Abstract
Background
Social inattention is common in children with autism whereas associative learning capabilities are considered a relative strength. Identifying early precursors of impairment associated with autism could lead to earlier identification of this disorder. The present study compared social and nonsocial visual attention patterns as well as associative learning in infant siblings of children with autism (AU sibs) and low risk (LR) infants at six months of age.
Methods
25 AU sibs and 25 LR infants were observed in a novel social-object learning task, within which attention to social and non-social cues was contrasted. Video recorded data were coded for percent duration of gaze to objects or caregiver. Movement rates to activate the toy within the associative learning task were also quantified.
Results
Both groups learned the association between moving a switch and activating a cause-effect toy. AU sibs spent less time looking at caregivers and more time looking at the toy or joystick when their caregivers made no attempts to engage their attention. However, response to caregiver-initiated social bids was comparable for both groups.
Conclusions
Infrequent self-initiated socially directed gaze may be an early marker of later social and communication delays.
Keywords: Attention, Autism, Infants, Social, Learning
Autism Spectrum Disorders (ASDs) represent a set of developmental disorders characterized by social and communication deficits, often along with repetitive behaviors and interests (American Psychiatric Association, 2000). The high prevalence of ASDs, 1 in 110 children, is a major public health issue (ADDM, 2009). There is an urgent need to detect ASDs as early as possible to permit earlier intervention, possibly improving outcome. ASDs are typically diagnosed between three and six years of age (Wiggins, Baio, & Rice, 2006). However, prospective, longitudinal research has been used to identify factors that allow for earlier detection of ASDs in infants at increased genetic risk (e.g., Landa & Garrett-Mayer, 2006; Landa, Holman, & Garrett-Mayer, 2007; Zwaigenbaum, et al., 2005). In addition to having an increased risk for ASDs, siblings of children with autism (AU sibs) are at increased genetic risk for related, milder social and communication impairments (Folstein, et al., 1999).
There is growing evidence from prospective studies that social communication development is disrupted between the ages of 14 to 24 months in most children later diagnosed with ASDs. Specifically, disruption in nonverbal communication is characterized by infrequent initiation of and response to joint attention cues of others (Charman et al., 2005; Chawarska, Klin, Paul, & Volkmar, 2007; Sullivan et al., 2006; Landa et al., 2007), infrequent reciprocal social interaction, poor integration of eye gaze within such interactions, and infrequent shared positive affect (Chawarska et al., 2007; Landa et al., 2007). However, little is known about whether similar signs of developmental disruption may be evident earlier in life for infants at risk for autism and related social and communication delays. Therefore, the main purpose of the present study was to compare precursors of social communication, specifically visual attention patterns, in six-month-old infant AU sibs and infants at low risk (LR infants) for ASDs.
The few prospective studies that have focused on the first year of life of AU sibs have provided mixed evidence of developmental disruption at this early age. In some studies, early signs of developmental disruption were noted in a subgroup of infant AU sibs. These signs include diminished social attention and eye contact (Zwaigenbaum et al. 2005), failure in orienting to name (Zwaigenbaum et al., 2005), and difficulty disengaging visual attention (Bryson, et al., 2007; Zwaigenbaum et al., 2005). Yet when AU sibs have been compared to LR infants as a group, only subtle differences have been identified. For example, four-month-old AU sibs exhibited more neutral affect, but no differences in visual attention, within the still-face procedure when compared to LR infants (Yirmiya et al., 2006). Another research group reported subtly reduced smiling in six-month-old AU sibs as compared to LR infants (Cassel et al. 2007). Lower rates of attention shifts between social and nonsocial stimuli in AU sibs as compared to LR infants have been reported, with no differences in total proportion of gaze to or away from caregivers (Ibanez et al., 2008). Moreover, Merin and colleagues (2007) reported no group differences in affect or attention between six-month-old AU sibs and LR infants within this same procedure. The inconsistent or modest findings of difference between infants at high and low risk for ASDs may be related to the dyadic nature of these tasks. Tasks requiring attention to a single stimulus within a dyadic interaction (e.g., caregiver-child) may have insufficiently challenged the infant AU sibs, and hence failed to elicit behavior that was notably different from that of typical development.
The approach taken in the present study was to introduce infants to a novel, multi-stimuli task that allowed for triadic interactions. A triadic interaction in infancy usually implies interactions involving a caregiver, infant, and object or event (Striano & Dahl, 2005). The triadic task employed in this study was a novel “social-object learning” task. The traditional associative learning paradigms do not involve social interactions with a caregiver (Rovee, Hayne, & Colombo, 2001). In contrast, our social-object learning task provided simultaneously available options for infants to attend to, and engage with, objects as well as their caregiver. The triadic interaction opportunity created within this task was designed as a probe for social orienting and social engagement. There were two primary motivations for the creation of this task. First, social orienting and engagement impairments are among the earliest behaviors to distinguish children with ASDs from those with other delays or typical development (Osterling & Dawson, 2002; Landa et al., 2007; Nadig et al., 2007). Second, these impairments may be precursors to later deficits in joint attention, a hallmark of ASDs (Mundy, Sigman, & Kasari, 1994). Thus, triadic interaction tasks could be particularly useful in identifying infants with vulnerability during the early emergence of social interactions.
