Abstract
This review article summarizes the main treatments for chronic obstructive pulmonary disease, their mechanisms, and the key evidence from trials supporting their use. Drug classes covered were short acting beta agonists (SABA), short acting muscarinic antagonists (SAMA), long acting beta agonists (LABA), long acting antimuscarinics (LAMA), inhaled corticosteroids (ICS), LABA/ICS combinations, specific phosphodiesterase (PDE4) inhibitors, non-specific PDE inhibitors, mucolytics, and oxygen. Non-specific therapies, such as opiates for relief of dyspnoea and therapies for smoking cessation, are also covered briefly. For each class of drug, mechanisms of action are described, key clinical trial results are reported, and available agents compared. Finally, the place of each drug in therapy is compared between current worldwide guidelines.
Keywords: chronic obstructive pulmonary disease, pharmacotherapies, disease management
Introduction
Chronic obstructive pulmonary disease (COPD) is a multi-component disease which is both preventable and treatable. It is currently the fourth leading cause of death worldwide and predicted to be the third by 2020.1 Globally the burden of disease is projected to increase in the coming decades due to continued exposure to COPD risk factors and an ageing population.1
COPD is characterized by airflow limitation that is progressive and not fully reversible; the latest severity categorization also includes exacerbation frequency and symptom burden as key features.1 COPD is associated with an enhanced chronic inflammatory response which is responsible for the airway abnormalities and architectural distortion of the lung parenchyma. In affected individuals lung function deteriorates progressively over several years, with increasing symptoms such as cough, sputum production, and dyspnoea. Acute exacerbations are defined by increased cough, dyspnea, or increased sputum purulence from baseline,2 and punctuate the disease process with a deleterious impact on patients’ daily activities and well-being.3 Frequent exacerbations are associated with more rapid decline of lung function4 and are one of the greatest costs to the health economy, partly through hospital admissions, and partly through loss of work days.5 Although mainly categorized by airflow limitation, in many patients the disease seems to be associated with several extra-pulmonary manifestations. What is unclear at present is whether these manifestations are directly related to COPD or are just an independent consequence of the exposure to common causal effects such as tobacco smoking and inactivity. The most widely recognized manifestations include the presence of concomitant cardiovascular disease, skeletal muscle dysfunction, osteoporosis, and clinical depression/anxiety.6 These co-morbidities interact to increase the risk of hospitalization and mortality in COPD patients, especially as the airway obstruction becomes more severe.7 The main goals in management of COPD are improving health status, reducing symptoms, preserving lung function decline, preventing exacerbations, and reducing mortality. This review outlines the pharmacological management of stable COPD.
Bronchodilators
Dyspnoea is one of the hallmark symptoms of COPD and one of the most common reasons for health resource utilization and increasing anxiety in affected patients.8 Dynamic hyperinflation as a result of increased lung volumes is a key reason why patients experience dyspnoea. Long acting bronchodilators reduce lung volumes by a reduction in air trapping and facilitate the emptying of the lungs.9 The subsequent improvement in inspiratory capacity leads to reduced dyspnoea and improved exercise tolerance.8 The available long acting bronchodilators include B2 agonists and anti-muscarinics.
Beta 2 adrenoceptor agonists (B2-agonists)
Mechanism of action
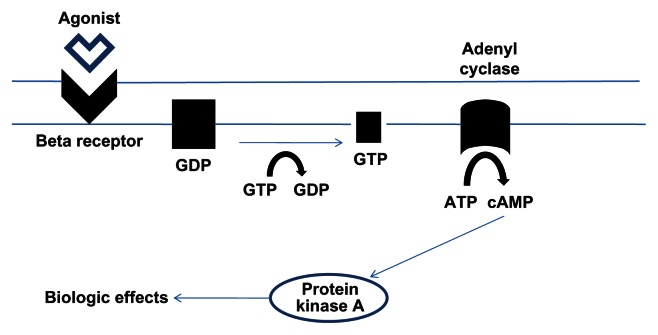
B2 adrenergic receptors (B2AR) are present in high density in airway smooth muscle cells. B2 agonists act by binding to the B2AR (Fig. 1). Interaction of the receptor with intracellular G proteins stimulates the production of intracellular cyclic adenosine monophosphate (cAMP). This leads to activation of protein kinase A, which results in phosphorylation of various targets mediating smooth muscle relaxation. The exact targets are unknown but probably involve myosin light chain kinase and calcium dependent potassium channels.10
Figure 1.
Mechanism of action of Beta agonists.
Notes: Binding of the agonist to the receptor results in a change in protein structure, which enables interaction with intracellular G proteins, production of cAMP and then protein kinase A, which mediates the bronchodilating effects via its actions on smooth muscle.
B2AR are also present in vascular endothelium, ciliated cells, circulating inflammatory cells (such as eosinophils), and sub-mucosal glands. The presence of the receptor on these cells explains some of the nonbronchodilator effects, including attenuation of mast cell mediator release, reduction of plasma exudation, and reduced activation of sensory nerves. Other beneficial effects include enhancement of mucociliary transport,11 attenuation of neutrophil recruitment,12 and inhibition of smooth muscle cell proliferation.13
Short acting B2AR agonists (SABAs)
Although many patients with COPD do not have reversible airflow obstruction, many have noted symptomatic improvement with the use of SABAs.14 SABAs are used both in acute and chronic management of COPD, the most commonly used being Salbutamol. Once administered, the onset of action is within 3 minutes with peak activity after 2.5 hours. The duration of action is between 4 and 6 hours.15 Salbutamol is mainly metabolized to a sulphate conjugate. Approximately 50% is excreted in this form with a smaller proportion as unchanged drug.16 The most recent Cochrane review showed that use of SABAs for at least seven days improved post bronchodilator lung function in patients with moderate to severe COPD. Patients were also less dyspnoeic and more likely to comply with treatment.14
Long acting B2AR agonists (LABAs)
This class of drug has recently been reviewed and compared to one of the long acting anti-muscarinic agents (LAMAs) by the Cochrane collaboration,17 although significant trial heterogeneity precluded meta-analysis, primarily due to the fact that indacaterol’s effect on health related quality of life (HRQoL) in particular differed from the other LABAs. Most of the primary studies of LABAs did not have exacerbation frequency as a primary outcome measure, although meta-analysis of their data has been carried out;18 in general there is a class effect showing reduction in exacerbations against placebo. Their place in therapy is generally considered as one of the options for maintenance therapy in patients that are symptomatic despite regular SABA use;1 treatment algorithms for the disease will be considered at the end of this article.
Salmeterol and formoterol
Salmeterol and formoterol are LABAs with extended duration of action maintained 12 hours after inhalation of a single dose,19 which has led to their twice daily dosing. Formoterol’s potency and speed of action make it effective in both quick relief and for prolonged effect. Despite these characteristics, it appears to be inferior to salmeterol in terms of health related quality of life (HRQoL) scores and its ability to reduce exacerbations, albeit in indirect comparisons only.17 Salmeterol and formoterol have some important differences which can be explained by their physicochemical properties. Both are lipophilic, with salmeterol being far more so than formoterol; the relative water solubility of formoterol enables it to diffuse rapidly to the B2AR and cause bronchodilation in between 1 to 3 minutes, similar to that of SABAs.20 Salmeterol’s onset of action is, at least 20 minutes, significantly longer.21 Both drugs have adequate lipophilic properties that allow them to remain in the airway tissues in close vicinity to B2AR, explaining the longer duration of action. There is also some evidence to suggest that salmeterol binds to an additional exosite within the receptor, potentiating the longer effect of the drug.22 Secondly the intrinsic efficacy of the drugs varies—formoterol is a full agonist whilst salmeterol is a partial agonist; hypothetically this implies formoterol should provide better bronchodilation.
Salmeterol xinafoate dissociates in solution to salmeterol and 1-hydroxy-2-naphthoic acid. These two compounds are then absorbed, distributed, metabolized, and excreted independently. The xinafoate moiety has no discernable pharmacological activity, is highly protein bound, and has a long elimination half-life of about 12 to 15 days in healthy individuals. The cytochrome P450 (CYP) isoform 3A4 is responsible for aliphatic oxidation of salmeterol base, which is extensively metabolized by hydroxylation, with the major metabolite being alpha- hydroxysalmeterol and with subsequent elimination predominantly in the feces.23 Formoterol is metabolized primarily in the liver by CYP450 enzymes and undergoes glucuronidation and O-demethylation. Elimination of formoterol is via the urine and feces in ratios of two thirds and one third respectively, with 10% of urinary excretion being of unchanged drug.24
Clinical trials pertaining to the efficacy of formoterol and salmeterol are shown in Table 1. Whilst no head to head trials of the 2 agents have been done, indirect comparison within the Cochrane review17 suggests that their effects are largely the same. Additionally, reduction in exacerbation rate was not maintained in patients on inhaled corticosteroids (ICS) and formoterol, unlike the other LABAs.18
Table 1.
Major randomized controlled trials (RCTs) of salmeterol and formoterol.
| Trial | Duration | Outcome | Comparator |
|---|---|---|---|
| Salmeterol | |||
| Boyd et al25 | 16 weeks | ↑ FEV1 | Placebo |
| Mahler et al26 | 12 weeks | ↑ FEV1 | Placebo, ipratropium* |
| Rennard et al27 | 12 weeks | ↑ FEV1 | Placebo |
| Calverley et al28 (TRISTAN) | 1 year | ↑ FEV1 ↓Exacerbations ↑ HRQoL |
Placebo, fluticasone, fluticasone/salmeterol |
| Calverley et al29 (TORCH) | 3 years | ↑ FEV1 ↓ Exacerbations ↑ HRQoL |
Placebo, fluticasone, salmeterol/fluticasone |
| Formoterol | |||
| Dahl et al30 | 12 weeks | ↑ FEV1 ↓Symptom scores |
Placebo, ipratropium* |
| De Rossi et al31 | 1 year | ↑ FEV1 | Placebo, theophylline* |
| Calverley et al32 | 1 year | ↔ FEV1 ↑ HRQoL |
Placebo, budesonide, budesonide/formoterol |
| Szafranski et al33 | 1 year | ↔ FEV1 ↔HRQoL |
Placebo, budesonide, budesonide/formoterol |
Notes: The table shows results against placebo; for results of the multicomponent trials against ICS and ICS/LABA please see text in relevant section.
Also significant result against active comparator.
