Abstract
OBJECTIVE
To estimate the cost-effectiveness of HIV screening strategies for the prevention of perinatal transmission in Uganda, a resource-limited country with high HIV prevalence and incidence.
STUDY DESIGN
We designed a decision-analytic model from a health care system perspective to assess the vertical transmission rates and cost-effectiveness of four different HIV screening strategies in pregnancy: 1) Rapid HIV antibody (Ab) test at initial visit (current standard of care); 2) Strategy 1 + HIV RNA at initial visit (adds detection of acute HIV); 3) Strategy 1 + repeat HIV Ab at delivery (adds detection of incident HIV); 4) Strategy 3 + HIV RNA at delivery (adds detection of acute HIV at delivery). Model estimates were derived from the literature and local sources, and life years saved were discounted at a rate of 3% per year. Based on World Health Organization guidelines, we defined our cost-effectiveness threshold as ≤3 times the gross domestic product per capita, which for Uganda was US$3300 in 2008.
RESULTS
Using base case estimates of 10% HIV prevalence among women entering prenatal care and 3% incidence during pregnancy, strategy 3 was incrementally the cost-effective option that led to the greatest total life years.
CONCLUSION
Repeat rapid HIV Ab testing at the time of labor is a cost-effective strategy even in a resource-limited setting such as Uganda.
Keywords: HIV, pregnancy, perinatal transmission, decision analysis, cost-effectiveness analysis
INTRODUCTION
Perinatal transmission of human immunodeficiency virus (HIV) during pregnancy, childbirth and breastfeeding is a public health crisis in sub-Saharan Africa. In 2008, the majority of the estimated 430,000 new HIV infections in children worldwide occurred in sub-Saharan Africa.1 Without timely diagnosis and subsequent antiretroviral therapy, vertical transmission rates of HIV have been shown to be as high as 25.5% in pregnancy with an additional 15% risk of transmission during breastfeeding.2,3 Furthermore, pregnant women have a higher risk of HIV acquisition and acute HIV infection is associated with much higher perinatal HIV transmission rates.4–6
The standard of care for HIV testing during pregnancy in sub-Saharan African countries such as Uganda has been one-time rapid HIV antibody testing at the initiation of prenatal care.7 The Uganda Ministry of Health has most recently recommended adding a repeat rapid HIV antibody test in the third trimester of pregnancy but most health clinics have not yet adopted these new guidelines due to limited testing kits.7 The former standard of one-time HIV antibody testing at the initiation of prenatal care failed to diagnose acute HIV infection because maternal antibodies to HIV are not yet detectable and it also failed to diagnose women who acquire HIV later in pregnancy. A new strategy of repeat rapid HIV antibody testing at the time of delivery with the addition of HIV RNA testing at the time of antibody testing may improve detection rates to allow timely medical interventions for the prevention of perinatal transmission. While it is reasonable to assume that additional HIV testing would likely decrease perinatal transmission, it is important to quantify the additional benefit and assess the best timing of such testing. Further, if such increased testing only leads to a small marginal improvement in clinical outcomes in a low-resource setting, it may not be worth the increased cost.
Thus, the purpose of this analysis is to assess the vertical transmission rates and cost-effectiveness of three different hypothetical HIV screening strategies for the prevention of perinatal transmission, allowing comparison to standard one-time testing at the initiation of prenatal care. In order to make the results relevant to sub-Saharan Africa, the center of the HIV epidemic, we chose to use a health care system perspective from Uganda, a resource-limited country with a high HIV prevalence and incidence.
MATERIALS AND METHODS
We developed a decision-analytic model with TreeAgePro 2009 software (Treeage Software Inc, Williamstown, MA) to compare the incremental costs and effectiveness of four different HIV screening strategies: (1) Rapid HIV antibody (Ab) at initial visit only (current standard of care); (2) Strategy 1 + HIV RNA at initial visit (adds detection of acute HIV); (3) Strategy 1 + repeat HIV Ab at delivery (adds detection of incident HIV); (4) Strategy 3 + HIV RNA at delivery (adds detection of acute HIV at delivery). This study is a theoretic decision-analytic model and is thus exempt from Institutional Board Review Approval since no human subjects were involved.
