Abstract
Background
Genetic factors in the pathogenesis of cardiomyopathies have received a lot attention during the past two decades. Angiotensin I converting enzyme (ACE) insertion/deletion (I/D) polymorphisms were found to be associated with cardiomyopathies. However, the previous results were inconsistent. The current meta-analysis aims to examine the association of ACE I/D polymorphisms and dilated cardiomyopathy (DCM) or hypertrophic cardiomyopathy (HCM).
Methods
Eight studies on DCM (1387 controls and 977 patients) and eight studies on HCM (1055 controls and 827 patients) were included in this meta-analysis.
Results
The overall data showed no significant association between ACE I/D polymorphism and DCM risk. Further subgroup analysis by ethnicity also did not find a significantly increased risk for D allele carriers among East Asians and Europeans. However, the overall analysis suggested that the D allele carriers might be associated with increased risk of HCM (DD/ID vs. II: OR = 1.69, 95% CI 1.04–2.74, P = 0.03).
Conclusion
In summary, the meta-analysis indicated that certain ACE I/D polymorphism might be associated with HCM but not DCM susceptibility. Given the limited sample sizes, further large multicenter case-control investigation is needed.
Introduction
Dilated cardiomyopathy (DCM), which is characterized by ventricular chamber enlargement and systolic dysfunction with normal left ventricular wall thickness, leads to progressive heart failure, arrhythmias, and sudden or heart failure related death. Previous family studies revealed that 20% to 50% of idiopathic DCM had a familial origin, suggesting genetic factors might play an important role in the disease pathogenesis. [1] On the other side, hypertrophic cardiomyopathy (HCM), which is diagnosed in the presence of left ventricular hypetrophy, is also reported to be genetically heterogenous. [2] During the past two decades, several genetic mutations were reported to cause DCM or HCM.
Several genes encoding the components of the renin-angiotensin-aldosterone system (RAAS) have been revealed to be associated with cardiovascular diseases, including hypertension, myocardial infarction, ischemic stroke, and cardiomyopathy. [3], [4], [5] As DCM to be considered, the insertion/deletion polymorphism in the angiotensin I converting enzyme gene (ACE I/D) has been commonly reported. [6], [7] However, the previous results were inconsistent. Raynolds MV et al found that compared with the DD frequency in the control population, the frequency of the ACE DD genotype was 48% higher in individuals with idiopathic DCM. [6] However, Montgomery HE et al reported that the ACE genotype distribution and allele frequencies were similar in patients and control subjects. [7] Furthermore, current evidence supported the inhibition of renin-angiotensin system might be beneficial to patients of DCM. Similarly, the association between ACE I/D polymorphisms and HCM was also inconsistently reported [8], [9].
To date, no large-scale studies have assessed the association between ACE I/D polymorphisms and DCM or HCM. This lack of knowledge emphasizes the importance of the present meta-analysis. Thus, we performed this meta-analysis to clarify this inconsistency between ACE I/D polymorphisms and DCM or HCM.
Materials and Methods
A computerized search of PubMed and the Cochrane Library published before November 2012 was conducted. Only studies published in English were considered. Furthermore, the references of the relevant studies were also searched. The google scholar website was also searched. When the same patient population was included in different reports, only the study with complete data was used in this meta-analysis. We used the following key words for searching for the relevant reports: angiotensin I converting enzyme, dilated cardiomyopathy, hypertrophic cardiomyopathy, variant and polymorphism. Two reviewers (J.M Yang and C Zhang) independently searched the titles, abstracts, and full-texts to determine whether the data met the inclusion criteria. Conflicts were resolved by consensus.
Inclusion Criteria
The studies included in the meta-analysis must meet all the following three criteria: (1) evaluating the association of ACE I/D polymorphism with DCM or HCM; (2) using case-control design; (3) providing sufficient data upon genotype counts.