Indacaterol is an ultra-long acting, once daily LABA; it is the only agent of this type currently on the market, although others are in development, including vilanterol.34 It is a partial B2 agonist which exhibits high intrinsic efficacy at the B2AR, more than two fold that of salbutamol and salmeterol.35 Partial agonists can exhibit antagonist behavior in the presence of an agonist with higher receptor efficacy, which could potentially result in inhibitory action of rescue medication, an effect which has been shown with salmeterol but not indacaterol.35 Studies using indacaterol have demonstrated a relatively rapid onset of action (5 minutes) with peak effect occurring at 15 minutes and lasting 24 hours.35 The rapid onset of action and length of duration of action is related to both the high affinity of indacaterol to the lipid raft domains within the B2AR membrane and its intrinsic efficacy at the receptor level.35 Following administration, the bioavailability of indacaterol is 43%, with a steady state reached after 12 to 14 days. It is primarily metabolized to a hydroxylated derivate by the CYP3A4 enzyme, and subsequent to this, to phenolic O glucuronides and other metabolites. Elimination occurs via the fecal route, with less than 2% being excreted unchanged in the urine.35
The clinical trials pertaining to indacaterol’s efficacy are shown in Table 2. Current evidence suggests that indacaterol is superior to other LABAs with regard to HRQoL.17 Sub-group analyses with regard to exacerbations showed that indacaterol had a greater effect on severe exacerbations (ie, hospitalized event rate).18
Table 2.
Major RCTs for indacaterol.
| Trial | Duration | Outcome | Comparator |
|---|---|---|---|
| Donohue et al36 (INHANCE) | 26 weeks | ↑ HRQoL ↓ Dyspnoea (TDI) |
Placebo, tiotropium |
| Dahl et al37 (INVOLVE) | I year | ↑ Bronchodilation (FEV1) ↑ HRQoL ↓ Dyspnoea (TDI) Prolonged time to exacerbation |
Placebo, formoterol |
| Buhl et al38 (INTENSITY) | 12 weeks | ↔ Bronchodilation (FEV1) ↑ HRQoL ↓ Dyspnoea (TDI) |
Tiotropium |
| Kornmann et al39 (INLIGHT-2) | 12 weeks | ↑ Bronchodilation (FEV1) ↑ HRQoL ↓ Dyspnoea (TDI) |
Placebo, salmeterol |
| Korn et al40 (INSIST) | 12 weeks | ↑ Bronchodilation (FEV1) ↓ Dyspnoea (TDI) |
Salmeterol |
Notes: The outcomes shown in the table are against the active comparator; good efficacy was also shown against placebo where this arm was used.
Abbreviations: TDI, transitional dyspnoea index.
Safety
In general, apart from the occasional episode of tachycardia and tremor, both SABAs and LABAs are well tolerated. However, here has been some concern with regard to cardiovascular safety and use of B2 agonists. Salpeter et al41 suggested increased cardiovascular risk with LABAs compared with placebo, a fact hotly debated but disproven in subsequent meta-analyses in COPD.42 It has also been suggested that tolerance/tachyphylaxis would render LABAs less efficacious over time. Data from Szafranski et al33 and TORCH29 has refuted this by demonstrating that the bronchodilator effect of LABA therapy is maintained at 1 and 3 years respectively.
Muscarinic antagonists
Mechanism of action
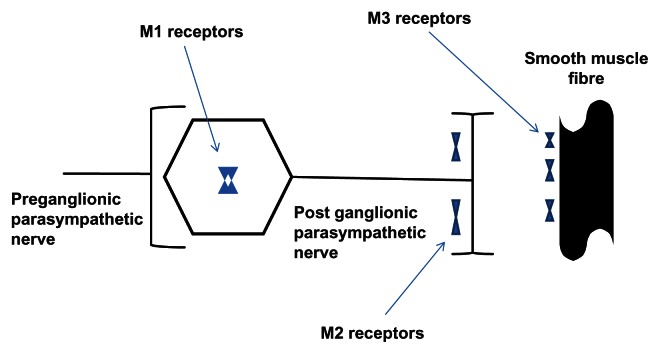
Para-sympathetic activity mediates both bronchial smooth muscle contraction and the release of mucus into the airway lumen through stimulation of muscarinic receptors, of which there are five distinct types (M1–M5).43 Whilst the role of subtypes M4 and M5 are unknown, subtypes M1–M3 are expressed in human lungs. M1 receptors are expressed in peribronchial ganglia, whilst M3 receptors are present on bronchial smooth muscle cells; their relationship to one another is shown in Figure 2. Both mediate bronchomotor tone and reflex bronchoconstriction. M2 receptors are located in the post ganglionic para-sympathetic nerve and act as auto receptors. Agonistic stimulation of these receptors provides feedback and inhibition of further acetylcholine release; thus, optimal inhibition of the parasympathetic pathway would be achieved by selectively blocking M1 and M3 receptors.43
Figure 2.
Location of anti-muscarinic receptors.
Notes: M1 receptors are expressed in peribronchial ganglia, whilst M3 are present on bronchial smooth muscle cells; both mediate bronchomotor tone and reflex bronchoconstriction. M2 receptors are located in the post ganglionic para-sympathetic nerve and act as auto receptors. The figure shows their relationship to one another. Adapted from Lipson DA (2006).
Short acting muscarinic antagonists (SAMAs)
Short acting muscarinic antagonists, like SABAs, are used both in the acute and chronic management of COPD. Ipratropium bromide is the most widely used following the discontinuation of Oxitropium bromide in 2004. Ipratropium blocks all muscarinic receptors without sub-type selectivity. Its onset of action is within minutes, with peak activity occurring between 1 and 2 hours and duration of action approximately 4 hours in the majority of patients.43 In a systematic review, combination of LABAs with ipratropium showed a small benefit in pre bronchodilator FEV1 of borderline statistical significance;14 however, for the most part LABAs were superior. Head to head comparison suggested that tiotropium was superior,44 and thus current guidance places LAMA ahead of SAMA for maintenance therapy.5
Long acting muscarinic antagonists (LAMAs)
In general, the place of LAMAs in therapy is the same as LABAs. Until recently there was only one LAMA on the market (tiotropium), but two other products have recently been licensed (glycopyrronium and aclidinium). The Cochrane review of LABAs versus LAMAs17 was only able to consider trials using tiotropium (due to their search dates) and concluded that its effects were superior to LABAs in terms of preventing exacerbations and disease related hospitalization. Nevertheless, drug choice for the individual patient may still suggest LABA as a good choice for some, ie, in patients with severe glaucoma; thus current guidance places the 2 classes of drug equal in therapy algorithms.1,5 Few head to head trials of LAMAs have been carried out, with the notable exception of GLOW2 (tiotropium versus glycopyrronium),45 which concluded that the products were equivalent in terms of trough FEV1, dyspnoea score, and exacerbation reduction. Aclidinium has been studied alongside tiotropium, but this trial was a small study of crossover design,46 and therefore larger, more definitive studies are still needed. A summary of clinical effects, as demonstrated by the various LAMA trials, is shown in Table 3; since most effects (with the exception of speed of onset) appear to be roughly equivalent between the 3 drugs they have been tabulated together.
Table 3.
Major RCTs for LAMAs.
| Trial | Duration | Outcome | Comparator |
|---|---|---|---|
| Tiotropium | |||
| Brusasco et al47 | 26 weeks | ↓Exacerbations ↑ HRQoL |
Placebo, salmeterol |
| Briggs et al48 | 12 weeks | ↑ FEV1 | Salmeterol* |
| Tashkin et al49 (UPLIFT) | 4 years | ↓Exacerbations ↑ HRQoL ↑ FEV1 |
Placebo |
| Vogelmeier et al50 (POET) | 1 year | ↓Exacerbations | Salmeterol* |
| Glycopyrronium | |||
| D’Urzo et al51 (GLOW1) | 26 weeks | ↑ Trough FEV1 ↑ HRQoL ↓Dyspnoea (TDI score) ↓Exacerbations |
Placebo |
| Kerwin et al45 (GLOW2) | 1 year | Similar to GLOW1 v placebo ↑ Bronchodilation on day 1 and week 26 v tiotropium |
Placebo, tiotropium* |
| Beeh et al8 (GLOW3) | 8 weeks | ↑ Endurance time ↑ Inspiratory capacity |
Placebo |
| Aclidinium | |||
| Jones et al52 (ACCLAIM I and II) | 1 year | ↑ Trough FEV1 ↑ HRQoL |
Placebo |
| Jones et al53 (ATTAIN) | 6 months | ↑ FEV1 ↑ HRQoL ↓ Dyspnoea (TDI) |
Placebo |
| Kerwin et al54 (ACCORD) | 12 weeks | ↑ FEV1 ↑ HRQoL ↓ Dyspnoea (TDI) |
Placebo |
| Fuhr et al46 | 15 days per treatment | Similar to ACCORD v placebo ↑ Morning FEV1 v tiotropium |
Placebo, tiotropium |
Notes: The table shows results against placebo unless otherwise stated.
Also significant result against active comparator.
Tiotropium
Tiotropium was the first available LAMA and is administered once daily. It binds to M1–M3 receptors and is 10 times more potent than ipratropium bromide. It dissociates slowly from M1 and M3 receptors, giving it its long acting effect, but dissociates relatively rapidly from the M2 receptor, giving it a unique kinetic selectivity.55 Once administered the onset of bronchodilation occurs within 30 minutes, with peak activity at 3 hours and sustained over more than 24 hours.43 Tiotropium has a low bioavailability of between 2%–3% and after regular once daily inhalation, it reaches a steady state after 2 weeks. The drug is not extensively metabolized but the portion that is undergoes hydrolysis into two major metabolites, a carboxylic acid derivative and an alcohol derivative, neither of which have any affinity for any of the muscarinic receptor subtype. A larger proportion of the drug is eliminated via renal secretion which means there is some accumulation in renal impairment.56
Glycopyrronium
Glycopyrronium is a novel once daily LAMA licensed for use in Europe in 2012. Like tiotropium it has a sustained 24 hour bronchodilator effect,45 and higher selectivity for the M3 receptor than the M2 receptor.57 Dissociation from the M3 receptor occurs four times faster than tiotropium57 and almost twice as fast as aclidinium.58 This suggests that glycopyrronium would have a more rapid onset of action, which has been confirmed in clinical studies.45,51 Glycopyrronium bromide undergoes mainly hydrolysis, which results in the formation of a carboxylic acid derivative, and to a lesser extent glucuronidation. Elimination is predominately via the kidneys.59
Aclidinium bromide
Aclidinium is a recently developed LAMA administered twice daily which was approved by the FDA in 2012. It is a potent and selective muscarinic antagonist which displays relative selectivity for the M3 receptor with a drug/receptor binding rate similar to that of ipratropium and 2.6 times faster than that of tiotropium.60 Aclidinium undergoes hydrolysis into two major metabolites, a carboxylic acid derivative and an alcohol derivative. Owing to its short plasma half-life and rapid metabolism, very little is excreted in the urine, such that no adjustments are required in renal disease.61 Once administered, onset of action occurs within 15 minutes with a peak activity at 2 hours.60
The clinical trials pertaining to aclidinium’s efficacy are shown in Table 4. Potential differences from other LAMAs include higher nocturnal FEV1 compared with tiotropium, resulting in better morning symptoms.46
Table 4.