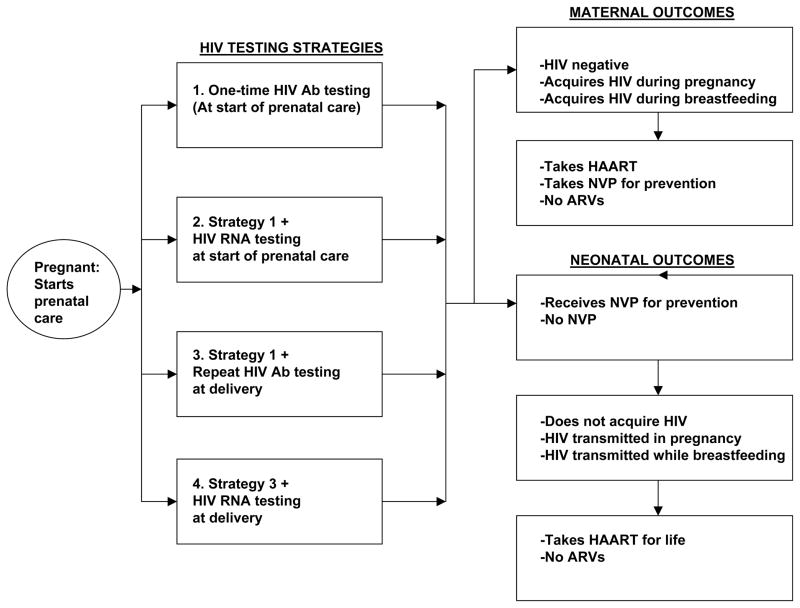
From a health care system perspective, we used our decision-analytic model to follow a hypothetical cohort of 10,000 Ugandan women presenting for prenatal care. Our outcomes included the estimated costs of each strategy, including the lifetime costs associated with HIV treatment, and life years saved. Women who were HIV negative at the time of enrollment could acquire new HIV infection during pregnancy, close to the time of labor, while breastfeeding, or could remain HIV negative. Women diagnosed as HIV positive could either receive highly active antiretroviral therapy (HAART) during pregnancy if applicable, take a one-time dose of nevirapine at the onset of labor and give their newborn a one-time dose of nevirapine (previously a recommended antiretroviral protocol for the prevention of vertical transmission in sub-Saharan Africa that is now no longer the standard of care), or not receive any antiretroviral therapy or infant prophylaxis for the prevention of vertical transmission. HIV positive women either had successful prevention of perinatal transmission, or had perinatal transmission to their neonates. The HIV positive women who did not have vertical transmission during pregnancy or labor could later go on to have vertical transmission during breastfeeding. A schematic of the decision tree is shown in Figure 1.
Figure 1.
Decision tree schematic
Probabilities
The input probabilities for our decision tree are displayed in Table 1. We estimated the probabilities of several HIV-related maternal and neonatal outcomes during pregnancy, delivery and breastfeeding, including the risk of perinatal transmission, based on the published literature. We obtained estimates for the model’s parameters from Uganda-specific data and/or sub-Saharan Africa-specific data when available. Otherwise, we used data from the international literature with an attempt to use estimates from other resource-limited countries. When we had no published data to guide our calculation of a model parameter, we utilized expert opinion and incorporated a wide range of uncertainty. Women enrolled in a given HIV screening strategy could either have established HIV infection, acute HIV infection, or be HIV negative at the time of the first HIV test. The sensitivity and specificity of each HIV test was incorporated into the decision tree. The sensitivity of HIV RNA testing is 100% with a false-positive rate of 2–5%, but with duplicate testing and considering tests < 5000 copies/ml as indeterminate results requiring additional testing, the number of false-positive HIV RNA tests can be reduced to zero.8,9 Furthermore, there is a very high level of viremia in the setting of acute HIV infection. Therefore, in our model we assumed 100% specificity for HIV RNA testing.
