterial extracellular symbionts of the genus Burkholderia [23]. The gut symbiont is not essential but beneficial for the host stinkbug: uninfected insects are able to become adult and reproduce, but their growth rate and body size are significantly reduced in comparison with symbiotic insects [24]. In addition to the putative nutritional role, some Burkholderia strains are capable of degrading organophophorus insecticides, thereby making their host insects resistant to the toxic chemicals [25]. The Riptortus-Burkholderia gut symbiosis is regarded as a promising model system for insect symbiosis studies in that (i) the symbiont is easily culturable on standard microbiological media, which is exceptional among insect symbiotic bacteria of beneficial nature, (ii) the symbiont is orally acquired by young nymphal stinkbugs from the soil environment every generation, (iii) both symbiotic and aposymbiotic insects are able to become adult and reproduce, and (iv) RNA interference of the host gene expression is feasible [24], [26], [27]. Owing to these features, symbiotic and aposymbiotic insects are easily compared experimentally.
In this study, we constructed expression sequence tag (EST) libraries of symbiotic and non-symbiotic midgut regions dissected from symbiotic and aposymbiotic individuals of R. pedestris, which provide the first transcriptomic data on gut symbiotic organ of a stinkbug, and unveil a number of insect genes including lysozyme gene, defensin-like protein gene, cathepsin protease genes, and cysteine rich secreted protein genes that are potentially involved in symbiotic interactions between the Burkholderia symbiont and the Riptortus host.
Results
Gut morphology of symbiotic and aposymbiotic insects
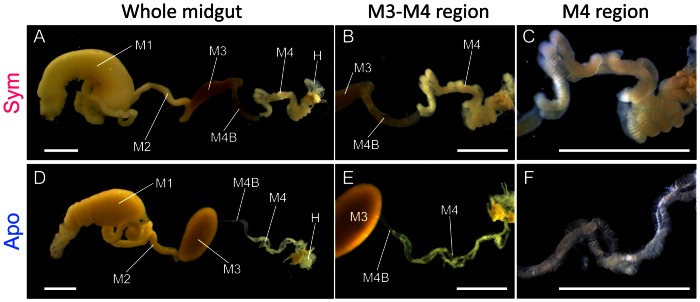
Figure 1 shows the midgut of R. pedestris consisting of several morphologically distinct regions: from anterior to posterior, stomach-like midgut first region (M1); tubular midgut second region (M2); expanded sac-like midgut third region (M3); and midgut fourth region (M4) with numerous crypts whose lumen is full of symbiotic Burkholderia cells. Between M3 and M4, there is a slightly enlarged, tubular portion, called anterior bulb of M4 or M4 bulb (M4B), which bears no crypts but contains the symbiotic bacteria [25], [28]. Among the midgut regions, M3, M4 and M4B exhibited remarkable morphological differences between symbiotic insects and aposymbiotic insects. M4 and M4B were enlarged in symbiotic insects (Fig. 1A–C), whereas the midgut regions were atrophied in aposymbiotic insects (Fig. 1D–F). By contrast, M3 was larger in aposymbiotic insects (Fig. 1D and E) than in symbiotic insects (Fig. 1A and B). These morphological differences were consistent across all individuals we examined.
Figure 1. Dissected midgut of R. pedestris three days after fifth instar molt.
(A–C) Midgut of symbiotic insect. (D–E) Midgut of aposymbiotic insect. Abbreviations: M1, midgut first region; M2, midgut second region; M3, midgut third region; M4, midgut fourth region with crypts; M4B, anterior bulb of midgut fourth section; H, hindgut. Bars show 2 mm.
Construction of midgut EST datasets
For a symbiotic insect and an aposymbiotic insect of the same isofemale line, we constructed cDNA libraries for each of the midgut region M3, M4B or M4. We used individuals at three days after fifth instar molt in this study because morphological differences of the midgut between symbiotic insects and aposymbiotic insects were conspicuous and suitable for dissecting each midgut region. In total, 6,924 clones were sequenced for the cDNA libraries of the symbiotic and aposymbiotic insects (DDBJ accession numbers HX275191-HX282114) (Table 1). From these ESTs, sequences corresponding to insect ribosomal RNA (DDBJ accession number AB725684), insect mitochondrial DNA (DDBJ accession number EU427344), and symbiont genes based on the draft genome sequence of the Burkholderia symbiont (Nikoh N et al., unpublished data) were eliminated at the criterion of E-value <10−20 under the BLASTn algorithm, which yielded 6,234 ESTs. These ESTs were subjected to automatic clustering by the Phred/Phrap/Consed software package (http://bozeman.mbt.washington.edu), and subsequently each cluster was inspected and corrected manually by dividing and reassembling putative chimeric sequences, which yielded 1,863 non-redundant EST clusters (Tables S1 and S2). Of these, 41 clusters were regarded either as isoforms or as premature forms of other clusters judging from their sequence identities (Table S2). Excluding these clusters, we obtained 1,822 non-redundant EST clusters/singletons (DDBJ accession numbers AB591382, AK416867- AK418687) (Table 1). Each of the clusters was assigned a serial identification number in the order of number of clones appearing in the total EST dataset (Table S1). Of the 1,822 clusters, 1,624 contained ORFs encoding predicted proteins no shorter than 50 amino acids, of which 1,194 and 1,173 exhibited significant sequence similarities to protein sequences of the fruit fly Drosophila melanogaster (Flybase ver. 5.42) and the aphid Acyrthosiphon pisum [29], respectively, at the cutoff threshold E-value of P<1e−10 by BLASTP search (Table S1).
Table 1. Summary of EST data sets.
| SymM3 | SymM4B | SymM4 | ApoM3 | ApoM4B | ApoM4 | Total | |
| Number of clones | 1,033 | 1,285 | 1,458 | 917 | 1,111 | 1,120 | 6,924 |
| Mitochondrial DNA | 67 | 49 | 38 | 48 | 43 | 31 | 276 |
| Ribosomal RNA | 195 | 46 | 26 | 65 | 42 | 31 | 405 |
| Burkholderia genes | 0 | 1 | 8 | 0 | 0 | 0 | 9 |
| Analyzed ESTs | 771 | 1,189 | 1,386 | 804 | 1,026 | 1,058 | 6,234 |
| No. of unique clusters | 440 | 367 | 532 | 482 | 505 | 487 | 1,822 |
Gene ontology terms of EST datasets from symbiotic and aposymbiotic insects
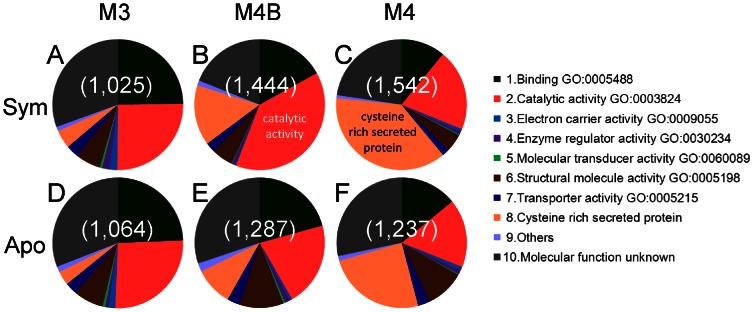
For each of the midgut EST datasets obtained from the M3, M4B and M4 regions of the symbiotic and aposymbiotic insects, the gene clusters were categorized into gene ontology (GO) molecular function terms that had been applied to Drosophila proteins based on Flybase ver. 5.42 (Fig. 2, Table S3). Besides the conventional GO terms, we adopted an additional category under the following criteria. Using the SignalP 4.0 program, we identified 465 genes with putative signal peptides, whose sequences are shown in Table S1. Among them, 97 genes had six or more cysteine residues and exhibited no sequence similarity to Drosophila and Acyrthosiphon genes. We categorized these genes as “cysteine-rich secreted protein” in this study (Fig. 2, Table S3).
Figure 2. Gene Ontology (GO) molecular function terms assigned to the midgut EST data of R. pedestris.
Ratio of number of EST clones representing each GO term per total number of EST clones is shown as each pie graph division. (A–C) Symbiotic insect. (D–F) Aposymbiotic insect. Number of total EST clones is indicated in parentheses. Because one cluster can be associated with more than one GO term, total number of EST clones shown in parentheses may be different from those shown in Table 1. Abbreviations: Sym, symbiotic insect; Apo, aposymbiotic insect; M3, midgut third region; M4: midgut fourth region with crypts; M4B, anterior bulb of midgut fourth section.
For the M3 region, the composition of GO terms of the symbiotic insect was quite similar to that of the aposymbiotic insect (Fig. 2A and D), which probably reflects the fact that the M3 region is not infected with the symbiont. For the M4B and M4 regions where the symbiont is localized, by contrast, the compositions of GO terms were remarkably different between the symbiotic insect and the aposymbiotic insect. In particular, the category “catalytic activity” was identified more frequently in the M4B region of the symbiotic insect than in the M4B region of the aposymbiotic insect (Fig. 2B and E), and the category “cysteine-rich secreted protein” was more represented in the M4 region of the symbiotic insect than in the M4 region of the aposymbiotic insect (Fig. 2C and F).