Historically, learning paradigms have been valuable for quantifying the ontogeny of learning and memory in pre-verbal infants (Rovee et al., 2001). Infants with typical development display robust and rapid action-based associative learning by three months of age, indicating the ability to learn cause-effect relationships (Rovee et al., 2001). Within these tasks, infants also display predictable patterns of attention shifting (Mast, Fagen, & Rovee-Collier, 1980), motor coordination (Heathcock et al., 2005), and affect variation (Alessandri, Sullivan, & Lewis, 1990). These tasks have been used to identify early impairments across a range of other populations such as infants with preterm birth (Rose, Feldman, & Jankowski, 2001; Heathcock et al., 2005; Heathcock et al., 2004), Down syndrome (Ohr & Fagen, 1993), and prenatal cocaine exposure (Alessandri et al., 1993). Although AU sibs have not been tested within learning tasks, preliminary evidence of abnormality in aspects of their visual attention may affect their performance (Bryson et al., 2007; Rose et al., 2001). In contrast, older children with autism, even those with intellectual disability, exhibit a relative strength in the ability to detect simple rule-based contingencies during object play with cause-effect toys (Klinger & Dawson, 2001). Overall, the aim of the present study was to compare patterns of social and non-social attention and learning in six-month-old AU sibs and LR infants when presented with the social-object learning task.
Method
Participants
Twenty five infant siblings of children with autism (AU sibs; males=14, females=11) and 25 typically developing, low risk infants with no family history of ASDs (LR infants; males=8, females=17; X2 =2.1, p>0.1; a non-significant gender difference between groups) were observed for one session at six months of age during the “social-object learning” task. The mean age (and standard deviation) of AU sibs and LR infants was 6.55 (0.66) and 6.67 (0.50) months, respectively with a non-significant group difference of p>0.1. Groups did not differ on raw scores from the Visual Reception scale of the Mullen Scales of Early Learning (Mullen, 1995) (AU sibs=9.28(1.20), LR infants=9.83(1.57), p>0.1), a measure of visually-based cognitive level. Participants were recruited as part of an ongoing larger, federally-funded study aimed at identifying early markers of ASDs using a prospective, longitudinal design. Participants were recruited through ASD advocacy groups, conferences, Kennedy Krieger Institute’s Center for Autism and Related Disorders, mailing invitations to families identified through public birth announcements, and word of mouth. We term our comparison group as “low risk” because the general population is at significantly lower risk to develop ASDs than younger siblings of children with ASD (Bailey et al., 1996). The probands with autism met the diagnostic criteria for autism on both the Autism Diagnostic Observation Schedule (ADOS) (Lord, Rutter, DiLavore, & Risi, 1999) and the Autism Diagnostic Interview-Revised (ADI-R) (Lord, Rutter, & Le Couteur, 1994). Exclusion criteria for both groups were: low birth weight (< 2500 grams), gestational age (< 35 weeks), birth trauma, head injury, prenatal illicit drug or excessive alcohol exposure, known genetic disorder that would confer increased risk of ASDs (e.g., fragile X). Infants were admitted to the study following informed parental consent as approved by the Johns Hopkins Institutional Review Board.
Experimental Set Up
The infants were seated in a custom infant chair for 10 minutes with a musical toy on their right side and caregivers on their left (Figure 1). The toy and caregiver were both located approximately 12 inches beyond the reach of the infant. A Sony mini DV camcorder placed in the front of the infant with an oblique view was used to record the infant’s body movements and visual attention as well as some portion of the toy.
Figure 1.
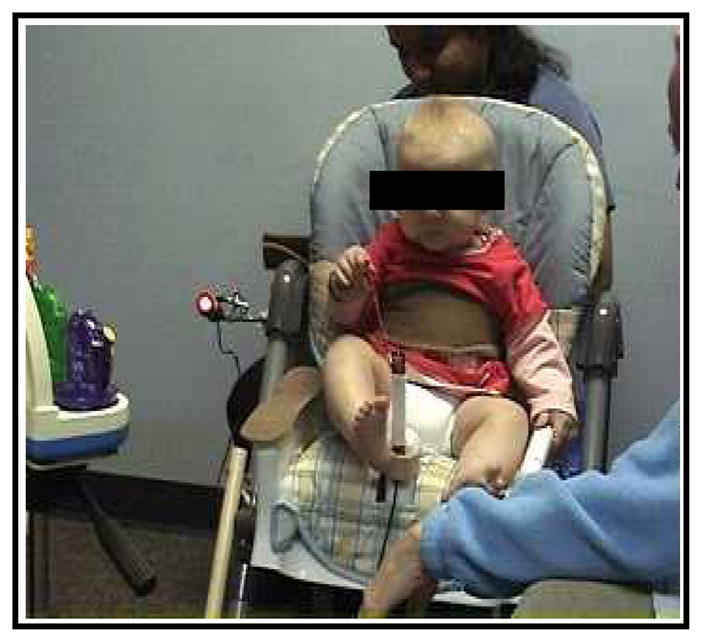
Experimental set-up for the social-object learning paradigm. It includes the infant, the musical toy on the infant’s right side, and the caregiver on the infant’s left side. The light activated by pulling the joystick during the baseline and extinction periods is also shown.