Exclusion Criteria
All the patients were excluded for ischemic cardiomyopathy and severe coronary obstruction for DCM, and potential stimulus such as hypertension, ischemic ischemic heart disease, valvular heart disease, congenital malformations of the heart or vessels, and intrinsic pulmonary disease for HCM.
Data Extraction
For each study, the following information was extracted: the first author’s name, publication date, region and ethnicity of participants, sample size of cases and controls, source of controls, myocardial biopsy, genotype distribution in cases and controls.
Statistical Methods
All the statistical analyses were performed by Review Manager version 5.1. Crude odds ratios (ORs) and 95% confidence intervals (CIs) were used to assess the association strength between ACE I/D polymorphism and DCM or HCM risk. We also tested the heterogeneity among the included reports and P<0.10 was considered to be significant heterogeneity. In this study, a random effects model was used because of the presence of heterogeneity. Because the number of included studies was <10, we did not assess the publication bias (www.cochranehandbook.org).
Results
Characteristics of the Included Studies
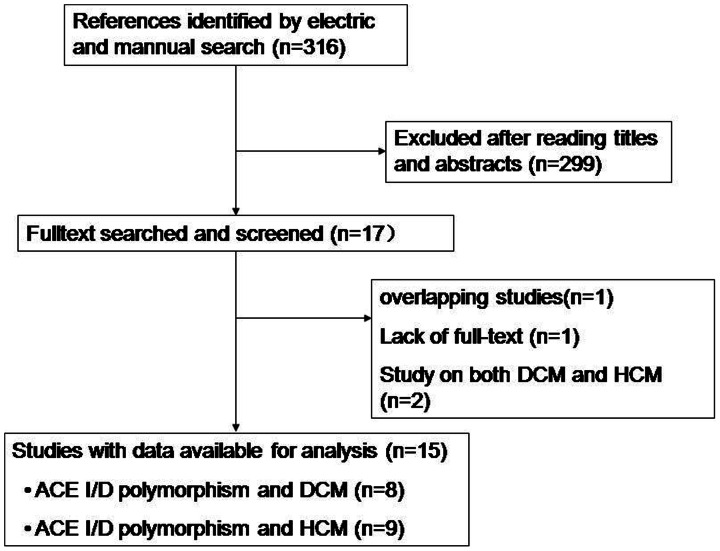
A total of 316 studies were screened and 299 studies were excluded after reading titles and abstracts. One study by Harn HJ et al. was excluded because of lacking full-text. For the two overlapping studies, [10], [11] the one published recently was included. [11] Two studies investigated the effect of ACE I/D polymorphisms on both DCM and HCM. [9], [12] Thus, eight studies on DCM (1387 controls and 977 patients, Table 1) and eight studies on HCM (1055 controls and 827 patients, Table 2) were included in this meta-analysis (Figure 1). Table 3 showed the genotype distribution in each included study.
Table 1. The characteristics of eligible studies on DCM considered in the meta-analysis.
| Author | Year | Region | Ethnicity | Sample size (case/control) | Source of controls | Myocardialbiopsy | Diagnostic criteria |
| Raynolds MV et al. [6] | 1993 | USA | Caucasian | 112/79 | Healthy donors | Yes | EF<40%, LV enlargement and normal coronary arteries |
| Montgomery HE et al. [7] | 1995 | England | English | 99/364 | Healthy subjects | Yes | EF<40%, LV dilation and normal coronary arteries |
| Sanderson JE et al. [20] | 1996 | China | Chinese | 100/100 | Healthy subjects | Yes | EF<40%, fractional shortening<25% and with no ischemic cardiomyopathy |
| Yamada Y et al. [9] | 1997 | Japan | Japanese | 88/122 | Healthy subjects | Yes | DCM without other potential stimulus |
| Tiret L et al. [17] | 2000 | French | NA | 422/387 | Healthy subjects | No | EF<40%, LV dilation and with no ≥50% artery obstruction |
| Rai TS et al. [12] | 2008 | India | Indian | 51/164 | Healthy subjects | No | EF<40% and LV end diastolic diameter>117% of normal value |
| Kucukarabaci B et al. [21] | 2008 | Turkey | Turkish | 29/20 | Healthy subjects | No | NA |
| Mahjoub S et al. [16] | 2010 | Tunisia | Tunisian | 76/151 | Healthy subjects | No | LV fractional shortening <25%, EF<45% and LVEDD>69 mm |
DCM, dilated cardiomyopathy; NA, not available; EF, ejection fraction; LV, left ventricular.