Dominant ESTs in the midgut cDNA libraries
In the midgut cDNA libraries, 6 genes were highly represented by more than 100 EST clones, and 20 genes were frequently represented by more than 30 EST clones (Table S1). Among them, 9 genes encoded cysteine-rich secreted proteins, 7 genes represented non-cysteine-rich, unknown secreted proteins, 3 genes represented cathepsin L proteases, 3 genes represented ferritin subunit proteins, and the remaining 4 genes encoded cathepsin B, zinc carboxypeptidase proteases, actin and c-type lysozyme, respectively (Table S1).
Symbiosis- and aposymbiosis-specific ESTs in the midgut regions
In the EST datasets, we identified 11 candidate symbiosis-specific genes that were represented by no less than 10 EST clones and detected exclusively in the symbiotic insect (Table 2). These genes exhibited the following patterns: (i) ten genes were preferentially expressed in the M4 and/or M4B regions of the symbiotic insect (except for glyoxal oxidase [Rped-0100]), (ii) six genes preferentially expressed in the M4 region encoded cysteine-rich secreted proteins (except for cathepsin L [Rped-0047] and unknown secreted protein [Rped-0090]), and (iii) two genes preferentially expressed in the M4B region encoded enzymes such as zinc carboxypeptidase [Rped-0023] and gpi-anchor transamidase [Rped-0031] (Table 2). These expression patterns were the main reason for different GO terms composition between symbiotic and aposymbiotic insects (Fig. 2).
Table 2. Symbiosis-associated genes identified in the EST analysis.
| Cluster ID | No. of clones | Annotation | SymM3 | SymM4B | SymM4 | ApoM3 | ApoM4B | ApoM4 | qRT-PCR* 1 |
| Rped-0008 | 62 | Cysteine-rich secreted protein | 1 | 0 | 61 | 0 | 0 | 0 | P<0.05 (M4) |
| Rped-0023 | 33 | Zinc carboxypeptidase | 0 | 32 | 1 | 0 | 0 | 0 | P<0.001 (M4) |
| Rped-0031 | 24 | gpi-anchor transamidase | 0 | 21 | 3 | 0 | 0 | 0 | N.S. |
| Rped-0043 | 19 | Cysteine-rich secreted protein | 0 | 2 | 17 | 0 | 0 | 0 | P<0.05 (M4) |
| Rped-0047 | 18 | CathepsinL | 0 | 0 | 18 | 0 | 0 | 0 | P<0.001 (M4) |
| Rped-0061 | 15 | Cysteine-rich secreted protein | 0 | 0 | 15 | 0 | 0 | 0 | N.S. |
| Rped-0077 | 12 | Cysteine-rich secreted protein | 0 | 0 | 12 | 0 | 0 | 0 | N.S. |
| Rped-0090 | 11 | Unknown secreted protein | 0 | 0 | 11 | 0 | 0 | 0 | P<0.001 (M4) |
| Rped-0095 | 10 | Cysteine-rich secreted protein | 0 | 0 | 10 | 0 | 0 | 0 | N.S. |
| Rped-0100 | 10 | Glyoxal oxidase | 10 | 0 | 0 | 0 | 0 | 0 | P<0.05 (M1,M2,M3) |
| Rped-0106 | 10 | Cysteine-rich secreted protein | 0 | 0 | 10 | 0 | 0 | 0 | P<0.05 (M4) |
1: Statistically significant differences between aposymbiotic and symbiotic insects in each midgut region by quantitative RT-PCR (t test; n = 8, see Figure 3B). N.S., not significant.
We also identified 7 candidate aposymbiosis-specific genes that were represented by no less than 10 EST clones and detected exclusively in the aposymbiotic insect (Table 3). These genes exhibited the patterns that (i) six genes were preferentially expressed in the M4B region (except for cathepsin B protease [Rped-0049]), (ii) two genes encoded defense-related proteins such as c-type lysozyme [Rped-0025] and defensin-like protein [Rped-0033], (iii) these defense-related proteins were expressed not only in the M4B region but also in the M3 region, and (iv) the other genes encoded unknown proteins (Table 3).
Table 3. Aposymbiosis-associated genes identified in the EST analysis.
| Cluster ID | No. of clones | Annotation | SymM3 | SymM4B | SymM4 | ApoM3 | ApoM4B | ApoM4 | qRT-PCR* 1 |
| Rped-0025 | 32 | c-type lysozyme | 0 | 0 | 0 | 5 | 26 | 1 | P<0.05 (M1,M2,M3,M4) |
| Rped-0033 | 23 | Defensin-like protein | 0 | 0 | 0 | 11 | 9 | 3 | P<0.05 (M1,M2,M3,M4) |
| Rped-0049 | 18 | Cathepsin B | 0 | 0 | 0 | 18 | 0 | 0 | N.S. |
| Rped-0053 | 17 | Unknown secreted protein | 0 | 0 | 0 | 0 | 17 | 0 | P<0.05 (M4) |
| Rped-0058 | 16 | Unknown protein | 0 | 0 | 0 | 0 | 14 | 2 | N.S. |
| Rped-0064 | 15 | Unknown protein | 0 | 0 | 0 | 6 | 7 | 2 | N.S. |
| Rped-0070 | 14 | Unknown secreted protein | 0 | 0 | 0 | 0 | 8 | 6 | P<0.001 (M4) |
1: Statistically significant differences between aposymbiotic and symbiotic insects in each midgut region by quantitative RT-PCR (t test; n = 8, see Figure 3B). N.S., not significant.
Expression analysis of symbiosis- and aposymbiosis-associated genes
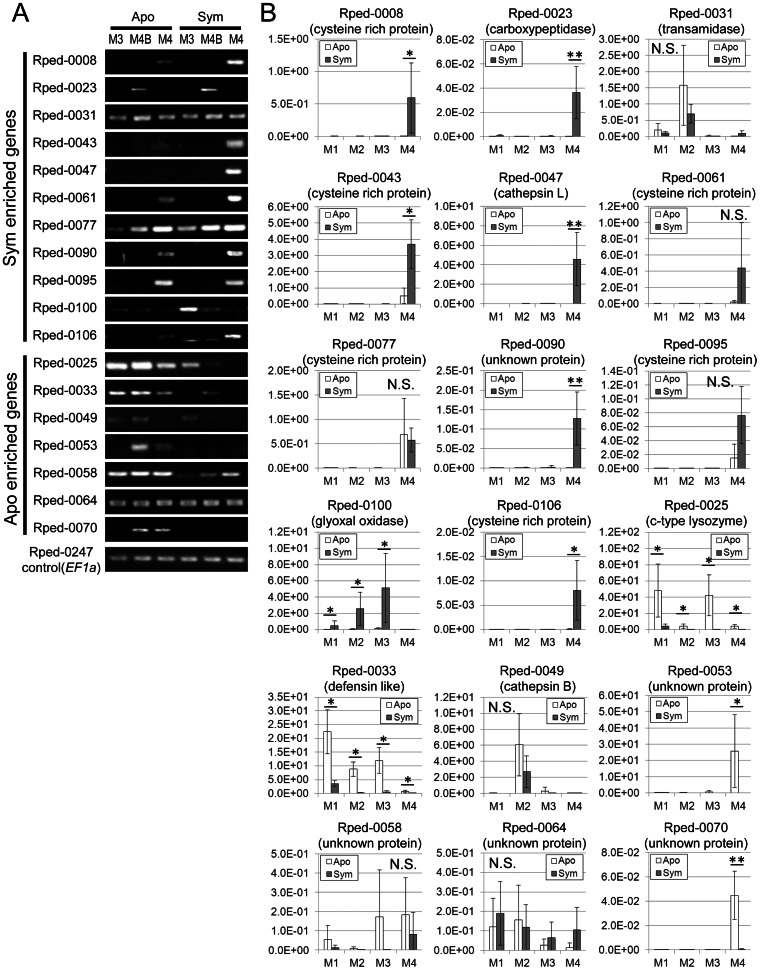
Genes representing 11 symbiosis-specific ESTs and 7 aposymbiosis-specific ESTs were subjected to semi-quantitative RT-PCR of M3, M4B, and M4 regions (Fig. 3A) and real-time quantitative RT-PCR of M1, M2, M3, and M4 (plus M4B) regions of symbiotic and aposymbiotic insects (Fig. 3B).
Figure 3. Relative expression levels of symbiosis- and aposymbiosis-associated gene candidates in R. pedestris.
(A) Semi-quantitative RT-PCR of candidate genes. Abbreviations are the same as in Figure 2. Elongation factor 1 alpha (EF1a) gene (Rped-0247) was used as an internal control. (B) Relative expression levels of candidate genes in four midgut parts, namely M1, M2, M3, and M4 (plus M4B). The expression levels were evaluated by quantitative RT-PCR in terms of each gene cDNA copies per EF1α cDNA copy. Means and standard deviations (n = 8) are shown. Statistically significant differences between aposymbiotic and symbiotic insects in each midgut region are shown by asterisks (t test; *, P<0.05; **, P<0.001). N.S. indicates no significant difference.