Table 1.
Probability and Cost Estimates
| Probability Variable | Value | Reference |
|---|---|---|
| HIV Prevalence | 0.1 | 7 |
| HIV Incidence: pregnancy | 0.03 | 10 |
| HIV Incidence: lactation (18 mo) | 0.04 | 11 |
| Rapid HIV Ab Sensitivity | 0.986 | 27 |
| Rapid HIV Ab Specificity | 0.999 | 27 |
| HIV RNA Sensitivity | 1 | 8,9 |
| HIV RNA Specificity | 1 | 8,9 |
| Lifetime probability of getting HIV diagnosed | 0.165 | 1 |
| Probability of getting HAART in pregnancy | 0.034 | 20 |
| Vertical transmission: pregnancy if no ARV | 0.255 | 2 |
| Vertical transmission: pregnancy if HAART | 0.01 | 12 |
| Vertical transmission: pregnancy if NVP in labor | 0.118 | 13 |
| Vertical transmission: labor if acute HIV | 0.35 | 12 |
| Vertical transmission: lactation (18 mo) | 0.20 | 3,14 |
| Vertical transmission: lactation (6 mo) | 0.16 | 3,14 |
| Vertical transmission: lactation if acute HIV | 0.36 | 15 |
| Vertical transmission: lactation if HAART (6 mo) | 0.05 | 16 |
| Cost Variable | Cost (US$) | Reference |
| Negative Rapid HIV Ab Test | $1.20 | 17 |
| Positive Rapid HIV Ab Test | $2.10 | 17 |
| HIV RNA Test | $32.11 | 18 |
| NVP in pregnancy | $8 | 19 |
| NVP for neonate | $4 | 19 |
| HAART in pregnancy | $68.25 | 20 |
| HAART during lactation | $45 | 20 |
| HAART: Woman for life | $8,000 | 21 |
| HAART: Child for life | $12,000 | 21 |
| Diagnostic HIV test for child | $22.70 | 28 |
We estimated a baseline prevalence of HIV among this theoretical cohort of pregnant Ugandan women to be 10%.7 We assumed an incidence of HIV during pregnancy to be 3% and during 18 months of lactation to be 4%.10,11 Based on a large randomized controlled trial from the Pediatric AIDS Clinical Trials Group 076, we used a vertical transmission rate of HIV infection during pregnancy to be 25.5% if no antiretrovirals were used.2 In the setting of incident HIV during pregnancy with a higher HIV viral load, we estimated a higher vertical transmission rate of 35%.12 Assuming the use of nevirapine for the prevention of vertical transmission in labor, we used a transmission rate of 11.8% based on the HIVNET 012 randomized controlled trial.13 In the setting of HAART use in pregnancy, we assumed a lowering of the vertical transmission rate down to 1%.12 Rates of vertical transmission in various possible scenarios during lactation were also estimated.3,14–16
Costs
The input cost estimates for our decision tree are presented in Table 1. Cost and utility estimates were also derived from published literature when possible, and local expert opinion as a default. All costs were expressed in U.S. dollars but were based on prices in Uganda for 2008. Costs were applied for the different types of HIV tests needed for each strategy (initial point of care rapid HIV antibody testing with additional confirmatory testing if positive, labor and delivery repeat testing with a rapid HIV antibody test, and acute HIV testing with a pooled HIV RNA test where applicable). Costs were also applied for the prevention of perinatal transmission with the local standard short course antiretroviral protocol using nevirapine, use of HAART in pregnancy and lactation, and life-time HAART treatment for a mother and/or child diagnosed with HIV.