Major RCTs of ICS in COPD.
| Trial | ICS | Duration | Outcome | Comparator |
|---|---|---|---|---|
| Burge et al76 (ISOLDE) | Fluticasone | 1 year | ↑ FEV1 ↓ Exacerbations |
Placebo |
| Calverley et al23 | Budesonide | 1 year | ↓ Exacerbations* ↑ HRQoL |
Placebo, formoterol, budesonide/formoterol |
| Szafranski et al33 | Budesonide | 1 year | ↑ FEV1 | Placebo, formoterol, budesonide/formoterol |
| Calverley et al28 (TRISTAN) | Fluticasone | 1 year | ↑ FEV1 ↓Exacerbations |
Placebo, salmeterol, salmeterol/fluticasone |
| Calverley et al29 (TORCH) | Fluticasone | 3 years | ↓Exacerbations ↑ FEV1 |
Placebo, salmeterol, salmeterol/fluticasone |
Notes: The table shows results for comparisons of ICS against placebo, although many trials used multiple comparators; see text and table in section on combined ICS/LABA for other results.
Only exacerbations requiring oral corticosteroids.
Safety
The adverse effects encountered with LAMAs are largely those attributed to its anti-cholinergic activity, with dry mouth being one of the most commonly reported side effects.49 Clinical trials involving all drugs have reported similar adverse event rates with the active intervention compared with placebo. In the ATTAIN and GLOW2 trials, the most common events reported in the aclidinium and glycopyrronium arm over placebo were nasopharyngitis.45,53 In the 4 year UPLIFT study, the most commonly encountered adverse events were due to lower respiratory causes; however, this was similar to the placebo arm.49
A study prior to the publication of UPLIFT suggested that use of tiotropium increased cardiovascular morbidity and mortality;62 data from UPLIFT refuted this demonstrating a non-significant reduction in all cause cardiovascular mortality.63 It is of note that UPLIFT used the Handihaler device, and excluded those with cardiovascular co-morbidity. Later analyses of trial data using the Respimat device (not licensed in the USA, but available in at least 55 countries worldwide) still suggested an adverse cardiovascular mortality effect with tiotropium.64 Much debate ensued after this, but a more recent Cochrane review65 and a further independent meta-analysis66 have confirmed the finding of increased mortality risk associated with the soft mist inhaler (Respimat). Ipratropium and tiotropium are actively transported through the bronchial epithelium using the OCTN2 transporter, which is also present in the human heart.67 Furthermore the Respimat device results in greater deposition in the lung than the Handihaler.68,69 Whilst such features may go some way to explaining the mechanism of cardiovascular morbidity and it’s apparent device specificity, this will require further study. In the meantime there has been a call in the UK for withdrawal of the Respimat device as a result of safety concerns.70
Inhaled Corticosteroids
It is clear that ICS reduce airway inflammation, airflow limitation, and symptoms in asthma and are therefore the mainstay of treatment.71 In COPD, however, the role of ICS is more controversial, predominantly because the pattern of inflammation differs. The inflammation in COPD is dominated by neutrophilic infiltration, with an increased numbers of macrophages and CD8 T lymphocytes; neutrophilic infiltration is not as responsive to steroids as the eosinophilic inflammation seen in asthma.72 Despite this, ICS were used in COPD before any real evidence of their efficacy was known. Their use in COPD has recently been reviewed by the Cochrane collaboration, which concluded that although use of ICS is associated with a reduction in exacerbation rates and possibly a reduced rate of decline in FEV1, these benefits need to be weighed against increased pneumonia risks and local side effects.73
Mechanism of action
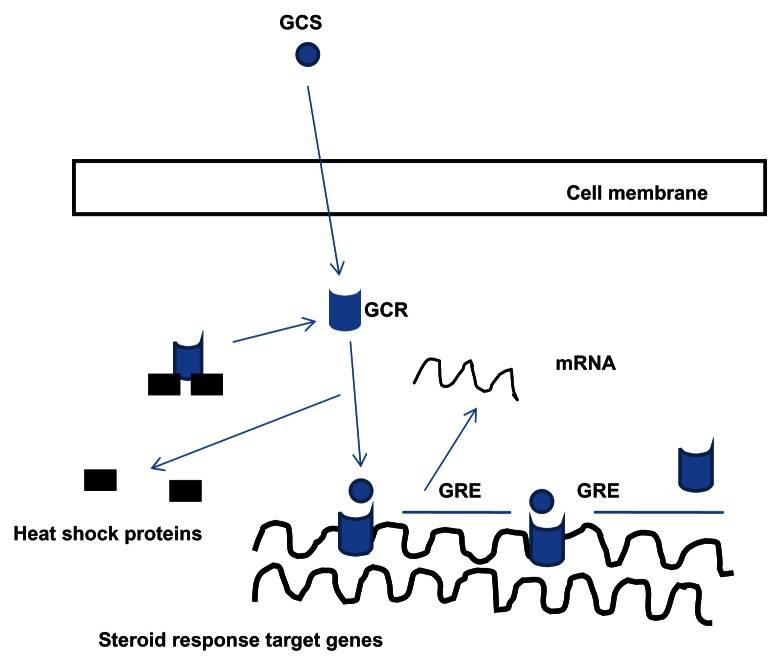
The primary actions of glucocorticoids arise by activation of specific glucocorticoid receptors (GCR) which are found in the cytoplasm of most mammalian cell types. The mechanism of action via the GCR is shown in Figure 3. Another method by which the GCR influences inflammatory processes in COPD is the interaction between histone deacetylase-2 (HDAC2) and nuclear factor kappa B (Nf-kB), a key transcription factor. Nf-kB is upregulated in many cell types during inflammation and favors the expression of pro inflammatory genes. HDAC2 mediated deacetylation of the GCR enables it to bind to Nf-kB and nullify its effect as a pro inflammatory molecule.74 HDAC2 modulation is a current area of interest in COPD therapy.75 The clinical trials pertaining to the efficacy of ICS are shown in Table 5.
Figure 3.
Mechanism of action of ICS.
Notes: When a glucocorticoid (GCS) binds to the GCR the heat shock proteins are detached resulting in a conformational change and formation of a GCR dimer. This dimerization is necessary for the binding of the GCR to the glucocorticoid response element (GRE) which is an area of DNA responsible for up or down regulating gene expression.
Table 5.
Relative therapeutic effects of ICS.
| Fluticasone | Budesonide | |
|---|---|---|
| Oral bioavailability (%) | <1 | 11 |
| Pulmonary deposition (%) | 16 | 28 |
| Receptor binding affinity | 1800 | 935 |
| Protein binding (%) | 90 | 88 |
| Half life (hrs) | 7.8 | 2.8 |
| Clearance (l/h) | 69 | 84 |
Note: 100% is the affinity of dexamethasone, to which the above are compared in the table.
Budesonide and fluticasone
Budesonide and fluticasone are the two ICS which have been used most extensively in clinical trials pertaining to COPD. Whilst the potency—and therein pharmacological response—of ICS is related to its affinity at the GCR, other factors such as particle size and pulmonary deposition will determine its therapeutic effect. Table 5 outlines the pharmacokinetic and pharmacodynamic features of both drugs.77
Budesonide is several times less lipophilic than fluticasone and, as a result, dissolves more readily in airway mucus and is more rapidly absorbed into the airway tissue and systemic circulation. Fluticasone, being more lipophilic and thus less water soluble, is more likely to be retained in the lumen of the airways; it therefore has a greater chance of being removed from the airways by mucociliary clearance and cough.78 Recent studies have shown higher proportions of fluticasone expectorated in the sputum of patient with COPD and fluticasone plasma concentration is more affected by airflow limitation than budesonide.78
Safety
The adverse effects of ICS are related to its local and systemic absorption. The amount of inhaled steroid reaching the systemic circulation is the sum of the pulmonary and orally bioavailable fractions. Given that most ICS have relatively low oral bioavailability, it is largely the absorption from the pulmonary vasculature which determines the systemically available steroid. Although adrenal suppression is a theoretical risk, it has not been reported in COPD, though it has been seen in bronchiectasis.79 Local effects, namely dysphonia and oral candidiasis. are more frequently observed adverse effects demonstrated in the trials in Table 4. One of the main concerns in patients using ICS is the significantly increased risk of pneumonia which was demonstrated in the TORCH trial29 and noted in the recent Cochrane review of ICS.73
Combination Therapy
There are currently two combination inhalers used in the treatment of patients with COPD: Seretide/Advair (salmeterol/fluticasone) and Symbicort (budesonide/formoterol). Both are a combination of a LABA with an ICS; thus, for the purposes of this article, the term combination therapy is synonymous with these drugs. It is important to note that many new combinations of drugs are in development for COPD, eg, LABA/LAMA,80 such that in future we may not use the term in the same way. The pharmacokinetics and pharmacodynamics of the component drugs have been discussed in the preceding sections. LABA/ICS combinations have been shown to improve lung function and HRQoL and to reduce exacerbations in COPD patients (Table 6). Current national and international guidelines advocate the use of combination inhalers in severe and very severe disease.
Table 6.
Major RCTs of ICS/LABA.
| Trial | ICS/LABA | Duration | Outcome | Comparator |
|---|---|---|---|---|
| Calverley et al23 | Budesonide/formoterol | 1 year | ↓Exacerbations v P and F ↔ FEV1 v all ↑ HRQoL v all |
Placebo, formoterol, budesonide |
| Szafranski et al33 | Budesonide/formoterol | 1 year | ↓Exacerbations v P and F ↔ FEV1 v P and B ↑ HRQoL v P and B |
Placebo, formoterol, budesonide |
| Calverley et al28 (TRISTAN) | Salmeterol/fluticasone | 1 year | ↑ FEV1 v all ↑ HRQoL v all ↓ Exacerbations v P |
Placebo, salmeterol, fluticasone |
| Calverley et al29 (TORCH) | Salmeterol/fluticasone | 3 years | ↓Exacerbations v all ↑ HRQoL v P |
Placebo, salmeterol, fluticasone |
| Wedzicha et al88 (INSPIRE) | Salmeterol/fluticasone | 2 years | ↔ Exacerbations ↑ HRQoL ↓ Mortality ↑ Pneumonia |
Tiotropium |
Notes: The table shows all results for ICS/LABA. See also ICS and LABA sections for individual component results.
Abbreviations: P, placebo; F, formoterol; S, salmeterol; B, budesonide.