Of the 11 candidate symbiosis-associated genes, six genes, namely Rped-0008 (cysteine-rich secreted protein), Rped-0023 (carboxypeptidase), Rped-0043 (cysteine-rich secreted protein), Rped-0047 (cathepsin L protease), Rped-0090 (unknown secreted protein) and Rped-0106 (cysteine-rich secreted protein), exhibited specifically and significantly increased expression in the M4 plus M4B region of symbiotic insects relative to the same region of aposymbiotic individuals, whereas one gene, Rped-0100 (glyoxal oxidase), exhibited significantly higher expression levels in the M1, M2 and M3 regions of symbiotic insects but little expression in the M4 plus M4B region. Rped-0061, Rped-0077 and Rped-0095 (all cysteine-rich secreted proteins) were preferentially expressed in the M4 plus M4B region, but the differences between symbiotic insects and aposymbiotic insects were not statistically significant (Fig. 3B).
Of the 7 candidate aposymbiosis-associated genes, two genes, namely Rped-0025 (c-type lysozyme) and Rped-0033 (defensin-like protein), exhibited consistently and significantly higher expression levels in all the midgut regions of aposymbiotic insects, whereas two genes, Rped-0053 and Rped-0070 (both unknown proteins), were specifically and highly expressed in the M4 plus M4B region of aposymbiotic insects (Fig. 3B).
Aposymbiosis-associated and other lysozyme genes
Diverse lysozyme genes are phylogenetically classified into chicken (c-), goose (g-), invertebrate (i-), bacterial and other types [30]. The lysozyme gene Rped-0025, which was highly expressed in the midgut of aposymbiotic insects but scarcely expressed in the midgut of symbiotic insects (Fig. 3; Table 3), was placed in the c-type lysozyme clade (Fig. S1).
Besides the highly-expressed and aposymbiosis-associated c-type lysozyme gene Rped-0025 (32 ESTs), two lysozyme transcripts, representing a bacterial type lysozyme gene Rped-0028 (28 ESTs) and a c-type lysozyme gene Rped-0069 (14 ESTs), were identified (Fig. S1), although expression of these genes were found in both the symbiotic and aposymbiotic insects (Table S4).
Aposymbiosis-associated defensin-like gene
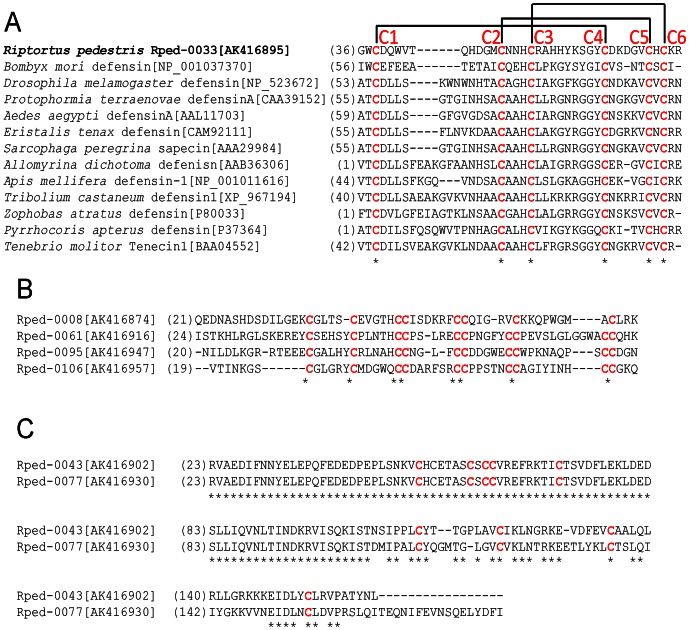
Insect defensins are cationic antimicrobial peptides consisting of 34–46 amino acid residues with molecular masses ranging from 2 to 6 kDa, in which the positions of six cysteine residues forming three intramolecular disulfide bridges are conserved and essential for expressing antimicrobial activities [31], [32]. The defensin-like gene Rped-0033, which was highly expressed in the midgut of aposymbiotic insects but scarcely expressed in the midgut of symbiotic insects (Fig. 3; Table 3), retained six cysteine residues but the other regions were not similar to conventional defensins (Fig. 4A).
Figure 4. Cysteine-rich protein genes identified in the EST data of R. pedestris.
(A) Amino acid sequence of defensin-like gene of R. pedestris compared with sequences of defensins from other insects. (B) Amino acid sequences of four cysteine-rich secretion proteins of short type. (C) Amino acid sequences of two cysteine-rich secretion proteins of long type. Conserved cysteine residues are highlighted in red. Estimated disulphide bridges are shown by solid line in (A). Accession numbers are in brackets, and first amino acid positions are in parentheses.
Besides the highly-expressed and aposymbiosis-associated defensin-like gene Rped-0033 (23 ESTs), no other defensin-like gene was detected in the cDNA libraries, although multiple defensin-like genes have been identified in other heteropteran bugs [33], [34].
Symbiosis-associated and other cysteine-rich secreted protein genes
Recent accumulation of genomic and transcriptomic data revealed that cysteine-rich secreted proteins, which are structurally similar to defensins in that they are cationic secreted peptides with 6–8 conserved cysteine residues that are predicted to form intramolecular disulfide bridges, are ubiquitously found across diverse organisms [35]. In the cDNA libraries of R. pedestris, we identified several cysteine-rich secreted protein genes whose expression patterns were strongly associated with the midgut region M4 of symbiotic insects (Fig. 3; Table 2). Some of them encoded relatively small peptides with around 70–90 residues (Fig. 4B), while others encoded larger peptides with about 150–170 residues (Fig. 4C).
In total, as many as 97 cysteine-rich secreted protein genes (including the symbiosis-associated genes listed in Table 2) were identified in the cDNA libraries, which accounted for 5.3% (97/1,822) of the genes and 21.0% (1,307/6,234) of the ESTs identified in the cDNA libraries. Notably, even when their expression was not associated with symbiotic status, many, if not all, of them exhibited preferential expression in the midgut M4 and/or M4B regions (ex. Rped-0001, Rped-0003, Rped-0009, Rped-0017, Rped-0026, Rped-0035, Rped-0037, Rped-0039, Rped-0048, Rped-0056, and others) (Table S5). These genes exhibited no significant sequence similarities to cysteine-rich secreted proteins of other organisms deposited in the public DNA and protein databases.
Symbiosis-associated and other cathepsin protease genes
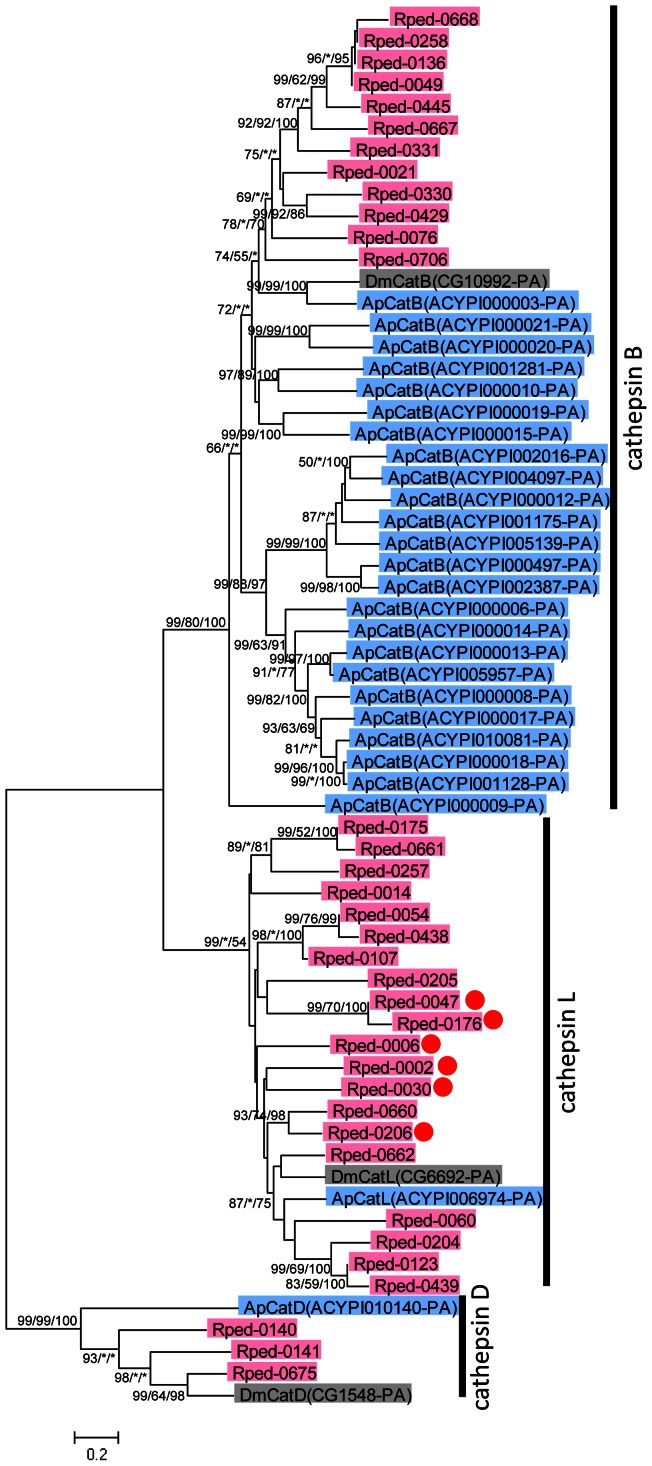
Several protease genes, namely Rped-0023 encoding zinc carboxypeptidase and Rped-0047 encoding cathepsin L protease, were highly and specifically expressed in the midgut region M4 of symbiotic insects (Fig. 3; Table 2). In the cDNA libraries of R. pedestris, notably, we identified a large number of cathepsin protease genes: 21 cathepsin L genes, 13 cathepsin B genes, and 3 cathepsin D genes (Table S6). In the genome of the fruit fly D. melanogaster, cathepsin L, cathepsin B and cathepsin D genes are all single-copied [36]. On the other hand, in the genome of the aphid A. pisum, cathepsin B genes are amplified to 27 copies via repeated gene duplications, whereas cathepsin L and cathepsin D genes are single-copied [29], [37]. Molecular phylogenetic analysis showed that (i) cathepsin B genes, cathepsin L genes and cathepsin D genes of R. pedestris constitute distinct monophyletic groups in the cathepsin phylogeny, respectively, (ii) cathepsin B genes of R. pedestris formed a cluster distinct from the cluster of cathepsin B genes of A. pisum, (iii) thus, cathepsin genes were probably amplified in the stinkbug lineage and in the aphid lineage independently, and (iv) cathepsin L genes were also amplified in the stinkbug lineage (Fig. 5).
