Abstract
Over the past decade, electrocorticography (ECoG) has been used for a wide set of clinical and experimental applications. Recently, there have been efforts in the clinic to adapt traditional ECoG arrays to include smaller recording contacts and spacing. These devices, which may be collectively called “micro-ECoG” arrays, are loosely defined as intercranial devices that record brain electrical activity on the submillimeter scale. An extensible 3D-platform of thin film flexible micro-scale ECoG arrays appropriate for Brain-Computer Interface (BCI) application, as well as monitoring epileptic activity, is presented. The designs utilize flexible film electrodes to keep the array in place without applying significant pressure to the brain and to enable radial subcranial deployment of multiple electrodes from a single craniotomy. Deployment techniques were tested in non-human primates, and stimulus-evoked activity and spontaneous epileptic activity were recorded. Further tests in BCI and epilepsy applications will make the electrode platform ready for initial human testing.
Keywords: Brain-Computer Interface, Electrocorticography, Electroencephalography, Epilepsy Monitoring, 3D-Platform
Introduction
Electrocorticography (ECoG) has become an increasingly standard clinical tool that has also spurred experimental exploration. Clinically approved ECoG arrays are relatively large and new smaller scale devices (micro-ECoG) are being developed using a variety of fabrication methods, materials, and designs.1,2 Micro-ECoG arrays are poised to improve on both the degree of invasiveness and signal quality by utilizing flexible electrode deployment through smaller craniotomies and by increasing the spatial and spectral resolution with higher density and smaller electrodes.3 To date, most clinical micro-scale ECoG devices have been built upon existing macro-ECoG platforms, and have not leveraged contemporary microfabrication processes, which afford the advantages of mass production, reproducibility and flexibility. While there have been a number of previous studies that have described several micro-fabrication techniques for producing micro-ECoG electrodes for high resolution mapping experiments,3 there remain a number of hurdles towards the adoption of this technology in a clinical setting.
Standard ECoG fabrication, involving discrete electrodes and wires embedded in silicone, has been pushed to its practical limits to approach the micro-ECoG spatial scale.4-7 Further advances may involve bio-microelectromechanical systems (bio-MEMS).1-3,8 Because the development of BioMEMS devices utilizes photolithographic techniques, many electrode variations can be produced in one batch, greatly reducing the time required for the development and testing cycle. The interface or connection between the electrode array and the amplifier is another important consideration. Both awkwardly large connectors1 and meticulous hand soldering2,8 have been used in previous systems. An easily assembled platform with high-density solderless connections that leverage the flexibility, and other material properties of thin-film micro-ECoG arrays, would be a crucial component of a BioMEMS-based electrode array. In this paper we describe the clinical application-specific design, fabrication, flexible deployment, and in vivo testing of a custom micro-ECoG platform in an animal model. Multiple electrode designs derived from the same platform were explored to demonstrate the creative synthesis of technology and clinical insight.
Electrode Platform Overview
With multiple clinical and experimental applications in mind, a general-purpose electrode platform was designed to address the unique properties and constraints of brain tissue and the subcranial space. The surface of the brain is not static. It shifts and deforms continuously, particularly following surgery,9 so flexible circuit technology that can move with the brain is an excellent match. Built on a layer of polymer that is tens of micrometers thick, thin-film electrodes can be designed to conform to a shifting brain. There are a wide range of available polymers (parylene C,10 Polyimide,11 Liquid-crystal polymer12), metals (platinum,2 gold13) and possible electrode geometries, and we created a central platform that is extendable to a wide range of materials and enables rapid geometrical design iteration.
The transcranial platform design centralizes all external connections, and can be assembled quickly with flexible electrodes using press-fit sockets. The platform has three parts: a printed circuit board (PCB) for connections to an amplifier and other circuit components; a plastic adapter ring for anchoring the platform to the skull; and a flexible electrode array to interface mechanically and electrically with the brain (Figure 1). The electrode is made with gold and platinum on polyimide, a bio-compatible polymer that has several advantageous properties. Polyimide is hydrophilic which allows it to adhere strongly to moist brain tissue, flexible to accommodate the gyrated cortex, and durable for long-term implantation durations. The electrode array can be placed either epidurally or subdurally. A PCB mates the electrode to small high-density connectors (Mill-max and Omnetics in this work), and a plastic adapter ring holds the PCB and electrode array in place over the craniotomy. The housing ring is milled out of an autoclavable X-ray transparent plastic, Ultem (McMaster-Carr), to fit snugly in a 19 mm burr hole, similar to that used in many standard clinical neurosurgical procedures.
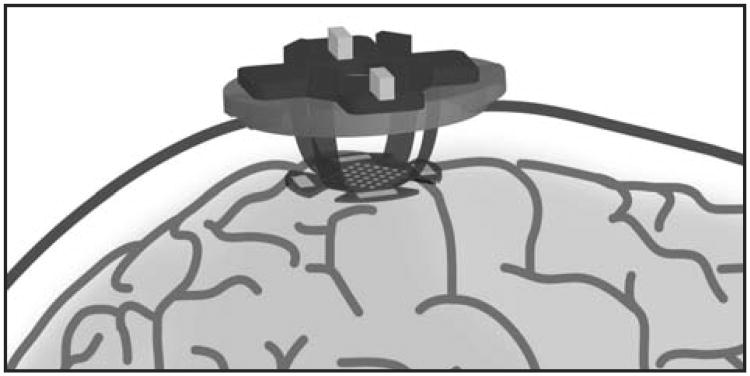
Figure 1.
