Abstract
Na-HCO3 cotransport (NBC) regulates intracellular pH (pHi) and HCO3 secretion in rat colon. NBC has been characterized as a 5,5′-diisothiocyanato-2-2′-stilbene (DIDS)-sensitive transporter in several tissues, while the colonic NBC is sensitive to both amiloride and DIDS. In addition, the colonic NBC has been identified as critical for pHi regulation as it is activated by intravesicular acid pH. Molecular studies have identified several characteristically distinct NBC isoforms [i.e. electrogenic (NBCe) and electroneutral (NBCn)] that exhibit tissue specific expression. This study was initiated to establish the molecular identity and specific function of NBC isoforms in rat colon. Northern blot and reverse transcriptase PCR (RT-PCR) analyses revealed that electrogenic NBCe1B or NBCe1C (NBCe1B/C) isoform is predominantly expressed in proximal colon, while electroneutral NBCn1C or NBCn1D (NBCn1C/D) is expressed in both proximal and distal colon. Functional analyses revealed that amiloride-insensitive, electrogenic, pH gradient-dependent NBC activity is present only in basolateral membranes of proximal colon. In contrast, amiloride-sensitive, electroneutral, [H+]-dependent NBC activity is present in both proximal and distal colon. Both electrogenic and electroneutral NBC activities are saturable processes with an apparent Km for Na of 7.3 and 4.3 mM, respectively; and are DIDS-sensitive with apparent Ki of 8.9 and 263.8 µM, respectively. In addition to Na-H exchanger isoform-1 (NHE1), pHi acidification is regulated by a HCO3-dependent mechanism that is HOE694-insensitive in colonic crypt glands. We conclude from these data that electroneutral, amiloride-sensitive NBC is encoded by NBCn1C/D and is present in both proximal and distal colon, while NBCe1B/C encodes electrogenic, amiloride-insensitive Na-HCO3 cotransport in proximal colon. We also conclude that NBCn1C/D regulates HCO3-dependent HOE694-insensitive Na-HCO3 cotransport and plays a critical role in pHi regulation in colonic epithelial cells.
Introduction
Na-HCO3 cotransporters (NBC) are critical for regulating intracellular pH (pHi), HCO3 absorption and HCO3 secretion [1], [2], [3], [4], [5], [6]. NBC have been classified as electroneutral (NBCn) and electrogenic (NBCe) that mediate Na and HCO3 cotransport with a stoichiometry of 1∶1 and 1:>1, respectively (Table-1) [1], [4], [6]. Both NBCn and NBCe have been shown responsible for HCO3 movement and pHi regulation in both epithelial and nonepithelial cells [1], [4], [6]. In general, NBC have been characterized as a DIDS (5,5′-diisothiocyanato-2-2′-stilbene)-sensitive (and amiloride-insensitive) transporter in several tissues [1], [4], [6]. However, we demonstrated a novel NBC that is sensitive to both amiloride and DIDS in basolateral membrane vesicles (BLMV) of rat distal colon [7]. Although both NBC and Na-H exchange functions are present in colonic BLMV, NBC has been identified as critical for pHi regulation since acid intravesicular pH activated the former but not the latter [8].
Table 1. Splice variants of NBCe and NBCn isoforms and their alternative names.
Molecular studies have isolated several splice variants of NBCe (i.e., NBCe1A, NBCe1B and NBCe1C) and NBCn (NBCn1A, NBCn1B, NBCn1C and NBCn1D) isoforms that exhibit tissue-specific expression patterns [9], [10], [11], [12], [13], [14], [15], [16]. A recent study has shown NBCn1, but not NBCe2 as major pHi regulator in mouse duodenum [17]. However, the molecular identity and the specific function of colonic NBC remain unknown. Studies were, therefore, initiated to identify whether the characteristically distinct colonic NBC is encoded by any of the existing NBC specific transcripts or by one or more totally distinct isoforms. The results presented in this study indicate that the colonic Na-HCO3 cotransport activities are encoded by NBCn- and NBCe-like proteins that are expressed in a tissue-specific pattern, as only NBCn-like transcripts are localized in distal colon, while both NBCn-like and NBCe-like transcripts are present in proximal colon. In addition, this study also demonstrates that HOE694 (a specific NHE1-inhibitor)-insensitive NBC plays a major role in pHi regulation.
Methods
Ethics Statement
The rats (Sprague-Dawley, 201–225 g) were given an anesthesia using intraperitoneal (i.p) injection of pentobarbital (40–50 mg/kg body wt). Following removal of the colon, the animals were euthanized by injecting 100 mg/kg pentobarbital. The experimental protocols used in this study were approved by the West Virginia University Institutional Animal Care and Use Committee (IACUC #11-0801).
Northern Blot Analyses
Total RNA from proximal and distal colonic epithelial cells, whole brain, heart and kidney were isolated using TRIzol Reagent (Invitrogen, Carlsbad, CA). mRNA was purified using Oligotex mRNA Midi Kit (Qiagen, Valencia, CA). Northern blot analyses were performed by standard molecular technique, as described previously [18]. In brief, 5 µg mRNA electrophoresed on 1% agarose gel was transferred to a nylon membrane and fixed using Stratalinker (Stratagene, CA). Following a 1–2 h prehybridization period in 50% formamide containing 20% dextransulfate, 2 M NaCl and 1% SDS, the blots were hybridized with the indicated cDNA probes that were labeled using dCT32P and the random primer labeling kit (Roche, Mannheim, Germany). Overnight hybridized blots were washed with 2×SSC (sodium chloride, sodium citrate) and 0.1% SDS for 30 min at 45°C. The blots were exposed to Hyper film MP (Amersham Pharmacia Biotech, Little Chalfont, UK) that were incubated at −80°C and were developed after 15 h. The blots were stripped by boiling in 0.1×SSC and 0.5% SDS and used on more than one occasion for hybridization with different probes. Densitometry was performed using Multi-Analyst Software (Bio-Rad, Hercules, CA).
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
NBCe1A-, NBCe1B/C- and NBCn1-specific segments were amplified by RT-PCR using mRNA from heart, brain, kidney, proximal colon and distal colon. Primers were designed from published sequences (Table 2). RT-PCR was performed using mRNA and OneStep RT-PCR Kit (Qiagen, Valencia, CA). The veracity of RT-PCR products was confirmed by sequencing (Keck Foundation, Yale University, New Haven, CT).
Table 2. Gene specific primers used for RT-PCR analyses: bNBC1 (brain NBC1), kNBC (kidney NBC1), NBC2 (NBCn-1b) and NBC3 (NBCn1-d) isoform specific primers were designed from published sequences.
| Gene | Primer | Position | Accession number |
| NBCe1A | Sense: 5′-GGCACAGAGAGAGGAGGCTT-3′ Antisense: 5-TGTCTTCCCAATGTCAGCCAG-3′ | 17–36 593–613 | AF027362 |
| NBCe1B | Sense: 5′-ACTGGAGGAGCGACGGAAG-3′ Antisense: 5′-TGTCTTCCCAATGTCAGCCAG-3′ | 27–45 730–750 | AF210250 |
| NBCn1 | Sense 5′-ATCTACCTCCGCTATGTCC-3′ Antisense: 5′-ACTCACAGGCTTTTCAGGGC-3′ | 3424–3442 3881–3900 | AF080106 |
Immunofluorescence Studies
Rat proximal and distal colon were flushed with 0.9% saline to remove fecal contents and then rapidly frozen in liquid nitrogen-cooled Freon. Five to ten micron frozen sections were prepared and placed on poly-lysine coated microscope slides. Tissue sections were permeabilized and fixed in acetone at –20°C for 10 minutes, air-dried and rehydrated in PBS. Non-specific sites were blocked with 1% BSA in PBS-0.05% Triton X100. Primary antibodies to NBCe1 [19] and NBCn1 [20] (kindly provided by Dr. Walter F. Boron Yale University, New Haven, Connecticut) were diluted in the blocking buffer and incubated on the tissue for 2 hrs at room temperature. Primary antibodies were subsequently detected with Alexa 594 anti-rabbit IgG (Molecular Probes, Eugene, OR). Microscopy was performed on a Zeiss 510 LSM (Thornwood, NJ) confocal microscope, and the images were processed using Adobe PhotoShop.