We used $2.80 for the baseline cost of a single rapid HIV antibody test, and $5.60 if the test was positive and additional confirmatory testing was needed, which reflects the cost of the test kit(s) only and not the cost of the health care provider administering the test or the facilities cost.17 For acute HIV testing using HIV RNA testing, we assumed a cost of $32.11.18 The use of nevirapine in labor for the mother and immediately postpartum for the neonate for the prevention of perinatal transmission costs approximately $8 for the mother and $4 for the neonate.19 The cost of HAART during pregnancy and 6 months of lactation were estimated as $68.25 and $45 respectively.20 The cost of HAART for life for a woman and the cost of HAART for life for a child were estimated as $8,000 and $12,000 respectively.21
Life Years Saved
The life expectancy estimates for our model are presented in Table 2. When calculating the life years saved, we assumed a 3% discount rate per year. We assumed that the average age of a pregnant woman in Uganda was 25 years and the overall life expectancy in Uganda to be 50 years, thus the additional life expectancy in our study for a woman without HIV was calculated as 25 years.7,11 For women in the cohort who were diagnosed with HIV and started on HAART, we assumed a similar life expectancy compared to women without HIV.22 For a child with perinatal transmission of HIV who goes on HAART for life, we estimated a life expectancy approximately two-thirds that of a child without HIV.23 We also assumed that the life expectancy for a child who acquired HIV perinatally but never received treatment with HAART had an even lower life expectancy of less than two-thirds that of a child with HIV on HAART.
Table 2.
Life Expectancy Estimates
| Life Expectancies- | Value (Years) | Reference |
|---|---|---|
| Mother without HIV* | 25 | 11 |
| Mother diagnosed with HIV, on HAART | 25 | 22 |
| Mother diagnosed with HIV, no ARVs | 11.4 | 29 |
| Child without HIV | 50 | 30 |
| Child with perinatal HIV, on HAART | 33 | 23 |
| Child with perinatal HIV, no ARVs | 16 | Assumption |
Average age of pregnant woman is 25, assume life expectancy of 50 for women in Uganda, so additional 25 years of life expectancy
Analysis
First, the costs and life years saved for each HIV testing strategy were calculated. Next, we calculated the incremental cost-effectiveness of the HIV testing strategies. In order to do this, the strategies were ranked in terms of total life years expected and compared incrementally to each other. Based on World Health Organization guidelines, we defined our cost-effective threshold as ≤3 times the gross domestic product per capita, which for Uganda was US$3,300 in 2008. In order to evaluate the robustness of our conclusions, univariate sensitivity analysis on all probabilities, life expectancies and costs in our model were performed with ranges from one-third to three times the baseline estimates.
RESULTS
Using our decision-analytic model for a theoretical cohort of 10,000 women entering prenatal care with base case estimates of 10% HIV prevalence and 3% HIV incidence during pregnancy, we found that the combined maternal and child life years saved for each HIV testing strategy are: 414,227 (strategy 1), 414,296 (strategy 2), 415,765 (strategy 3), and 415,794 (strategy 4). With respects to the cost of each strategy, strategy 1 was the least expensive at US$4.11 million, followed by strategy 2 at US$4.46 million, and strategy 3 at US$5.02 million. Finally, strategy 4 was the most costly at US$5.31 million.
For the calculation of incremental cost-effectiveness, the HIV testing strategies 2, 3 and 4 were each incrementally compared to baseline strategy 1. Strategy 2 was dominated by a blend of strategy 1 and 3 with a coefficient of inequity between 0.616 and 0.955 (extended dominance). Compared to strategy 1, strategies 3 and 4 had incremental cost-effectiveness of US$379 and $10,267 per life year, respectively. Using the World Health Organization definition of ≤3 times the gross domestic product per capita as the cost-effectiveness threshold, which for Uganda was US$3,300 in 2008, we found that HIV testing strategy 3 (repeat HIV antibody testing at the time of delivery) was incrementally the cost-effective option that led to the greatest total life years (Table 3).
Table 3.