A schematic representation of the 3D-electrode platform on a primate brain with the alternative ground and reference pads shown surrounding the perimeter of the electrode array.
General Method
Electrode
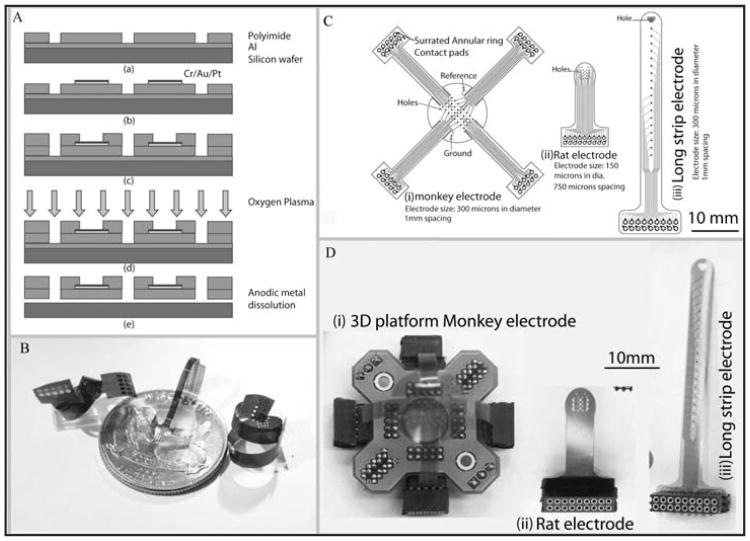
The micro-ECoG electrode array was fabricated in a class 100 cleanroom. The electrode fabrication process must yield a biocompatible and non-toxic electrode array, and the entire process was designed to avoid toxic chemicals. A photodefinable polyimide substrate was chosen over other processes that use harmful Hydrofluoric acid (HF) as an etchant for patterning. Additionally, photodefinable polymide reduces the number of overall steps in the lithographic process. Polyimide serves as the insulating substrate for the electrode and gold traces which connect to each electrode site. Platinum was deposited on the traces and electrode sites to improve electrical characteristics, including decreasing the electrode impedance. Each electrode array consisted of 16 or 32 electrode sites with the expectation that in some instances multiple electrode arrays could be implanted in a single craniotomy. The photolithographic transparency mask sets were designed in Adobe Illustrator, and were printed on high-resolution transparency masks (Fineline-Imaging, Colorado Springs, CO). The shapes and sizes of electrodes were systematically varied, yielding a wide array of electrode designs (Figure 2C).
Figure 2.
A) Electrode fabrication process using lithography to pattern Polyimide, metal evaporation to deposit the traces and electrode sites, and anodic metal dissolution to release the electrodes. B) Various electrode designs that show the flexibility of the polyimide polymer substrate, making it suitable for a wide range of applications. C) The layout of a variety of electrode designs with different shapes, electrode sizes and inter-electrode spacing. D) The realization of the design layout in (C) with corresponding platform and connectors.
Briefly, the electrode fabrication process (Figure 2A) follows. After cleaning a 4-inch silicon wafer with acetone, a 200 nm thick layer of aluminum was evaporated (CHA-600 E-beam Evaporator) onto the surface as a sacrificial layer. Polyimide (HD 4110, HD Microsystems) was spin coated and patterned using photolithography (Mask aligner: MA6 Suss MicroTec) following the manufacturer's recommendation to form a 12 μm thick base layer of polyimide (Figure 2A(a)). The polyimide was subsequently cured at 375 °C under a nitrogen atmosphere for 2 hours (Cooke Oven). A metallization step (10 nm Cr/200 nm Au/20 nm Pt) defining the interconnection pads, connecting traces, and electrode sites was performed using electron beam evaporation (CHA-600 E-beam Evaporator) and a lift-off process. The second layer of polyimide was then spin coated, patterned and cured under the same conditions as the first base layer. Before releasing the device, oxygen plasma (RIE Unaxis 790) was used to clean the surface for 2 minutes (250 W, 20 Torr) ensuring that the remaining polyimide and solvent residue on the electrode sites and contact pads were completely removed (Figure 2A(d)). The thin film devices were released using an anodic metal dissolution technique. In brief, the wafer was immersed in a sodium chloride solution (2 M) with Pt counter electrodes under an applied voltage of 0.8 V that allowed the dissolution of the aluminum layer and release of the polyimide film. To complete the process, the devices were soaked in deionized water, cleaned and dried.
Thin-film electrodes naturally roll into coils, form into springs, and fold into 3-dimensional geometries (Figure 2B). These deformable structures are well suited to interface with the nervous system at many levels, including peripherally, via a catheter delivery system, intracortically, or on the cortical surface with or without an intact dura mater. Thin-film electrodes were first applied to peripheral nerves rolled into a conduit or perforated to allow nerve fibers to regrow through the electrode.14 Other neural targets include deep brain structures, to which the devices may be delivered via catheter, bundled with electrodes and other sensors,15 or intracortical targets using flexible substrates,16 similar to the traditional silicon based intracortical electrode.17 The micro-ECoG array depicted in Figure 2D(i) uses spring action to interface epi- or subdurally while attached to the platform that fits in a 19 mm burr hole.