Basolateral Membrane Vesicle (BLMV) Preparation
BLMV were isolated from both proximal and distal colon of normal rats. BLMV were prepared by the sucrose-density gradient and differential centrifugation method, as described previously [7]. In brief, colonic segments harvested from anesthetized rats were filled with 4 mM Hepes-Tris buffer (pH 7.4) containing 5 mM EDTA and 0.5 mM dithiothreitol (DTT). Following 30 min incubation in the same solution the colonic sacs were emptied and the mucosa was scraped using glass slides. The mucosa resuspended in 10 mM Tris-HCl buffer (pH 7.4) containing 250 mM sucrose was homogenized at full speed for 40 strokes using loose fit Teflon homogenizers (Potter-Elvhjem). The homogenates loaded onto continuous (13%–30%) sucrose gradient were isokinetically centrifuged (SW 40.1 rotor, Beckman L7-525 ultracentrifuge) for 7 min at 29,000 rpm. The Na,K-ATPase-rich fractions (top 2–5 ml fractions) from continuous sucrose-gradients were collected and homogenized with 10 strokes at full speed. The homogenates were layered onto continuous-discontinuous sucrose (12%–15%/35% and 55%) gradients and centrifuged for 2 hrs. Membrane fractions collected at the junction between 12%–15% and 35% sucrose were diluted with deionized water (10 times) and centrifuged at 50,000 rpm (Ti 50.1 rotor, Beckman L7-525 ultracentrifuge). Final BLMV pellets were resuspended in appropriate volume of 50 mM MES-Tris buffer (pH 5.5) containing 150 mM K-gluconate and stored at −80°C until used. All solutions used were ice cold, and all preparations were performed at 4°C. Membrane purity was periodically assessed by 10–12 fold enrichment of Na,K-ATPase activity compared to homogenate. Na,K-ATPase activity was measured by the method of Forbush et al [21], as described earlier [22]. Protein assay was performed using the Lowry method [23].
Uptake Studies
Outward proton and inward HCO3 (proton/HCO3) gradient-driven and intravesicular positive membrane potential-dependent HCO3 gradient-driven 22Na (Amersham, Chicago, IL) uptake in BLMV was performed by a rapid filtration technique, as described previously [7]. Proton/HCO3 gradient-driven 22Na uptake. BLMV loaded with 150 mM K-gluconate and 50 mM MES-Tris buffer (pH 5.5) were incubated at room temperature for 6 sec. in medium containing 150 mM KHCO3, 0.1 mM 22Na and 50 mM HEPES-Tris buffer (pH 7.5). Membrane potential-dependent HCO3 gradient-driven 22Na uptake. BLMV loaded with 150 mM NMG-gluconate and 50 mM HEPES-Tris buffer (pH 7.5) were incubated at room temperature for 6 sec in medium containing 150 mM KHCO3, 0.1 mM22Na, 50 mM HEPES-Tris buffer (pH 7.5) and 25 µM valinomycin. Uptake was arrested with ice-cold stop solution containing 150 mM K-gluconate and 50 mM HEPES-Tris buffer (pH 7.5) and BLMV were filtered on 0.4 µm filter and washed with 4 ml stop solution. 22Na trapped in BLMV collected on filters was dissolved in Optifluor and counted in Beckman counter and used for calculation.
Crypt Gland Isolation and pHi Studies
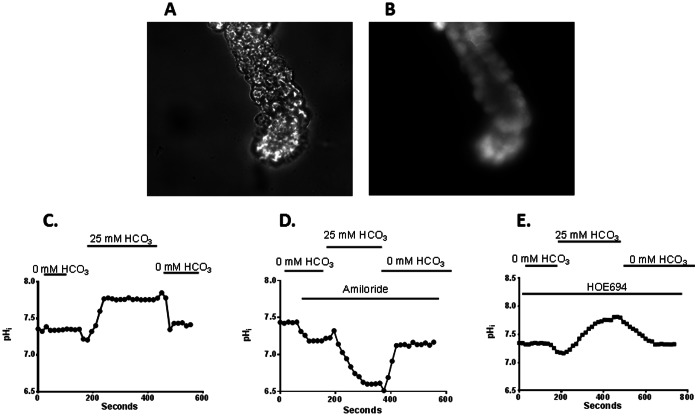
Colonic crypt glands were isolated by the divalent chelation method of Lomax et al [24], as described previously [25]. In brief, distal and/or proximal colon removed from anaesthetized rats filled with HEPES buffer (in mM: 115 NaCl, 5 KCl, 1.2 CaCl2, 1.2 MgCl2, 2 NaH2PO4, 32.2 Hepes and 10 glucose; pH: 7.4) were placed in HEPES buffer containing 5 mM EDTA and incubated at 37°C for15 min. The dispersed crypt glands were precipitated by centrifugation at 200–250 rpm (SorvallRC5B, SS34 rotor) for 1 min and the crypts were resuspended in HEPES buffer. Crypt glands were placed on cover-slips coated with the biological adhesive Cell Tak attached to a JG-23 perfusion chamber (Warner Instruments, Hamden, CT). The crypts were then perfused with a HEPES buffer containing BCECF-AM and incubated in the darkness for 30 min. The perfusion chamber was mounted onto the stage of an inverted Olympus IX50 microscope. The chamber was maintained at 37°C using a thermostat feedback system attached to a continuous chamber perfusion system for constant laminar flow. Following loading the chamber was perfused for 5 min. at 37°C to remove any deesterified dye on the surface of the crypt. The cell incorporated BCECF was excited at 490 nm and 440 nm and the emission signal was monitored at 530 nm (Figures 1A and 1B). Initial studies examined the effect of bath HCO3 on pHi in the presence and absence of amiloride and HOE694. Under basal condition, bath (i.e., basolateral) HCO3 increased pHi, an indication for the presence of HCO3-dependent H+ extrusion and/or HCO3 uptake process on the basolateral membranes of proximal crypt glands (Figure 1C). In the absence of HCO3, bath amiloride slightly decreased pHi, an indication for basolateral NHE (i.e., NHE1) mediated H+ extrusion in crypts (Figure 1D). In the continued presence of amiloride, bath HCO3 substantially reduced the pHi, while the HCO3-generated acid pHi partially recovered following bath HCO3 removal (Figure 1D). These observations indicate that amiloride-sensitive H+ extrussion is regulated by both HCO3-dependent and HCO3-independent pathways that might be mediated by amiloride-sensitive NBC and NHE1, respectively. To distinguish NBC-dependent H+ extrusion from NHE1, the effect of bath HCO3 on pHi was also examined in the presence of bath HOE694 (a NHE1 specific inhibitor) [26]. In contrast to amiloride, bath HCO3 in the presence of HOE694 increased the pHi, an indication for the presence of HCO3-dependent H+ extrusion process on the basolateral membranes (Figure 1E). These observations indicate that both HOE694-sensitive/HCO3-independent (i.e., NHE1) and HOE694-insensitive/HCO3-dependent (i.e., NBC) H+ extrusion processes are present on the basolateral membranes of proximal crypt glands.
Figure 1. Effect of extracellular HCO3 in the presence and absence of HOE694 on intracellular pH change in crypt glands from proximal colon.
Images of a crypt gland before [A] and after BCECF loading [B]. The BCECF loaded crypts were excited at 490 nm and 440 nm and the emission signal was monitored at 535 nm to derive intracellular pH (pHi). [C] Under basal condition, bath HCO3 increased pHi, while the increased pHi was completely reversed by HCO3 removal. [D] In the presence of amiloride, bath HCO3 reduced the pHi, while the reduced pHi was partially reversed by HCO3 removal. [E] In the presence of HOE694, bath HCO3 increased pHi, while the increased pHi was completely reversed by HCO3 removal. Typical tracing presented is representative of observations in 4 different crypts from 5 different rats.
In additional studies, crypt glands were sequentially perfused with HEPES buffer, HEPES buffer containing HOE694 (1 µM) and HEPES buffer containing HCO3 (25 mM) plus HOE694 (1 µM). Crypts were also sequentially perfused with HEPES buffer containing HCO3 plus HOE694, HEPES containing HOE694 and HEPES buffer containing HCO3 plus HOE694. Before termination of the experiments with high-K+ calibration the crypts were exposed to HEPES buffer containing HOE694. The data recorded as the ratio between 490/440 nm was converted to intracellular pH (pHi) after calibration with the high K buffer (in mM: 105 KCl, 1.2 CaCl2, 1.2 MgCl2, Hepes 32.2, NMDG-32.2 and mannitol 5; pH 7.0) and Nigericin. Osmolality of all solutions used was adjusted to 295–300 mOsm.
Statistical Analysis
Data presented represent mean ± SE of triplicate assays of three different membrane preparations for uptake studies, and 3 crypts from three different distal colon. ANOVA statistical analyses were performed using the SPSS program. p<0.05 were considered statistically significant.