Figure 5. Diversity of cathepsin protease genes identified in the EST data of R. pedestris.
A neighbor-joining phylogeny inferred from 428 aligned amino acid sites is shown, while maximum likelihood and Bayesian phylogenies exhibited substantially the same topologies. On each node, statistical support values are indicated in the order of [bootstrap value of neighbor-joining]/[bootstrap value of maximum likelihood]/[posterior probability of Bayesian]. Asterisks indicate support values lower than 50%. Clades of cathesin B, cathepsin L and cathepsin D are indicated on the right side. Genes of R. pedestris, A. pisum and D. melanogaster are colored in red, blue and gray, respectively. Red circles indicate genes preferentially expressed in symbiotic insects (EST clones more than tenfold in symbiotic insect and no less than 5 EST clones).
Symbiosis-associated expression and activity of cathepsin L proteases
In the cDNA libraries of R. pedestris, cathepsin L genes accounted for as much as 7.5% of the total ESTs (466/6,234), whereas cathepsin B genes and cathepsin D genes represented only 1.4% (90/6,234) and 0.2% (15/6,234), respectively. In particular, the cathepsin L genes Rped-0002 (194 ESTs), Rped-0006 (101 ESTs) and Rped-0030 (24 ESTs) were notable in that they are not only highly represented in the cDNA libraries (319/6,234 = 5.1%) but also exclusively expressed in the midgut M4B region of symbiotic insects (Table S6). Consequently, most of the cathepsin L ESTs were represented in the cDNA library of the M4B region of the symbiotic insect (Fig. 6A).
Figure 6. Expression and activity of cathepsin L proteases in midgut regions of R. pedestris.
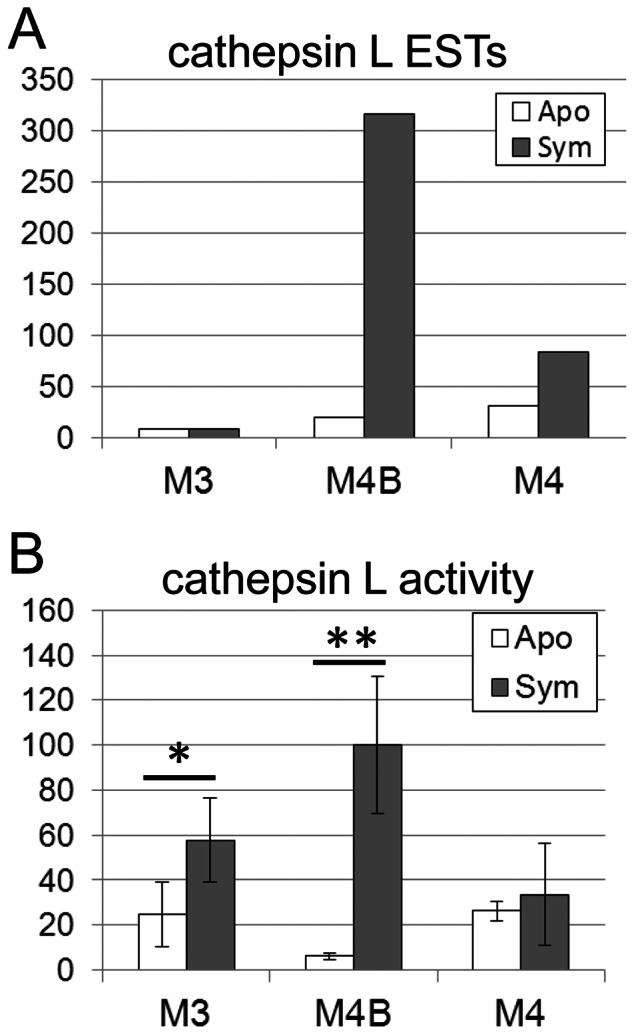
(A) Total number of EST clones representing cathepsin L genes in the midgut EST libraries. (B) Relative enzymatic activities of cathepsin L proteases in extracts of the midgut regions. Means and standard deviations (n = 10) are shown. Statistically significant differences are indicated by asterisks (t test; *, P<0.05; **, P<0.001). Abbreviations are as in Figure 2.
By making use of the synthetic fluorescent substrate, Z-Val-Val-Arg-MCA, that is specifically hydrolyzed by cathepsin L-like proteases [38], we enzymatically measured cathepsin L activities in homogenates of dissected M3, M4B and M4 regions of symbiotic and aposymbiotic insects. In agreement with the EST results (Fig. 6A), the highest cathepsin L activities were found in the M4B region of symbiotic insects, which were significantly higher than the activities in the M4B region of aposymbiotic insects (Fig. 6B). Meanwhile, being unexpected from the EST results (Fig. 6A), considerable cathepsin L activities were also detected in the M3 region of both symbiotic and aposymbiotic insects, and the activities in symbiotic insects were significantly higher than the activities in aposymbiotic insects (Fig. 6B).
Symbiosis- and aposymbiosis-associated secreted protein genes of unknown function
Rped-0053 and Rped-0070 were specifically expressed in the M4 region of aposymbiotic insects (Table 3; Fig. 3), whereas Rped-0090 was specifically expressed in the M4 region of symbiotic insects (Table 2; Fig. 3). These genes were with signal peptide sequences at their 5′ end, and exhibited no sequence similarity to known proteins in the public DNA and protein databases. Instead of being cysteine-rich, Rped-0053, Rped-0070 and Rped-0090 were lysine-, serine- and leucine-rich proteins, respectively (Table S1).
Discussion
In this study, we constructed EST libraries of symbiotic and non-symbiotic regions of the midgut dissected from symbiotic and aposymbiotic insects of R. pedestris, and the EST data revealed a number of intriguing candidate genes whose expression patterns are correlated to symbiosis/aposymbiosis with the Burkholderia gut symbiont. The symbiosis-related transcriptomic data provide valuable basic information as well as initial clues to the molecular mechanisms underlying the host-symbiont interactions. Hereafter, we discuss potential biological roles of the symbiosis- and aposymbiosis-associated genes of R. pedestris identified in this study. Needless to say, the arguments based on the EST data and previous relevant literatures are speculative, but they will provide working hypotheses directing toward future experimental studies.
Identification of many cysteine-rich secretion protein genes: candidate effector molecules involved in host-symbiont interactions
Identification of 97 cysteine-rich secretion protein genes, many of which are preferentially expressed in the symbiotic midgut regions and some of which are expressed in a symbiosis-associated manner, comprises the most interesting finding in this study, on the ground that recent studies have highlighted biological importance of cysteine-rich secreted proteins in plant and insect endosymbiotic systems. In the legume-Rhizobium nitrogen-fixing symbiosis, many cysteine-rich secreted protein genes are preferentially expressed in root nodules, and at least some of them exhibit antimicrobial activities in vitro and induce irreversible differentiation of the symbiont cells into bacteroids in planta [19], [39]. In the aphid-Buchnera nutritional symbiosis, a number of cysteine-rich secreted protein genes are expressed in a bacteriocyte-specific manner, although their biological roles are elusive [7]. Furthermore, recent accumulation of genomic and transcriptomic data has revealed abundant occurrences of cysteine-rich secreted proteins in other organisms including Arabidopsis thaliana [40] and other plants [41], and also corals [42]. These relatively short peptides are structurally related to antimicrobial peptides like defensins in that they are cysteine-rich and cationic, and many of them are thought to have antimicrobial activities [43]. Identification of many cysteine-rich secretion protein genes expressed in the gut symbiotic organ of R. pedestris highlights an unexpected molecular commonality among endocellular and extracellular symbiotic associations in plants and insects.