The 2-D array depicted in Figures 2D(i), 3A and 3B has a rectangular grid of 32 electrode sites (300 μm diameter) spaced 1 mm apart. The impedances of these electrodes in this array are nominally 5-10 kOhms at 1 kHz. The traces run up 4 flexible legs to connect to the PCB. At the PCB, each electrode site has a corresponding multi-leafed contact that is sandwiched between a pair of electrical sockets (Mill-Max) (inset of Figure 3E). The leaves bend under pressure from the conical shoulder of the socket pins, avoiding high stress regions that would arise if the contact were simply annular, and that would result in cracking of the contact pad metal layer. In principle only a single leaf is needed, so the 6-leaf design adds redundancy to each contact, increasing the yield. Mechanical connectors like these are especially necessary when assembling the electrode into a 3-dimensional platform, since soldering would be a challenging alternative due to the difficulties inherent in aligning and maintaining the individual arms while under bending stress.
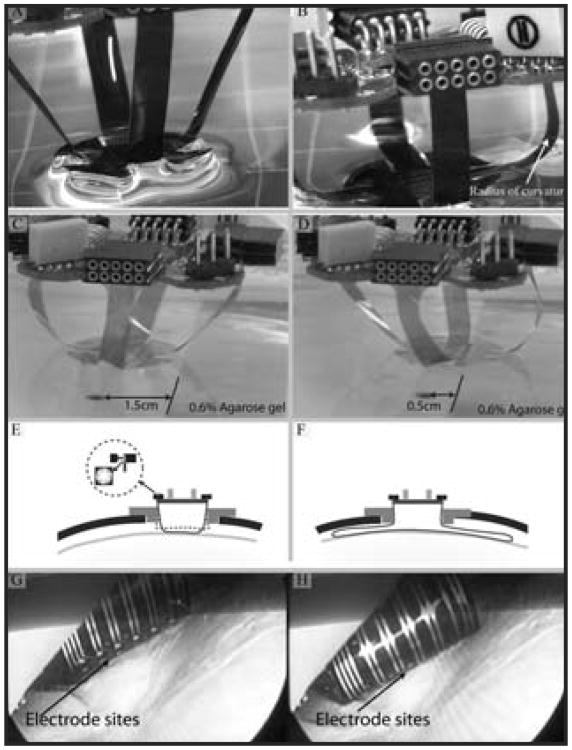
Figure 3.
A) Four legs allow the 2-D array to translate along 3 axes and rotate about an additional 2 axes. The flexible legs maintain only a minimal amount of pressure to ensure close contact between the electrode and tissue. B) The hydrophilicity of the polyimide helps the electrode adhere to the dura or pia mater. C) and D) An x- axis translation of the brain tissue phantom (0.6% agarose gel) up to 1 cm, representing the constant contact of the electrode array with dura due to small brain movement in all x-, y-, z- and 3 rotational axes in vivo. E) A cross-section of the electrode platform shows an assembled electrode and PCB sitting atop a plastic adapter ring inside a craniotomy. The adapter ring can be temporarily fixed to the cranium. The inset shows the multi-leafed electrode contact to the electrical sockets on the PCB. F) Diagram illustrating the flexible legs (longer leg cable than those shown in A-E), which flare out radially under the skull to cover a larger area of cortex. G) and H) Electrode sites come in contact with the dura as the electrode is deployed. The dura can be left intact (pictured) or removed.
Animal and Surgery
Terminal surgery
Terminal surgeries were performed for initial electrode deployment tests and to obtain acute recordings in rhesus or cynomolgus macaques (n = 5). All terminal and survival animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Wisconsin-Madison. In this experiment, the electrode was deployed through one craniotomy, while a video was recorded with an endoscope through a second craniotomy (Figure 4A). Due to the flexible nature of the electrode, the array was inserted using a small guide wire, which attaches to the front of the electrode (see small hole at the distal end of the electrode in Figure 4D) and subsequently retracted after the electrode is in place. This electrode design has the potential of being used as a presurgical epilepsy diagnostic tool to find a focal area before initiating the resection treatment.18
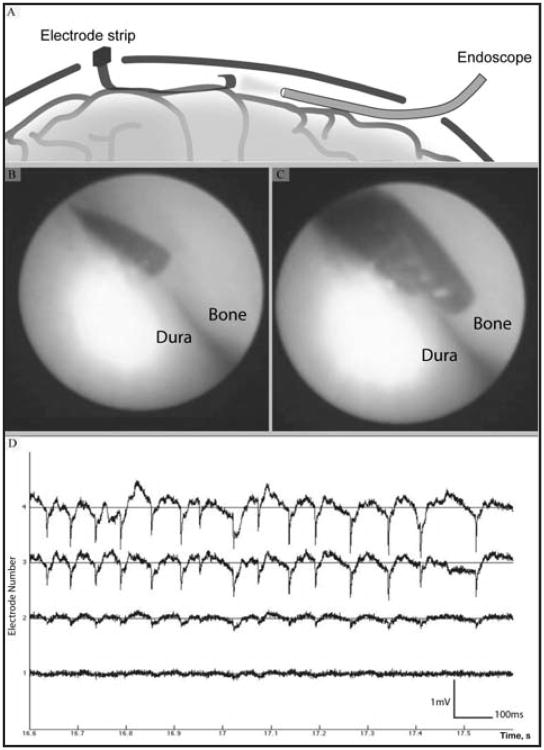
Figure 4.