Results
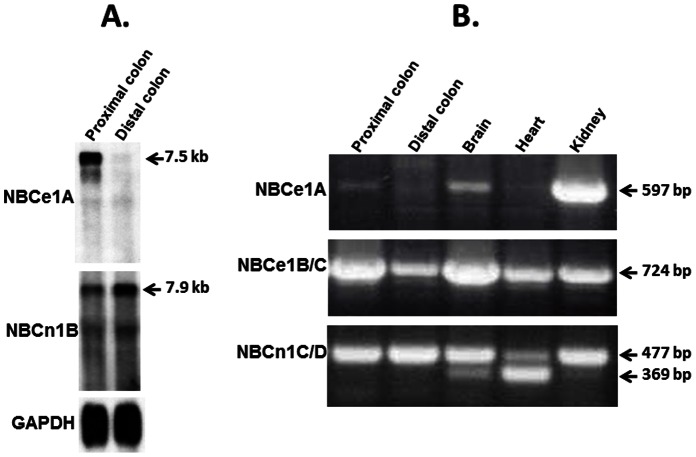
To identify whether any existing NBC-like isoforms are expressed in the rat colon, northern blot analyses of proximal and distal colon mRNA were performed using full length NBCe1A (also known as kNBC1) and NBCn1A (also known as NBC3) cDNA as probes [1], [14], [15]. As shown in Figure 2, NBCe1A cDNA hybridized with a 7.5 kb transcript in proximal colon, but barely identified any mRNA in distal colon (Figure 2A). In contrast, the NBCn1 probe hybridized with a 7.9 kb transcript in both proximal colon and distal colon (Figure 2A). These observations indicate that both NBCe1- and NBCn1-like mRNAs are expressed in proximal colon, while, in contrast, only NBCn1-like mRNA is identified in distal colon.
Figure 2. Northern blot and RT-PCR analyses of mRNA from rat colon.
[A] Northern blot analyses indicate that NBCe1A (7.5 kb)-like mRNA is predominantly expressed in proximal colon, while NBCn1B (7.9 kb)-like mRNA is expressed in both proximal and distal colon. Full length NBCe1A and NBCn1A cDNAs were used as probe. The blots were also probed with a GAPDH cDNA fragment. Results presented are from an individual blot that was stripped and probed sequentially with each probe (NBCe1A, NBCn1A and GAPDH). [B] RT-PCR analyses indicate that NBCe1B/C and NBCn1C/D specific transcripts are predominantly expressed in epithelial cells from proximal and distal colon. NBC isoform specific fragments were amplified by RT-PCR using isoform specific primers (Table 2) and mRNA from proximal colon, distal colon, brain, heart and kidney. RT-PCR products were separated on 1% agarose gels and stained with ethidium bromide. cDNA size markers are indicated on the right side.
In additional studies, RT-PCR analyses were performed to establish the specific NBCe1 and NBCn1 isoforms that are expressed in these colonic segments as at least three splice variants of NBCe1 [NBCe1A (kidney), NBCe1B (pancreas and heart), and NBCe1C (brain)] [9], [13], [15], [27] and four splice variants of NBCn1 [NBCn1A, NBCn1B, NBCn1C and NBCn1D (Table-1), according to the terminology by Choi et al [12] have been reported from various tissues [1], [12], [14]. RT-PCR analyses were performed using mRNA from both proximal and distal colon, and compared to RT-PCR analyses of mRNA from brain, heart and kidney. As shown in Figure 2B, the 725 bp fragment that is common in 5′-region of both NBCe1B and NBCe1C (NBCe1B/C) that is amplified in brain, heart and kidney, is also amplified in both proximal and distal colon. NBCe1B and NBCe1C isoforms differ only at 3′-end [1]. This is in contrast to the northern blot findings which did not reveal any NBCe1 expression in distal colon. A possible explanation is that RT-PCR is more sensitive and can identify even smallest amounts of mRNA in a given tissue. However, there is no experimental support for NBCe1 function in distal colon [8]. In contrast to NBCe1B/C, the NBCe1A-specific fragment, which is predominantly amplified from brain and kidney, is absent in distal colon (Figure 2B). These results demonstrate that either NBCe1B or NBCe1C is likely to be the predominant NBCe1 variant in both proximal and distal colon. However, from these results the presence of a different yet unidentified NBCe1 variant cannot be excluded. The NBCn1-specific 477 bp fragment that is amplified from both proximal and distal colon corresponds to the “B cassette” as seen by Choi et al [12] in NBCn1C/D indicating that either one or both of these or a third yet unidentified isoform containing the “B cassette” is present in rat colon (Figure 2B). The 369 bp fragment amplified in brain and heart corresponds to NBCn1B which is missing the “B cassette”. The NBCn1B specific fragment was not amplified from either proximal or distal colon (data not shown).
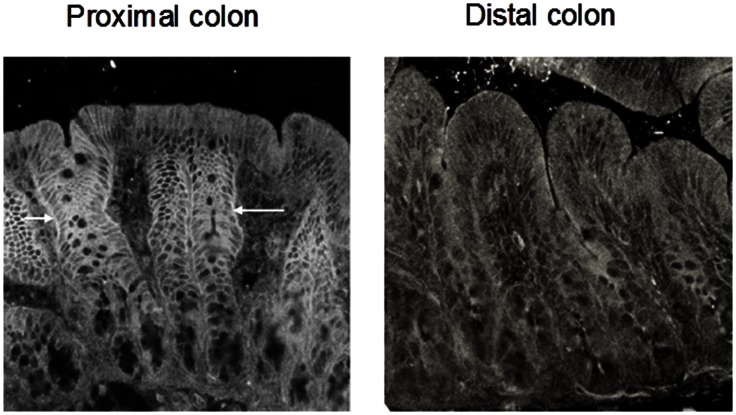
Although northern blot analyses indicated that NBCe-like mRNA expression is not present in distal colon (Figure 2A), RT-PCR analyses provided evidence that NBCe1B/C isoform specific mRNA may be present in distal colon (Figure 2B). Therefore, immunofluorescence studies were performed in both proximal and distal colon using a polyclonal anti-peptide antibody raised against a fusion protein that is common to all NBCe isoforms [19]. As shown in Figure 3, NBCe-like proteins are predominantly localized on the basolateral membranes of mid-crypt regions of proximal colon, but are only weakly present in the surface cells. In contrast, NBCe-like proteins are not localized in epithelial cells of distal colon (Figure 4). These observations are consistent with the northern blot analyses (Figure 2A) that NBCe-like NBC is present only in proximal colon but not in distal colon. The RT-PCR amplification of NBCe1B/C-like transcripts from distal colon (Figure 2B) may be due to either cross contamination of proximal mRNA or nominal expression of NBCe1B/C transcripts in distal colon. Thus, in the proximal colon both NBCe1B/C and NBCn1C/D specific mRNAs are present while in the distal colon NBCn1C/D specific mRNA is primarily expressed.
Figure 3. Immunocytochemical localization of NBCe1-like proteins in rat proximal and distal colon.
[A] Rat proximal colon demonstrates strong basolateral staining for NBCe1 in epithelial cells of the mid-crypt region (arrows) and weaker staining in surface cells. [B] Rat distal colon shows no localization of NBCe1 in the epithelial cells. Magnification 40x.
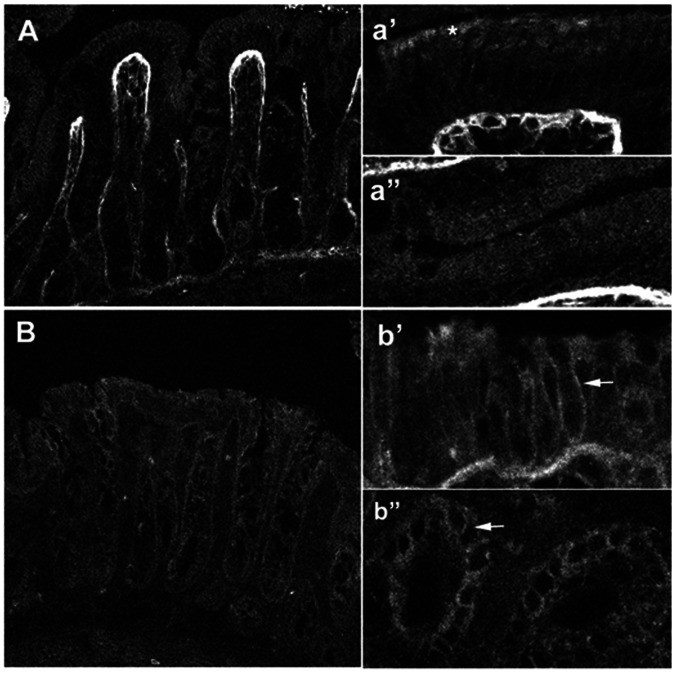
Figure 4. Immunocytochemical localization of NBCn1-like proteins in rat proximal and distal colon.
[A, a’, a”] Rat proximal colon demonstrates weak epithelial staining for NBCn1 along the crypt to surface axis. The connective tissue underlying the epithelial cells is strongly fluorescent. Higher magnification of the surface cells [a’] shows a rather punctuate apical signal [*]. High magnification of epithelial cells in the crypt region (a”) demonstrates a weak diffuse localization. [B, b’, b”] Rat distal colon shows stronger staining for NBCn1. High magnifications of surface cells [b’] and crypt cells [b”] clearly demonstrate basolateral membrane localization [arrows]. Smooth muscle cells were also strongly stained (data not shown).