Biological functions of the cysteine-rich proteins in the Riptortus-Burkholderia gut symbiosis are currently unknown. In the legume-Rhizobium symbiosis, the nodule-specific cysteine-rich proteins target the bacterial membrane and cytosol within the symbiosome, and act as plant effectors to direct the bacteroids into a terminally differentiated state [19], [44]. It is conceivable, although speculative, that similarly, the cysteine-rich proteins may be secreted from the intestinal epithelial cells into the gut lumen, and act on proliferation and/or physiology of the symbiont cells. Experimental studies in vivo (suppression of the cysteine-rich proteins by RNA interference) and in vitro (incubation of the symbiont cells with the cysteine-rich proteins) are to be conducted to verify this hypothesis.
Aposymbiosis-associated expression of lysozyme and defensin-like genes: possible biological role in the context of symbiosis
Lysozymes are the enzymes that destroy bacterial cell walls by degrading peptideglycans, thereby showing antibacterial activities and playing important roles in defense against bacterial infections [45]. Conventionally, lysozymes have been, together with an array of antimicrobial peptides, regarded as inducible bactericidal proteins that are highly expressed in response to microbial infections and accumulate in the insect hemolymph [31], [46]. However, the lysozyme gene Rped-0025 and the defensin-like gene Rped-0033 of R. pedestris were highly expressed in aposymbiotic insects but scarcely expressed in symbiotic insects (Fig. 3; Table 3), suggesting a unique regulation of these defense-related genes in the context of host-symbiont interactions. It should be noted that, recently, a variety of correlations between lysozyme gene expression and symbiont infection have been reported in other symbiotic systems: expression of a c-type lysozyme gene is down-regulated in the ovary of Wolbachia-infected parasitic wasp Asobara tabida [16]; expression of an i-type lysozyme gene is down-regulated in Wolbachia-infected pill bug Armadillidium vulgare [15]; in the grain weevils Sitophilus zeamais and S. oryzae, expression of an i-type lysozyme gene is down-regulated in the bacteriocytes harboring Sodalis-allied symbiotic bacteria endocellularly [10], [11]; and in the pea aphid Acyrthosiphon pisum, strikingly, two i-type lysozyme genes are specifically expressed in the bacteriocytes harboring Buchnera, which represent the most abundant transcripts in the symbiotic cells [8]. Meanwhile, in the Sitophilus weevils, permanent infection of bacteriocytes with Sodalis-allied primary endosymbiont leads to up-regulation of an antimicrobial peptide, coleoptericin-A, whose function is to restrict the endosymbiont infection to the bacteriocytes [47]. Coleoptericin-A contains no cysteine residue, which is different from the defensin-like proteins [32].
Biological function of the aposymbiosis-associated lysozyme and defensin-like gene in the midgut of R. pedestris is currently elusive. In saprophagous insects like D. melanogaster, a part of amplified lysozyme genes are preferentially expressed in the midgut [48], [49], which are suggested to function for digesting bacteria-rich fermented foods [46]. Digestive roles of gut-associated lysozyme genes have also been suggested for the house fly Musca domestica [50], mosquitoes [51] and termites [52]. However, it seems unlikely that the midgut lysozyme of R. pedestris plays a digestive role because (i) the food of the stinkbug, plant sap, is not bacteria-rich, (ii) food digestion must be necessary for both symbiotic and aposymbiotic insects, and (iii) if the symbiotic bacteria in the midgut are digested and utilized, suppressed expression of the lysozyme gene in symbiotic insects does not make sense.
In holometabolous insects, up-regulated immune functions including lysozyme production have been detected in the midgut of mature larvae before pupation, which are presumably vulnerable to bacterial infections during the radical developmental reorganization of metamorphosis [49], [53]–[55]. In several hemimetabolous insects and ticks, up-regulated lysozyme expression is observed immediately after molting [56], [57]. In the midgut epithelial cells of the tobacco hornworm Manduca sexta, lysozyme granules are stored and released into the gut lumen just before metamorphosis [53]. Here, these gut lysozymes may have a defensive role in the course of insect development, particularly against potentially virulent gut microbes at the immune-compromised metamorphosis and molting stages [46].
Considering the similar expression patterns of lysozyme gene and defensin-like gene (Fig. 3B), we suggest the possibility that the lysozyme and defensin-like gene product may be involved in suppression of improper bacterial infections in the midgut. In this context, it may be relevant that synergistic bactericidal effects of lysozyme and other antimicrobial peptides including defensin have been reported [58], [59]. Whether expression of these and other defense-related genes are induced by infection with non-symbiotic bacteria in R. pedestris is of interest and deserves future studies.
Over-expression of cathepsin L protease genes in the midgut M4B region: candidate molecules involved in regulation over symbiont population
Cathepsins are lysosomal acidic proteases ubiquitously found in animals and other organisms, which are classified into approximately a dozen families, like cathepsin A, cathepsin B, cathepsin C and others, based on their structure, catalytic mechanism and substrate specificity [60]. While many cathepsin proteases are thought to be mainly involved in intracellular protein turnover, remarkable cathepsin protease activities have been detected and characterized in the midgut of diverse hemiptaran, lepidopteran, coleopteran and other groups of insects, where they presumably function as digestive enzymes [61]–[64].
In this study, we found that several cathepsin L genes are highly and preferentially expressed in the midgut M4B region of symbiotic insects (Fig. 6A; Table S7), which was also confirmed by measuring cathepsin L activities in the midgut region (Fig. 6B). These results suggest that these cathepsin L genes play some biological roles in the M4B region of symbiotic insects. In the midgut of R. pedestris, the voluminous M4 region bears a number of crypts whose cavity is full of the symbiont cells, whereas the tubular M4B region is, although directly connected to the M4 region, devoid of crypts (Fig. 1) and exhibits much weaker symbiont signals than the M4 region [25]. Over-expression of cathepsin L protease genes in the M4B region may function to make substrates accessible to the symbiotic bacteria. Alternatively, the cathepsin L proteases in the M4B region may function to digest the symbiont cells overflowed from the adjacent M4 region, by which the host insect may control the symbiont population and/or utilize the symbiont-derived nutritional resources. Whether or not RNA interference of these cathepsin L genes results in accumulation of the symbiont cells in the M4B region will be a critical test for the hypothesis, which should be addressed in future studies.
In the aphid bacteriocytes, lysosomal activities are suggested to play important roles in controlling the obligate endosymbiont Buchnera, wherein lysosomes fuse to host-derived symbiosomes and degrade the symbiont cells therein [65]. Here it should be noted that not only lysosomal cathepsin proteases but also lysozymes and antimicrobial peptides are stored in endocellular granules [46], [60], which can be delivered to bacterial targets through membrane trafficking mechanisms: to endocellular bacteria via fusion to the symbiosome, and to extracellular bacteria via fusion to the cell membrane [53], [65]. In this context, we suggest that involvement of membrane trafficking for delivering effector molecules may underlie some molecular aspects commonly found among various endocellular and extracellular host-symbiont associations.
Highly expressed ferritin genes in the midgut
Although neither related to the symbiotic insect nor to the M4 and M4B regions, it is notable that several ferritin subunit genes are highly expressed in the midgut of R. pedestris (Table S1). Ferritin is a ubiquitous globular protein of 450 kDa consisting of 24 subunits, which stores iron and releases it in a controlled fashion [66]. What biological roles the ferritin genes play in the midgut of R. pedestris is totally unknown, but, meaningfully, previous studies on diverse insects, crustaceans and nematodes reported that Wolbachia infections influence iron metabolism of their host organisms, affect host's fitness components in an iron-dependent manner, and up-/down-regulate ferritin gene expression [15], [16], [18], [67], [68].
Genes related to innate immunity
The genome project of the aphid A. pisum revealed its peculiar innate immune system: while most insects possess three major immune gene cascades, the Toll pathway, the IMD pathway, and the JAK/STAT pathway [69], the aphid genome lacks IMD pathway genes, many antimicrobial peptides and c-type lysozyme [29], [70]. R. pedestris and A. pisum belong to the same insect order Hemiptera, but our EST analyses identified c-type lysozyme gene, defensin-like gene, and relish gene (Rped-1145, 1 clone) (Table S1) which is a key transcription factor in the IMD pathway [71], in R. pedestris. Hence, it is suggested that the lack of IMD pathway genes has evolved in the aphid lineage specifically.
Effects of symbiosis on morphogenesis of the midgut symbiotic organ
Finally, we note that in the Riptortus-Burkholderia gut symbiosis, morphogenesis of the host symbiotic organ is remarkably affected by the symbiont infection: in the symbiotic insects, the midgut M4 and M4B regions become larger than those in aposymbiotic insects, while the midgut M3 region was smaller (Fig. 1). Enlargement of the M4 and M4B regions in symbiotic insects should reflect induction/suppression of many genes and functions involved in symbiosis, while enlargement of the M3 region in aposymbiotic insects may, although speculative, be due to resource allocation between the adjacent midgut regions. Because the morphological differences between the aposymbiotic midgut and the symbiotic midgut must have established before the fifth nymphal instar, gene expression for gut morphogenesis should be different at earlier developmental stages. The detailed observation on effect of symbiosis on midgut morphogenesis deserves future studies. Symbiont-induced morphogenesis of host symbiotic organ has been well documented in legume-Rhizobium nitrogen-fixing symbiosis [72], [73] and squid-Vibrio luminescent symbiosis [74], [75].