Long strip electrode suitable for acute recording. A) A representation of a strip electrode inserted through a very small (10 mm) burr hole simultaneously with an endoscope for real time imaging of electrode position. B) and C) Real time images of electrodes in contact with the dura, taken from the scope which was situated on the opposite side of the location of electrode entry. D) Epileptic activity observed from an acute recording using the same long strip electrode array. Spontaneous epileptic activity recorded under anethesia from 4 neighboring electrode sites shows the signal amplitude of up to 2 mV, droping over a spatial scale resolvable only by micro-ECoG arrays.
Chronic implant surgery
Survival surgery procedures approved by the University of Wisconsin-Madison IACUC were done in rhesus macaques (n = 3) using the most promising designs and deployment methods. Rhesus macaques were chronically implanted with a miniaturized electrode array over the right sensorimotor cortex region, centered 14 mm anterior of the intra-aural line and 14 mm laterally off the sagittal suture (Figure 5A). The monkeys were first anesthetized with Ketamine (10 mg/kg) for surgical preparation including vital monitor positioning and intubation. Isoflurane (0.5-5%) was used for anesthetic induction and maintenance during the non-recording parts of the procedure. Following anesthetic induction, they were placed into a stereotaxic frame, and the scalp was shaved and prepped with alternating povidone iodine and alcohol. After the initial incision, exposed skull was cleaned and dried. To obtain interoperative recordings, a continuous rate infusion of ketamine: fentanyl at a dosage of 10-20 mg/kg/hr for ketamine and 0.0001-0.001 mg/kg/hr for the fentanyl was initiated just before the skull was opened and isoflurane concentration maintained at 0.5% or less. A small hole was drilled through the skull until dura was exposed, and a stainless steel screw (size 4-40) was used for attachment of a ground wire. Then a small craniotomy (19 mm in diameter) was made using a spherical burr (stryker) designed for use with a high-speed surgical drill. After exposing the dura, the micro-ECoG array was placed epidurally onto the sensorimotor area of the cortex. GelFoam (Pharmacia and Upjohn Co, New York, NY) was placed on top of the array.
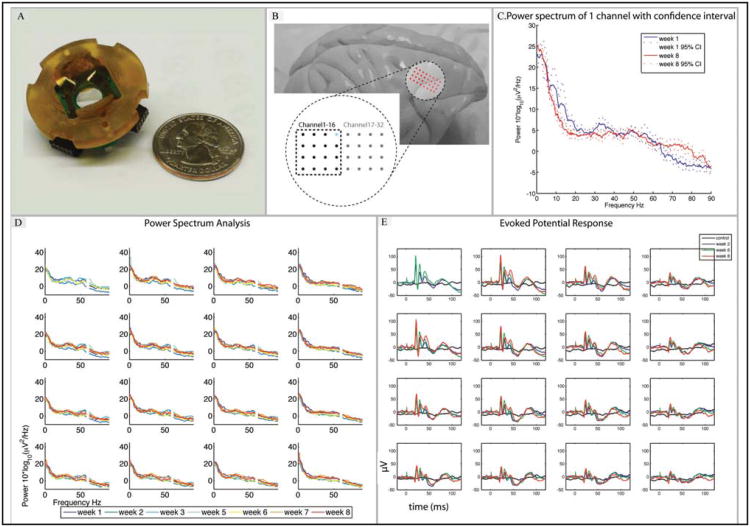
Figure 5.
Long-term micro-ECoG recording comparison. A) An electrode array and platform design that fits a 19 mm craniotomy in comparison with a quarter coin. B) Position of the electrode array on a non-human primate brain (Rhesus macaque), situated over sensorimotor cortex. C) Power spectrum (with 95% confidence intervals) of an example electrode site (highlighted in blue in (Figure 5B)) showing stability of the recorded baseline signal over an 8 week period. D) Power spectra of channels 1-16 over 8 weeks show long-term stability in long-term implant (60 Hz line noise omitted). E) Evoked potential response due to contralateral hind limb electrical stimulation (2.5 mA, 2 ms) compared to control (no stimulus) (week 2, 6 and 8).
Results
Coupling device and brain
To harness the spring-action of the polymer, and allow the brain to move freely without damage, a multi-leg electrode design was used. Very small forces cause the legs to bend, and these same forces help keep the device in contact with the dura or pia mater without breaking electrode contact with the brain surface. This is important for chronic ECoG monitoring, as in clinical applications the brain can move relative to the skull. In addition to the forces imparted by the electrodes legs, the hydrophilic nature of the polyimide substrate helps the electrode maintain its position on the brain. Polyimide is unique in that it becomes increasingly hydrophilic with repeated contact to water.19 Thus, the initial hydrophilicity of polyimide allows the electrode to be placed easily during deployment, and the electrode then stays in place as the polyimide substrate's hydrophilic structure nature binds reversibly to moist tissues such as the dura or pia mater (Figure 3A and 3B). The multi-leg design leverages the flexible and hydrophilic properties of polyimide to maintain contact even as the brain translates relative to the cranium in 6 axes (x-, y-, z-, and 3 rotational axes). Figures 3C and 3D illustrate the translation in the x-axis for approximately 1 cm while the electrode array remains in contact with the brain tissue phantom model (0.6% agarose gel20). The y- and z- and 3 rotational axes showed similar results to those shown in Figures 3C and 3D by moving the brain model in different axes while keeping the electrode array stationary. Sudden translations of 5 mm did not affect electrical properties of the electrode nor did rotations (data not shown). The device has greatest translational flexibility in the axis normal to the electrode surface, and somewhat reduced rotational flexibility along the same axis, but this matches the expected movements of a patient's brain. The legs of the electrode exert a tiny amount of pressure to keep the electrode surface in contact with the brain. Only an estimated 0.52 Pa is applied when the legs are in the compressed position (Figure 3B), which is equivalent to 52 mg of force per square centimeter. As can be observed from Figure 3B, this force is insufficient to even break the surface tension of water, and is unlikely to cause any depression of the brain surface. It does however produce enough of a downward force to maintain the electrode in contact with the cortical surface during relative brain motion.