Table 2. The characteristics of eligible studies on HCM considered in the meta-analysis.
| Author | Year | Region | Ethnicity | Sample size (case/control) | Source of controls | Myocardialbiopsy | Diagnostic criteria |
| Marian AJ et al. [22] | 1993 | USA | NA | 100/106 | Familiar healthy subjects | No | septal or ventricular thickness≥13 mm without other potential causes |
| Pfeufer A et al. [23] | 1996 | German | Caucasian | 50/50 | Healthy subjects | No | septal or ventricular thickness≥13 mm without hypertension and valvular heart disease |
| Yamada Y et al. [9] | 1997 | Japan | Japanese | 71/122 | Healthy subjects | Yes | LV hypertrophy without other potential causes |
| Ogimoto A et al. [24] | 2002 | Japan | Japanese | 138/205 | Healthy subjects | No | LV hypertrophy without other potential causes |
| Kawaguchi H et al. [11] | 2003 | Japan | Japanese | 80/88 | Familiar healthy subjects | No | LV hypertrophy without other potential causes |
| Rai TS et al. [12] | 2008 | India | Indian | 118/164 | Healthy subjects | No | Unexplained LV hypertrophy ≥13 mm or >2 standard deviations |
| Kaya CT et al. [8] | 2010 | Turkey | Turkish | 63/20 | Healthy subjects | No | LV hypertrophy ≥13 mm without other hypertrophic stimulus |
| Coto E et al. [19] | 2010 | Spain | Caucasian | 207/300 | Healthy subjects | No | LV hypertrophy ≥13 mm without other hypertrophic stimulus |
HCM, hypertrophic cardiomyopathy; NA, not available; LV, left ventricular.
Figure 1. Flow chart of studies selection.
Table 3. The distribution of polymorphism for cases and controls.
| Genotypes (n) | Alleles (n) | |||||||||||
| Cases | Controls | I | D | |||||||||
| DCM | II | ID | DD | II | ID | DD | Case | Control | Case | Control | ||
| Raynolds MV et al. [6] | 1993 | 72 | 40 | 60 | 19 | NA | NA | NA | NA | |||
| Montgomery HE et al. [7] | 1995 | 18 | 50 | 31 | 84 | 168 | 112 | 86 | 334 | 112 | 292 | |
| Sanderson JE et al. [20] | 1996 | 39 | 49 | 12 | 39 | 48 | 13 | 127 | 126 | 73 | 74 | |
| Yamada Y et al. [9] | 1997 | 36 | 35 | 17 | 50 | 55 | 17 | 107 | 155 | 69 | 89 | |
| Tiret L et al. [17] | 2000 | 94 | 200 | 128 | 71 | 190 | 126 | 388 | 332 | 456 | 442 | |
| Rai TS et al. [12] | 2008 | 8 | 33 | 10 | 47 | 87 | 30 | 49 | 181 | 53 | 147 | |
| Kucukarabaci B et al. [21] | 2008 | 5 | 18 | 6 | 7 | 9 | 4 | 28 | 23 | 30 | 17 | |
| Mahjoub S et al. [16] | 2010 | 12 | 38 | 26 | 46 | 83 | 22 | 62 | 175 | 90 | 127 | |
| HCM | ||||||||||||
| Marian AJ et al. [22] | 1993 | 7 | 49 | 44 | 22 | 46 | 38 | 63 | 90 | 137 | 122 | |