During minutes 0 to 2 (Baseline), the infant’s right hand was tethered to a joystick located in the front and midline of the infant (Table 1 and Figure 1). Each time the infant bent the joystick, it activated a light attached to the chair. No contingent activation of the toy was provided in this period. During minutes 2 to 8 (Acquisition), a toy located in the front and right side of the infant was activated for 5 seconds, producing colorful lights and music, each time the infant moved the joystick. During minutes 8 to 10 (Extinction), the association between the joystick and toy was broken as the toy was not activated when the joystick moved. Each period was equally divided into spontaneous and social phases, with the spontaneous phase always preceding the social phase. During spontaneous phases, the caregiver remained silent. Thus, any social orientation of attention exhibited by the infant was infant-initiated. Parents were allowed to smile back to the child if he/she looked, but otherwise not respond. During the social phase, the caregiver initiated social engagement with the infant using a standardized script, as described below. During the Baseline period, the animals and colors on the toy were described. During the Acquisition period, the infant was asked to play the music (“Infant’s name, can you play the music? Where did it go?”), and provided verbal reinforcement (“Yeahh! You played the music!”) upon activating the toy. During the Extinction period, caregivers continued to ask the infants to play the music but no reinforcement was provided, as the toy was not activated. Caregivers were always the infant’s mother except in the case of one LR infant (father) and two AU sibs (father and grandmother).
Table 1.
Summary of the social-object learning paradigm and percent duration of caregiver feedback in the social phases
| Duration | Periods | Association | Phases | Social engagement initiated by caregiver: Mean percent (SD) |
|---|---|---|---|---|
| Minutes 0–2 | Baseline | Bending of joystick causes no toy activation | Spontaneous | No |
| Social | Yes % Feedback: AU sibs=52.9% (12.3), LR infants=54.0% (16.5) |
|||
| Minutes 2–8 | Acquisition | Bending of joystick causes toy activation | Spontaneous | No |
| Social | Yes % Feedback: AU sibs=58.1% (11.8), LR infants=64.2% (17.0) |
|||
| Minutes 8–10 | Extinction | Bending of joystick causes no toy activation | Spontaneous | No |
| Social | Yes % Feedback: AU sibs=48.1% (16.9), LR infants=51.9% (18.8) |
We also coded the duration of caregivers’ verbal feedback across all periods/phases to examine whether caregivers in both groups responded differently to their infants. In the spontaneous phase, no such feedback was provided. For the social phase data, we conducted a Group (AU sibs, LR infants) x Period (Baseline, Acquisition, Extinction) ANOVA with groups as the between-subjects factor and Period as the within-subjects factor. The ANOVA revealed a main effect of Period (F(2,96)=8.12, p<0.001, η2 =0.1) with no other group effects or group x period interaction effects (p>0.1). Three post hoc comparisons revealed greater duration of caregiver feedback in the Acquisition period than the Baseline (p<0.01) or Extinction (p<0.001) (see data in Table 1). No group differences were found in proportion of caregiver feedback during the social phases.
Data Analyses and Dependent Variables
The Observer coding software (NOLDUS, Inc) was used to code dependent variables from videotapes, frame by frame. Intra- and inter-rater reliability was computed by one-way random intra-class correlations for each variable. Inter-rater reliability of above 95% was achieved by the primary coder (1st author) with a blinded secondary coder for one-third of the total data (i.e., 16 infants or 160 minutes) across all variables (see definitions below). Intra-rater reliability was above 97% for all variables.
Learning was defined as a significant increase in the rates of toy activation during the Extinction period as compared to the Baseline period (Rovee et al., 2001). Rates of toy activation were coded per minute for each period of the social-object learning task.
Duration of attention to social and non-social stimuli was defined as time in seconds during which eye gaze was focused onto either the “caregiver’s face” on the left, the “musical toy” on the right, the “joystick” in front, or anywhere else in the testing room coded as “other”. These duration codes were calculated for each phase of the learning task.
Statistical Analyses
Pearson’s correlation was used to determine associations between variables. Each variable was subjected to a full model ANOVA using SPSS software (SPSS, Inc) with groups (AU sibs and LR infants) as the between-subjects factor and learning periods (Baseline, Acquisition, Extinction) and phases (spontaneous, social) as the two within-subjects factors. Based on preliminary analyses of data skewness, the toy activation rates were not transformed and a square root transformation was performed for the percent duration of gaze to caregiver and to toy and joystick to normalize their distributions. Transformed variables were used in the statistical analyses but raw variables are presented in the tables and figures. We report p- and F-values after performing the Greenhouse-Geisser correction which corrects for inequality of variance. Lastly, post hoc comparisons using paired or independent t-tests were performed with a focus on group by period/phase interactions. Statistical significance for the ANOVAs was defined as p-values < 0.05. Because this is a pilot study with a relatively small sample size, statistical trends were reported. A statistical trend was defined as p-values > 0.05 but < 0.1. For post-hoc analyses, we report original p-values; however, results are only reported to be significant if they meet the criteria based on Bonferroni corrections.