Using an unfurling technique (Figure 3F), the electrode array could be deployed several centimeters distal from the craniotomy if the cable arms are designed to be longer than that shown in Figure 3A-3E. This technique would allow the electrodes to be deployed from a craniotomy away from the site of injury, minimizing further trauma and scarring at the injury site. During unfurling, the electrode never came close to the critical radius of curvature that would damage its electrical integrity. Bench tests showed that more than 95% of electrode sites maintained electrical continuity at a 1 mm radius of curvature (data not shown).
Images of the unfurling electrode deployment method depicted in Figure 3E are shown in Figures 3G and H. These images, taken during a terminal procedure in a cynomolgus macaque, show that the electrode sites come into contact with the brain surface as the electrode strip unfurls. With the same principle in mind, a multi-leg flared electrode was also prototyped (image not shown). Multi-leg electrodes can flare out radially from a small 5 mm burr hole to cover an area of cortex 3 times as large as the unfurling electrode, and enable a minimally invasive surgical procedure for mapping or diagnosing any abnormal brain function in a specific area or provide for a large area of coverage in a chronic neural recording application.
Alternative electrode designs and deployment methods for clinical applications
Weighed against a more extensive craniectomy, deployment through a moderately sized craniotomy is a less invasive option. For example, to keep the craniotomy relatively small, but extend the spatial reach of the electrode, a 16-site strip electrode was designed (Figure 2C(iii) and 2D(iii)) that may be deployed epidurally. This electrode might be appropriate for monitoring epileptic activity (Figure 4D) in a large area if its radial position were changed. With some practice, it can be deployed leaving the dura intact. After removing any dural adhesions that may be present, the electrode can be inserted with the aid of an elevator. These deployment techniques were tested in a nonhuman primate spontaneous epilepsy model.
During the non-human primate epilepsy model procedure, recordings from the micro-ECoG were able to detect spontaneous epileptic activity in an anesthetized rhesus macaque that had a previous open-chambered cranial implant for several years. The implant was removed and the animal was used as a breeder for three years prior to terminal recording. The monkey had no history of grand mal seizures, but less severe epileptic episodes may have been present. Spontaneous seizure activity under ketamine/fentanyl continuous rate infusion (ketamine, 20 mg/kg/hr and fentanyl, 1.5 μg/kg/hr), the anesthetic combination used in this study, was characterized by large depolarizations (maximum of 2 mV) seen across multiple sites. Interestingly, the signal amplitude decreased over the span of four neighboring electrode sites spaced 1 mm apart—a spatial scale unresolvable by traditional ECoG or EEG technology (Figure 4D).
Multiple strip electrodes, combined with a 2-D array, could be deployed from a single craniotomy, resulting in electrode densities superior to the current standard of care. As a thin and flexible polymer device, it was easily removed when recordings were completed. This electrode design utilizes the flexibility of the electrode to reduce the required craniotomy size. The flexibility of the electrode also helps reduce the risk of dural bleeding and reduce other surgical complications. The high resolution of the microECoG spacing could allow precise localization of pathological neural activity and could help inform a neurosurgical team in clinical applications. For clinical applications, especially epilepsy monitoring, a device that has higher spatial representation, such as micro-ECoG where the focal change of cortical physiology may span as few as 1-2 electrode sites, could allow the diagnosis to be more accurate. Recent studies have suggested that macro-epileptic seizure activity might be the result of the coordinated synchrony of numerous spatially distinct “micro-seizure” domains, whose activity may proceed the large scale activity traditionally used as clinical evidence during seizure monitoring.21
Chronic in vivo tests
Initial signal recording procedures were obtained using standard methods to elicit stimulus-evoked potentials, both interoperatively (as described above) and chronically.22 For chronic recordings, light anesthesia (Ketamine, 10-15 mg/kg) and analgensia (Buprenorphine, 0.005-0.03 mg/kg) were administered intramuscularly or subcutaneously 30-60 minutes before the start of an intramuscular stimulation recording session and electrical stimulation evoked potentials were recorded. Multiple (n = 60) short cathodic electrical stimulation (2 ms pulse) of current 2-4 mA was applied with 25 gauge needle electrodes. Neural activity was sampled at 3051 Hz using an RZ2 amplifier (Tucker-Davis Technologies, Alachua, FL). A band-pass filter was set at 0.1 to 200 Hz. Longitudinal baseline recording for power spectrum analysis of the micro-ECoG over 8 weeks was performed. The power spectra of channels 1-16, within the area of interest for the foot electrical stimulation, are illustrated in Figure 5B. Baseline recordings, evoked potentials and electrode impedance were obtained for 5 minutes weekly to ensure the long-term stability of the micro-ECoG device. The baseline power spectrum was calculated using the Matlab toolbox Chronux 2.023 from 30 seconds of data from each recording session. A 1.57 second window, a time-bandwidth product of 3, and 5 tapers were used to calculate the power spectrum. The 95% confidence intervals were found with the jackknife resampling method to compare weeks 1 and 8 (highlighted in blue in Figure 5B). The power spectrum appeared to be stable in most of the electrode sites (Figure 5D). In sensorimotor rhythm, a mu band (8-12 Hz) and gamma band (30-40 Hz) were present and recorded by the ECoG device. Note that the frequencies of 58 to 62 Hz were omitted in Figure 5D as they represent main electrical frequency noise. The power spectra of channels 17-32 are not shown for space considerations, but also appear to be stable.