Conclusion and perspective
In conclusion, using a conventional EST approach with relatively small amount of sequence data, we successfully identified some intriguing host genes that exhibit symbiosis-associated expression patterns in the Riptortus-Burkholderia gut symbiotic system. Recently, high-throughput next generation sequencing technologies have become readily available [76], which will enable much broader and deeper understanding of the host-symbiont interactions in a genomic/transcriptomic perspective. In R. pedestris, infection with the Burkholderia symbiont establishes at the second instar stage via oral ingestion [27]. Therefore, the EST analyses of fifth instar nymphs in this study must have unveiled consequences rather than processes of symbiotic influence on the host gene expression. In this context, transcriptomic comparisons between symbiotic and aposymbiotic insects at the post-infection, second-third instar stages are of interest. In R. pedestris and other heteropteran bugs, RNA interference generally works effectively [26], which provides a straightforward approach to functional understanding of the symbiosis-associated host insect genes. Future studies in these lines will shed light on the commonality and the diversity among various insect-microbe symbiotic systems ranging from bacteriome-specific obligate associations through systemic facultative associations to gut extracellular associations.
Materials and Methods
Insects and symbiotic bacteria
R. pedestris was collected from fields of the soybean Glycine max at Tsukuba, Ibaraki, Japan, and maintained in the laboratory. The locations are not privately-owned or protected in any way, and no specific permits were required. The field studies did not involve endangered or protected species. An isofemale line, TKS-1, was established and used for experiments. The insects were reared on soybean seeds and distilled water containing 0.05% ascorbic acid (DWA) at 25°C under a long-day regimen of 16 h light and 8 h dark. The Burkholderia symbiont strain RPE75 was used in this study, which is a spontaneous rifampin-resistant mutant derived from the strain RPE64 originally isolated from the midgut crypts of R. pedestris [27]. The symbiont was cultured with YG medium (5 g/l yeast extract, 4 g/l glucose, 1 g/l of NaCl) containing 10 mg/l of rifampicin (YG-RIF) at 150 rpm in broth or on 1.5% ager plates at 26°C.
Oral administration of cultured symbiont
Hatchlings of R. pedestris were divided into two experimental groups: one was symbiotic (infected) group and the other was aposymbiotic (uninfected) group. In the aposymbiotic group, the nymphs were reared with symbiont-free DWA from hatching to fifth instar. In the symbiotic group, the nymphs were orally administrated with cultured Burkholderia symbiont as described [27]. The symbiont strain RPE75 was grown to an early log phase in YG-RIF medium on a gyratory shaker (150 rpm) at 26°C. Colony forming units (CFU) were estimated by plating the cultured media on YG-RIF agar plates. The symbiont cells were harvested by centrifugation, resuspended in DWA, and adjusted to 107 CFU/ml. Each nymph was fed with the symbiont-containing water during the first two days of second instar stage. After the symbiont treatment, the water was replaced by symbiont-free DWA, and insects were reared until fifth instar.
Construction of cDNA library and sequencing of EST clones
In order to construct cDNA libraries, a symbiotic insect and an aposymbiotic insect at the fifth instar were collected and dissected three days after molting. Three parts of the midgut (M3, M4B and M4) were dissected in phosphate buffered saline (PBS; 137 mM NaCl, 8.10 mM Na2HPO4, 2.68 mM KCl and 1.47 mM KH2PO4, pH 7.4), and total RNAs were immediately extracted from the tissues by using RNAiso plus (Takara), which were subjected to construction of cDNA libraries using SMART™ cDNA Library Construction Kit (Clontech) and Gigapack III Gold Packaging Extract (Agilent Technologies). The cDNAs ligated to λ phage vector were transformed with Escherichia coli BM25.8 (Clontech), in which the λ DNA was converted into a plasmid. The plasmids were amplified using Illustra Templiphi Amplification Kit (GE Healthcare) from a single colony of the E. coli, and sequenced using an ABI prism 3130 Genetic Analyzer (Applied Biosystems, Foster City, USA). All EST sequences have been deposited into the DDBJ database with accession numbers HX275191-HX282114. SignalP 4.0 [http://www.cbs.dtu.dk/services/SignalP/] was used for signal peptide prediction.
Quantitative RT-PCR
Quantitative reverse transcription PCR (RT-PCR) was performed to evaluate the expression levels of candidate symbiosis-associated genes of R. pedestris. From each fifth instar nymph, four midgut parts (M1, M2, M3 and M4 [M4 + M4B]) were dissected in PBS, and total RNA was extracted by using RNAiso plus. The RNA samples were reverse transcribed with random primers (N6) and first-strand cDNA synthesis kit (GE Healthcare), and subjected to real-time quantitative PCR using a Stratagene Mx3000P (Stratagene, La Jolla, CA). Each of the PCR mixtures consisted of 2 µl of 10×TaqGold buffer (Applied Biosystems), 1.2 µl of 25 mM MgCl2, 2 µl of nucleotide mixture solution (2 mM each of dATP, dTTP, dGTP and dCTP), 0.2 µl of SYBR Green I (1/1,000-diluted solution) (Molecular Probes), 0.3 µl of primer mixture solution (10 µM each of forward and reverse primers), 0.1 µl of AmpliTaqGold DNA polymerase (Applied Biosystems), 8.9 µl of distilled water, 0.8 µl of dimethyl sulfoxide, and 4 µl of DNA sample solution. The PCR temperature profile was 94°C for 1 min, 35 cycles of 94°C for 1 min, 53°C for 1.5 min and 72°C for 1.5 min, followed by 72°C for 7 min. The primers are listed in Table S7. We used the standard curve method to calculate relative gene expression levels and used elongation factor 1 alpha (EF1a) gene of R. pedestris (accession number AB591382) as an internal control gene. For semi-quantitative RT-PCR, the cDNA samples were adjusted to the same concentration of EF1a cDNA copies using a Stratagene Mx3000P, and subjected to PCR amplification with the same primers.
Assay of cathepsin L protease activity
Measurement of cathepsin L protease activity was performed as described [77]. Three parts of intact midgut (M3, M4B and M4) were dissected from symbiotic and aposymbiotic fifth instar nymphs, and individually homogenized in a lysis buffer (20 mM acetate buffer [pH 4.0], 50 mM NaCl, 5 mM EDTA, 5 mM 2-mercaptoethanol, 0.5% [vol/vol] Nonidet P-40). After centrifugation, 50 µl of the supernatant was combined with 445 µl of reaction buffer (0.1 M citrate buffer [pH 6.0], 75 mM NaCl, 5 mM EDTA, 2 mM cysteine), preincubated at 27°C for 5 min, and then mixed with 5 µl of 10 mM Z-Val-Val-Arg-MCA, a synthetic substrate for cathepsins L and S (Peptide Institute). After incubation at 27°C for 15 min, the reaction was stopped by adding 750 µl of 17% acetic acid. The protease activity was measured by a spectrofluorophotometer (RF-5300PC, Shimadzu) with excitation and emission wavelengths of 380 and 460 nm, respectively. As a negative control, samples were heat-inactivated at 95°C for 2 min prior to the enzymatic reaction.
Molecular phylogenetic analysis
Amino acid sequences were aligned using Clustal_X [78]. Molecular phylogenetic analyses were conducted by three methods, neighbor-joining method using MEGA5 [79], maximum likelihood method using MEGA5 [79], and Bayesian with MrBayes v3.1.2 [80]. Bootstrap values for neighbor-joining and maximum likelihood phylogenies were obtained by 1000 resamplings. In total 7,500 trees were generated for each Bayesian analysis (ngen = 1,000,000, samplefreq = 100, burn in = 2,500).
Supporting Information
Molecular phylogenetic analysis of lysozyme genes. A neighbor-joining phylogeny inferred from 1,380 aligned amino acid sites is shown, while maximum likelihood and Bayesian phylogenies exhibited substantially the same topologies. On each node, statistical support values are indicated in the order of [bootstrap value of neighbor-joining]/[bootstrap value of maximum likelihood]/[posterior probability of Bayesian]. Asterisks indicate support values lower than 50%. Red boxes indicate the R. pedestris genes.
(TIF)
List of nonredundant EST clusters obtained from midgut cDNA libraries of R. pedestris .
(XLS)
List of EST clusters from R. pedestris representing either putative isoforms or premature transcripts.
(XLS)
Assignment of Gene Ontology (GO) molecular function terms to the midgut EST data sets of R. pedestris .
(XLS)
List of lysozyme genes.
(XLS)
List of cysteine-rich secreted protein genes.
(XLS)
List of cathepsin protease genes.
(XLS)
Primer sets for quantitative RT-PCR.
(XLS)
Funding Statement
This study was funded by the Program for Promotion of Basic and Applied Researches for Innovations in Bio-oriented Industry (BRAIN), the Global Research Laboratory (GRL) program, and the Japan Society for the Promotion of Science (JSPS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Moran NA, McCutcheon JP, Nakabachi A (2008) Genomics and evolution of heritable bacterial symbionts. Annu Rev Genet 42: 165–190. [DOI] [PubMed] [Google Scholar]
- 2. Oliver KM, Degnan PH, Burke GR, Moran NA (2010) Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu Rev Entomol 55: 247–266. [DOI] [PubMed] [Google Scholar]
- 3.Buchner P (1965) Endosymbiosis of animals with plant microorganisms. New York, NY.: Interscience.