Results
Associations among Learning and Visual Attention Measures
All correlations described below were measured separately for each group, for each period and phase. Toy activation rates did not correlate with any visual attention variables in any of the periods or phases (r values ranged from −0.2 to 0.3). A significant negative correlation was found between the percent duration of gaze to caregiver and percent duration of gaze to toy or joystick for the majority of the periods and phases (LR infants, r=−0.69±0.19, AU sibs, r=−0.77±0.08, ps<0.01) except for the spontaneous phase of the Extinction period in LR infants (r=−0.34, p<0.1). There was no one-to-one correspondence between learning and attention variables. However, longer looking durations at the toy or joystick were associated with shorter looking durations at the caregiver for the majority of the phases/periods. There was no one-to-one correspondence between learning and attention variables.
Learning
Both AU sibs and LR infants learned the association between body movements and toy activations (see Table 2). Rates of toy activation by pressing the joystick were strikingly similar for both groups over the various learning periods. A full model ANOVA for rates of toy activation revealed a main effect of Period (F(2,96)=22.63, p<0.001, η2 =0.3), a main effect of Phase (F(1,48)=6.99, p<0.05, η2 =0.1), and a Period × Phase interaction (F(2,96)=4.87, p<0.05, η2 =0.1) with no group or group x period/phase interaction effects. Four post hoc comparisons were conducted after pooling data across groups to examine learning based on the Period x Phase interaction. Per Bonferroni corrections, toy activation rates were significantly greater during both phases of the Extinction period as compared to their respective Baselines (p’s <0.001). In addition, toy activation rates were significantly greater during the spontaneous phase of Acquisition versus its Baseline (p<0.01) with no differences in the social phase of Acquisition versus its Baseline. Overall, both groups displayed similar associative learning abilities based on the traditional definition of learning (i.e.; Extinction must differ from Baseline).
Table 2.
Within-group comparisons for AU sibs and LR infants: Learning as defined by rates of toy activation in the Spontaneous phase of the Baseline versus the Spontaneous phase of the Extinction periods, and in the Social phase of the Baseline versus the Social phase of the Extinction periods.
| Periods | Phases | Mean (SD) of Rate of Toy Activation | ||
|---|---|---|---|---|
| AU sibs | LR infants | p-values for within-group comparisons: Baseline v. Extinction or Baseline v. Acquisition | ||
| Baseline | Spontaneous | 6.2 (3.5) | 6.2 (3.3) | NA |
| Social | 6.4 (3.7) | 6.5 (4.9) | NA | |
| Acquisition | Spontaneous | 7.7 (5.3) | 8.6 (4.8) | p<0.01* |
| Social | 6.9 (5.4) | 7.9 (3.7) | p>0.05 | |
| Extinction | Spontaneous | 11.8 (8.5) | 12.6 (7.3) | p<0.001* |
| Social | 9.2 (6.7) | 9.0 (5.1) | p<0.001* | |
Original, uncorrected, p-values that meet criteria for significance based on Bonferoni correction. Analyses were done after pooling data across the two groups
Gaze to the Caregiver
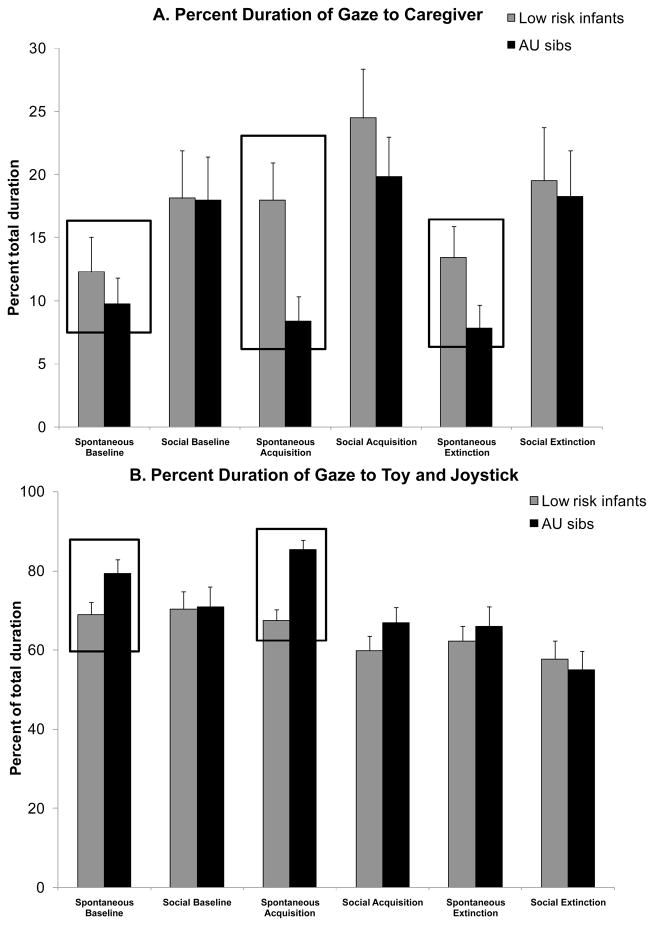
The percent duration of gaze directed to the caregiver was examined to compare attention to social cues (Figure 2A and Table 3A). A full model ANOVA revealed a main effect of Period (F(2,96) = 3.36, p<0.05), η2 = 0.1), a main effect of Phase (F(1,48) = 24.59, p<0.001, η2 =0.3), a statistical trend for Phase x Group interaction (F(1,48) = 3.09, p<0.08, η2 =0.1) and no other interaction effects. First, we conducted three post hoc comparisons to examine the main effect of Period. Per Bonferroni corrections, the percent duration of gaze to caregiver was significantly greater during the Acquisition period as compared to the Baseline (p=0.01) and Extinction (p<0.001). Infants also spent significantly more time looking to their caregivers during the social phase than the spontaneous phase. Lastly, we conducted two post hoc comparisons to examine the Phase x Group interaction. Per Bonferroni corrections, the percent duration of gaze to caregiver was significantly lower in AU sibs as compared to LR infants during the spontaneous phases (p<0.001, η2 =0.6) with no group differences in the social phases (p>0.1). These results suggest that the context of learning and contrasting social stimuli evoke different visual attention patterns in infants. Most importantly, AU sibs tended to show diminished self-initiation of attention to caregivers as compared to LR infants during the spontaneous phases of learning and not the social phases; which is when caregivers were directly bidding to their infants.