With 1 mm spacing, our non-human primate electrode arrays showed a distinctive evoked potential amplitude difference between adjacent electrode sites when the contralateral foot was electrically stimulated (Figure 5C). Higher evoked potentials were observed over the stimulated part of the cortex and then faded out slowly towards the anterior (channels 17-32). Repeated electrical pulse (2.5 mA, 2 ms) stimulation evoked potentials up to 100 μV in amplitude. This was large compared to control baseline recordings in which no stimulus was applied. Over 8 weeks, the evoked potential responses were similar in both amplitude and waveform (Figure 5E). This verifies that our epidural micro-ECoG has an acceptable signal quality and a high spatial resolution, and does not have signal properties that are drastically different than previously reported from both micro- and macro-electrode recordings.
Discussion
Thin-film neural interfaces are becoming a significant research technology with many potential applications in clinical settings including advanced neuroprosthetics. With their high degree of flexibility, thin-film electrodes can flex with the brain, assemble into 3-D structures, and deploy through reduced surgical openings. Precise fabrication tools utilizing photolithographic techniques enable high electrode densities for greater spatial resolution than standard ECoG arrays.
Micro-ECoG arrays may some day replace existing clinical ECoG technologies for many applications as the current generation of ECoG arrays with discretely wired electrode sites in silicone have reached practical size limits. Electrodes of many sizes and shapes are easily fabricated in parallel using thin-film processes using rapid prototyping process, and so the set of possible electrode designs, the “design space,” is quite large. Many electrode designs can be developed with the same fabrication process to fit any application including rodent and non-human primate studies, and eventual human clinical trials. Based on signal considerations, the electrode site size and spacing can be matched to the spectral and spatial characteristics of the signal, respectively, or to the anatomical layout of the gyri. This may someday make it possible to customize electrodes for individual patient needs in clinical applications. On the mechanical side, the thickness of the polyimide substrate can be increased for added strength during deployment, or reduced for deployment strategies requiring additional electrode flexibility. Theoretical and computational modeling can help guide the design process, but due to the rapid prototyping nature of the manufacture process, there are also opportunities to directly test new devices. 3-D electrode designs have not yet been utilized in the micro-ECoG community, but 3-D electrodes can make superior contact with a moving brain. To keep the electrode array in constant contact with the brain surface (i.e., to self seat), the highly hydrophilic nature of the material is crucial for accommodating the brain movement inside the skull. In addition, electrodes can be easily repositioned, as the adhesion mechanism is reversible. This platform also allows the surgeon to deploy the device precisely and safely with minimal difficulty, because the whole platform can be placed without having to directly handle the delicate electrode array. The electrode itself does not need to be directly manipulated as is the case with most penetrating MEMS electrodes. High-yield solderless connections can also reduce the man-hours required for assembly of multiple devices, providing a viable pathway towards the scale-up necessary for clinical manufacturing. It would be very difficult to assemble the device shown in Figure 3, if the connections at the end of each of the arms had to be individually soldered or epoxied.
The combination of the polyimide surface properties and its flexibility allows for tight coupling of the electrode with the convoluted cortical surface. As this technology moves towards clinical applications, the degree of flexibility is important to accommodate the gyro-cephalic brain.24 Other polymers (e.g., parylene C10 and silk fibroin24) also have desirable material properties. The optical transparency of parylene is desirable in a research setting where imaging or perhaps optogenetics25 may be used.
We have demonstrated that efficient recordings of abnormal electrical activity may be obtained from a very small area of the brain with a long strip electrode (Figure 4). Traditional epilepsy monitoring requires temporarily implanting an FDA approved standard clinical ECoG array that involves a potentially risky surgical procedure. The strip electrode can be applied not only for epilepsy monitoring, but also for cortical mapping. A large cortical area can be mapped from a small burr hole by changing the long strip electrodes radial position. None of the previous studies has exploited this promising feature and integrated it into a particularly versatile micro-ECoG design. Other studies1-2 proposed larger micro-ECoG arrays that cover up to a half of the hemisphere. These designs may be practical for cortical mapping, but for neuroprosthetics, a small electrode array that covers the sensorimotor cortex may be sufficient to decode limb movement activities.26-27 In addition, the 3-D platform electrode array also demonstrated long-term signal recording stability in a chronic rhesus macaque model. The power spectra of the baseline recording and the evoked potentials of all sites were stable over 8 weeks. Before moving forward to clinical trials, an acceptable chronic implant electrode has to address issues related to robustness, signal quality and stability, and biocompatibility. Further work will be necessary to fully characterize the relationships between signal quality, electrochemical measures and histological reactions to the presence of the device.