Synergistic mechanism of action
The two components of ICS/LABA may have an additive or synergistic effect; there is greater evidence of synergy in allergic inflammation than in COPD patients.81 Animal studies suggest that the combination of ICS plus LABA behaves synergistically,82 as ICS may regulate the coupling of β receptors to G proteins and hence cAMP activation, and overall response to LABA. Exposure to exogenous LABA (or SABA) leads to uncoupling by phosphorylation of the receptor via various pathways, which theoretically can lead to drug tolerance. Exposure to corticosteroids restores receptors to their previously sensitized state.83 Chronic LABA or SABA exposure will also lead to reduced β receptor numbers, as they are internalized and degraded; ICS reverse this effect because the activation of GRE causes gene transcription and hence synthesis of these receptors.84
The main RCTS for combination inhalers are shown in Table 6. A number of secondary analyses of TORCH data have also been published, detailing determinants of change in health status,85 beneficial effects on FEV1 decline,86 and stratifying analysis for disease stage.87
Safety
The side effect profiles of LABA/ICS combinations are effectively the sum of their component parts, as described above. Similar to the use of ICS, the most significant adverse event is the risk of pneumonia with combination inhalers.89 The risk is significantly higher than placebo or LABA but not so when compared with ICS. A recent systematic review and indirect comparison of trials looking at Symbicort and Seretide suggests that the risk of pneumonia is greater with Seretide, perhaps due to overall elevation in steroid load. The caveat to this is that data from the TORCH trial had a large bearing on the overall findings.90
Phosphodiesterase Inhibitors
Mechanism of action
Roflumilast is a selective phosphodiesterase 4 (PDE4) inhibitor; PDE4 is an enzyme which is expressed in many pro-inflammatory cells. The therapeutic effects of roflumilast are thought to be mediated via increased levels of cellular cAMP and include inhibition of microvascular leakage, inhibition of trafficking, release of cytokines, and chemokines from inflammatory cells, and mild bronchodilation.91 Roflumilast has a high oral bioavailability and is largely protein bound (98%). Peak plasma concentrations occur within one hour, mean elimination half-life is 17 hours, and a steady state is achieved after 4 days. The major metabolic pathway for roflumilast elimination after oral administration is pyridine N-oxidation, with the formation of roflumilast N-oxide; this metabolite is an active compound with pharmacokinetics distinct from its parent compound. Peak plasma concentrations occur after 4 hours and its elimination half is approximately 27 hours. Although the major elimination route for both the drug and its metabolite is the kidney, studies have shown that dose adjustment is not required in renal impairment.92 Other selective PDE4 inhibitors were developed and went through trials,93 but roflumilast is the only one currently on the market.
Theophylline is a non-selective phosphodiesterase inhibitor. Through the enzymatic inhibition of phosphodiesterase, levels of cAMP and cGMP are increased giving it its weak bronchodilator effect.94 To achieve this, however, fairly high concentrations of theophylline are needed. Recent evidence has shown that theophylline has some anti-inflammatory action and maybe able to modulate inflammatory gene expression by its interaction with the histone deacetylase.76 Theophylline like Roflumilast has a high oral bioavailability. Peak plasma concentration occurs between 1 and 2 hours, and it has an elimination half-life of 8 hours.75,77 Metabolism is largely by the liver, whereby the drug undergoes hydroxylation, N-demethylation, and N methylation to various compounds.75,77 The main RCTs for PDE4 inhibitors are shown in Table 10. Roflumilast appears to work best in the subset of COPD patients who have chronic bronchitis.95 Despite widespread use, there have been few parallel group studies of oral theophyllines; the Cochrane review in 2002 included 20 relatively small crossover studies and concluded that there were moderate beneficial effects on lung function, with the caveat that results may not be generalizable.96
Safety
Treatment-related adverse events were higher in the roflumilast treatment arms of the clinical trials in Table 7. Gastrointestinal side effects, namely weight loss and nausea, were the most common, followed by neuropsychiatric effects. Although dropout rates overall were similar between roflumilast and placebo, more patients in the roflumilast arm dropped out within the first few weeks. Theophylline has been used for a number of years in airway disease and still has a role in the management of COPD.1 The main adverse effects encountered include tachycardia, nausea, and tremor. Unfortunately it has a narrow therapeutic index and drug monitoring should be undertaken at regular intervals. The propensity for theophylline to interact with other drugs is another drawback to its use.
Table 7.
Major RCTs of phophodiesterase inhibitors.
| Trial | Drug | Duration | Outcome | Comparator |
|---|---|---|---|---|
| ZuWhallack et al97 | Theophylline | 12 weeks | ↑ FEV1** ↓ Symptoms** |
Salmeterol |
| Rabe et al98 | Roflumilast | 24 weeks | ↑ FEV1 ↓ Exacerbations |
Placebo |
| Calverley et al99 | Roflumilast | 1 year | ↑ FEV1 ↓Exacerbations* |
Placebo |
| Calverley et al100 | Roflumilast | 1 year | ↑ FEV1 ↓Exacerbations |
Placebo |
| Fabbri et al101 | Roflumilast | 1 year | ↑ FEV1 | Placebo |
Notes:
In GOLD stage 4 patients only;
in combination with salmeterol.
Mucolytics
Mucus hypersecretion and resultant chronic cough can often be a feature of COPD. Mucolytics have been used to reduce sputum viscosity and to aid expectoration; those most widely used are carbocysteine and N-acetylcysteine (NAC). Others include erdosteine and ambroxol. NAC and to a lesser extent carbocysteine have antioxidant properties. The awareness that oxidative stress and the formation of reactive oxygen species play a role in COPD, especially during exacerbations, has suggested that treatment with mucolytics may be able to influence exacerbation rates.102
Carbocysteine is a blocked thiol derivative of the amino acid L-cysteine. While the mechanism of action is not completely understood, it has the ability to split glycoprotein bonds in mucus. In vitro studies also suggest a mucoregulatory mechanism. Carbocysteine is well absorbed and peak serum concentration as achieved at between 1 and 1.7 hours after ingestion. Approximately 30%–60% of the drug is excreted unchanged in the urine. The fraction of the drug not excreted in this manner undergoes metabolism via varying pathways with great inter-individual variability.103
NAC is recognized as a mucolytic agent with direct and indirect antioxidant properties. It differs from carbocysteine in that it has a free thiol group capable of interacting with reactive oxygen species, leading to NAC disulfide as an end product. NAC’s indirect properties arise from its role as a glutathione precursor. It is rapidly absorbed following oral administration with peak plasma concentrations occurring between 2 to 3 hours and has a plasma half-life of 6.3 hours. NAC undergoes mainly hepatic metabolism.104
The most recent systematic review of clinical effectiveness of mucolytics included 30 studies and demonstrated a small but significant reduction in exacerbations in treated patients with COPD.105 Mucolytic agents however do not seem to have any effect on lung function. One of the largest trials with the power to demonstrate this was undertaken by Decramer et al. The use of NAC had no effect on lung function in those with mild or more severe COPD; additionally, mucolytics had no effect on HRQoL.106
Oxygen
Overt or relative hypoxia is one of the hallmarks of COPD, especially in the latter stages of the disease. Oxygen therapy to ameliorate this has been proven to be effective for patients who have severe resting hypoxia, and it is considered a prescription intervention in many countries. Whilst it is beyond the scope of this article to review all evidence pertaining to oxygen in detail, some key points and major studies can be included.
The basis for LTOT (>15 hours daily) is derived from two landmark RCTs: the NOTT trial which compared 12 hour (nocturnal) and 24 hour oxygen therapy,107 and the MRC trial which compared > 15 hour oxygen therapy to placebo.108 The main outcome in both trials was improved survival in patients receiving oxygen for at least 15 hours daily, though this improved survival was not seen in the MRC trial until one year after the initiation of oxygen therapy. The NOTT trial also demonstrated a fall in mean pulmonary artery pressure (PAP). Whilst a fall in mean PAP was not shown in the MRC trial, increases in PAP seen in the control arm did not occur in the patients undergoing oxygen therapy. LTOT is indicated for patients in a clinically stable state who have PaO2 < 7.3 kPa (55 mmHg) or 7.3–8 kPa (55–60 mmHg) in the presence of pulmonary hypertension, nocturnal hypoxia, or secondary polycythaemia when assessed on two separate occasions. Aside from LTOT, two other modes of oxygen therapy exist: ambulatory and short burst (SBOT); both have been reviewed relatively recently.109 Ambulatory oxygen is indicated in mobile patients who meet the LTOT criteria and is commonly considered in other COPD patients who exhibit exertional desaturation to less than 90%. SBOT criteria are poorly defined, and in general no benefits are seen;110 it should therefore be used only in a palliative setting.
Smoking Cessation
Tobacco smoke is arguably the most important etiological factor for the development of COPD in the developed world. The impact of continued smoking and its cessation on lung function (FEV1) is well known and illustrated via the Fletcher-Peto Curve.111 Despite the continued advances in pharmacotherapies for COPD, smoking cessation remains the best way to delay progression of COPD at all stages of the disease.
Over the last few years the neurochemical basis of nicotine addiction has been better understood; it is thought that the addictive properties of nicotine are a result of dopamine release mediated via stimulation of the alpha4beta2 nicotinic receptors.112 Currently, the most common therapeutic strategies employ the use of Varenicline, Bupropion, and Nicotine replacement therapy (NRT). Varenicline is a selective nicotinic receptor partial agonist. It mimics the effects of nicotine on dopamine release in the nucleus accumbens when given alone but attenuates this response to a subsequent nicotine challenge and reduces nicotine self-administration.112 Bupropion inhibits the reuptake of dopamine, noradrenaline, and serotonin in the central nervous system.112 During nicotine withdrawal, levels of these neurotransmitters fall, leading to withdrawal symptoms. Therefore, in limiting reuptake the symptoms of withdrawal are attenuated. All three modalities113–115 have proven to be effective compared to placebo in smoking cessation, with available evidence favoring the superiority of Varenicline over Bupropion. There is limited direct evidence between Varenicline and NRT, and only a few heterogeneous trials comparing Bupropion to NRT; overall it is likely that both are superior to NRT.113–115 Post-marketing surveillance has raised subsequent concerns about possible between Varenicline and suicidal ideation, such that a recent systematic review (mainly of evidence in populations unselected for psychiatric disease) advised using it only with caution in patients with pre-existing psychiatric problems.116 Conversely a recent RCT specific to schizophrenic patients found if effective and safe.117
Opiates
Despite optimal management of COPD with the above pharmacotherapies, dyspnoea can be problematic, especially in the advanced stages of the disease process. It pervades all aspects of patients’ life leading to a detrimental effect on its quality; opiates are widely used in a palliative setting to reduce the sensation of dyspnoea.118 Endogenous opioids might modulate dyspnoea by a reduction in ventilatory drive in response to carbon dioxide,119 hypoxia,119 and exercise.120 This decrease in respiratory effort may lead to a reduction in breathlessness. In addition, the sedative effect of opiates may reduce anxiety. The use of opiates to manage dyspnoea was the subject of a systematic review in 2002.121 This demonstrated a significant beneficial effect of oral and parenteral opiates over placebo. This benefit was also seen when the subgroup analysis for patients solely with COPD was undertaken. One of the major concerns and possibly barriers to opiate usage is respiratory depression, especially in opioid naive patients. However, when appropriately administered and monitored at lower doses, they do not appear to cause significant respiratory depression.122
Other Potential Pharmacotherapies
The multicomponent nature of COPD has meant that therapeutic strategies have been trialed in an attempt to gain additional benefit to the interventions outlined above. Ameliorating sequelae such as pulmonary hypertension (PH), improving nutrition, and trying to reduce systemic inflammation all have theoretical merits.