Figure 2.
A & B. AU sibs spent significantly less time looking at the caregiver and more time looking at the toy and joystick during the spontaneous phases versus social phases. Error bar denotes standard error. In both figures, the spontaneous phases that distinguished the two groups are highlighted.
Table 3.
Between-group comparisons for percent of gaze directed to caregiver and toy or joystick in AU sibs and LR infants.
| Phases | Periods | A. Mean (SD) of Percent Gaze to Caregiver | ||
|---|---|---|---|---|
| AU sibs | LR infants | p-values for between-group comparisons | ||
| Spontaneous | Baseline | 9.7 (10.3) | 12.3 (13.8) | p<0.001* |
| Acquisition | 8.4 (9.8) | 17.9 (14.9) | ||
| Extinction | 7.8 (9.2) | 13.4 (12.4) | ||
| Social | Baseline | 17.9 (16.9) | 18.1 (18.8) | p>0.1 |
| Acquisition | 19.8 (15.9) | 24.5 (19.5) | ||
| Extinction | 18.3 (18.3) | 19.5 (21.0) | ||
| Phases | Periods | B. Mean (SD) of Percent Gaze to Non-social Stimuli | ||
| AU sibs | LR infants | p-values for between-group comparisons | ||
| Spontaneous | Baseline | 79.5 (16.9) | 68.9 (15.9) | p<0.001* |
| Acquisition | 85.3 (12.0) | 67.4 (13.9) | ||
| Extinction | 66.1 (24.2) | 62.2 (19.6) | ||
| Social | Baseline | 70.9 (24.9) | 70.3 (21.8) | p>0.1 |
| Acquisition | 66.9 (18.9) | 59.9 (17.4) | ||
| Extinction | 54.9 (23.8) | 57.7 (22.6) | ||
p-value original, uncorrected, p-values that meet criteria for significance based on Bonferoni correction. The two groups were compared for each phase after collapsing data across the three periods.
Gaze to objects comprising the learning paradigm apparatus
The combined percent duration of gaze to the toy or joystick within the learning task was examined. In general, the AU sibs spent more time looking at non-social stimuli compared to the LR infants (Figure 2B and Table 3B). A full model ANOVA for percent duration of looking at non-social stimuli revealed a main effect of Period (F(2,96) = 12.96, p<0.001, η2 =0.2), a main effect of Phase (F(1,48) = 19.57, p<0.001, η2 =0.3), a Phase × Group interaction effect (F(1,48) = 4.72, p<0.05, η2 =0.1) and no other interaction effects. First, we conducted three post hoc comparisons to examine the main effect of Period. Per Bonferroni corrections, the percent duration of gaze to toy and joystick was significantly greater during the Baseline (p<0.001) and Acquisition period (p<0.001) as compared to Extinction. This indicates that regardless of risk for ASD, infants find the relevant, non-social stimuli less compelling when the contingency between their action and the toy activation no longer exists. Gaze to the toy and joystick was also greater during the spontaneous phases than the social phases. Next, we performed two post hoc comparisons to examine the Phase x Group interaction. Per Bonferroni corrections, the percent duration of gaze to non-social stimuli was significantly greater in AU sibs as compared to LR infants during the spontaneous phases (p<0.01, η2 =0.5) with no group differences in the social phases (p>0.1). The AU sibs’ propensity to orient attention towards non-social stimuli was greater in the absence of caregiver overtures as compared to LR infants. These findings confirm that, in the absence of socially directed attention, AU sibs were attending to relevant, non-social stimuli (joystick or toy) rather than peripheral stimuli (e.g., ceiling lights, video camera) or staring blankly into space.
Discussion
The present study examined learning and visual attention in AU sibs using a cause-effect learning task with intervals of social engagement initiated by their caregiver. Performance of the AU sibs was compared to a matched group of LR infants. We found no evidence of impaired associative learning among the AU sibs, but AU sibs exhibited less time than LR infants looking to their caregivers and more time looking at non-social stimuli such as the toy or joystick during the spontaneous phases of the learning task.
Associative Learning
Both groups showed associative learning abilities (Table 2), as they increased their toy activation rates during the Extinction period as compared to Baseline. The social-object associative learning task developed in this study is an adaptation of other similar learning paradigms (Rovee et al., 2001; Alessandri et al., 1991; Lobo & Galloway, 2008) but is the first of its kind to contrast social and object cues in order to detect early developmental disruption in infants at risk for ASDs. We used the traditional definition of learning from the associative learning literature, wherein the activation rate of a group of infants during the Extinction period is expected to be above Baseline (Rovee et al., 2001). Based on this definition, both groups appear to have learned the association spontaneously, without improvement when encouragement from the caregiver was offered.