Micro-ECoG arrays may someday rival the utility of silicon and micro wire based neuroprosthetics interfaces that are currently employed. Arm movements in 3-D have been decoded from spiking data28 but similar 3-D movement decoding has not yet been achieved with micro-ECoG. A better understanding of the micro-ECoG signal and its relationship to other brain signals may improve its efficacy in BCI applications. Its overall utility is still promising though, since penetrating electrodes often lose the ability to isolate spikes over a period of weeks or months.16,29
EEG and ECoG have traditionally had broader clinical application than penetrating electrodes, because of the relatively high risk encountered by penetrating electrodes. Relatively less invasive micro-ECoG devices may someday replace existing macro-ECoG technologies. The flexibility, customizability, and quick assembly of the platform presented above leads to many clinical and experimental paths. BCIs rely on high quality information-dense signals extracted from the cortex. Our nonhuman primate data show similar utility for supporting results for evoked activity applications, such as P300 or similar motor cortical BCI and epileptic monitoring. Further validation in animal models of epilepsy and BCI will help to prepare the electrode platform for initial human testing.
Advances in the thin-film electronics industry have created new opportunities for micro-ECoG devices. The next puzzle-piece borrowed from industry could be integrated amplifier circuits housed directly on the electrode substrate. A complete micro-ECoG platform will someday include the electrode sites, amplifiers,3 analog to digital converters, wireless transmission and power all on a single flexible thin-film device which can be inserted subcranially through a very small craniotomy. In principle, all of the components are ready, but skilled technology integration and collaboration will be required to continue to push this promising technology forward.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NIH NIBIB 1R01EB009103-01 and 2R01EB000856-06) and in parts by the Wallace H Coulter Foundation Institutional Translational Partnership and a refinement grant from the Center for Alternatives to Animal Testing (terminal experiments) to LKH.
Footnotes
Disclosure and Conflict of Interest: S. Thongpang, T.J. Richner, S.K. Brodnick, A. Schendel, J. Kim, J.A. Wilson, J. Hippensteel, L. Krugner-Higby, D. Moran, A.S. Ahmed, D. Neimann, K. Sillay, J.C. Williams have no conflicts of interest in relation to this article.
The online version of this article can be found at http://eeg.sagepub.com/content/42/4/259
References
- 1.Hollenberg B, Richards C, Richards R, Bahr D, Rector D. A MEMS fabricated flexible electrode array for recording surface field potentials. J Neurosci Methods. 2006;153(1):147–153. doi: 10.1016/j.jneumeth.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Rubehn B, Bosman C, Oostenveld R, Fries P, Stieglitz T. A MEMS-based flexible multichannel ECoG-electrode array. J Neural Eng. 2009;6(3):036003. doi: 10.1088/1741-2560/6/3/036003. [DOI] [PubMed] [Google Scholar]
- 3.Viventi J, Kim D, Moss J, Kim Y, Blanco J, Annetta N, et al. A conformal, bio-interfaced class of silicon electronics for mapping cardiac electrophysiology. Sci Transl Med. 2010;2(24):24ra22. doi: 10.1126/scitranslmed.3000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kellis S, House P, Thomson K, Brown R, Greger B. Human neocortical electrical activity recorded on nonpenetrating microwire arrays: applicability for neuroprostheses. Neurosurg Focus. 2009;27(1):E9. doi: 10.3171/2009.4.FOCUS0974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanco J, Stead M, Krieger A, Viventi J, Marsh W, Lee K, et al. Unsupervised classification of high-frequency oscillations in human neocortical epilepsy and control patients. J Neurophysiol. 2010;104(5):2900–2912. doi: 10.1152/jn.01082.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leuthardt E, Freudenberg Z, Bundy D, Roland J. Microscale recording from human motor cortex: implications for minimally invasive electrocorticographic brain-computer interfaces. Neurosurg Focus. 2009;27(1):E10. doi: 10.3171/2009.4.FOCUS0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pei X, Leuthardt EC, Gaona CM, Brunner P, Wolpaw JR, Schalk G. Spatiotemporal dynamics of electrocorticographic high gamma activity during overt and covert word repetition. Neuroimage. 2011;54(4):2960–2972. doi: 10.1016/j.neuroimage.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toda H, Suzuki T, Sawahata H, Majima K, Kamitani Y, Hasegawa I. Simultaneous recording of ECoG and intracortical neuronal activity using a flexible multichannel electrode-mesh in visual cortex. NeuroImage. 2011;54:203–212. doi: 10.1016/j.neuroimage.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Nimsky C, Ganslandt O, Cerny S, Hastreiter P, Greiner G, Fahlbusch R. Quantification of, visualization of, and compensation for brain shift using intraoperative magnetic resonance imaging. Neurosurgery. 2000;47(5):1070–1079. doi: 10.1097/00006123-200011000-00008. discussion 1079-1080. [DOI] [PubMed] [Google Scholar]