Decision Analysis Results
| Cohort of 10,000 Pregnant Women | |||
|---|---|---|---|
| Strategies | Cost US$ (Million) | Life Years Saved | Incremental C/E |
| 1. Current standard: 1 HIV test | 4.11 | 414,227 | --- |
| 2. Add HIV RNA to strategy #1 | 4.46 | 414,296 | (Extended Dominance) |
| 3. Repeat HIV test at delivery | 5.02 | 415,765 | $379 |
| 4. Add HIV RNA to strategy #3 | 5.31 | 415,794 | $10,267 |
Sensitivity Analysis
Strategy 3 remained a cost-effective HIV screening option in univariate sensitivity analysis of HIV incidence (Figure 2). However, when the incidence of HIV exceeds 8%, strategy 4 (addition of HIV RNA testing at the time of repeat testing) became the cost-effective strategy with the greatest total life years, meeting the cost-effectiveness threshold of < $3,300 per life-year gained as compared, incrementally, to strategy 3. We also conducted additional univariate sensitivity analysis of all the probabilities, cost estimates, and life expectancies down to one-third and up to three times their baseline estimates without alteration in the cost-effectiveness outcomes of the different HIV testing strategies. Specifically, varying the prevalence of HIV from its baseline estimate of 10% (range 3% to 30%) and the incidence of HIV from its base-case estimate of 3% (range 1% to 9%) did not significantly affect outcomes.
Figure 2. Incremental Cost-Effectiveness of Four HIV Testing Strategies by Incidence of HIV*.
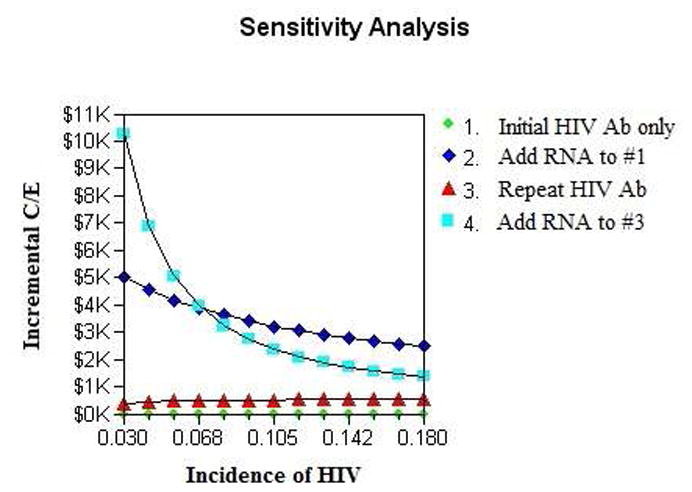
*Based on World Health Organization guidelines, we defined our cost-effective thres hold as ≤3 times the gross domestic product per capita, which for Uganda was US$3300 in 2008.
DISCUSSION
Based on the World Health Organization definition of cost-effectiveness in a resource-limited setting as ≤3 times the gross domestic product per capita, which for Uganda was US$3,300 per life year in 2008, our results show that repeat rapid HIV testing at the end of pregnancy is cost-effective. Our decision-analytic model is based on a theoretical cohort of 10,000 pregnant women in Uganda, a low-resource setting with a high prevalence of HIV similar to other countries in sub-Saharan Africa where HIV is epidemic. Our findings are robust to univariate sensitivity analysis of all the probabilities, life expectancies, and costs varied from down to one-third and up to three times the base-case estimates.
Although the life years saved may seem small on the individual level, sub-Saharan Africa had a population of 836 million people in 2009 (Population Reference Bureau), so the effect of repeat HIV testing in pregnancy should be considered within the context of the entire population. Among our theoretical cohort of only 10,000 pregnant women, there was already a significant number of life years saved (1,538 life years) if repeat HIV testing (strategy 3) was implemented as compared to the current standard of care of a single HIV test at the initiation of prenatal care (strategy 1).