- 4. Werren JH, Baldo L, Clark ME (2008) Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol 6: 741–751. [DOI] [PubMed] [Google Scholar]
- 5.Bourtzis K, Miller TA (2003) Insect Symbiosis. Boca Raton, FL: CRC.
- 6. Douglas AE (1989) Mycetocyte symbiosis in insects. Biol Rev Camb Philos Soc 64: 409–434. [DOI] [PubMed] [Google Scholar]
- 7. Shigenobu S, Stern DL (2013) Aphids evolved novel secreted proteins for symbiosis with bacterial endosymbiont. Proc Biol Sci 280: 20121952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nakabachi A, Shigenobu S, Sakazume N, Shiraki T, Hayashizaki Y, et al. (2005) Transcriptome analysis of the aphid bacteriocyte, the symbiotic host cell that harbors an endocellular mutualistic bacterium, Buchnera . Proc Natl Acad Sci U S A 102: 5477–5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hansen AK, Moran NA (2011) Aphid genome expression reveals host-symbiont cooperation in the production of amino acids. Proc Natl Acad Sci U S A 108: 2849–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anselme C, Perez-Brocal V, Vallier A, Vincent-Monegat C, Charif D, et al.. (2008) Identification of the Weevil immune genes and their expression in the bacteriome tissue. BMC Biol 6. [DOI] [PMC free article] [PubMed]
- 11.Vigneron A, Charif D, Vincent-Monegat C, Vallier A, Gavory F, et al. (2012) Host gene response to endosymbiont and pathogen in the cereal weevil Sitophilus oryzae. BMC Microbiol 12. [DOI] [PMC free article] [PubMed]
- 12. Moriyama M, Koga R, Hosokawa T, Nikoh N, Futahashi R, et al. (2012) Comparative transcriptomics of the bacteriome and the spermalege of the bedbug Cimex lectularius (Hemiptera: Cimicidae). Appl Entomol Zool 47: 233–243. [Google Scholar]
- 13. Nakamura Y, Gotoh T, Imanishi S, Mita K, Kurtti TJ, et al. (2011) Differentially expressed genes in silkworm cell cultures in response to infection by Wolbachia and Cardinium endosymbionts. Insect Mol Biol 20: 279–289. [DOI] [PubMed] [Google Scholar]
- 14. Burke GR, Moran NA (2011) Responses of the pea aphid transcriptome to infection by facultative symbionts. Insect Mol Biol 20: 357–365. [DOI] [PubMed] [Google Scholar]
- 15. Chevalier F, Herbiniere-Gaboreau J, Charif D, Mitta G, Gavory F, et al. (2012) Feminizing Wolbachia: a transcriptomics approach with insights on the immune response genes in Armadillidium vulgare . BMC Microbiol 12 Suppl 1S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kremer N, Charif D, Henri H, Gavory F, Wincker P, et al. (2012) Influence of Wolbachia on host gene expression in an obligatory symbiosis. BMC Microbiol 12 Suppl 1S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xi ZY, Gavotte L, Xie Y, Dobson SL (2008) Genome-wide analysis of the interaction between the endosymbiotic bacterium Wolbachia and its Drosophila host. BMC Genomics 9. [DOI] [PMC free article] [PubMed]
- 18. Kremer N, Voronin D, Charif D, Mavingui P, Mollereau B, et al. (2009) Wolbachia interferes with ferritin expression and iron metabolism in insects. PLoS Pathog 5: e1000630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van de Velde W, Zehirov G, Szatmari A, Debreczeny M, Ishihara H, et al. (2010) Plant peptides govern terminal differentiation of bacteria in symbiosis. Science 327: 1122–1126. [DOI] [PubMed] [Google Scholar]
- 20.Schuh RT, Slater JA (1995) True bugs of the world (Hemiptera: Heteroptera). New York: Cornell University Press.
- 21.Kikuchi Y, Hosokawa T, Fukatsu T (2008) Diversity of bacterial symbiosis in stinkbugs. In: Dijk TV, editor. Microbial ecology research trends. New York: Nova Science Publishers, Inc,.
- 22. Glasgow H (1914) The gastric caeca and the caecal bacteria of the Heteroptera. Biol Bull 3: 101–171. [Google Scholar]
- 23. Kikuchi Y, Meng XY, Fukatsu T (2005) Gut symbiotic bacteria of the genus Burkholderia in the broad-headed bugs Riptortus clavatus and Leptocorisa chinensis (Heteroptera: Alydidae). Appl Environ Microbiol 71: 4035–4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kikuchi Y, Hosokawa T, Fukatsu T (2007) Insect-microbe mutualism without vertical transmission: a stinkbug acquires a beneficial gut symbiont from the environment every generation. Appl Environ Microbiol 73: 4308–4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kikuchi Y, Hayatsu M, Hosokawa T, Nagayama A, Tago K, et al. (2012) Symbiont-mediated insecticide resistance. Proc Natl Acad Sci U S A 109: 8618–8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Futahashi R, Tanaka K, Matsuura Y, Tanahashi M, Kikuchi Y, et al. (2011) Laccase2 is required for cuticular pigmentation in stinkbugs. Insect Biochem Mol Biol 41: 191–196. [DOI] [PubMed] [Google Scholar]
- 27. Kikuchi Y, Hosokawa T, Fukatsu T (2011) Specific developmental window for establishment of an insect-microbe gut symbiosis. Appl Environ Microbiol 77: 4075–4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goodchild AJP (1963) Studies on the functional anatomy of the intestines of Heteroptera. Proc Zool Soc London 141: 851–910. [Google Scholar]
- 29. International Aphid Genomics Consortium (2010) Genome sequence of the pea aphid Acyrthosiphon pisum . PLoS Biol 8: e1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bachali S, Jager M, Hassanin A, Schoentgen F, Jolles P, et al. (2002) Phylogenetic analysis of invertebrate lysozymes and the evolution of lysozyme function. J Mol Evol 54: 652–664. [DOI] [PubMed] [Google Scholar]
- 31. Hoffmann JA, Hetru C (1992) Insect defensins: inducible antibacterial peptides. Immunol Today 13: 411–415. [DOI] [PubMed] [Google Scholar]
- 32. Bulet P, Hetru C, Dimarcq JL, Hoffmann D (1999) Antimicrobial peptides in insects; structure and function. Dev Comp Immunol 23: 329–344. [DOI] [PubMed] [Google Scholar]
- 33. Araujo CA, Waniek PJ, Stock P, Mayer C, Jansen AM, et al. (2006) Sequence characterization and expression patterns of defensin and lysozyme encoding genes from the gut of the reduviid bug Triatoma brasiliensis . Insect Biochem Mol Biol 36: 547–560. [DOI] [PubMed] [Google Scholar]
- 34. Lopez L, Morales G, Ursic R, Wolff M, Lowenberger C (2003) Isolation and characterization of a novel insect defensin from Rhodnius prolixus, a vector of Chagas disease. Insect Biochem Mol Biol 33: 439–447. [DOI] [PubMed] [Google Scholar]
- 35. Maroti G, Kereszt A, Kondorosi E, Mergaert P (2011) Natural roles of antimicrobial peptides in microbes, plants and animals. Res Microbiol 162: 363–374. [DOI] [PubMed] [Google Scholar]
- 36. Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, et al. (2000) The genome sequence of Drosophila melanogaster . Science 287: 2185–2195. [DOI] [PubMed] [Google Scholar]
- 37. Rispe C, Kutsukake M, Doublet V, Hudaverdian S, Legeai F, et al. (2008) Large gene family expansion and variable selective pressures for cathepsin B in aphids. Mol Biol Evol 25: 5–17. [DOI] [PubMed] [Google Scholar]
- 38. Barrett AJ, Kirschke H (1981) Cathepsin-B, Cathepsin-H, and Cathepsin-L. Methods in Enzymology 80: 535–561. [DOI] [PubMed] [Google Scholar]
- 39. Mergaert P, Nikovics K, Kelemen Z, Maunoury N, Vaubert D, et al. (2003) A novel family in Medicago truncatula consisting of more than 300 nodule-specific genes coding for small, secreted polypeptides with conserved cysteine motifs. Plant Physiol 132: 161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Silverstein KAT, Graham MA, Paape TD, VandenBosch KA (2005) Genome organization of more than 300 defensin-like genes in arabidopsis. Plant Physiol 138: 600–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Silverstein KAT, Moskal WA, Wu HC, Underwood BA, Graham MA, et al. (2007) Small cysteine-rich peptides resembling antimicrobial peptides have been under-predicted in plants. Plant Journal 51: 262–280. [DOI] [PubMed] [Google Scholar]
- 42.Sunagawa S, DeSalvo MK, Voolstra CR, Reyes-Bermudez A, Medina M (2009) Identification and gene expression analysis of a taxonomically restricted cysteine-rich protein family in reef-building corals. PLoS One 4. [DOI] [PMC free article] [PubMed]
- 43. Marshall E, Costa LM, Gutierrez-Marcos J (2011) Cysteine-Rich Peptides (CRPs) mediate diverse aspects of cell-cell communication in plant reproduction and development. J Exp Bot 62: 1677–1686. [DOI] [PubMed] [Google Scholar]
- 44. Haag AF, Baloban M, Sani M, Kerscher B, Pierre O, et al. (2011) Protection of Sinorhizobium against host cysteine-rich antimicrobial peptides is critical for symbiosis. PLoS Biol 9: e1001169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Callewaert L, Michiels CW (2010) Lysozymes in the animal kingdom. J Biosci 35: 127–160. [DOI] [PubMed] [Google Scholar]
- 46.Hultmark D (1996) Insect lysozymes. In: Jolles P, editor. Lysozymes: Model Enzymes in Biochemistry and Biology. Switzerland: Birkhauser Verlag Basel. 87–102.