Our finding that infants at increased risk for ASDs showed typical performance in the learning task is compatible with past reports that associative learning is a relative strength in older individuals with autism. For example, Minshew and Goldstein (2001) showed that adolescents and adults with autism performed as well as healthy controls during simple paired associative learning tasks and short-term memory tasks. However, performance on complex tasks such as list learning, maze learning, and span tasks was impaired. Many treatment approaches in autism, such as highly structured applications of the principles of Applied Behavioral Analysis (Baglio et al., 1996), capitalize on the strength of associative learning. It is possible that, like older children, infants at high genetic risk for ASDs may benefit from frequent exposure to simple but highly salient cause-effect learning contexts to facilitate development.
Initiation of Social Attention
In general, infants’ duration of gaze to their caregivers was greater in the condition where they learned novel cause-effect relationships (Acquisition versus Baseline or Extinction, Figure 2A), when their own actions activated the toy. Self-initiated social sharing may be more likely within contexts that promote discovery of such relationships. This finding has implications for the early presence of infant social sharing behavior in the context of novelty. Specifically, during the spontaneous phases, the AU sibs spent less time attending to their caregiver and more time attending to non-social stimuli, such as the joystick or the toy, as compared to the LR infants (Figure 2 & Table 3). That is, infant AU sibs spontaneously attended less often to their caregivers when the caregiver was quiet and passive (and the infant was engaged in object-based play). This finding is consistent with reports by Zwaigenbaum et al. (2005) and Landa et al. (2007) in which 12- to 14-month-old AU sibs displayed fewer initiations and responses to engage with others. It is also consistent with findings from retrospective studies that infants or toddlers later diagnosed with autism exhibited decreased frequency of gaze to caregiver’s faces and failure to orient to name (Osterling & Dawson, 1994, 2002) and increased visual interest in objects than people (Maestro et al., 2002). Ibanez and colleagues (2008) reported a similar tendency in AU sibs for diminished attention to caregivers during the unresponsive phase of the still face paradigm as compared to LR infants.
Our finding that AU sibs exhibited diminished gaze to caregivers during the spontaneous phases of the learning task may indicate a disruption in early developing processes involved in initiation of joint attention. Initiation of joint attention (IJA) is the ability to use gaze and gesture to direct the attention of others to spontaneously share experiences (Mundy & Newell, 2007) and is impaired in children with ASDs (Mundy et al., 1986). In contrast to the attenuated levels of self-initiated social gaze during spontaneous phases, AU sibs exhibited typical levels of social gaze when their caregivers actively engaged them. Together, these findings may indicate a greater vulnerability in the developing social initiation system compared to the social responsiveness system at this early age in infants at increased risk for ASDs. This interpretation is supported by earlier reports of greater impairment in initiation of joint attention than in response to joint attention in older children with ASDs (Zwaigenbaum et al., 2005; Mundy et al., 1994).
The finding of improved social orienting in the infant AU sibs during the social phase of the learning task revealed that the attention of infants at risk for ASDs can be readily recruited toward their caregiver. Identifying contexts that maximize socially directed attention is important for infants at increased risk for ASDs given the pivotal role that socially oriented attention may play in many facets of development, including joint attention, affective reciprocity, face processing, and early language development (Mundy, Fox, & Card, 2003).
Limitations
Several limitations are acknowledged for this initial study of involving the social-object learning task. The sample size is relatively small, follow-up outcomes were not yet available, and advanced measures such as eye tracking systems were not used thereby limiting our interpretation of the visual attention patterns. For example, when a child looked in the direction of the caregiver’s face, we could not distinguish the specific facial features at which the child was looking. Furthermore, the specificity to ASD of the visual attention patterns observed in AU sibs requires further research involving comparison groups such as infants with Down syndrome or infants born preterm. Lastly, not all LR infants had an older sibling; hence, our data may be more variable due to the developmental differences attributed to birth order.
Conclusions
In summary, we compared learning and visual attention patterns of 6-month-old siblings of children with autism to that of same-age low risk infants using a novel social-object learning task. Shorter durations of self-initiated gaze to caregivers distinguished AU sibs from LR infants. We also described an assessment context that contrasted infants’ attention between social and non-social cues, which may prove useful for identifying risk for autism or milder associated impairments in the first year of life.
Atypical visual attention patterns such as reduced social gaze is found in children with ASDs. However, there is mixed evidence about whether these deficits are present in young infants at risk for ASDs (AU sibs).
We showed that context is critical to identifying early differences in attention patterns of AU sibs. They showed reduced social attention when caregivers were not actively bidding to them. Moreover, it may be possible to examine early disruptions related to ASDs by six months.
It is important to evaluate infant AU sibs in contexts contrasting social initiation versus social responsiveness. Novel learning contexts are also valuable in assessing social development early in life.