Immunofluorescence studies were also performed to establish the cell- and membrane-specific localization of NBCn-like proteins in proximal and distal colon. Figure 4 shows that NBCn-like proteins are weakly present in basolateral membranes of both surface and crypt cells in proximal colon (Figures 4A, 4a’, 4a”). At higher magnification NBCn-like proteins are also localized in apical membranes of proximal colon (Figure 4a’). Strong fluorescence is also present in connective tissue underlying the epithelial cells. In contrast, NBCn-like protein expression is stronger in basolateral membranes of both surface (Figure 4b’) and crypt cells (Figure 4b”) of distal colon. NBCn-like proteins are not localized in apical membranes of distal colon (Figure 4B). NBCn-like proteins are also localized in smooth muscle cells (data not shown). It has to be noted here that the human NBCn2A, which has been shown to exhibit ethylisopropylamiloride (EIPA; an amiloride analogue)-sensitive Na-HCO3 cotransport activity in an oocyte expression system was originally cloned form skeletal muscle [14]. These observations suggest that NBCn-like isoforms with significant homology may be present in basolateral membranes of both proximal and distal colon.
The immunofluorescence studies establish that both NBCe-like and NBCn-like proteins are present in basolateral membranes of proximal colon, while only NBCn-like proteins are present in basolateral membranes of distal colon (Figures 3 and 4). Therefore, experiments were designed to identify whether functionally distinct Na-HCO3 cotransporters are present in basolateral membrane vesicles (BLMV) of colon. In these studies, similar to our prior study with distal colon [7], 22Na uptake measured in presence of both outward proton and inward HCO3 (proton/HCO3) gradients provided evidence for Na-HCO3 cotransport in both proximal and distal BLMV (Figure 5). It is important to emphasize that neither the presence nor the absence of Cl affected proton/HCO3 gradient-driven 22Na uptake in these BLMV (unpublished observations). In initial studies the effect of 1 mM amiloride, 1 mM DIDS and 1 µM HOE694 on proton/HCO3 gradient-driven 22Na uptake was examined. Similar to our earlier observations, both amiloride and DIDS almost completely inhibited proton/HCO3 gradient-driven 22Na uptake in distal BLMV (Figure 5B) [7]. In contrast, although DIDS inhibited proton/HCO3 gradient-driven 22Na uptake by similar magnitude, amiloride only partially (44%) inhibited proton/HCO3 gradient-driven 22Na uptake in proximal BLMV (Figure 5B). Since amiloride-sensitive 22Na uptake could represent Na-H exchange as NHE1 isoform is also present in proximal BLMV [18], the effect of HOE694 was also examined. Since prior studies established HOE694 to inhibit NHE1 with a kinetic constant of 0.16 µM, 50 µM HOE694 was used for complete inhibition of NHE1 function in BLMV [28]. As shown in Figures 5A and 4B, proton/HCO3 gradient-driven 22Na uptake was not significantly inhibited by HOE694. The HOE694-insensitive proton/HCO3 gradient-driven 22Na uptake was inhibited by 95% by the addition of DIDS (Figure 5). These observations establish that HOE694-insensitive proton/HCO3 gradient-driven 22Na uptake occurs via a DIDS-sensitive transport that consists of both amiloride-sensitive and amiloride-insensitive Na-HCO3 cotransport processes in the basolateral membranes of proximal colon. These observations that both amiloride-sensitive and amiloride-insensitive components are present in proximal colon but only amiloride-sensitive component is present in distal colon combined with the molecular studies showing the presence of NBCe1B/C and NBCn1C/D in proximal colon but only NBCn1C/D in distal colon raises the possibility that NBCn1C/D encodes the amiloride-sensitive NBC activity, while NBCe1B/C encodes amiloride-insensitive Na-HCO3 cotransport activity.
Figure 5. Effect of inhibitors on Na-HCO3 cotransport activities in basolateral membrane vesicles from proximal and distal colon.
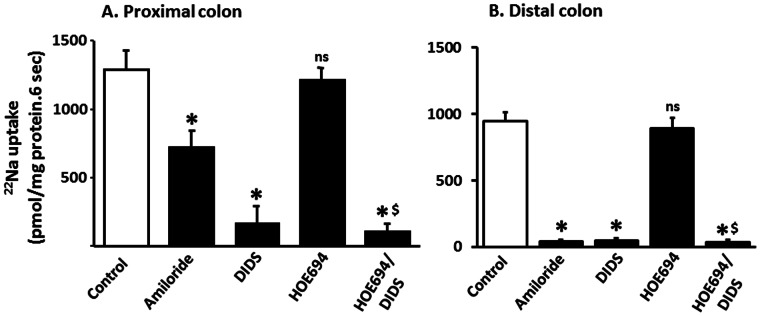
Proton/HCO3 gradient-driven 22Na uptake (0.1 mM) was measured in the absence (Control) and presence of inhibitors (1 mM amiloride; 1 mM DIDS; 1 µM HOE694) in basolateral membrane vesicles (BLMV) from rat proximal [A] and distal [B] colon, as described in the method section. Amiloride partially inhibited, while DIDS almost completely inhibited the Na-HCO3 cotransport activity in BLMV from proximal colon. Both amiloride and DIDS inhibited the Na-HCO3 cotransport activity in BLMV from distal colon. HOE694 did not significantly inhibit Na-HCO3 cotransport in BLMV from either proximal or distal colon. *p<0.001 - compared to control; $ p<0.001 - compared to HOE694; ns: not significant compared to control.
Kinetic studies were performed to distinguish the characteristics of amiloride-sensitive and amiloride-insensitive Na-HCO3 cotransport in proximal BLMV. As shown in Figure 6, increasing extra-vesicular Na concentrations (1–50 mM) saturate both amiloride-insensitive and amiloride-sensitive Na-HCO3 cotransporters with Km for Na of 7.3±0.6 and 4.3±0.3 mM, respectively. Increasing extravesicular DIDS concentrations inhibit both amiloride-insensitive and amiloride-sensitive Na-HCO3 cotransport with apparent half-maximal inhibitory concentrations (Ki) of 8.9±1.2 and 263.8±26.4 µM, respectively (Figure 7). In additional studies, the effect of intravesicular positive membrane potential (generated with an inward K gradient and valinomycin) was examined as electrogenic Na-HCO3 cotransporters have also been shown to mediate the movement of two or more HCO3 for each Na ion [1], [4], [6]. As shown in Figure 8, intravesicular positive membrane potential substantially stimulated amiloride-insensitive Na-HCO3 cotransport in proximal BLMV. In contrast, amiloride-sensitive Na-HCO3 cotransport was not altered by the intravesicular positive membrane potential. These observations indicate that amiloride-sensitive Na-HCO3 cotransport is electroneutral and relatively less sensitive to DIDS, while amiloride-insensitive Na-HCO3 cotransport is electrogenic and highly DIDS-sensitive, and further establish that amiloride-sensitive and amiloride-insensitive Na-HCO3 cotransporters are distinct and separate carrier-mediated transport processes in basolateral membranes of rat proximal colon. These functional studies are consistent with the molecular experiments that concluded that in the proximal colon both NBCn1C/D and NBCe1B/C were present, while in the distal colon where NBC activity was predominantly electroneutral and amiloride-sensitive, only NBCn1C/D was identified.
Figure 6. Kinetics of Na-HCO3 cotransport activities in basolateral membrane vesicles from proximal colon.
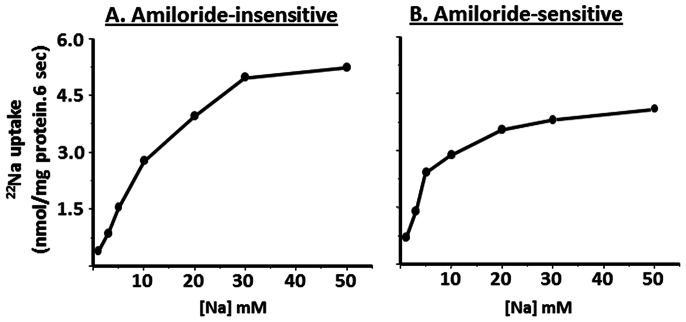
Increasing extravesicular Na concentrations (1–50 mM) saturate both amiloride-insensitive and amiloride-sensitive proton/HCO3 gradient-driven 22Na uptake with apparent Km for Na of 7.3±0.6 and 4.3±0.3 mM, respectively. Proton/HCO3 gradient-driven 22Na uptake was measured as described in the Method section with increasing extravesicular Na concentrations both in presence and absence of inhibitors (either 1 mM amiloride or 1 µM HOE694). Uptake obtained in presence of amiloride represented amiloride-insensitive proton/HCO3 gradient-driven uptake. Amiloride-sensitive uptake (which also includes HOE694 sensitive Na-H exchange) was calculated by subtracting uptake obtained in presence of amiloride from that of uptake in the absence of inhibitors. HOE694-sensitive uptake was calculated by subtracting uptake obtained in presence of HOE694 from that of uptake in the absence of inhibitors. Amiloride-sensitive proton/HCO3 gradient-driven uptake was calculated by subtracting HOE694-sensitive uptake from that of amiloride-sensitive uptake. Best fit curves were drawn using Michaelis-Menten equation. The kinetic constants were calculated using a nonlinear regression with the EnzFitter software (Biosoft, Cambridge, UK).