- 47. Login FH, Balmand S, Vallier A, Vincent-Monegat C, Vigneron A, et al. (2011) Antimicrobial peptides keep insect endosymbionts under control. Science 334: 362–365. [DOI] [PubMed] [Google Scholar]
- 48. Kylsten P, Kimbrell DA, Daffre S, Samakovlis C, Hultmark D (1992) The lysozyme locus in Drosophila melanogaster; Different genes are expressed in midgut and salivary glands. Mol Gen Genet 232: 335–343. [DOI] [PubMed] [Google Scholar]
- 49. Daffre S, Kylsten P, Samakovlis C, Hultmark D (1994) The lysozyme locus in Drosophila melanogaster: an expanded gene family adapted for expression in the digestive tract. Mol Gen Genet 242: 152–162. [DOI] [PubMed] [Google Scholar]
- 50. Lemos FJA, Ribeiro AF, Terra WR (1993) A bacteria digesting midgut lysozyme from Musca domestica (Diptera) larvae; purification, properties and secretory mechanism. Insect Biochem Mol Biol 23: 533–541. [Google Scholar]
- 51. Li B, Calvo E, Marinotti O, James AA, Paskewitz SM (2005) Characterization of the c-type lysozyme gene family in Anopheles gambiae . Gene 360: 131–139. [DOI] [PubMed] [Google Scholar]
- 52. Fujita A (2004) Lysozymes in insects: what role do they play in nitrogen metabolism? Physiol Entomol 299: 305–310. [Google Scholar]
- 53. Russell VW, Dunn PE (1991) Lysozyme in the midgut of Manduca sexta during metamorphosis. Arch Insect Biochem Physiol 17: 67–80. [DOI] [PubMed] [Google Scholar]
- 54. Mittapalli O, Shukle RH, Sardesai N, Giovanini MP, Williams CE (2006) Expression patterns of antibacterial genes in the Hessian fly. J Insect Physiol 52: 1143–1152. [DOI] [PubMed] [Google Scholar]
- 55. Zhang Y, Huang J, Zhou B, Zhang C, Liu W, et al. (2009) Up-regulation of lysozyme gene expression during metamorphosis and immune challenge of the cotton bollworm, Helicoverpa armigera . Arch Insect Biochem Physiol 70: 18–29. [DOI] [PubMed] [Google Scholar]
- 56. Kopacek P, Vogt R, Jindrak L, Weise C, Safarik I (1999) Purification and characterization of the lysozyme from the gut of the soft tick Ornithodoros moubata . Insect Biochem Mol Biol 29: 989–997. [DOI] [PubMed] [Google Scholar]
- 57. Kollien AH, Fechner S, Waniek PJ, Schaub GA (2003) Isolation and characterization of a cDNA encoding for a lysozyme from the gut of the reduviid bug Triatoma infestans . Arch Insect Biochem Physiol 53: 134–145. [DOI] [PubMed] [Google Scholar]
- 58. Chalk R, Townson H, Natori S, Desmond H, Ham PJ (1994) Purification of an insect defensin from the mosquito, Aedes aegypti . Insect Biochem Mol Biol 24: 403–410. [DOI] [PubMed] [Google Scholar]
- 59. Engstrom P, Carlsson A, Engstrom A, Tao ZJ, Bennich H (1984) The antibacterial effect of attacins from the silk moth Hyalophora cecropia is directed against the outer membrane of Escherichia coli . EMBO J 3: 3347–3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Turk B, Turk D, Turk V (2000) Lysosomal cysteine proteases: more than scavengers. Biochim Biophys Acta 1477: 98–111. [DOI] [PubMed] [Google Scholar]
- 61. Cristofoletti PT, Ribeiro AF, Deraison C, Rahbe Y, Terra WR (2003) Midgut adaptation and digestive enzyme distribution in a phloem feeding insect, the pea aphid Acyrthosiphon pisum . J Insect Physiol 49: 11–24. [DOI] [PubMed] [Google Scholar]
- 62. Koiwa H, Shade RE, Zhu-Salzman K, D'Urzo MP, Murdock LL, et al. (2000) A plant defensive cystatin (soyacystatin) targets cathepsin L-like digestive cysteine proteinases (DvCALs) in the larval midgut of western corn rootworm (Diabrotica virgifera virgifera). FEBS Lett 471: 67–70. [DOI] [PubMed] [Google Scholar]
- 63. Girard C, Jouanin L (1999) Molecular cloning of cDNAs encoding a range of digestive enzymes from a phytophagous beetle, Phaedon cochleariae . Insect Biochem Mol Biol 29: 1129–1142. [DOI] [PubMed] [Google Scholar]
- 64. Houseman JG, Downe AER (1983) Cathepsin D-like activity in the posterior midgut of Hemipteran insects. Comp Biochem Physiol B 75: 509–512. [Google Scholar]
- 65. Nishikori K, Morioka K, Kubo T, Morioka M (2009) Age- and morph-dependent activation of the lysosomal system and Buchnera degradation in aphid endosymbiosis. J Insect Physiol 55: 351–357. [DOI] [PubMed] [Google Scholar]
- 66. Harrison PM, Arosio P (1996) The ferritins: molecular properties, iron storage function and cellular regulation. Biochim Biophys Acta 1275: 161–203. [DOI] [PubMed] [Google Scholar]
- 67. Brownlie JC, Cass BN, Riegler M, Witsenburg JJ, Iturbe-Ormaetxe I, et al. (2009) Evidence for metabolic provisioning by a common invertebrate endosymbiont, Wolbachia pipientis, during periods of nutritional stress. PLoS Pathog 5: e1000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wu B, Novelli J, Foster J, Vaisvila R, Conway L, et al. (2009) The heme biosynthetic pathway of the obligate Wolbachia endosymbiont of Brugia malayi as a potential anti-filarial drug target. PLoS Negl Trop Dis 3: e475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Welchman DP, Aksoy S, Jiggins F, Lemaitre B (2009) Insect immunity: from pattern recognition to symbiont-mediated host defense. Cell Host Microbe 6: 107–114. [DOI] [PubMed] [Google Scholar]
- 70. Gerardo NM, Altincicek B, Anselme C, Atamian H, Barribeau SM, et al. (2010) Immunity and other defenses in pea aphids, Acyrthosiphon pisum . Genome Biol 11: R21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hedengren M, Asling B, Dushay MS, Ando I, Ekengren S, et al. (1999) Relish, a central factor in the control of humoral but not cellular immunity in Drosophila . Mol Cell 4: 827–837. [DOI] [PubMed] [Google Scholar]
- 72. Schultze M, Kondorosi A (1998) Regulation of symbiotic root nodule development. Annu Rev Genet 32: 33–57. [DOI] [PubMed] [Google Scholar]
- 73. Stougaard J (2000) Regulators and regulation of legume root nodule development. Plant Physiol 124: 531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. McFall-Ngai MJ (2002) Unseen forces: the influence of bacteria on animal development. Dev Biol 242: 1–14. [DOI] [PubMed] [Google Scholar]
- 75. Nyholm SV, McFall-Ngai MJ (2004) The winnowing: establishing the squid-vibrio symbiosis. Nat Rev Microbiol 2: 632–642. [DOI] [PubMed] [Google Scholar]
- 76. Tautz D, Ellegren H, Weigel D (2010) Next generation molecular ecology. Mol Ecol 19 Suppl 11–3. [DOI] [PubMed] [Google Scholar]
- 77. Kutsukake M, Shibao H, Nikoh N, Morioka M, Tamura T, et al. (2004) Venomous protease of aphid soldier for colony defense. Proc Natl Acad Sci U S A 101: 11338–11343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25: 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Molecular phylogenetic analysis of lysozyme genes. A neighbor-joining phylogeny inferred from 1,380 aligned amino acid sites is shown, while maximum likelihood and Bayesian phylogenies exhibited substantially the same topologies. On each node, statistical support values are indicated in the order of [bootstrap value of neighbor-joining]/[bootstrap value of maximum likelihood]/[posterior probability of Bayesian]. Asterisks indicate support values lower than 50%. Red boxes indicate the R. pedestris genes.
(TIF)
List of nonredundant EST clusters obtained from midgut cDNA libraries of R. pedestris .
(XLS)
List of EST clusters from R. pedestris representing either putative isoforms or premature transcripts.
(XLS)
Assignment of Gene Ontology (GO) molecular function terms to the midgut EST data sets of R. pedestris .
(XLS)
List of lysozyme genes.
(XLS)
List of cysteine-rich secreted protein genes.
(XLS)
List of cathepsin protease genes.
(XLS)
Primer sets for quantitative RT-PCR.
(XLS)