Acknowledgments
We would like to thank the infants and families who participated in this study. We thank Dr. Michele Lobo at the University of Delaware and Dr. Margaret Sullivan at the University of Medicine and Dentistry of New Jersey for their valuable suggestions during the development of the learning task. We also thank Elizabeth Stuart for her statistical advice. We thank the various undergraduate, graduate, and doctoral students as well as clinical research staff at the Kennedy Krieger REACH laboratory who contributed to this project through scheduling, data organization, data collection, and data processing: Marguerite Adams, Julianna Finelli, Dana Herman, Christine Hess, James Mancini, Alison Marvin, Allison Nelson, Allison O’Neill, Amy Reese, Julie Rusyniak, and Melissa Warren. We also thank Derek Monette for his contribution with coding these data. AB and RL thank Cure Autism Now and Karma Foundation for the mentor based, Young Investigator Award awarded to AB. RL thanks the National Institutes of Mental Health for support of her research through grants MH59630 and 154MH066417.
Abbreviations
- AU sibs
Infants at risk for autism
- LR infants
Low risk infants
References
- Alessandri SM, Sullivan MW, Lewis M. Violation of expectancy and frustration in early infancy. Developmental Psychology. 1990;26(5):738–744. [Google Scholar]
- Alessandri SM, Sullivan MW, Imaizumi S, Lewis M. Learning and emotional responsivity in cocaine-exposed infants. Developmental Psychology. 1993;29(6):989–997. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-IV-TR) 4. Washington, DC: 2000. [Google Scholar]
- Baglio C, Benavidiz D, Compton L, Matson J, Paclawskyj T. Behavioral treatment of autistic persons: A review of research from 1980 to present. Research in Developmental Disabilities. 1996;17:433–465. doi: 10.1016/s0891-4222(96)00030-3. [DOI] [PubMed] [Google Scholar]
- Bailey A, Phillips W, Rutter M. Autism: Towards an integration of clinical, genetic, neuropsychological, and neurobiological perspectives. Journal of Child Psychology and Psychiatry. 1996;37:89–126. doi: 10.1111/j.1469-7610.1996.tb01381.x. [DOI] [PubMed] [Google Scholar]
- Bryson S, Zwaigenbaum L, Brian J, Roberts W, Szatmari P, Rombough V, McDermott C. A prospective case series of high-risk infants who developed autism. Journal of Autism and Developmental Disorders. 2007;37:12–24. doi: 10.1007/s10803-006-0328-2. [DOI] [PubMed] [Google Scholar]
- Cassel T, Messinger DS, Ibanez LV, Haltigan JD, Acosta S, Buchman AC. Early social and emotional communication in the infant siblings of children with autism spectrum disorders: An examination of the broad phenotype. Journal of Autism and Developmental Disorders. 2007;37:122–132. doi: 10.1007/s10803-006-0337-1. [DOI] [PubMed] [Google Scholar]
- Charman T, Taylor E, Drew A, Cocerill H, Brown J, Baird G. Outcome at 7 years of age of children diagnosed with autism at age 2: predictive valitdity of assessments conducted at 2 and 3 years of age and pattern of symptom change over time. Journal of Child Psychology and Psychiatry. 2005;46(5):500–513. doi: 10.1111/j.1469-7610.2004.00377.x. [DOI] [PubMed] [Google Scholar]
- Chawarska K, Klin A, Paul R, Volkmar F. Autism spectrum disorder in the second year: Stability and change in syndrome expression. Journal of Child Psychology and Psychiatry. 2007;48(2):128–138. doi: 10.1111/j.1469-7610.2006.01685.x. [DOI] [PubMed] [Google Scholar]
- Folstein SE, Santangelo SL, Gilman SE, Piven J, Landa RL, Lainhart J, Hein J, Wzorek M. Predictors of cognitive test patterns in autism families. Journal of Child Psychology and Psychiatry. 1999;40:1117–1128. [PubMed] [Google Scholar]
- Heathcock JH, Bhat A, Lobo MA, Galloway JC. Relative kicking frequency of infants born full-term and preterm during learning, short-term, and long-term memory periods of the mobile paradigm. Physical Therapy. 2005;85(1):8–18. [PubMed] [Google Scholar]
- Heathcock JH, Bhat A, Lobo MA, Galloway JC. The performance of infants born preterm and fullterm in the mobile paradigm: learning and memory. Physical Therapy. 2004;84(9):808–821. [PubMed] [Google Scholar]
- Ibanez LV, Messinger D, Newell L, Lambert B, Sheskin M. Visual disengagement in the infant siblings of children with an autism spectrum disorder (ASD) Autism. 2008;12(5):473–485. doi: 10.1177/1362361308094504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger LG, Dawson G. Prototype formation in autism. Development and Psychopathology. 2001;13:111–124. doi: 10.1017/s0954579401001080. [DOI] [PubMed] [Google Scholar]
- Landa R, Garrett-Mayer E. Development in infants with autism spectrum disorders A prospective study. Journal of Child Psychology and Psychiatry. 2006;47(6):629–638. doi: 10.1111/j.1469-7610.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- Landa R, Holman K, Garrett-Mayer E. Social and communication development in toddlers with early and later diagnosis of autism spectrum disorders. Archives of General Psychiatry. 2007;64(7):853–864. doi: 10.1001/archpsyc.64.7.853. [DOI] [PubMed] [Google Scholar]