Figure 7. DIDS-inhibition kinetics of Na-HCO3 cotransport activities in basolateral membrane vesicles from proximal colon.
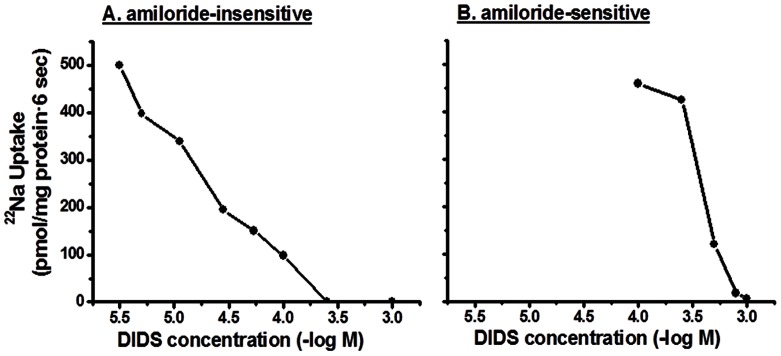
Increasing DIDS concentrations inhibit both amiloride-insensitive and amiloride-sensitive proton/HCO3 gradient-driven 22Na uptake with half maximal inhibitory concentration (Ki) of approximately 8.9±1.2 and 263.8±26.4 µM, respectively. Proton/HCO3 gradient-driven 22Na uptake (0.1 mM) was measured as described in the Method section with increasing extravesicular DIDS concentrations both in presence and absence of inhibitors (either 100 µM EIPA or 1 µM HOE694). Uptake obtained in presence of amiloride represented amiloride-insensitive proton/HCO3 gradient-driven uptake. Amiloride-sensitive uptake (which also includes HOE694 sensitive Na-H exchange) was calculated by subtracting uptake obtained in presence of EIPA from that of uptake in the absence of inhibitors. HOE694-sensitive uptake was calculated by subtracting uptake obtained in presence of HOE694 from that of uptake in the absence of inhibitors. Amiloride-sensitive proton/HCO3 gradient-driven uptake was calculated by subtracting HOE694-sensitive uptake from that of EIPA-sensitive uptake. The inhibitory constants were calculated using a nonlinear regression with the EnzFitter software (Biosoft, Cambridge, UK).
Figure 8. Effect of intravesicular membrane potential on Na-HCO3 cotransport activities in basolateral membrane vesicles from proximal colon.
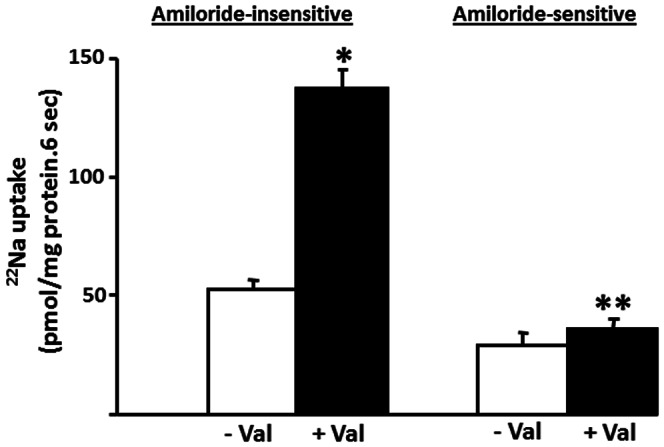
Intravesicular positive membrane potential [generated using inward K+ gradient and valinomycin (Val)] dependent HCO3-gradient-driven 22Na (0.1 mM) uptake was measured as described in the Method section both in presence and absence of 1 mM amiloride. Intravesicular positive potential stimulated amiloride-insensitive HCO3 gradient-driven 22Na uptake. Intravesicular positive potential did not affect amiloride-sensitive Na-HCO3 cotransport. Uptake obtained in presence of amiloride represented amiloride-insensitive uptake, while amiloride-sensitive uptake was calculated by subtracting the amiloride-insensitive uptake from that of uptake in the absence of amiloride. *p<0.001 compared to –Val in amiloride-insensitive; **p<0.05 compared to –Val in amiloride-sensitive.
Studies were designed to establish whether amiloride-insensitive and amiloride-sensitive Na-HCO3 cotransport of proximal BLMV are regulated by proton concentration and/or by the magnitude of the pH gradient since amiloride-sensitive Na-HCO3 cotransport in rat distal colon is regulated by proton concentration [8]. In these studies, the effect of one- and two-unit outward acid pH gradient with a constant extravesicular pH (pHo/pHi: 7.5/6.5 vs 7.5/5.5), as well as a one-unit pH gradient at different pHs (pHo/pHi: 8.0/7.0 vs 7.0/6.0) was examined. Both amiloride-insensitive and amiloride-sensitive Na-HCO3 cotransport activities were minimal in the absence of a pH gradient (Figure 9). Imposition of a one-unit pH gradient significantly (12-fold) enhanced amiloride-insensitive Na-HCO3 cotransport, but there was no further enhancement in presence of a two-unit pH gradient (Figure 9). In contrast, amiloride-sensitive Na-HCO3 cotransport was only minimally enhanced by a one-unit pH gradient but was substantially (26-fold) stimulated by a two-unit pH gradient (Figure 9). These observations suggest that, although an outward proton gradient is important for maximal activation of both amiloride-insensitive and amiloride-sensitive Na-HCO3 cotransporters, amiloride-insensitive activity is maximally stimulated by a ten-fold proton concentration gradient while a hundred-fold proton concentration gradient is required for stimulation of amiloride-sensitive NBC activity.
Figure 9. Effect of varying outward acid pH-gradients on Na-HCO3 cotransport activities in basolateral membrane vesicles from proximal colon.
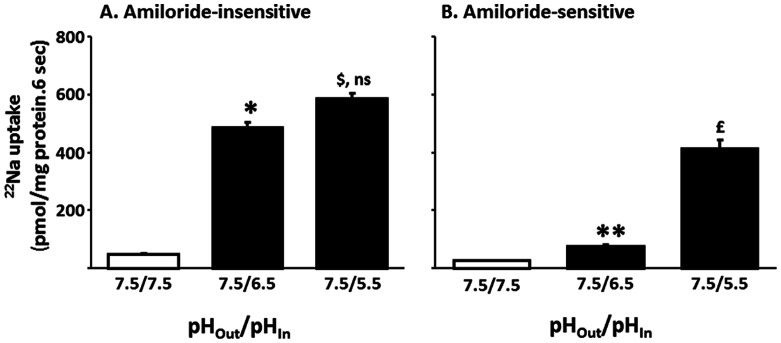
Proton/HCO3 gradient-driven 22Na uptake (0.1 mM) was measured as described in the Method section with different extravesicular pH in presence and absence of inhibitors (1 mM amiloride or 1 µM HOE694). Both one-unit (pHo/pHi: 7.5/6.5) and two-unit (pHo/pHi: 7.5/5.5) outward acid pH-gradients stimulated the amiloride-insensitive Na-HCO3 cotransport. A two-unit, but not a one-unit outward acid pH gradient stimulated amiloride-sensitive Na-HCO3 cotransport. Uptake obtained in presence of amiloride represented amiloride-insensitive proton/HCO3 gradient-driven uptake. Amiloride-sensitive uptake (which also includes HOE694 sensitive Na-H exchange) was calculated by subtracting uptake obtained in presence of amiloride from that of uptake in the absence of inhibitors. HOE694-sensitive uptake was calculated by subtracting uptake obtained in presence of HOE694 from that of uptake in the absence of inhibitors. Amiloride-sensitive proton/HCO3 gradient-driven uptake was calculated by subtracting HOE694-sensitive uptake from that of amiloride-sensitive uptake. *p<0.02 compared to 7.5/7.5 (pHo/pHi) in amiloride-insensitive; $p<0.002 compared to 7.5/7.5 (pHo/pHi) in amiloride-insensitive; **p<0.004 compared to 7.5/7.5 (pHo/pHi) in amiloride-sensitive; *p<0.001 compared to 7.5/7.5 (pHo/pHi) in amiloride-sensitive; ns: not significant compared to 7.5/6.5 (pHo/pHi) in amiloride-insensitive.
Since the results presented in Figure 9 do not distinguish between absolute proton concentration and magnitude of the proton concentration gradient, the effect of a one-unit outward acid pH gradient at different pHs was examined on amiloride-insensitive and amiloride-sensitive Na-HCO3 cotransport. As shown in Figure 10, the amiloride-insensitive Na-HCO3 cotransport activity which is maximally stimulated by a one-unit pH gradient is identical at two different pHs (i.e., 8.0/7.0 vs 7.0/6.0) indicating that the absolute proton concentration is not critical. In contrast, amiloride-sensitive Na-HCO3 cotransport activity is approximately 4-fold higher in presence of a one-unit pH gradient at 7.0/6.0 than in presence of a one-unit pH gradient at 8.0/7.0 (Figure 10). It is also possible that amiloride-sensitive and amiloride-insensitive NBC may reflect different intravesicular and extravesicular pH sensitivity, and that the amiloride-sensitive NBC may be highly sensitive at intravascular pH ≤6.0. The observations that the activation of amiloride-insensitive and amiloride-sensitive Na-HCO3 cotransport activities by pH gradients and by proton concentration gradients differ substantially suggest that these two HCO3 transporters regulate different cellular functions in rat proximal colon.