| Pfeufer A et al. [23] | 1996 | 26 | 24 | 36 | 14 | NA | NA | NA | NA | |||
| Yamada Y et al. [9] | 1997 | 31 | 32 | 8 | 50 | 55 | 17 | 94 | 155 | 48 | 89 | |
| Ogimoto A et al. [24] | 2002 | 53 | 64 | 21 | 83 | 95 | 27 | 170 | 261 | 106 | 149 | |
| Kawaguchi H et al. [11] | 2003 | 26 | 41 | 13 | 43 | 28 | 17 | 93 | 114 | 67 | 62 | |
| Rai TS et al. [12] | 2008 | 11 | 63 | 44 | 47 | 87 | 30 | 85 | 181 | 151 | 147 | |
| Kaya CT et al. [8] | 2010 | 8 | 34 | 21 | 5 | 9 | 6 | 50 | 19 | 76 | 21 | |
| Coto E et al. [19] | 2010 | 35 | 100 | 72 | 46 | 135 | 119 | 170 | 227 | 242 | 373 | |
DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; NA, not available.
Association of ACE I/D Polymorphisms and DCM Susceptibility
Although the overall results demonstrated that the D allele carriers might be more susceptible to DCM, the risk did not reach a statistical significance. (DD/ID vs. II: OR = 1.34, 95% CI 0.92–1.95, P = 0.13; DD vs. ID/II: OR = 1.27, 95% CI 0.93–1.74, P = 0.13; DD vs. II: OR = 1.44, 95% CI 0.88–2.36, P = 0.14). Similarly, in the subgroup analysis by ethnicity, no significantly increased risk was found for D allele carriers among East Asians and Europeans. (Table 4).
Table 4. Summary of pooled ORs according to ACE I/D polymorphisms.
| DCM | HCM | ||||
| Comparison | Total | East Asian | European | Total | Japanese |
| Study (n) | 8 | 2 | 3 | 8 | 3 |
| 2/2 versus 1/1 | |||||
| OR(95% CI) | 1.44(0.88–2.36) | 1.16(0.64–2.11) | 0.93(0.57–1.53) | 1.67(0.90–3.11) | 1.10(0.69–1.74) |
| P value for heterogeneity | 0.01 | 0.51 | 0.18 | 0.0006 | 0.68 |
| 2/2 versus 1/1+1/2 | |||||
| OR(95% CI) | 1.27(0.93–1.74) | 1.20(0.69–2.08) | 1.02(0.81–1.29) | 1.27(0.88–1.82) | 0.96(0.63–1.48) |
| P value for heterogeneity | 0.06 | 0.40 | 0.18 | 0.02 | 0.67 |
| 2/2+1/2 versus 1/1 | |||||
| OR(95% CI) | 1.34(0.92–1.95) | 1.00(0.67–1.49) | 0.91(0.68–1.22) | 1.69(1.04–2.74) | 1.20(0.88–1.63) |
| P value for heterogeneity | 0.02 | 0.99 | 0.11 | 0.0009 | 0.17 |
| 1/2 versus 1/1 | |||||
| OR(95% CI) | 1.10(0.88–1.37) | 0.95(0.62–1.45) | 0.93(0.68–1.27) | 1.62(1.06–2.46) | 1.29(0.76–2.20) |
| P value for heterogeneity | 0.12 | 0.74 | 0.12 | 0.01 | 0.09 |
| 2/2 versus 1/2 | |||||
| OR(95% CI) | 1.08(0.87–1.35) | 1.22(0.68–2.20) | 0.96(0.73–1.25) | 0.99(0.79–1.26) | 0.85(0.54–1.34) |
| P value for heterogeneity | 0.20 | 0.36 | 0.90 | 0.26 | 0.36 |
ACE, angiotensin I converting enzyme; DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy;
1/1, homozygosity for I allele; 1/2, heterozygosity; 2/2, homozygosity for D allele; CI, confidence interval; OR, odds ratio.