It is also important to consider the marginal benefit produced by strategy 4 as compared to strategy 3. In our analysis, we utilized a cost-effectiveness threshold of only US$3,300 per life year gained. In our baseline analysis, strategy 4 was not cost effective by this standard as it produced an additional 29 life years (in our 10,000 person cohort) as compared to strategy 3 at an incremental cost of approximately $290,000, or $10,267 per life year. To be fair, this would be considered incrementally cost effective in the U.S., but not in a resource-limited country such as Uganda. The ethical issues related to such decision making are important to thoughtfully explore when attempting to create health policies that will lead to better health around the globe.
Similar to any cost-effectiveness analysis, our study is limited in its inability to perfectly model the complexities of clinical medicine and accurately estimate probabilities and costs. For example, certain urban areas of sub-Saharan Africa may have a higher prevalence and incidence of HIV infection among pregnant women, and other rural areas may have much lower numbers. Another limitation of our study is that our model does not reflect the most updated Ugandan protocols for antiretroviral treatment during pregnancy and in newborns, since these have recently changed in response to the newest WHO guidelines.7,24 However, our study is still relevant since there is an inherent lag time in implementing policy changes and our model still reflects the current practice in much of the country and the rest of sub-Saharan Africa. A major strength of our study is that our findings were still robust in univariate sensitivity analysis in which we varied the prevalence and incidence of HIV infection as previously described from their baseline estimates.
Furthermore, other potential limitations of our model include difficulty in accurately estimating life expectancies. For example, we assumed women in the cohort who were diagnosed with HIV and started on HAART had a similar life expectancy compared to women without HIV, but this may not always be the case in areas of sub-Saharan Africa where co-morbidities such as malaria or tuberculosis infection may be highly prevalent.25 Likewise, we estimated life expectancy for a child with perinatal transmission of HIV who goes on HAART for life to be approximately two-thirds that of a child without HIV, but again, this may be highly variable.23 A major strength of our study is that in order to account for these limitations, we did vary these life expectancies in univariate sensitivity analysis and found no effect on which strategy the model found to be optimal.
Another limitation of our study is that we did not include the newest generation of HIV enzyme immunoassay (EIA) tests that can detect HIV p24 antigen in addition to HIV antibody in our decision-analytic model. The detection of HIV p24 antigen would potentially allow for earlier diagnosis of infection during the “window period” of time prior to HIV antibody production.26 We did not include this new test in any of our four HIV screening strategies since it has not been available for wide-spread use in Uganda. However, we believe that this new generation HIV test is an important new screening tool and should be assessed in a subsequent cost-effectiveness study of HIV screening during pregnancy in resource-limited countries of sub-Saharan Africa. The ability to improve the timely detection of new HIV infection is especially important during pregnancy since pregnant women are at an increased risk of acquiring HIV infection and acute HIV infection is associated with a higher risk of perinatal transmission.4–6
In summary, repeat rapid HIV antibody testing at the end of pregnancy is a cost-effective strategy even in a resource-limited setting such as Uganda. Additionally, adding acute HIV testing at the time of the repeat HIV rapid antibody test also becomes cost-effective when the incidence of HIV exceeds 8%. This data will hopefully inform public health policy decisions throughout sub-Saharan Africa regarding the implementation of repeat HIV testing during pregnancy for the detection of incident HIV and prevention of perinatal HIV transmission.
Acknowledgments
Funding information: Dr. Lena Kim was supported by the National Institutes of Health 5T32 HD007162-29 (Graduate Research Training in Perinatal Biology Award).
Footnotes
Conflicts of Interest: None
Presented at the Society for Maternal-Fetal Medicine 2010 30th Annual Meeting, February 1-6, 2010, Chicago, IL.