Figure 10. Effect of one-unit pH-gradient with varying proton concentrations on Na-HCO3 cotransport activities in basolateral membrane vesicles from proximal colon.
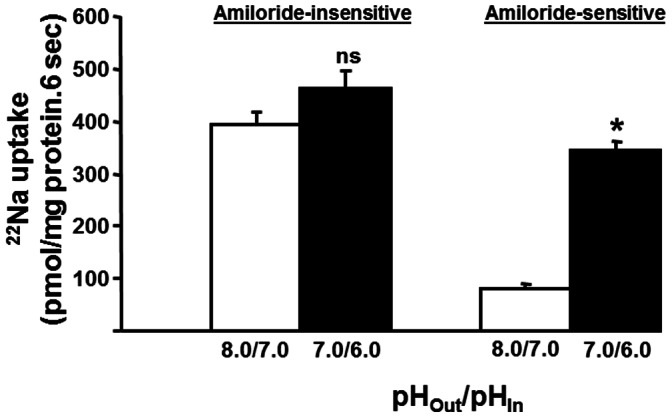
Proton/HCO3 gradient-driven 22Na uptake (0.1 mM) was measured as described in the Method section with one unit acid outward pH gradient at different extravesicular and intravesicular pH in presence and absence of inhibitors (1 mM amiloride or 1 µM HOE694). One-unit outward acid pH-gradients with both low (pHo/pHi: 8.0/7.0) and high (pHo/pHi: 7.0/6.0) proton concentrations stimulated amiloride-insensitive Na-HCO3 cotransport, while amiloride-sensitive Na-HCO3 cotransport was substantially stimulated only at high proton concentrations. Uptake obtained in presence of amiloride represented amiloride-insensitive proton/HCO3 gradient-driven uptake. Amiloride-sensitive uptake (which also includes HOE694 sensitive Na-H exchange) was calculated by subtracting uptake obtained in presence of amiloride from that of uptake in the absence of inhibitors. HOE694-sensitive uptake was calculated by subtracting uptake obtained in presence of HOE694 from that of uptake in the absence of inhibitors. Amiloride-sensitive proton/HCO3 gradient-driven uptake was calculated by subtracting HOE694-sensitive uptake from that of amiloride-sensitive uptake. *p<0.001 - compared to 8.0/7.0 (pHo/pHi) in amiloride-sensitive; ns: not significant - compared to 8.0/7.0 (pHo/pHi) in amiloride-insensitive.
Since amiloride-sensitive Na-HCO3 cotransport is regulated by [H+]-gradients (Figures 9 and 10), experiments were designed to identify whether amiloride-sensitive Na-HCO3 cotransport plays any role in the regulation of pHi in the colon. In this study the role of Na-HCO3 cotransport on pHi regulation was determined by measuring pHi in crypt glands isolated from proximal colon (Figures 1A and 1B). Partial pHi acidification (dpHi/dt; control vs –HCO3∶ 0.437±0.013 vs 0.320±0.011; p<0.001) by bath HCO3 removal indicates the presence of HCO3-independent H+ extrusion pathway in crypt cells from proximal colon (Figure 11A). Since HCO3 diffusion would have contributed to the pHi acidification in HCO3-free medium, the effect of HOE694 was examined on pHi in presence of bath HCO3. Similar to HCO3 removal, the NHE1 inhibitor HOE694 also only partially acidified the pHi in crypt cells from proximal colon (dpHi/dt control vs +HOE694∶ 0.440±0.012 vs 0.274±0.011; p<0.001) (Figure 11B). These observations indicate that both HCO3-dependent (i.e., HOE694-insensitive) and HCO3-independent (i.e., HOE694-sensitive) H+-extrusion pathways are present in basolateral membranes of crypt cells from proximal colon.
Figure 11. Effect of extracellular HCO3 and HOE694 on the rate of intracellular pH (pHi; proton extrusion) in crypt glands from proximal colon.
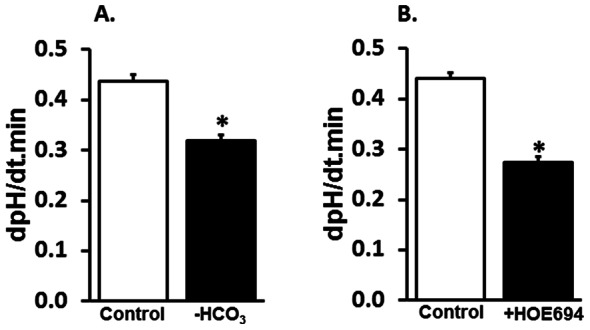
[A] Rate of change in pHi was examined in presence (Control) and absence (-HCO3) of extracellular HCO3. [B] Rate of change in pHi in the presence of extracellular HCO3 was examined in presence (HOE694) and absence (Control) of 1 µM HOE694. *p<0.001 - compared to respective control.
Studies were also designed to test whether amiloride-sensitive HCO3-dependent H+-extrusion plays a critical role in pHi regulation. In these studies, pHi regulation in presence of bath HCO3 was performed in crypt glands isolated from distal colon, which is devoid of NBCe expression (Figures 2A and 3) and amiloride-insensitive NBC activity [7]. As shown in Figure 12, HOE694, which inhibits NHE1-mediated H+-extrusion, only partially but significantly acidified the pHi in crypt cells (dpHi/dt control vs –HOE694∶ 0.427±0.011 vs 0.346±0.011; p<0.0003). This observation indicates that, in addition to HOE694-sensitive H+-extrusion (i.e., NHE1 mediated H+ extrusion), a HOE694-insensitive H+-extrusion pathway is also present in crypt from distal colon. Thus, to determine whether NBCn1 mediates the HOE694-insensitive fraction of H+ extrusion, the effect of amiloride (0.1 mM) was examined on pHi acidification. In the presence of HOE694, bath amiloride further acidified pHi in crypt cells from distal colon (dpHi/dt HOE694 vs HOE694/amiloride: 0.346±0.011 vs 0.124±0.011; p<0.0003). These observations indicate that amiloride-sensitive H+-extrusion consists of both HOE694-sensitive (i.e., NHE1-mediated) and HOE694-insensitive (i.e., NBCn mediated) H+-extrusion processes in basolateral membranes of crypt cells from distal colon. The substantially higher rate of pHi acidification by amiloride compared to that by HOE694 alone (dpHi/dt HOE694 vs HO694/amiloride: 0.081±0.017 vs 0.222±0.028; p<0.001) indicates that amiloride-sensitive H+ extrusion mediated via NBCn plays a major role in pHi regulation in colonic crypts.
Figure 12. Effect of HOE694 on intracellular pH (pHi; proton extrusion) in crypt gland from distal colon.
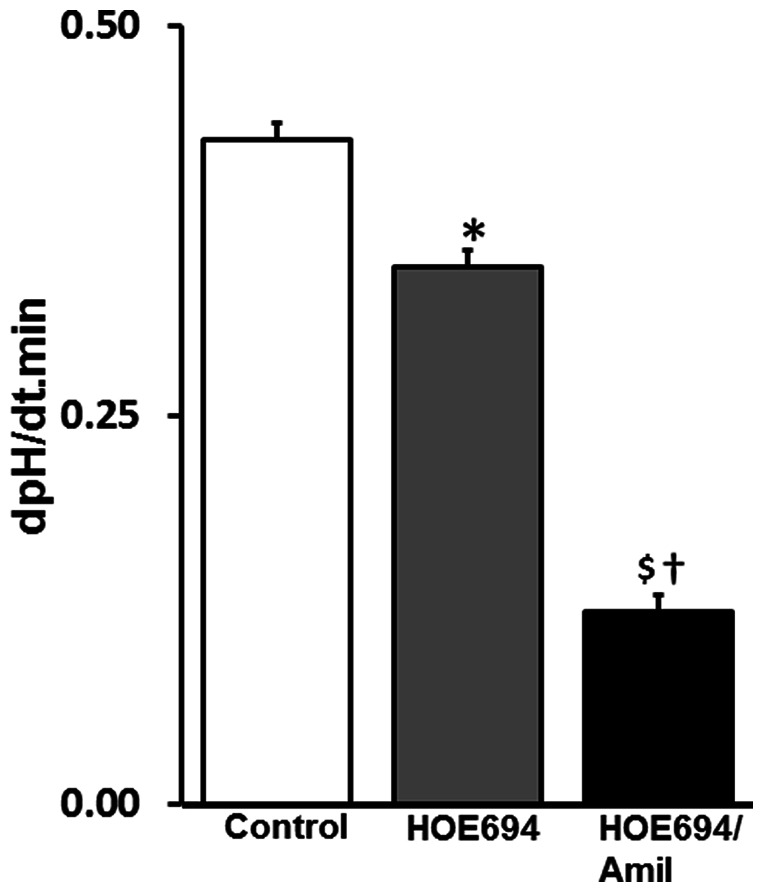
Rate of change in pHi in presence of extracellular HCO3 was examined in the presence (HOE694) and absence (Control) of 1 µM HOE694. Rate of pHi in the presence of HOE694 was also measured in additional presence of 0.1 mM amiloride (HOE694/Amil). *p<0.0003 - compared to control; $ p<0.0001– compared to control; † p<0.0001– compared to HOE694.