Association of ACE I/D Polymorphisms and HCM Susceptibility
Overall, compared with ACE II genotype, patients with D allele showed a significant increased risk of HCM (DD/ID vs. II: OR = 1.69, 95% CI 1.04–2.74, P = 0.03). However, patients with DD genotype did not showed significant risk of HCM compared with II genotype (DD vs. II: OR = 1.67, 95% CI 0.90–3.11, P = 0.10) or I allele carriers (DD vs. ID/II: OR = 1.27, 95% CI 0.88–1.82, P = 0.20). In the subgroup analysis by ethnicity, no statistically increased risk was found for D allele carriers among Japanese. (Table 4).
Discussion
To our best knowledge, our present study is the first meta-analysis to assess the association between ACE I/D polymorphism and DCM or HCM risk. Overall, our meta-analysis suggested that ACE I/D polymorphisms might be associated with HCM susceptibility but not DCM.
The angiotensin I converting enzyme enhances the synthesis of angiotensin II (Ang II), which induces cell proliferation, migration and hypertrophy, and enhances the proinflammatory cytokines and matrix metalloproteinases. Thus, overexpression of Ang II plays a powerful role in cardiomyopathy. Studies have demonstrated blocking Ang II is beneficial to patients with cardiomyopathy or heart failure. Previous studies have found that ACE I/D polymorphisms are related with plasma Ang II levels. ACE I/D polymorphisms have been extensively examined for a variety of clinical endpoints, such as hypertension, coronary artery disease [13], cough [14] and cancer [15]. The ACE I/D polymorphisms also modulate the phenotype in patients with DCM and HCM. However, studies from different populations have demonstrated conflicting data. Mahjoub S et al. found that DD genotype and D allele of angiotensin-converting enzyme I/D gene polymorphism are associated with increased risk of dilated cardiomyopathy in a Tunisian population, [16] while Tiret L et al did not find this correlation. [17] In the present meta-analysis, our overall results did not show significant association between ACE I/D polymorphisms and DCM risk. Furthermore, the subgroup analysis also did not find a statistically increased risk in D allele carriers among East Asians and Europeans, suggesting ACE I/D polymorphisms might not be associated with DCM risk. The previous data of ACE I/D polymorphisms in HCM patients also did not reach a consistency. Rai TS et al found that D allele of ACE I/D polymorphism significantly influences the HCM phenotypes. [12] However, Yamada Y et al reported that the ACE I/D polymorphisms are not related to HCM in a Japanese population. [9] Our current meta-analysis found that compared with ACE II genotype, patients with D allele showed a significant increased risk of HCM, suggesting ACE I/D polymorphisms might attribute to HCM risk.
Several limitations should be considered. First, some genetic defects were known in HCM or DCM. However, all the included studies did not present such information, which might affect the analysis results. Second, heterogeneity among the included studies may affect the interpretation of the results of the meta-analysis. Third, most of the sample sizes of the referenced studies are relatively small, which might weaken the meta-analysis results. Furthermore, lamin A/C mutations in DCM and sarcomeric mutations in HCM contribute a lot to cardiomyopathy risk. [18] The study by Coto E et al found a possible association between AT1R A1166C but not ACE I/D polymorphisms and HCM. [19] Unfortunately, little was known about the role of ACE I/D polymorphisms in lamin A/C and sarcomeric mutations risk. Further study is needed to confirm this association.
Overall, the present meta-analysis suggested that certain ACE I/D polymorphism might be associated with HCM but not DCM susceptibility. The findings of the current study may add benefit to risk stratification strategies in patients with HCM and may encourage further study focusing on the effect of ACE I/D polymorphisms on HCM and risk. These results also suggest a potential treatment approach by regulating RASS in HCM patients. However, given the small sample sizes in this meta-analysis, further large random case-control studies are needed for further confirmation.