References
- 1.World Health Oranization/UNAIDS. Joint United Nations Programme on HIV/AIDS and World Health Organization; 2009. Geneva, Switzerland: 2009. AIDS Epidemic Update: December 2009. [Google Scholar]
- 2.Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331:1173–80. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]
- 3.De Cock KM, Fowler MG, Mercier E, et al. Prevention of mother-to-child HIV transmission in resource-poor countries: translating research into policy and practice. Jama. 2000;283:1175–82. doi: 10.1001/jama.283.9.1175. [DOI] [PubMed] [Google Scholar]
- 4.Gray RH, Li X, Kigozi G, et al. Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study. Lancet. 2005;366:1182–8. doi: 10.1016/S0140-6736(05)67481-8. [DOI] [PubMed] [Google Scholar]
- 5.Pilcher CD, Tien HC, Eron JJ, Jr, et al. Brief but efficient: acute HIV infection and the sexual transmission of HIV. J Infect Dis. 2004;189:1785–92. doi: 10.1086/386333. [DOI] [PubMed] [Google Scholar]
- 6.Keating MA, Hamela G, Miller WC, Moses A, Hoffman IF, Hosseinipour MC. High HIV incidence and sexual behavior change among pregnant women in Lilongwe, Malawi: implications for the risk of HIV acquisition. PLoS One. 2012;7:e39109. doi: 10.1371/journal.pone.0039109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ministry of Health, Republic of Uganda. Uganda Clinical Guidelines 2010, National Guidelines on Common Conditions. 2010. [Google Scholar]
- 8.Hecht FM, Busch MP, Rawal B, et al. Use of laboratory tests and clinical symptoms for identification of primary HIV infection. AIDS. 2002;16:1119–29. doi: 10.1097/00002030-200205240-00005. [DOI] [PubMed] [Google Scholar]
- 9.Lelie PN, van Drimmelen HA, Cuypers HT, et al. Sensitivity of HCV RNA and HIV RNA blood screening assays. Transfusion. 2002;42:527–36. doi: 10.1046/j.1537-2995.2002.00101.x. [DOI] [PubMed] [Google Scholar]
- 10.Moodley D, Esterhuizen TM, Pather T, Chetty V, Ngaleka L. High HIV incidence during pregnancy: compelling reason for repeat HIV testing. Aids. 2009 doi: 10.1097/QAD.0b013e32832a5934. [DOI] [PubMed] [Google Scholar]
- 11.Morrison CS, Wang J, Van Der Pol B, Padian N, Salata RA, Richardson BA. Pregnancy and the risk of HIV-1 acquisition among women in Uganda and Zimbabwe. Aids. 2007;21:1027–34. doi: 10.1097/QAD.0b013e3280f00fc4. [DOI] [PubMed] [Google Scholar]
- 12.Cooper ER, Charurat M, Mofenson L, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr. 2002;29:484–94. doi: 10.1097/00126334-200204150-00009. [DOI] [PubMed] [Google Scholar]
- 13.Jackson JB, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: 18-month follow-up of the HIVNET 012 randomised trial. Lancet. 2003;362:859–68. doi: 10.1016/S0140-6736(03)14341-3. [DOI] [PubMed] [Google Scholar]
- 14.Coovadia HM, Rollins NC, Bland RM, et al. Mother-to-child transmission of HIV-1 infection during exclusive breastfeeding in the first 6 months of life: an intervention cohort study. Lancet. 2007;369:1107–16. doi: 10.1016/S0140-6736(07)60283-9. [DOI] [PubMed] [Google Scholar]
- 15.Liang K, Gui X, Zhang YZ, Zhuang K, Meyers K, Ho DD. A Case Series of 104 Women Infected with HIV-1 via Blood Transfusion Postnatally: High Rate of HIV-1 Transmission to Infants through Breast-Feeding. J Infect Dis. 2009;200:682–6. doi: 10.1086/605123. [DOI] [PubMed] [Google Scholar]