PH in COPD adversely affects survival and exercise capacity. Although the use of oxygen therapy has a protective effect on the progression of PH in patients with advanced COPD, its use is limited to patients who meet the LTOT criteria. Vasoactive compounds used in primary PH (sildenafil, bosentan, and nitric oxide) have been investigated in COPD and are shown in Table 8. Since some trials have shown worse HRQoL in treated patients, these drugs are not used routinely, although trial periods to see if benefit is seen are sometimes employed in selected patients by specialist centers.123
Table 8.
RCTs of drugs for PH in COPD.
| Trial | Drug | Duration | Outcome | Comparator |
|---|---|---|---|---|
| Vonbank et al124 | Nitric oxide + oxygen | 3 months | ↓PAP ↔ PaO2 |
Oxygen |
| Stolz et al125 | Bosentan | 3 months | ↓ PaO2 ↓HRQoL ↔ 6MWT distance |
Placebo |
| Valerio et al126 | Bosentan | 18 months | ↓PAP* ↑ 6MWT distance* |
Placebo |
| Rao et al127 | Sildenafil | 12 weeks | ↓PAP ↑ 6MWT distance |
Placebo |
| Lederer et al128 | Sildenafil | 4 weeks per intervention | ↔ 6MWT distance ↓HRQoL |
Placebo |
Notes:
Statistically significant improvement over baseline in treated patients; comparison between groups was not reported.
Over the last few years a number of studies have shown a relationship between vitamin D deficiency and severe COPD, whereby its deficiency seems to correlate with the degree of airflow obstruction.129,130 Clearly on this basis it would seem reasonable to replenish vitamin D levels. In a yearlong RCT, Lehouck et al131 used high dose vitamin D supplementation in patients with COPD. However, no benefit in lung function or exacerbations was seen in the treatment arm, except in the severely deficient in whom supplementation would be indicated for other reasons. Some studies have used other forms of nutritional supplementation in the context of pulmonary rehabilitation,132 albeit with limited benefits. Consequently, nutritional supplementation is not routinely used therapeutically in COPD.
Treatment Algorithms in COPD
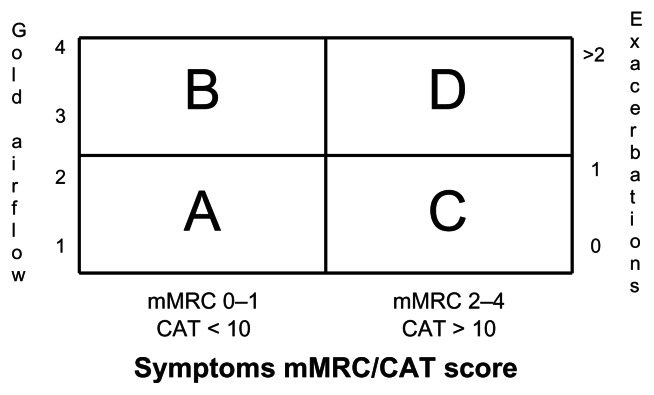
The updated GOLD guidance1 has made some changes to the way that the therapies detailed above are recommended. Other national guidance (eg, British NICE guidance5) differs markedly in the order of which drugs are used. In order to interpret the GOLD guidance, disease stage, symptoms, HRQoL and exacerbation frequency must all be taken into account; this is shown in Figure 4. Table 9 compares the main differences between guidelines, some of which are described below.
Figure 4.
Classifying disease severity by GOLD.
Notes: Patients are categorised by the combination of severity of spirometric impairment (GOLD airflow) or exacerbations and mMRC or CAT. Whilst it is recognised that spirometry or exacerbations and mMRC or CAT may result in different groups for some patients, GOLD recommends that treatment should be according to whichever method chosen results in the higher risk.
Table 9.
Comparison of GOLD and UK NICE guidance for COPD management.
| FEV1 | GOLD category | GOLD 1st line | GOLD 2nd line | NICE guidance |
|---|---|---|---|---|
| >50% | A | SABA/SAMA | LAMA/LABA/SAMA/SABA Theophylline |
SAMA/SABA LABA/LAMA |
| B | LAMA/LABA | LAMA+LABA | LABA+ICS* | |
| <50% | C | LABA+ICS/LAMA | LAMA+LABA PD4 Theophylline |
LABA+ICS LAMA+LABA ICS+LABA+LAMA |
| D | LABA+ICS/LAMA | LAMA+LABA ICS+LAMA ICS+LABA+LAMA ICS+LABA+PD4 LAMA+PD4 Theophylline |
Notes: First choice treatment shown in italics.
Only if persistent symptoms.
First line treatment
The use of bronchodilators is central to the management of COPD and, although not extensively discussed, the use of short acting agents are best used for rescue of symptoms. Both LAMAs and LABAs in the trials above were used across the full spectrum of disease severity and have all been shown to have significant effects on outcome measures in COPD. Consistent clinically and statistically significant improvements in lung function, HRQoL, and reduction in exacerbations in the region of 15%–20% have been demonstrated. International and national guidelines suggest that either a LABA or LAMA be used as maintenance therapy for GOLD2 disease. The choice of which agent depends on patient preference, tolerability, and cost.
Based on current evidence, tiotropium and indacaterol are essentially equivalent, with indacaterol being slightly more favorable at influencing HRQoL. The place of aclidinium and glycopyrronium is likely to be as an alternative to tiotropium or LABA. Thus, when it comes to prescribing maintenance therapy for patients with COPD, a trial of either indacaterol or tiotropium should be used in the first instance as they provide significantly better bronchodilation and reduce the risk of severe exacerbations.
Additions if remains symptomatic
The role of ICS in COPD is contentious. In most of the trials discussed, although FEV1 was superior to placebo the magnitude of improvement in FEV1 with ICS was inferior to that of the LABA used. TORCH was the only trial to show slower decline in FEV1 against placebo when fluticasone was used as a monotherapy. What has been proven to be relatively consistent is their ability to reduce exacerbations. This benefit is largely in patients with FEV1 < 50% (ie, GOLD ¾). The tradeoff for this benefit is an increased risk of local side effects and pneumonia. It is in these patients who suffer recurrent exacerbations that the risk benefit ratio is greatest. Their use in management of COPD cannot be advocated earlier as both LAMA and LABA monotherapy produce significant increases in lung function outcomes as well as a reduction in exacerbations. Current national guidelines in the UK do not suggest using ICS as monotherapy but in addition to a LABA; GOLD guidelines only recommend ICS monotherapy used with a LAMA as a second choice in patients with severe disease who suffer recurrent exacerbations and have poor functional capacity.
Whilst this section of the review focuses on the use of ICS in COPD, a growing body of evidence suggests that an overlap syndrome of COPD and asthma exists,133–136 although the syndrome is not clearly defined. However, the general consensus is that patients with incompletely reversible airflow obstruction exhibit sputum eosinophilia, history of atopy, and, following bronchodilator, either improvement in FEV1 > 12% (200 mls), or improvement in FEV1 > 15% (400 mls). In this subset of patients ICS therapy may have greater benefit and should be employed earlier.135,136
Other options
The use of roflumilast in patients has been largely confined to GOLD3/4 disease. In most of the clinical trials patients were selected on the basis of chronic bronchitis, recurrent exacerbations requiring oral corticosteroids, or the need for hospital admission. Its use provided modest but significantly improved FEV1 compared with placebo and a reduction in exacerbation rates when used with a long acting bronchodilator. International guidelines advocate its use as second line in GOLD 4 patients or as an adjunct to treatment in GOLD 3 patients who are already on LABA/ICS combination inhalers. UK guidance advocates its use in GOLD3 patients with a history of recurrent bronchitis or exacerbations. The place of theophylline is seemingly more variable. International guidelines advocate its use as an alternative to inhaled bronchodilators in mild disease, but also as an adjunct from moderate to very severe disease. UK guidelines only suggest its use after a trial of short or long acting bronchodilators or in those who cannot tolerate inhaled therapy.
Conclusion
The pharmacological management of COPD is driven by symptoms. As a progressive disease it is clear that over time increasing intervention will be required to manage symptoms appropriately. This review has outlined the key pharmacological treatments used. Unfortunately however, no treatment has been shown conclusively to reduce mortality and very few slow the rate of decline in lung function in COPD. ICS have not proven to be as effective as in asthma and what benefits are derived need to be balanced against the increased risk of pneumonia. Since bronchodilators are the mainstay of current management the search to improve existing bronchodilators will continue; so also will the search for novel anti-inflammatory agents and therapeutic strategies to reverse the corticosteroid resistance seen in COPD.
Footnotes
Author Contributions
Wrote the first draft of the manuscript: SE. Contributed to the writing of the manuscript: AMT. Agree with manuscript results and conclusions: AMT, SE. Jointly developed the structure and arguments for the paper: AMT, SE. Made critical revisions and approved final version: AMT. All authors reviewed and approved of the final manuscript.
Disclosures and Ethics
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests. Provenance: the authors were invited to submit this paper.
Funding
Neither author received funds specific to the writing of this review. AMT has current funds from the MRC, NIHR, Linde REAL fund, Hospital Infection Society and Alpha 1 Foundation.
Competing Interests
AMT has received speaker or advisory board fees totaling less than $3000 over the last 5 years from each of Novartis, GSK, Boehringer and AstraZeneca, all of whom make inhalers for use in COPD. AMT has non-commercial research funding of approximately $70,000 from the Linde group, who manufacture oxygen products in Europe.
References
- 1.Global Initiative for Obstructive Lung Disease. [Accessed Sep 21, 2012]. http://www.goldcopd.com.
- 2.Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106(2):196–204. doi: 10.7326/0003-4819-106-2-196. [DOI] [PubMed] [Google Scholar]
- 3.Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1418–22. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- 4.Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–52. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Institute for Health and Clinical Excellence. Chronic Obstructive Pulmonary Disease. Jun, 2010. http://www.nice.org.uk/CG1012010.