- Landry R, Bryson S. Impaired disengagement of attention in young children with autism. Journal of Child Psychology and Psychiatry. 2004;45(6):1115–1122. doi: 10.1111/j.1469-7610.2004.00304.x. [DOI] [PubMed] [Google Scholar]
- Lobo MA, Galloway JC. Experience matters: The relationship between experience, exploration, and the emergence of means-end performance. Child Development. 2008;79(6):1869–1890. doi: 10.1111/j.1467-8624.2008.01231.x. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised (ADI-R): A revised version of diagnostic interview for caregivers of individuals with pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule (ADOS) Los Angeles: Western Psychological Services; 1999. [Google Scholar]
- Maestro S, Muratori F, Cavallaro MC, Pei F, Stern D. Attentional skills during the first 6 months of age in autism spectrum disorder. Journal of American Academy of Child Adolescent Psychiatry. 2002;41:1239–1245. doi: 10.1097/00004583-200210000-00014. [DOI] [PubMed] [Google Scholar]
- Mast VK, Fagen JW, Rovee-Collier CK. Immediate and long-term memory for reinforcement context: The development of learned expectancies in early infancy. Child Development. 1980;51(3):700–707. [PubMed] [Google Scholar]
- Merin N, Young GS, Ozonoff S, Rogers SJ. Visual fixation patterns during reciprocal social interaction distinguish a subgroups of 6-month-old infants at-risk for autism from comparison infants. Journal of Autism and Developmental Disorders. 2007;37:108–121. doi: 10.1007/s10803-006-0342-4. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Goldstein G. The pattern of intact and impaired memory functions in autism. Journal of Child Psychology and Psychiatry. 2001;42(8):1095–1101. doi: 10.1111/1469-7610.00808. [DOI] [PubMed] [Google Scholar]
- Mullen EM. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service; 1995. [Google Scholar]
- Mundy P, Sigman M, Ungerer J, Sherman T. Defining the social deficits of autism: The contribution of nonverbal communication measures. Journal of Child Psychology and Psychiatry. 1986;27:657–669. doi: 10.1111/j.1469-7610.1986.tb00190.x. [DOI] [PubMed] [Google Scholar]
- Mundy P, Sigman M, Kasari C. Joint attention, developmental level, and symptom presentation in children with autism. Development and Psychopathology. 1994;6:389–401. [Google Scholar]
- Mundy P, Card J, Fox N. EEG correlates of the development of infant joint attention skills. Developmental Psychobiology. 2000;36:325–338. [PubMed] [Google Scholar]
- Mundy P, Newell L. Attention, Joint Attention, and Social Cognition. Current Directions in Psychological Science. 2007;16(5):269–274. doi: 10.1111/j.1467-8721.2007.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadig AS, Ozonoff S, Young GS, Rozga A, Sigman M, Rogers SJ. A prospective study of response to name in infants at risk for autism. Archives of Pediatric and Adolescent Medicine. 2007;161:378–83. doi: 10.1001/archpedi.161.4.378. [DOI] [PubMed] [Google Scholar]
- Ohr PS, Fagen JW. Temperment, conditioning, and memory in 3-month-old infants with Down syndrome. Journal of Applied Developmental Psychology. 1993;14:175–190. [Google Scholar]
- Osterling J, Dawson G. Early recognition of 1-year-old infants with autism spectrum disorder versus mental retardation. Development and Psychopathology. 2002;14:239–251. doi: 10.1017/s0954579402002031. [DOI] [PubMed] [Google Scholar]
- Osterling J, Dawson G. Early recognition of children with autism: A study of first birthday home videotapes. Journal of Autism and Developmental Disorders. 1994;24(3):247–258. doi: 10.1007/BF02172225. [DOI] [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ. Attention and recognition memory in the 1st year of life: A longitudnal study of preterm and full-term infants. Developmental Psychology. 2001;31:135–151. [PubMed] [Google Scholar]
- Rovee CK, Hayne H, Colombo M. The development of implicit and explicit memory. Vol. 24. Amsterdam, Philadelphia: John Benjamins Publishing Company; 2001. [Google Scholar]
- Striano T, Stahl D. Sensitivity to triadic attention in early infancy. Developmental Science. 2005;8(4):333–343. doi: 10.1111/j.1467-7687.2005.00421.x. [DOI] [PubMed] [Google Scholar]
- Sullivan M, Finelli J, Marvin A, Garrett-Mayer E, Bauman M, Landa R. Response to joint attention in toddlers at risk for autism spectrum disorder: A prosepctives study. Journal of Autism and Developmental Disorders. 2006;37:37–48. doi: 10.1007/s10803-006-0335-3. [DOI] [PubMed] [Google Scholar]
- Wiggins LD, Baio J, Rice C. Examination of the time between first evaluation and first autism spectrum diagnosis in a population-based sample. Journal of Developmental and Behavioral Pediatrics. 2006;27(2):S79–S87. doi: 10.1097/00004703-200604002-00005. [DOI] [PubMed] [Google Scholar]
- Yirmiya N, Gamliel I, Pillowsky T, Feldman R, Baron-Cohen S, Sigman M. The development of siblings of children with autism at 4 and 14 months: social engagement, communication, and cognition. Journal of Child Psychology and Psychiatry. 2006;47(5):511–523. doi: 10.1111/j.1469-7610.2005.01528.x. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Neuroscience. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]