- 16.Kilewo C, Karlsson K, Ngarina M, et al. Prevention of mother-to-child transmission of HIV-1 through breastfeeding by treating mothers with triple antiretroviral therapy in Dar es Salaam, Tanzania: the Mitra Plus study. J Acquir Immune Defic Syndr. 2009;52:406–16. doi: 10.1097/QAI.0b013e3181b323ff. [DOI] [PubMed] [Google Scholar]
- 17.Wilkinson D, Wilkinson N, Lombard C, et al. On-site HIV testing in resource-poor settings: is one rapid test enough? Aids. 1997;11:377–81. doi: 10.1097/00002030-199703110-00016. [DOI] [PubMed] [Google Scholar]
- 18.Sources and prices of selected medicines and diagnostics for people living with HIV/AIDS. A Joint UNICEF-UNAIDS-WHO-MSF Project. 2005 Jun; [Google Scholar]
- 19.Rely K, Bertozzi SM, Avila-Figueroa C, Guijarro MT. Cost-effectiveness of strategies to reduce mother-to-child HIV transmission in Mexico, a low-prevalence setting. Health Policy Plan. 2003;18:290–8. doi: 10.1093/heapol/czg035. [DOI] [PubMed] [Google Scholar]
- 20.UNAIDS. 2008 Report on the global AIDS epidemic: Joint United Nations Programme on HIV/AIDS. 2008 Aug [Google Scholar]
- 21.Kahn JG, Marseille E, Auvert B. Cost-effectiveness of male circumcision for HIV prevention in a South African setting. PLoS Med. 2006;3:e517. doi: 10.1371/journal.pmed.0030517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Sighem AI, Gras LA, Reiss P, Brinkman K, de Wolf F. Life expectancy of recently diagnosed asymptomatic HIV-infected patients approaches that of uninfected individuals. Aids. 2010;24:1527–35. doi: 10.1097/QAD.0b013e32833a3946. [DOI] [PubMed] [Google Scholar]
- 23.Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372:293–9. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: Recomendations for a public health approach, 2010 version. Geneva: World Health Organization; 2010. [PubMed] [Google Scholar]
- 25.Cardiello PG, van Heeswijk RP, Hassink EA, et al. Simplifying protease inhibitor therapy with once-daily dosing of saquinavir soft-gelatin capsules/ritonavir (1600/100 mg): HIVNAT 001. 3 study. J Acquir Immune Defic Syndr. 2002;29:464–70. doi: 10.1097/00042560-200204150-00006. [DOI] [PubMed] [Google Scholar]
- 26.Bentsen C, McLaughlin L, Mitchell E, et al. Performance evaluation of the Bio-Rad Laboratories GS HIV Combo Ag/Ab EIA, a 4th generation HIV assay for the simultaneous detection of HIV p24 antigen and antibodies to HIV-1 (groups M and O) and HIV-2 in human serum or plasma. J Clin Virol. 2011;52 (Suppl 1):S57–61. doi: 10.1016/j.jcv.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 27.Mayhood MK, Afwamba IA, Odhiambo CO, et al. Validation, performance under field conditions, and cost-effectiveness of Capillus HIV-1/HIV-2 and determine HIV-1/2 rapid human immunodeficiency virus antibody assays using sequential and parallel testing algorithms in Tanzania. J Clin Microbiol. 2008;46:3946–51. doi: 10.1128/JCM.01045-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khamadi S, Okoth V, Lihana R, et al. Rapid identification of infants for antiretroviral therapy in a resource poor setting: the Kenya experience. J Trop Pediatr. 2008;54:370–4. doi: 10.1093/tropej/fmn036. [DOI] [PubMed] [Google Scholar]
- 29.Glynn JR, Sonnenberg P, Nelson G, Bester A, Shearer S, Murray J. Survival from HIV-1 seroconversion in Southern Africa: a retrospective cohort study in nearly 2000 gold-miners over 10 years of follow-up. Aids. 2007;21:625–32. doi: 10.1097/QAD.0b013e328017f857. [DOI] [PubMed] [Google Scholar]
- 30.WHO. Uganda: Summary country profile for HIV/AIDS treatment scale-up. World Health Organization; 2005. Dec, [Google Scholar]