- 6.Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. J Assoc Physicians India. 2009;33(5):1165–85. doi: 10.1183/09031936.00128008. [DOI] [PubMed] [Google Scholar]
- 7.Mannino DM, Thorn D, Swensen A, Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J. 2008;32(4):962–9. doi: 10.1183/09031936.00012408. [DOI] [PubMed] [Google Scholar]
- 8.Beeh KM, Wagner F, Khindri S, Drollmann AF. Effect of indacaterol on dynamic lung hyperinflation and breathlessness in hyperinflated patients with COPD. COPD. 2011;8(5):340–5. doi: 10.3109/15412555.2011.594464. [DOI] [PubMed] [Google Scholar]
- 9.Cooper CB. Airflow obstruction and exercise. Resp Med. 2009;103(3):325–34. doi: 10.1016/j.rmed.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Hanania NA, Sharafkhaneh A, Barber R, Dickey BF. Beta-agonist intrinsic efficacy: measurement and clinical significance. Am J Respir Crit Care Med. 2002;165(10):1353–8. doi: 10.1164/rccm.2109060. [DOI] [PubMed] [Google Scholar]
- 11.Fazio F, Lafortuna C. Effect of inhaled salbutamol on mucociliary clearance in patients with chronic bronchitis. Chest. 1981;80(Suppl 6):827–30. doi: 10.1378/chest.80.6.827. [DOI] [PubMed] [Google Scholar]
- 12.Maris NA, van der Sluijs KF, Florquin S, et al. Salmeterol, a beta2-receptor agonist, attenuates lipopolysaccharide-induced lung inflammation in mice. Am J Physiol Lung Cell Mol Physiol. 2004;286(6):L1122–8. doi: 10.1152/ajplung.00125.2003. [DOI] [PubMed] [Google Scholar]
- 13.Tomlinson PR, Wilson JW, Stewart AG. Inhibition by salbutamol of the proliferation of human airway smooth muscle cells grown in culture. Br J Pharmacol. 1994;111(2):641–7. doi: 10.1111/j.1476-5381.1994.tb14784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Appleton S, Jones T, Poole P, et al. Ipratropium bromide versus long-acting beta-2 agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;3:CD006101. doi: 10.1002/14651858.CD006101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Noord JA, Smeets JJ, Maesen FP. A comparison of the onset of action of salbutamol and formoterol in reversing methacholine-induced bronchoconstriction. Res Med. 1998;92(12):1346–51. doi: 10.1016/s0954-6111(98)90140-8. [DOI] [PubMed] [Google Scholar]
- 16.Morgan DJ, Paull JD, Richmond BH, Wilson-Evered E, Ziccone SP. Pharmacokinetics of intravenous and oral salbutamol and its sulphate conjugate. Br J Clin Pharmacol. 1986;22(5):587–93. doi: 10.1111/j.1365-2125.1986.tb02939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chong J, Karner C, Poole P. Tiotropium versus long-acting beta-agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;9:CD009157. doi: 10.1002/14651858.CD009157.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Nie B, Xiong W, Xu Y. Effect of long-acting beta-agonists on the frequency of COPD exacerbations: a meta-analysis. J Clin Pharm Ther. 2012;37(2):204–11. doi: 10.1111/j.1365-2710.2011.01285.x. [DOI] [PubMed] [Google Scholar]
- 19.Ball DI, Brittain RT, Coleman RA, et al. Salmeterol, a novel, long-acting beta 2-adrenoceptor agonist: characterization of pharmacological activity in vitro and in vivo. Br J Pharmacol. 1991;104(3):665–71. doi: 10.1111/j.1476-5381.1991.tb12486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benhamou D, Cuvelier A, Muir JF, et al. Rapid onset of bronchodilation in COPD: a placebo-controlled study comparing formoterol (Foradil Aerolizer) with salbutamol (Ventodisk) Resp Med. 2001;95(10):817–21. doi: 10.1053/rmed.2001.1161. [DOI] [PubMed] [Google Scholar]
- 21.Celik G, Kayacan O, Beder S, Durmaz G. Formoterol and salmeterol in partially reversible chronic obstructive pulmonary disease: a crossover, placebo-controlled comparison of onset and duration of action. Respiration. 1999;66(5):434–9. doi: 10.1159/000029427. [DOI] [PubMed] [Google Scholar]
- 22.Green SA, Spasoff AP, Coleman RA, Johnson M, Liggett SB. Sustained activation of a G protein-coupled receptor via “anchored” agonist binding. Molecular localization of the salmeterol exosite within the 2-adrenergic receptor. J Biol Chem. 1996;271(39):24029–35. doi: 10.1074/jbc.271.39.24029. [DOI] [PubMed] [Google Scholar]
- 23.Manchee GR, Barrow A, Kulkarni S, et al. Disposition of salmeterol xinafoate in laboratory animals and humans. Drug Metab Dispos. 1993;21(6):1022–8. [PubMed] [Google Scholar]
- 24.Rosenborg J, Larsson P, Tegner K, Hallstrom G. Mass balance and metabolism of [(3)H]Formoterol in healthy men after combined i.v. and oral administration-mimicking inhalation. Drug Metab Dispos. 1999;27(10):1104–16. [PubMed] [Google Scholar]
- 25.Boyd G, Morice AH, Pounsford JC, Siebert M, Peslis N, Crawford C. An evaluation of salmeterol in the treatment of chronic obstructive pulmonary disease (COPD) Eur Respir J. 1997;10(4):815–21. [PubMed] [Google Scholar]
- 26.Mahler DA, Donohue JF, Barbee RA, et al. Efficacy of salmeterol xinafoate in the treatment of COPD. Chest. 1999;115(4):957–65. doi: 10.1378/chest.115.4.957. [DOI] [PubMed] [Google Scholar]
- 27.Rennard SI, Anderson W, ZuWallack R, et al. Use of a long-acting inhaled beta2-adrenergic agonist, salmeterol xinafoate, in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163(5):1087–92. doi: 10.1164/ajrccm.163.5.9903053. [DOI] [PubMed] [Google Scholar]
- 28.Calverley P, Pauwels R, Vestbo J, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003;361(9356):449–56. doi: 10.1016/S0140-6736(03)12459-2. [DOI] [PubMed] [Google Scholar]
- 29.Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–89. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 30.Dahl R, Greefhorst LA, Nowak D, et al. Inhaled formoterol dry powder versus ipratropium bromide in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(5):778–84. doi: 10.1164/ajrccm.164.5.2007006. [DOI] [PubMed] [Google Scholar]
- 31.Rossi A, Kristufek P, Levine BE, et al. Comparison of the efficacy, tolerability, and safety of formoterol dry powder and oral, slow-release theophylline in the treatment of COPD. Chest. 2002;121(4):1058–69. doi: 10.1378/chest.121.4.1058. [DOI] [PubMed] [Google Scholar]
- 32.Calverley PM, Boonsawat W, Cseke Z, Zhong N, Peterson S, Olsson H. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. Eur Respir J. 2003;22(6):912–9. doi: 10.1183/09031936.03.00027003. [DOI] [PubMed] [Google Scholar]
- 33.Szafranski W, Cukier A, Ramirez A, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J. 2003;21(1):74–81. doi: 10.1183/09031936.03.00031402. [DOI] [PubMed] [Google Scholar]
- 34.Kempsford R, Norris V, Siederer S. Vilanterol trifenatate, a novel inhaled long-acting beta2 adrenoceptor agonist, is well tolerated in healthy subjects and demonstrates prolonged bronchodilation in subjects with asthma and COPD. Pulm Pharmacol Ther. 2013 Apr;26(2):256–64. doi: 10.1016/j.pupt.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Battram C, Charlton SJ, Cuenoud B, et al. In vitro and in vivo pharmacological characterization of 5-[(R)-2-(5,6-diethyl-indan-2-ylamino)-1-hydroxyethyl]-8-hydroxy-1H-quinolin-2-o ne (indacaterol), a novel inhaled beta(2) adrenoceptor agonist with a 24-h duration of action. J Pharmacol Exp Ther. 2006;317(2):762–70. doi: 10.1124/jpet.105.098251. [DOI] [PubMed] [Google Scholar]
- 36.Donohue JF, Fogarty C, Lotvall J, et al. Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med. 2010;182(2):155–62. doi: 10.1164/rccm.200910-1500OC. [DOI] [PubMed] [Google Scholar]
- 37.Dahl R, Chung KF, Buhl R, et al. Efficacy of a new once-daily long-acting inhaled beta2-agonist indacaterol versus twice-daily formoterol in COPD. Thorax. 2010;65(6):473–9. doi: 10.1136/thx.2009.125435. [DOI] [PubMed] [Google Scholar]
- 38.Buhl R, Dunn LJ, Disdier C, et al. Blinded 12-week comparison of once-daily indacaterol and tiotropium in COPD. Eur Respir J. 2011;38(4):797–803. doi: 10.1183/09031936.00191810. [DOI] [PubMed] [Google Scholar]
- 39.Kornmann O, Dahl R, Centanni S, et al. Once-daily indacaterol versus twice-daily salmeterol for COPD: a placebo-controlled comparison. Eur Respir J. 2011;37(2):273–9. doi: 10.1183/09031936.00045810. [DOI] [PubMed] [Google Scholar]
- 40.Korn S, Kerwin E, Atis S, Amos C, Owen R, Lassen C. Indacaterol: once-daily provides superior efficacy to salmeterol twice-daily in COPD: a 12-week study. Respiratory Medicine. 2011;105(5):719–26. doi: 10.1016/j.rmed.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 41.Salpeter SR. Cardiovascular safety of beta(2)-adrenoceptor agonist use in patients with obstructive airway disease: a systematic review. Drugs and Aging. 2004;21(6):405–14. doi: 10.2165/00002512-200421060-00005. [DOI] [PubMed] [Google Scholar]
- 42.Rodrigo GJ, Nannini LJ, Rodriguez-Roisin R. Safety of long-acting beta-agonists in stable COPD: a systematic review. Chest. 2008;133(5):1079–87. doi: 10.1378/chest.07-1167. [DOI] [PubMed] [Google Scholar]
- 43.Lipson DA. Tiotropium bromide. Int J Chron Obstruct Pulmon Dis. 2006;1(2):107–14. doi: 10.2147/copd.2006.1.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Noord JA, Bantje TA, Eland ME, Korducki L, Cornelissen PJ. A randomised controlled comparison of tiotropium nd ipratropium in the treatment of chronic obstructive pulmonary disease. The Dutch Tiotropium Study Group. Thorax. 2000;55(4):289–94. doi: 10.1136/thorax.55.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kerwin E, Hebert J, Gallagher N, et al. Efficacy and safety of NVA237 versus placebo and tiotropium in patients with COPD: the GLOW2 study. Eur Respir J. 2012;40(5):1106–14. doi: 10.1183/09031936.00040712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fuhr R, Magnussen H, Sarem K, et al. Efficacy of aclidinium bromide 400 mug twice daily compared with placebo and tiotropium in patients with moderate to severe COPD. Chest. 2012;141(3):745–52. doi: 10.1378/chest.11-0406. [DOI] [PubMed] [Google Scholar]
- 47.Brusasco V, Hodder R, Miravitlles M, Korducki L, Towse L, Kesten S. Health outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with COPD. Thorax. 2003;58(5):399–404. doi: 10.1136/thorax.58.5.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Briggs DD, Jr, Covelli H, Lapidus R, Bhattycharya S, Kesten S, Cassino C. Improved daytime spirometric efficacy of tiotropium compared with salmeterol in patients with COPD. Pulmonary Pharmacology and Therapeutics. 2005;18(6):397–404. doi: 10.1016/j.pupt.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 49.Tashkin DP, Celli B, Senn S, et al. A 4 year trial of tiotropium in COPD. N Engl J Med. 2008;359:1543–54. doi: 10.1056/NEJMoa0805800. [DOI] [PubMed] [Google Scholar]