Funding Statement
This work was supported by the National Natural Science Foundation of China Grants (No. 81100207, 30900607, 81270350 and 81100206). No additional external funding received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Michels VV, Moll PP, Miller FA, Tajik AJ, Chu JS, et al. (1992) The frequency of familial dilated cardiomyopathy in a series of patients with idiopathic dilated cardiomyopathy. N Engl J Med 326: 77–82. [DOI] [PubMed] [Google Scholar]
- 2. Kaufman BD, Auerbach S, Reddy S, Manlhiot C, Deng L, et al. (2007) RAAS gene polymorphisms influence progression of pediatric hypertrophic cardiomyopathy. Hum Genet 122: 515–523. [DOI] [PubMed] [Google Scholar]
- 3. Zhang H, Sun ML, Peng J, Sun T, Zhang Y, et al. (2011) Association of the angiotensin type 1 receptor gene A1166C polymorphisms with myocardial infarction: a meta-analysis. J Thromb Haemost 9: 1258–1260. [DOI] [PubMed] [Google Scholar]
- 4. Zhang H, Sun M, Sun T, Zhang C, Meng X, et al. (2011) Association between angiotensin II type 1 receptor gene polymorphisms and ischemic stroke: a meta-analysis. Cerebrovasc Dis 32: 431–438. [DOI] [PubMed] [Google Scholar]
- 5. Orenes-Pinero E, Hernandez-Romero D, Jover E, Valdes M, Lip GY, et al. (2011) Impact of polymorphisms in the renin-angiotensin-aldosterone system on hypertrophic cardiomyopathy. J Renin Angiotensin Aldosterone Syst 12: 521–530. [DOI] [PubMed] [Google Scholar]
- 6. Raynolds MV, Bristow MR, Bush EW, Abraham WT, Lowes BD, et al. (1993) Angiotensin-converting enzyme DD genotype in patients with ischaemic or idiopathic dilated cardiomyopathy. Lancet 342: 1073–1075. [DOI] [PubMed] [Google Scholar]
- 7. Montgomery HE, Keeling PJ, Goldman JH, Humphries SE, Talmud PJ, et al. (1995) Lack of association between the insertion/deletion polymorphism of the angiotensin-converting enzyme gene and idiopathic dilated cardiomyopathy. J Am Coll Cardiol 25: 1627–1631. [DOI] [PubMed] [Google Scholar]
- 8. Kaya CT, Gurlek A, Altin T, Kilickap M, Karabulut HG, et al. (2010) The relationship between angiotensin converting enzyme gene I/D polymorphism and QT dispersion in patients with hypertrophic cardiomyopathy. J Renin Angiotensin Aldosterone Syst 11: 192–197. [DOI] [PubMed] [Google Scholar]
- 9. Yamada Y, Ichihara S, Fujimura T, Yokota M (1997) Lack of association of polymorphisms of the angiotensin converting enzyme and angiotensinogen genes with nonfamilial hypertrophic or dilated cardiomyopathy. Am J Hypertens 10: 921–928. [DOI] [PubMed] [Google Scholar]
- 10. Yoneya K, Okamoto H, Machida M, Onozuka H, Noguchi M, et al. (1995) Angiotensin-converting enzyme gene polymorphism in Japanese patients with hypertrophic cardiomyopathy. Am Heart J 130: 1089–1093. [DOI] [PubMed] [Google Scholar]
- 11. Kawaguchi H (2003) Angiotensin-converting enzyme and angiotensinogen gene polymorphism in hypertrophic cardiomyopathy. Exp Clin Cardiol 8: 155–159. [PMC free article] [PubMed] [Google Scholar]
- 12. Rai TS, Dhandapany PS, Ahluwalia TS, Bhardwaj M, Bahl A, et al. (2008) ACE I/D polymorphism in Indian patients with hypertrophic cardiomyopathy and dilated cardiomyopathy. Mol Cell Biochem 311: 67–72. [DOI] [PubMed] [Google Scholar]