Discussion
HCO3 secretion is an essential colonic function that is present in both health and diarrhea. HCO3 secretion requires intracellular HCO3 accumulation as a result of either cell metabolism and/or HCO3 uptake across colonocytes’ basolateral membranes and an exit mechanism across their apical membranes. In recent studies, we have identified three distinct mechanisms for HCO3 movement across the apical membranes of rat distal colon: 1) Cl-dependent HCO3 secretion (i.e., Cl-HCO3 exchange); 2) short-chain-fatty acid (SCFA)-dependent HCO3 secretion (i.e., SCFA-HCO3 exchange); and 3) cAMP-induced HCO3 secretion (via anion channels) [29]. In these studies we also have demonstrated that all three of these HCO3 secretory processes require the presence of HCO3 in the serosal bath solution indicating the importance of a basolateral HCO3 uptake process for HCO3 secretion [29]. Studies with BLMV have identified both Cl-HCO3 exchange and Na-HCO3 cotransport processes as possible mechanisms for HCO3 uptake across basolateral membranes of rat distal colon [7], [30]. In support of a role for Na-HCO3 cotransport in HCO3 movement, basolateral Na-HCO3 cotransporters have been shown to mediate both HCO3 secretion and absorption in renal proximal tubules and pancreatic ducts [1], [2], [31], [32]. However, Na-HCO3 cotransport is also important for the regulation of pHi [33]. Thus, molecular studies have isolated several electrogenic and electroneutral Na-HCO3 cotransport isoforms which have been shown to be expressed in a tissue-specific pattern [1], [4], [6]. However, although Cl-HCO3 exchange has been identified as an anion exchange isoform-2 (AE2) in rat distal colon [30], the molecular identities of Na-HCO3 cotransport in the rat colon have not as yet been identified.
Our present northern blot analyses indicate that both NBCe- and NBCn-like transcripts are expressed in proximal colon, while only NBCn- and not NBCe-like mRNAs and proteins are expressed in distal colon (Figures 2, 3 and 4). RT-PCR analyses confirmed that NBCe- and NBCn-like proteins might be encoded by NBCe1B/C- and NBCn1C/D- (or yet unidentified isoforms containing the amplified fragments) specific transcripts in colon, respectively (Figure 2B). It is not known whether these two isoforms mediate identical (or similar) HCO3-linked functions or whether these two isoforms regulate two distinct HCO3-linked activities.
These studies also established that NBC activities in the proximal and distal colon were not identical in that amiloride-sensitive NBC activity was present in both proximal and distal colon, while amiloride-insensitive activity was only present in the proximal colon (Figure 5) [7]. The observation of both the expression of NBCn specific transcripts and the presence of amiloride-sensitive Na-HCO3 cotransport activity in BLMV of proximal and distal colon suggests that NBCn1 transcripts encode amiloride-sensitive Na-HCO3 cotransport in colonic basolateral membranes (Figures 2A, 3, 4 and 5) [7]. Human NBCn1A, which is 89–92% identical to the rat NBCn1 isoforms expressed in Xenopus oocytes has been shown to exhibit EIPA-, an amiloride analogue, sensitive Na-HCO3 cotransport activity which support the hypothesis that colonic NBCn1 transcripts encode amiloride-sensitive Na-HCO3 cotransport activity in both proximal and distal BLMV [14]. On the other hand, the observations that NBCe-specific mRNA and amiloride-insensitive Na-HCO3 cotransport activity are present only in BLMV of proximal, but not in distal colon indicate that NBCe1B/C specific Na-HCO3 cotransport mediates amiloride-insensitive Na-HCO3 cotransport in the basolateral membrane of proximal colon. This conclusion is consistent with other studies that observed decreased cAMP-stimulated HCO3 secretion and SITS-sensitive current in the colon of NBC1−/− mice indicating that NBC1 activity is a component of basolateral HCO3 uptake during cAMP-stimulated anion secretion in the proximal colon [34] and the involvement of NBCe and NBCn in basolateral electroneutral and electrogenic HCO3 transport in proximal duodenal villus cells [35].
Our studies revealed that amiloride-insensitive Na-HCO3 cotransport requires a pH gradient, whereas the magnitude of amiloride-sensitive Na-HCO3 cotransport is dependent on high proton concentration (Figures 9 and 10). As discussed, NBCe1B/C-like and NBCn1C/D-like transcripts encode amiloride-insensitive and amiloride-sensitive Na-HCO3 cotransport functions in rat colon, respectively. Thus, we propose that amiloride-insensitive NBCe1B/C mediates transcellular transport of HCO3, while NBCn1C/D regulates pHi homeostasis. The conclusion that NBCe1B/C mediates transcellular transport of HCO3 is supported by the demonstrations that: 1) amiloride-insensitive Na-HCO3 cotransport activity is predominantly present in proximal, but not in distal colon; and 2) NBCe1B/C-like mRNA is localized in a tissue (proximal colon) specific pattern (Figures 2A and 2B). Rat kidney NBCe has been shown to be activated by intracellular acid pH in a Xenopus oocyte expression system [36] and mouse NBCe has been shown to contribute to pHi regulation in parotid acinar cells [37]. However, there are no studies that characterized the effect of pHi on NBCn isoforms. It should, therefore, be of interest to compare and contrast the pH dependency of NBCe and NBCn isoforms in an expression system to define their specific role.
Our study also demonstrates that a HCO3-dependent amiloride-sensitive transporter plays a major role in pHi regulation in epithelial cells of the colonic crypt. This conclusion is supported by the observations that there is a 2.7-fold higher rate of pHi acidification in presence of amiloride than that found in the presence of HOE694 alone (Figure 12). Although both NHE1 and amiloride-sensitive NBC are present, bath amiloride-sensitive H+ extrusion (i.e., pHi acidification) has always been presumed to be mediated only by NHE1 [28], [38]. The present study used HOE694 to distinguish amiloride-sensitive NBC (i.e., NBCn1)-mediated H+ extrusion from that of NHE1-mediated H+ extrusion in colonic crypts. NBCn1 has also been shown as the major pHi regulator in mouse duodenal epithelial cells [17]. In that study, Chen et al have used a NBCn1 knockout mouse to demonstrate the role of NBCn1 mediated pHi neutralization as a defensive mechanism against cellular acidification generated by gastric acid in duodenum [17].
These studies identified two distinct NBC (NBCe1B/C and NBCn1C/D) isoforms in proximal colon, but only one isoform (NBCn1C/D) in distal colon. The transport characteristics of these two isoforms have been established: NBCe1B/C is amiloride-insensitive, highly DIDS-sensitive (Ki: 8.9±1.2 µM), not activated by high proton concentrations, but activated by a proton gradient. In contrast, NBCn1C/D is amiloride-sensitive, less sensitive to DIDS (Ki: 263.8±26.4 µM) and activated by high proton concentrations. HCO3 secretion has been identified in both proximal and distal colon [39]. Detailed studies of HCO3 secretion in the distal colon demonstrate that basolateral HCO3 movement is required but suggest that this uptake process involves NKCC (Na-K-2Cl cotransport) and not NBC. Although parallel studies of HCO3 secretion have not been performed in the proximal colon, we speculate that NBCe1B/C may be responsible for HCO3 uptake and that the molecular mechanism of HCO3 secretion in proximal and distal colon differs. In addition, studies are in progress to clone NBCe1B/C-like and NBCn1C/D-like cDNAs from rat colon to establish whether these isoforms exhibit amiloride-insensitive and amiloride-sensitive Na-HCO3 cotransport in an in vitro expression system, respectively.