- 10.Hassler C, Von Metzen RP, Ruther P, Stieglitz T. Characterization of parylene c as an encapsulation material for implanted neural prostheses. J Biomed Mater Res B Appl Biomater. 2010;93(1):266–274. doi: 10.1002/jbm.b.31584. [DOI] [PubMed] [Google Scholar]
- 11.Rubehn B, Stieglitz T. In vitro evaluation of the long-term stability of polyimide as a material for neural implants. Biomaterials. 2010;31(13):3449–3458. doi: 10.1016/j.biomaterials.2010.01.053. [DOI] [PubMed] [Google Scholar]
- 12.Lee SW, Seo JM, Ha S, Kim ET, Chung H, Kim SJ. Development of microelectrode arrays for artificial retinal implants using liquid crystal polymers. Invest Ophthalmol Vis Sci. 2009;50(12):5859–5866. doi: 10.1167/iovs.09-3743. [DOI] [PubMed] [Google Scholar]
- 13.Yeager JD, Phillips DJ, Rector DM, Bahr DF. Characterization of flexible ecog electrode arrays for chronic recording in awake rats. J Neurosci Methods. 2008;173(2):279–285. doi: 10.1016/j.jneumeth.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stieglitz T, Beutel H, Meyer J. A flexible, light-weight multichannel sieve electrode with integrated cables for interfacing regenerating peripheral nerves. Sensors and Actuators A: Physical. 1997;60(1-3):240–243. [Google Scholar]
- 15.Li C, Wu PM, Jung WS, Ahn CH, Shutter LA, Narayan RK. A novel lab-on-a-tube for multimodality neuromonitoring of patients with traumatic brain injury (TBI) Lab Chip. 2009;9(14):1988–1990. doi: 10.1039/b900651f. [DOI] [PubMed] [Google Scholar]
- 16.Rousche PJ, Pellinen DS, Pivin DP, Williams JC, Vetter RJ, Kipke DR. Flexible polyimide-based intracortical electrode arrays with bioactive capability. IEEE Trans Biomed Eng. 2008;48(3):361–371. doi: 10.1109/10.914800. [DOI] [PubMed] [Google Scholar]
- 17.Kipke DR, Vetter RJ, Williams JC, Hetke JF. Silicon-substrate intracortical microelectrode arrays for long-term recording of neuronal spike activity in cerebral cortex. IEEE Trans Neural Syst Rehabil Eng. 2003;11(2):151–155. doi: 10.1109/TNSRE.2003.814443. [DOI] [PubMed] [Google Scholar]
- 18.Iida K, Otsubo H, Arita K, Andermann F, Olivier A. Cortical resection with electrocorticography for intractable porencephaly-related partial epilepsy. Epilepsia. 2005;46(1):76–83. doi: 10.1111/j.0013-9580.2005.28704.x. [DOI] [PubMed] [Google Scholar]
- 19.Hennig A, Eichhorn K, Staudinger U, Sahre K, Rogalli M, Stamm M, et al. Contact angle hysteresis: study by dynamic cycling contact angle measurements and variable angle spectroscopic ellipsometry on polyimide. Langmuir. 2004;20(16):6685–6691. doi: 10.1021/la036411l. [DOI] [PubMed] [Google Scholar]
- 20.Chen ZJ, Gillies GT, Broaddus WC, Prabhu SS, Fillmore H, Mitchell RM, et al. A realistic brain tissue phantom for intraparenchymal infusion studies. J Neurosurg. 2004;101(2):314–322. doi: 10.3171/jns.2004.101.2.0314. [DOI] [PubMed] [Google Scholar]
- 21.Stead M, Bower M, Brinkmann BH, Lee K, Marsh WR, Meyer B, et al. Microseizures and the spatiotemporal scales of human partial epilepsy. Brain. 2010;133(9):2789–2797. doi: 10.1093/brain/awq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nunez PL, Srinivasan R, editors. Electric Field of the Brain. 2nd. New York: Oxford University Press; 2006. p. 10. [Google Scholar]
- 23.Mitra P, Bokil H, editors. Observed Brain Dynamics. New York: Oxford University Press; 2008. [Google Scholar]
- 24.Kim DH, Viventi J, Amsden J, Xiao J, Vigeland L, Kim YS, et al. Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. Nat Mater. 2010;9(6):511–517. doi: 10.1038/nmat2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deisseroth K. Optogenetics. Nat Methods. 2011;8(1):26–29. doi: 10.1038/nmeth.f.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rouse AG, Moran DW. Neural adaptation of epidural electrocorticographic (eecog) signals during closed-loop brain computer interface (BCI) tasks. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:5514–5517. doi: 10.1109/IEMBS.2009.5333180. [DOI] [PubMed] [Google Scholar]
- 27.Wang W, Chan SS, Heldman DA, Moran DA. Motor cortical representation of hand translation and rotation during reaching. J Neurosci. 2010;30(3):958–962. doi: 10.1523/JNEUROSCI.3742-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vargas-Irwin C, Shakhnarovich G, Yadollahpour P, Mislow J, Black M, Donoghue J. Decoding complete reach and grasp actions from local primary motor cortex populations. J Neurosci. 2010;30(29):9659–9669. doi: 10.1523/JNEUROSCI.5443-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams JC, Hippensteel JA, Dilgen J, Shain W, Kipke DR. Complex impedance spectroscopy for monitoring tissue responses to inserted neural implants. J Neural Eng. 2007;4(4):410–423. doi: 10.1088/1741-2560/4/4/007. [DOI] [PubMed] [Google Scholar]