- 13. Kato N, Tatara Y, Ohishi M, Takeya Y, Onishi M, et al. (2011) Angiotensin-converting enzyme single nucleotide polymorphism is a genetic risk factor for cardiovascular disease: a cohort study of hypertensive patients. Hypertens Res 34: 728–734. [DOI] [PubMed] [Google Scholar]
- 14. Nishio K, Kashiki S, Tachibana H, Kobayashi Y (2011) Angiotensin-converting enzyme and bradykinin gene polymorphisms and cough: A meta-analysis. World J Cardiol 3: 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sun M, Liu C, Wei F, Zhong J, Sun Y (2011) Association of angiotensin I converting enzyme insertion/deletion polymorphism with breast cancer: a meta-analysis. J Renin Angiotensin Aldosterone Syst 12: 611–616. [DOI] [PubMed] [Google Scholar]
- 16. Mahjoub S, Mehri S, Bousaada R, Ouarda F, Zaroui A, et al. (2010) Association of ACE I/D polymorphism in Tunisian patients with dilated cardiomyopathy. J Renin Angiotensin Aldosterone Syst 11: 187–191. [DOI] [PubMed] [Google Scholar]
- 17. Tiret L, Mallet C, Poirier O, Nicaud V, Millaire A, et al. (2000) Lack of association between polymorphisms of eight candidate genes and idiopathic dilated cardiomyopathy: the CARDIGENE study. J Am Coll Cardiol 35: 29–35. [DOI] [PubMed] [Google Scholar]
- 18. Malhotra R, Mason PK (2009) Lamin A/C deficiency as a cause of familial dilated cardiomyopathy. Curr Opin Cardiol 24(3): 203–208. [DOI] [PubMed] [Google Scholar]
- 19. Coto E, Palacin M, Martin M, Castro MG, Reguero JR, et al. (2010) Functional polymorphisms in genes of the Angiotensin and Serotonin systems and risk of hypertrophic cardiomyopathy: AT1R as a potential modifier. J Transl Med 8: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sanderson JE, Young RP, Yu CM, Chan S, Critchley JA, et al. (1996) Lack of association between insertion/deletion polymorphism of the angiotensin-converting enzyme gene and end-stage heart failure due to ischemic or idiopathic dilate cardiomyopathy in the Chinese. Am J Cardiol 77: 1008–1010. [DOI] [PubMed] [Google Scholar]
- 21. Kucukarabaci B, Birdane A, Gunes HV, Ata N, Degirmenci I, et al. (2008) Association between angiotensin converting enzyme (ACE) gene I/D polymorphism frequency and plasma ACE concentration in patients with idiopathic dilated cardiomyopathy. Anadolu Kardiyol Derg 8: 65–66. [PubMed] [Google Scholar]
- 22. Marian AJ, Yu QT, Workman R, Greve G, Roberts R (1993) Angiotensin-converting enzyme polymorphism in hypertrophic cardiomyopathy and sudden cardiac death. Lancet 342: 1085–1086. [DOI] [PubMed] [Google Scholar]
- 23. Pfeufer A, Osterziel KJ, Urata H, Borck G, Schuster H, et al. (1996) Angiotensin-converting enzyme and heart chymase gene polymorphisms in hypertrophic cardiomyopathy. Am J Cardiol 78: 362–364. [DOI] [PubMed] [Google Scholar]
- 24. Ogimoto A, Hamada M, Nakura J, Miki T, Hiwada K (2002) Relation between angiotensin-converting enzyme II genotype and atrial fibrillation in Japanese patients with hypertrophic cardiomyopathy. J Hum Genet 47: 184–189. [DOI] [PubMed] [Google Scholar]