Funding Statement
This study was supported by the Wellcome Trust and National Institute of Diabetes and Digestive and Kidney Diseases Research grants DK-018777. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Boron WF (2001) Sodium-coupled bicarbonate transporters. JOP 2: 176–181. [PubMed] [Google Scholar]
- 2. Boron WF, Boulpaep EL (1983) Intracellular pH regulation in the renal proximal tubule of the salamander. Basolateral HCO3- transport. J Gen Physiol 81: 53–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dart C, Vaughan-Jones RD (1992) Na(+)-HCO3- symport in the sheep cardiac Purkinje fibre. J Physiol 451: 365–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kurtz I, Petrasek D, Tatishchev S (2004) Molecular mechanisms of electrogenic sodium bicarbonate cotransport: structural and equilibrium thermodynamic considerations. J Membr Biol 197: 77–90. [DOI] [PubMed] [Google Scholar]
- 5. Marino CR, Jeanes V, Boron WF, Schmitt BM (1999) Expression and distribution of the Na(+)-HCO(-)(3) cotransporter in human pancreas. Am J Physiol 277: G487–494. [DOI] [PubMed] [Google Scholar]
- 6. Soleimani M, Burnham CE (2001) Na+:HCO(3–) cotransporters (NBC): cloning and characterization. J Membr Biol 183: 71–84. [DOI] [PubMed] [Google Scholar]
- 7. Rajendran VM, Oesterlin M, Binder HJ (1991) Sodium uptake across basolateral membrane of rat distal colon. Evidence for Na-H exchange and Na-anion cotransport. J Clin Invest 88: 1379–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rajendran VM, Binder HJ (1994) Differential modulation of Na-HCO3 cotransport and Na-H exchange by pH in basolateral membrane vesicles of rat distal colon. J Biol Chem 269: 156–160. [PubMed] [Google Scholar]
- 9. Abuladze N, Lee I, Newman D, Hwang J, Boorer K, et al. (1998) Molecular cloning, chromosomal localization, tissue distribution, and functional expression of the human pancreatic sodium bicarbonate cotransporter. J Biol Chem 273: 17689–17695. [DOI] [PubMed] [Google Scholar]
- 10. Bevensee MO, Apkon M, Boron WF (1997) Intracellular pH regulation in cultured astrocytes from rat hippocampus. II. Electrogenic Na/HCO3 cotransport. J Gen Physiol 110: 467–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burnham CE, Amlal H, Wang Z, Shull GE, Soleimani M (1997) Cloning and functional expression of a human kidney Na+:HCO3- cotransporter. J Biol Chem 272: 19111–19114. [DOI] [PubMed] [Google Scholar]
- 12. Choi I, Aalkjaer C, Boulpaep EL, Boron WF (2000) An electroneutral sodium/bicarbonate cotransporter NBCn1 and associated sodium channel. Nature 405: 571–575. [DOI] [PubMed] [Google Scholar]
- 13. Choi I, Romero MF, Khandoudi N, Bril A, Boron WF (1999) Cloning and characterization of a human electrogenic Na+-HCO-3 cotransporter isoform (hhNBC). Am J Physiol 276: C576–584. [DOI] [PubMed] [Google Scholar]
- 14. Pushkin A, Abuladze N, Lee I, Newman D, Hwang J, et al. (1999) Cloning, tissue distribution, genomic organization, and functional characterization of NBC3, a new member of the sodium bicarbonate cotransporter family. J Biol Chem 274: 16569–16575. [DOI] [PubMed] [Google Scholar]
- 15. Romero MF, Fong P, Berger UV, Hediger MA, Boron WF (1998) Cloning and functional expression of rNBC, an electrogenic Na(+)-HCO3- cotransporter from rat kidney. Am J Physiol 274: F425–432. [DOI] [PubMed] [Google Scholar]
- 16. Romero MF, Hediger MA, Boulpaep EL, Boron WF (1997) Expression cloning and characterization of a renal electrogenic Na+/HCO3- cotransporter. Nature 387: 409–413. [DOI] [PubMed] [Google Scholar]
- 17. Chen M, Praetorius J, Zheng W, Xiao F, Riederer B, et al. (2012) The electroneutral Na+:HCO3- cotransporter NBCn1 is a major pHi regulator in murine duodenum. J Physiol 590: 3317–3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ikuma M, Kashgarian M, Binder HJ, Rajendran VM (1999) Differential regulation of NHE isoforms by sodium depletion in proximal and distal segments of rat colon. Am J Physiol 276: G539–549. [DOI] [PubMed] [Google Scholar]
- 19. Schmitt BM, Biemesderfer D, Romero MF, Boulpaep EL, Boron WF (1999) Immunolocalization of the electrogenic Na+-HCO-3 cotransporter in mammalian and amphibian kidney. Am J Physiol 276: F27–38. [DOI] [PubMed] [Google Scholar]
- 20. Vorum H, Kwon TH, Fulton C, Simonsen B, Choi I, et al. (2000) Immunolocalization of electroneutral Na-HCO(3)(–) cotransporter in rat kidney. Am J Physiol Renal Physiol 279: F901–909. [DOI] [PubMed] [Google Scholar]
- 21. Forbush B (1983) Assay of Na,K-ATPase in plasma membrane preparations: increasing the permeability of membrane vesicles using sodium dodecyl sulfate buffered with bovine serum albumin. Anal Biochem 128: 159–163. [DOI] [PubMed] [Google Scholar]
- 22. Del CastilloJR, Rajendran VM, Binder HJ (1991) Apical membrane localization of ouabain-sensitive K(+)-activated ATPase activities in rat distal colon. Am J Physiol 261: G1005–1011. [DOI] [PubMed] [Google Scholar]
- 23. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275. [PubMed] [Google Scholar]
- 24. Lomax RB, McNicholas CM, Lombes M, Sandle GI (1994) Aldosterone-induced apical Na+ and K+ conductances are located predominantly in surface cells in rat distal colon. Am J Physiol 266: G71–82. [DOI] [PubMed] [Google Scholar]
- 25. Rajendran VM, Geibel J, Binder HJ (1995) Chloride-dependent Na-H exchange. A novel mechanism of sodium transport in colonic crypts. J Biol Chem 270: 11051–11054. [DOI] [PubMed] [Google Scholar]
- 26. Counillon L, Scholz W, Lang HJ, Pouyssegur J (1993) Pharmacological characterization of stably transfected Na+/H+ antiporter isoforms using amiloride analogs and a new inhibitor exhibiting anti-ischemic properties. Mol Pharmacol 44: 1041–1045. [PubMed] [Google Scholar]
- 27. Bevensee MO, Schmitt BM, Choi I, Romero MF, Boron WF (2000) An electrogenic Na(+)-HCO(–)(3) cotransporter (NBC) with a novel COOH-terminus, cloned from rat brain. Am J Physiol Cell Physiol 278: C1200–1211. [DOI] [PubMed] [Google Scholar]
- 28. Yun CH, Tse CM, Nath SK, Levine SA, Brant SR, et al. (1995) Mammalian Na+/H+ exchanger gene family: structure and function studies. Am J Physiol 269: G1–11. [DOI] [PubMed] [Google Scholar]
- 29. Vidyasagar S, Rajendran VM, Binder HJ (2004) Three distinct mechanisms of HCO3- secretion in rat distal colon. Am J Physiol Cell Physiol 287: C612–621. [DOI] [PubMed] [Google Scholar]
- 30. Ikuma M, Geibel J, Binder HJ, Rajendran VM (2003) Characterization of Cl-HCO3 exchange in basolateral membrane of rat distal colon. Am J Physiol Cell Physiol 285: C912–921. [DOI] [PubMed] [Google Scholar]
- 31. Ishiguro H, Steward MC, Lindsay AR, Case RM (1996) Accumulation of intracellular HCO3- by Na(+)-HCO3- cotransport in interlobular ducts from guinea-pig pancreas. J Physiol 495 (Pt 1): 169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhao H, Star RA, Muallem S (1994) Membrane localization of H+ and HCO3- transporters in the rat pancreatic duct. J Gen Physiol 104: 57–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Boron WF, Boulpaep EL (1983) Intracellular pH regulation in the renal proximal tubule of the salamander. Na-H exchange. J Gen Physiol 81: 29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gawenis LR, Bradford EM, Prasad V, Lorenz JN, Simpson JE, et al. (2007) Colonic anion secretory defects and metabolic acidosis in mice lacking the NBC1 Na+/HCO3- cotransporter. J Biol Chem 282: 9042–9052. [DOI] [PubMed] [Google Scholar]
- 35. Praetorius J, Hager H, Nielsen S, Aalkjaer C, Friis UG, et al. (2001) Molecular and functional evidence for electrogenic and electroneutral Na(+)-HCO(3)(-) cotransporters in murine duodenum. Am J Physiol Gastrointest Liver Physiol 280: G332–343. [DOI] [PubMed] [Google Scholar]
- 36. Grichtchenko, II, Romero MF, Boron WF (2000) Extracellular HCO(3)(-) dependence of electrogenic Na/HCO(3) cotransporters cloned from salamander and rat kidney. J Gen Physiol 115: 533–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim YB, Yang BH, Piao ZG, Oh SB, Kim JS, et al. (2003) Expression of Na+/HCO3- cotransporter and its role in pH regulation in mouse parotid acinar cells. Biochem Biophys Res Commun 304: 593–598. [DOI] [PubMed] [Google Scholar]
- 38. Zachos NC, Tse M, Donowitz M (2005) Molecular physiology of intestinal Na+/H+ exchange. Annu Rev Physiol 67: 411–443. [DOI] [PubMed] [Google Scholar]
- 39. Umesaki Y, Yajima T, Yokokura T, Mutai M (1979) Effect of organic acid absorption on bicarbonate transport in rat colon. Pflugers Arch 379: 43–47. [DOI] [PubMed] [Google Scholar]