- 50.Vogelmeier C, Hederer B, Glaab T, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364(12):1093–103. doi: 10.1056/NEJMoa1008378. [DOI] [PubMed] [Google Scholar]
- 51.D’Urzo A, Ferguson GT, van Noord JA, et al. Efficacy and safety of once-daily NVA237 in patients with moderate-to-severe COPD: the GLOW1 trial. Respir Res. 2011;12:156. doi: 10.1186/1465-9921-12-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones PW, Rennard SI, Agusti A, et al. Efficacy and safety of once-daily aclidinium in chronic obstructive pulmonary disease. Respir Res. 2011;12:55. doi: 10.1186/1465-9921-12-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jones PW, Singh D, Bateman ED, et al. Efficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN study. Eur Respir J. 2012;40(4):830–6. doi: 10.1183/09031936.00225511. [DOI] [PubMed] [Google Scholar]
- 54.Kerwin EM, D’Urzo AD, Gelb AF, Lakkis H, Garcia Gil E, Caracta CF. Efficacy and safety of a 12-week treatment with twice-daily aclidinium bromide in COPD patients (ACCORD COPD I) COPD. 2012;9(2):90–101. doi: 10.3109/15412555.2012.661492. [DOI] [PubMed] [Google Scholar]
- 55.Haddad EB, Mak JC, Barnes PJ. Characterization of [3H]Ba 679 BR, a slowly dissociating muscarinic antagonist, in human lung: radioligand binding and autoradiographic mapping. Molecul Pharmacol. 1994;45(5):899–907. [PubMed] [Google Scholar]
- 56.Turck D, Weber W, Sigmund R, et al. Pharmacokinetics of intravenous, single-dose tiotropium in subjects with different degrees of renal impairment. J Clin Pharmacol. 2004;44(2):163–72. doi: 10.1177/0091270003261315. [DOI] [PubMed] [Google Scholar]
- 57.Sykes DA, Dowling MR, Leighton-Davies J, et al. The Influence of receptor kinetics on the onset and duration of action and the therapeutic index of NVA237 and tiotropium. J Pharmacol Exp Ther. 2012;343(2):520–8. doi: 10.1124/jpet.112.194456. [DOI] [PubMed] [Google Scholar]
- 58.Casarosa P, Bouyssou T, Germeyer S, Schnapp A, Gantner F, Pieper M. Preclinical evaluation of long-acting muscarinic antagonists: comparison of tiotropium and investigational drugs. J Pharmacol Exp Ther. 2009;330(2):660–8. doi: 10.1124/jpet.109.152470. [DOI] [PubMed] [Google Scholar]
- 59.Sechaud R, Renard D, Zhang-Auberson L, Motte Sde L, Drollmann A, Kaiser G. Pharmacokinetics of multiple inhaled NVA237 doses in patients with chronic obstructive pulmonary disease (COPD) Int J Clin Pharmacol Ther. 2012;50(2):118–28. doi: 10.5414/cp201612. [DOI] [PubMed] [Google Scholar]
- 60.Gavalda A, Miralpeix M, Ramos I, et al. Characterization of aclidinium bromide, a novel inhaled muscarinic antagonist, with long duration of action and a favorable pharmacological profile. J Pharmacol Exp Ther. 2009;331(2):740–51. doi: 10.1124/jpet.109.151639. [DOI] [PubMed] [Google Scholar]
- 61.Schmid K, Pascual S, Gil EG, Ortiz S, Jansat JM. Pharmacokinetics and safety of aclidinium bromide, a muscarinic antagonist, in adults with normal or impaired renal function: A phase I, open-label, single-dose clinical trial. Clin Ther. 2010;32(10):1798–812. doi: 10.1016/j.clinthera.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 62.Singh S, Loke YK, Furberg CD. Inhaled anticholinergics and risk of major adverse cardiovascular events in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2008;300(12):1439–50. doi: 10.1001/jama.300.12.1439. [DOI] [PubMed] [Google Scholar]
- 63.Celli B, Decramer M, Kesten S, Liu D, Mehra S, Tashkin DP. Mortality in the 4 year trial of tiotropium (UPLIFT) in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180:948–55. doi: 10.1164/rccm.200906-0876OC. [DOI] [PubMed] [Google Scholar]
- 64.Singh S, Loke YK, Enright PL, Furberg CD. Mortality associated with tiotropium mist inhaler in patients with chronic obstructive pulmonary disease: systematic review and meta-analysis of randomised controlled trials. BMJ. 2011;342:d3215. doi: 10.1136/bmj.d3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Karner C, Chong J, Poole P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;7:CD009285. doi: 10.1002/14651858.CD009285.pub2. [DOI] [PubMed] [Google Scholar]
- 66.Dong YH, Lin HH, Shau WY, Wu YC, Chang CH, Lai MS. Comparative safety of inhaled medications in patients with chronic obstructive pulmonary disease: systematic review and mixed treatment comparison meta-analysis of randomised controlled trials. Thorax. 2013;68(1):48–56. doi: 10.1136/thoraxjnl-2012-201926. [DOI] [PubMed] [Google Scholar]
- 67.Nakamura T, Nakanishi T, Haruta T, Shirasaka Y, Keogh JP, Tamai I. Transport of ipratropium, an anti-chronic obstructive pulmonary disease drug, is mediated by organic cation/carnitine transporters in human bronchial epithelial cells: implications for carrier-mediated pulmonary absorption. Mol Pharm. 2010;7(1):187–95. doi: 10.1021/mp900206j. [DOI] [PubMed] [Google Scholar]
- 68.Brand P, Meyer T, Weuthen T, et al. Lung deposition of radiolabeled tiotropium in healthy subjects and patients with chronic obstructive pulmonary disease. J Clin Pharmacol. 2007;47(10):1335–41. doi: 10.1177/0091270006295788. [DOI] [PubMed] [Google Scholar]
- 69.Brand P, Hederer B, Austen G, Dewberry H, Meyer T. Higher lung deposition with Respimat Soft Mist inhaler than HFA-MDI in COPD patients with poor technique. Int J Chron Obstruct Pulmon Dis. 2008;3(4):763–70. [PMC free article] [PubMed] [Google Scholar]
- 70.Beasley R, Singh S, Loke YK, Enright P, Furberg CD. Call for worldwide withdrawal of tiotropium Respimat mist inhaler. BMJ. 2012;345:e7390. doi: 10.1136/bmj.e7390. [DOI] [PubMed] [Google Scholar]
- 71.British Thoracic Society. British Guideline on the Management of Asthma. 2008. http://thorax.bmj.com/content/63/Suppl_4/iv1.full.pdf.
- 72.Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2008;8(3):183–92. doi: 10.1038/nri2254. [DOI] [PubMed] [Google Scholar]
- 73.Yang IA, Clarke MS, Sim EH, Fong KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;7:CD002991. doi: 10.1002/14651858.CD002991.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ito K, Yamamura S, Essilfie-Quaye S, et al. Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-kappaB suppression. J Exp Med. 2006;203(1):7–13. doi: 10.1084/jem.20050466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barnes PJ. Development of New Drugs for COPD. Curr Med Chem. 2012 Sep 3; doi: 10.2174/0929867311320120005. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 76.Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA, Maslen TK. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ. 2000;320(7245):1297–303. doi: 10.1136/bmj.320.7245.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Winkler J, Hochhaus G, Derendorf H. How the lung handles drugs: pharmacokinetics and pharmacodynamics of inhaled corticosteroids. Proc Am Thorac Soc. 2004;1(4):356–63. doi: 10.1513/pats.200403-025MS. [DOI] [PubMed] [Google Scholar]
- 78.Dalby C, Polanowski T, Larsson T, Borgstrom L, Edsbacker S, Harrison TW. The bioavailability and airway clearance of the steroid component of budesonide/formoterol and salmeterol/fluticasone after inhaled administration in patients with COPD and healthy subjects: a randomized controlled trial. Respir Res. 2009;10:104. doi: 10.1186/1465-9921-10-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Holme J, Tomlinson JW, Stockley RA, Stewart PM, Barlow N, Sullivan AL. Adrenal suppression in bronchiectasis and the impact of inhaled corticosteroids. Eur Respir J. 2008;32(4):1047–52. doi: 10.1183/09031936.00016908. [DOI] [PubMed] [Google Scholar]
- 80.van Noord JA, Buhl R, Laforce C, et al. QVA149 demonstrates superior bronchodilation compared with indacaterol or placebo in patients with chronic obstructive pulmonary disease. Thorax. 2010;65(12):1086–91. doi: 10.1136/thx.2010.139113. [DOI] [PubMed] [Google Scholar]
- 81.Johnson M. Interactions between corticosteroids and beta2-agonists in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2004;1(3):200–6. doi: 10.1513/pats.200402-010MS. [DOI] [PubMed] [Google Scholar]
- 82.Mattila MJ, Salonen RO. Modification by betamethasone of the effects of bronchodilator drugs on cholinergic bronchoconstriction in rats. Br J Pharmacol. 1984;83(3):607–14. doi: 10.1111/j.1476-5381.1984.tb16214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hui KK, Conolly ME, Tashkin DP. Reversal of human lymphocyte beta-adrenoceptor desensitization by glucocorticoids. Clin Pharmacol Ther. 1982;32(5):566–71. doi: 10.1038/clpt.1982.204. [DOI] [PubMed] [Google Scholar]
- 84.Mak JC, Nishikawa M, Barnes PJ. Glucocorticosteroids increase beta 2-adrenergic receptor transcription in human lung. Am J Physiol. 1995;268(1 Pt 1):L41–6. doi: 10.1152/ajplung.1995.268.1.L41. [DOI] [PubMed] [Google Scholar]
- 85.Jones PW, Anderson JA, Calverley PM, et al. Health status in the TORCH study of COPD: treatment efficacy and other determinants of change. Respir Res. 2011;12:71. doi: 10.1186/1465-9921-12-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Celli BR, Thomas NE, Anderson JA, et al. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med. 2008;178(4):332–8. doi: 10.1164/rccm.200712-1869OC. [DOI] [PubMed] [Google Scholar]
- 87.Jenkins CR, Jones PW, Celli B, et al. Efficacy of salmeterol and fluticasone propionate by GOLD stage of COPD: analysis from the randomised, placeo controlled TORCH study. Respir Res. 2009;10:59. doi: 10.1186/1465-9921-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wedzicha JA, Calverley PM, Seemungal TA, Hagan G, Ansari Z, Stockley RA. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med. 2008;177(1):19–26. doi: 10.1164/rccm.200707-973OC. [DOI] [PubMed] [Google Scholar]
- 89.Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;9:CD006829. doi: 10.1002/14651858.CD006829.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Halpin DM, Gray J, Edwards SJ, Morais J, Singh D. Budesonide/formoterol vs. salmeterol/fluticasone in COPD: a systematic review and adjusted indirect comparison of pneumonia in randomised controlled trials. Int J Clin Pract. 2011;65(7):764–74. doi: 10.1111/j.1742-1241.2011.02685.x. [DOI] [PubMed] [Google Scholar]
- 91.Giembycz MA, Field SK. Roflumilast: first phosphodiesterase 4 inhibitor approved for treatment of COPD. Drug Des Devel Ther. 2010;4:147–58. doi: 10.2147/dddt.s7667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bethke TD, Hartmann M, Hunnemeyer A, Lahu G, Gleiter CH. Influence of renal impairment on the pharmacokinetics of oral roflumilast: an open-label, parallel-group, single-center study. Int J Clin Pharmacol Ther. 2011;49(8):491–9. doi: 10.5414/cp201556. [DOI] [PubMed] [Google Scholar]
- 93.Rennard SI, Schachter N, Strek M, Rickard K, Amit O. Cilomilast for COPD: results of a 6-month, placebo-controlled study of a potent, selective inhibitor of phosphodiesterase 4. Chest. 2006;129(1):56–66. doi: 10.1378/chest.129.1.56. [DOI] [PubMed] [Google Scholar]
- 94.Rovei V, Chanoine F, Strolin Benedetti M. Pharmacokinetics of theophylline: a dose-range study. Br J Clin Pharmacol. 1982;14(6):769–78. doi: 10.1111/j.1365-2125.1982.tb02035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rennard SI, Calverley PM, Goehring UM, Bredenbroker D, Martinez FJ. Reduction of exacerbations by the PDE4 inhibitor roflumilast—the importance of defining different subsets of patients with COPD. Respir Res. 2011;12:18. doi: 10.1186/1465-9921-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ram FS, Jones PW, Castro AA, et al. Oral theophylline for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2002;4:CD003902. doi: 10.1002/14651858.CD003902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.ZuWallack RL, Mahler DA, Reilly D, et al. Salmeterol plus theophylline combination therapy in the treatment of COPD. Chest. 2001;119(6):1661–70. doi: 10.1378/chest.119.6.1661. [DOI] [PubMed] [Google Scholar]
- 98.Rabe KF, Bateman ED, O’Donnell D, Witte S, Bredenbroker D, Bethke TD. Roflumilast—an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2005;366(9485):563–71. doi: 10.1016/S0140-6736(05)67100-0. [DOI] [PubMed] [Google Scholar]
- 99.Calverley PM, Sanchez-Toril F, McIvor A, Teichmann P, Bredenbroeker D, Fabbri LM. Effect of 1-year treatment with roflumilast in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176(2):154–61. doi: 10.1164/rccm.200610-1563OC. [DOI] [PubMed] [Google Scholar]
- 100.Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374(9691):685–94. doi: 10.1016/S0140-6736(09)61255-1. [DOI] [PubMed] [Google Scholar]
- 101.Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet. 2009;374(9691):695–703. doi: 10.1016/S0140-6736(09)61252-6. [DOI] [PubMed] [Google Scholar]
- 102.Dekhuijzen PN. Antioxidant properties of N-acetylcysteine: their relevance in relation to chronic obstructive pulmonary disease. Eur Respir J. 2004;23(4):629–36. doi: 10.1183/09031936.04.00016804. [DOI] [PubMed] [Google Scholar]
- 103.Hooper C, Calvert J. The role for S-carboxymethylcysteine (carbocisteine) in the management of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2008;3(4):659–69. [PMC free article] [PubMed] [Google Scholar]
- 104.Dekhuijzen PN, van Beurden WJ. The role for N-acetylcysteine in the management of COPD. Int J Chron Obstruct Pulmon Dis. 2006;1(2):99–106. doi: 10.2147/copd.2006.1.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Poole P, Black PN, Cates CJ. Mucolytic agents for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;8:CD001287. doi: 10.1002/14651858.CD001287.pub4. [DOI] [PubMed] [Google Scholar]
- 106.Decramer M, Rutten-van Molken M, Dekhuijzen PN, et al. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): a randomised placebo-controlled trial. Lancet. 2005;365(9470):1552–60. doi: 10.1016/S0140-6736(05)66456-2. [DOI] [PubMed] [Google Scholar]
- 107.Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Nocturnal Oxygen Therapy Trial Group. Ann Intern Med. 1980;93(3):391–8. doi: 10.7326/0003-4819-93-3-391. [DOI] [PubMed] [Google Scholar]
- 108.Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working Party. Lancet. 1981;1(8222):681–6. [PubMed] [Google Scholar]
- 109.Stoller JK, Panos RJ, Krachman S, Doherty DE, Make B. Oxygen therapy for patients with COPD: current evidence and the long-term oxygen treatment trial. Chest. 2010;138(1):179–87. doi: 10.1378/chest.09-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.O’Neill B, Mahon JM, Bradley J. Short-burst oxygen therapy in chronic obstructive pulmonary disease. Respir Med. 2006 Jul;100(7):1129–38. doi: 10.1016/j.rmed.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 111.Fletcher C, Peto R. The natural history of chronic airflow obstruction. BMJ. 1977;1(6077):1645–8. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fagerstrom K, Balfour DJ. Neuropharmacology and potential efficacy of new treatments for tobacco dependence. Expert Opin Investig Drugs. 2006 Feb;15(2):107–16. doi: 10.1517/13543784.15.2.107. [DOI] [PubMed] [Google Scholar]
- 113.Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2007;1:CD000031. doi: 10.1002/14651858.CD000031.pub3. [DOI] [PubMed] [Google Scholar]
- 114.Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2012;4:CD006103. doi: 10.1002/14651858.CD006103.pub2. [DOI] [PubMed] [Google Scholar]
- 115.Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;11:CD000146. doi: 10.1002/14651858.CD000146.pub4. [DOI] [PubMed] [Google Scholar]
- 116.Ahmed AI, Ali AN, Kramers C, Harmark LV, Burger DM, Verhoeven WM. Neuropsychiatric adverse events of varenicline: a systematic review of published reports. J Clin Psychopharmacol. 2013;33(1):55–62. doi: 10.1097/JCP.0b013e31827c0117. [DOI] [PubMed] [Google Scholar]
- 117.Williams JM, Anthenelli RM, Morris CD, et al. A randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of varenicline for smoking cessation in patients with schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2012;73(5):654–60. doi: 10.4088/JCP.11m07522. [DOI] [PubMed] [Google Scholar]
- 118.Rocker G, Horton R, Currow D, Goodridge D, Young J, Booth S. Palliation of dyspnoea in advanced COPD: revisiting a role for opioids. Thorax. 2009;64(10):910–5. doi: 10.1136/thx.2009.116699. [DOI] [PubMed] [Google Scholar]
- 119.Weil JV, McCullough RE, Kline JS, Sodal IE. Diminished ventilatory response to hypoxia and hypercapnia after morphine in normal man. N Engl J Med. 1975;292(21):1103–6. doi: 10.1056/NEJM197505222922106. [DOI] [PubMed] [Google Scholar]
- 120.Santiago TV, Johnson J, Riley DJ, Edelman NH. Effects of morphine on ventilatory response to exercise. J Appl Physiol. 1979;47(1):112–8. doi: 10.1152/jappl.1979.47.1.112. [DOI] [PubMed] [Google Scholar]
- 121.Jennings AL, Davies AN, Higgins JP, Gibbs JS, Broadley KE. A systematic review of the use of opioids in the management of dyspnoea. Thorax. 2002;57(11):939–44. doi: 10.1136/thorax.57.11.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Abernethy AP, Currow DC, Frith P, Fazekas BS, McHugh A, Bui C. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ. 2003;327(7414):523–8. doi: 10.1136/bmj.327.7414.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hurdman J, Condliffe R, Elliot CA, et al. Pulmonary hypertension in COPD: results from the ASPIRE registry. Eur Respir J. 2012 Sep 27; doi: 10.1183/09031936.00079512. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 124.Vonbank K, Ziesche R, Higenbottam TW, et al. Controlled prospective randomised trial on the effects on pulmonary haemodynamics of the ambulatory long term use of nitric oxide and oxygen in patients with severe COPD. Thorax. 2003;58(4):289–93. doi: 10.1136/thorax.58.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Stolz D, Rasch H, Linka A, et al. A randomised, controlled trial of bosentan in severe COPD. Eur Respir J. 2008;32(3):619–28. doi: 10.1183/09031936.00011308. [DOI] [PubMed] [Google Scholar]
- 126.Valerio G, Bracciale P, Grazia D’Agostino A. Effect of bosentan upon pulmonary hypertension in chronic obstructive pulmonary disease. Ther Adv Respir Dis. 2009;3(1):15–21. doi: 10.1177/1753465808103499. [DOI] [PubMed] [Google Scholar]
- 127.Rao RS, Singh S, Sharma BB, Agarwal VV, Singh V. Sildenafil improves six-minute walk distance in chronic obstructive pulmonary disease: a randomised, double-blind, placebo-controlled trial. Indian J Chest Dis Allied Sci. 2011;53(2):81–5. [PubMed] [Google Scholar]
- 128.Lederer DJ, Bartels MN, Schluger NW, et al. Sildenafil for chronic obstructive pulmonary disease: a randomized crossover trial. COPD. 2012;9(3):268–75. doi: 10.3109/15412555.2011.651180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Janssens W, Bouillon R, Claes B, et al. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax. 2010;65(3):215–20. doi: 10.1136/thx.2009.120659. [DOI] [PubMed] [Google Scholar]
- 130.Wood AM, Bassford C, Webster D, et al. Vitamin D-binding protein contributes to COPD by activation of alveolar macrophages. Thorax. 2011;66(3):205–10. doi: 10.1136/thx.2010.140921. [DOI] [PubMed] [Google Scholar]
- 131.Lehouck A, Mathieu C, Carremans C, et al. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2012;156(2):105–14. doi: 10.7326/0003-4819-156-2-201201170-00004. [DOI] [PubMed] [Google Scholar]
- 132.Creutzberg EC, Wouters EF, Mostert R, Weling-Scheepers CA, Schols AM. Efficacy of nutritional supplementation therapy in depleted patients with chronic obstructive pulmonary disease. Nutrition. 2003;19(2):120–7. doi: 10.1016/s0899-9007(02)00841-9. [DOI] [PubMed] [Google Scholar]
- 133.Gibson PG, Simpson JL. The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax. 2009;64(8):728–35. doi: 10.1136/thx.2008.108027. [DOI] [PubMed] [Google Scholar]
- 134.Hardin M, Silverman EK, Barr RG, et al. The clinical features of the overlap between COPD and asthma. Respir Res. 2011;12:127. doi: 10.1186/1465-9921-12-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Soler-Cataluna JJ, Cosio B, Izquierdo JL, et al. Consensus document on the overlap phenotype COPD-asthma in COPD. Archivos de Bronconeumologia. 2012;48(9):331–7. doi: 10.1016/j.arbres.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 136.Kitaguchi Y, Komatsu Y, Fujimoto K, Hanaoka M, Kubo K. Sputum eosinophilia can predict responsiveness to inhaled corticosteroid treatment in patients with overlap syndrome of COPD and asthma. Int J Chron Obstruct Pulmon Dis. 2012;7:283–9. doi: 10.2147/COPD.S30651. [DOI] [PMC free article] [PubMed] [Google Scholar]