Abstract
The loudness-dependence of the auditory evoked potential (LDAEP) slope may be inversely related to serotonin (5-HT) neurotransmission. Thus, steep LDAEPs tend to predict a positive response to selective serotonin reuptake inhibitor (SSRI) antidepressants, which augment 5-HT. However, LDAEPs also predict outcome to antidepressants indirectly altering 5-HT (e.g. bupropion). Hence, the LDAEP’s predicative specificity and sensitivity to antidepressant response/outcome remains elusive. Scalp N1, P2 and N1/P2 LDAEP slopes and standardized low resolution brain electromagnetic tomography (sLORETA)-localized N1 and P2 LDAEP slopes were assessed in depressed individuals (N=51) at baseline, 1 and 12 weeks post-treatment with one of three antidepressant regimens [escitalopram (ESC) + bupropion (BUP), ESC or BUP]. Clinical response was greatest with ESC+BUP at week 1. Treatment responders had steep N1 sLORETA-LDAEP baseline slopes while non-responders had shallow ones. P2 sLORETA-LDAEP slope increases at 1 week existed in responders; decreases were noted in non-responders. Exploratory analyses indicated that more BUP and ESC responders versus non-responders had steep baseline N1 sLORETA-LDAEP slopes. Additionally, slight decreases in scalp P2 LDAEP by week 1 existed for ESC treatment, while slope increases existed with ESC+BUP treatment. Only baseline N1 sLORETA-LDAEP discriminated treatment responders/non-responders. This work confirms that certain LDAEP measures are associated with treatment outcome and appear to be differentially modulated with varying antidepressant drug regimens, though this should be confirmed using larger samples.
Keywords: antidepressants, classification, serotonin, major depressive disorder (MDD), loudness-dependence of auditory evoked potentials (LDAEP)
Introduction
Though many pharmacotherapies exist for treating major depressive disorder (MDD), the majority of patients do not remit with initial treatment (Thase, 2003). Additionally, those who do benefit from antidepressants typically experience weeks-long delays before symptom relief. Although selective serotonin reuptake inhibitors (SSRIs) continue to be the most commonly used antidepressants (Marcus & Olfson, 2010), their therapeutic response variability is high. Some evidence suggests that SSRI efficacy may be enhanced by co-administering other drugs, such as bupropion (Spier, 1998; Lam et al., 2004), and that remission rates can be increased and clinical improvement expedited if drug combinations are given at treatment initiation (Blier et al., 2010). Currently, no established markers exist for predicting response to specific antidepressant pharmacotherapies; such markers would aid in optimizing treatments. One such candidate may be electroencephalogram (EEG)-derived measures to an auditory challenge.
High 5-hydroxytryptamine (5-HT; serotonin) neurotransmission exists in primary sensory cortices, such as the auditory cortex, and is likely implicated in modulating sensory processing (Hegerl et al., 2001). Two EEG-derived auditory evoked potentials (AEPs), the N1 and P2, are generated in auditory cortices; their peak-to-peak amplitude (N1/P2) correlates positively with intensity. By plotting N1/P2 amplitude against intensity, a loudness dependence of the AEP (LDAEP; or intensity-dependent AEP, IDAEP) slope is constructed, which appears to be inversely related to 5-HT activity. As cortical hyper-activation with increasing intensity could be damaging, 5-HT activity may inhibit excess neural firing (Juckel et al., 1999). Thus, low dorsal raphe nucleus 5-HT pre-activation is thought to be reflected by steeper LDAEPs than when 5-HT pre-activation is high, and associated with shallow LDAEP slopes (Mulert et al., 2005).
Though this inverse relationship has been demonstrated pre-clinically (Juckel et al., 1997; 1999; Wutzler et al., 2008), evidence for LDAEP sensitivity to central 5-HT activity in humans is indirect and less consistent. For instance, acute tryptophan depletion (ATD), which lowers 5-HT levels, induced unaltered (Debener et al., 2002; Massey et al., 2004; O’Neill et al., 2008), increased (Norra et al., 2008) and even decreased (Dierks et al., 1999; Kähkönen et al., 2002) intensity-dependent N1/P2 amplitudes or LDAEP slopes. Studies probing acute SSRI effects on the LDAEP in healthy adults have also yielded mixed results, with reports of no LDAEP changes (Uhl et al., 2006; Guille et al., 2008) and the expected slope decreases following both acute and chronic SSRI administration (Nathan et al., 2006; Segrave et al., 2006; Simmons et al., 2011). Further evidence linking the 5-HT system with intensity-dependent AEPs comes from associations between altered LDAEP slopes and polymorphisms of terminal 5-HT1B autoreceptors (Juckel et al., 2008) and 5-HT transporters (Hensch et al., 2006; Lee et al., 2011). Nevertheless, clinical evidence for a strong link between the LDAEP/AEPs and central 5-HT activity is tenuous. Additionally, the purported sensitivity of the LDAEP/AEPs to 5-HT neurotransmission has been questioned, as evidence indicates LDAEP/AEPs alterations with other neurotransmitter system modulations (Juckel et al., 1997; Beucke et al., 2010; Lee et al., 2011, but see O’Neill et al., 2006, 2008; Oliva et al., 2010).
Individuals with aberrant 5-HT system function, as is thought to occur in depression, may be more likely to exhibit altered LDAEPs and intensity-dependent AEPs. Though greater N1/P2 amplitudes with increased intensity and steeper LDAEP slopes have been noted in MDD (Gopal et al., 2004; Manjarrez-Gutierrez et al., 2009), suggesting inefficient 5-HT neurotransmission, others have found no such alterations (Linka et al., 2007; Park et al., 2010). Additionally, LDAEP slope modulations may to be associated with specific MDD subtypes and features (Chen et al., 2005; Fitzgerald et al., 2009; Linka et al., 2009).
Despite these issues, baseline LDAEP slopes appear to be a strong predictor of antidepressant response, especially to 5-HT-targeting drugs. Presumably, individuals with steep pre-treatment LDAEPs have (at least to a certain extent) attenuated 5-HT neurotransmission and may be more likely to respond favorably to drugs that augment it, which indeed seems to be the case (P2 LDAEP: Paige et al., 1994; Gallinat et al., 2000; Mulert et al., 2002; 2007; intensity-dependent N1: Linka et al., 2004; Lee et al., 2005; Park et al., 2011). Conversely, those with shallow LDAEPs may benefit more from treatments indirectly targeting the 5-HT system (N1 LDAEP: Linka et al., 2005; Juckel et al., 2007; Mulert et al., 2007). However, some studies have also found that steep pre-treatment LDAEP slopes predict favorable response to bupropion (Paige et al., 1995) and lithium (Juckel et al., 2004). While both drugs affect 5-HT, their mechanisms of action differ from SSRIs and they substantially alter activity of other monoamines (Blier et al., 1987; Ghanbari et al., 2010). Thus, questions remain regarding the specificity of the LDAEP as predictive measures to particular antidepressant regimens. Furthermore, the predictive utility of LDAEP slopes constructed using the N1, the P2 or the amplitude between the N1 and P2 (N1/P2) has not been systematically probed.
Few studies have also examined whether the LDAEP changes with antidepressant treatment. Previous work noted no LDAEP changes with chronic SSRI or bupropion treatment in depressed adults (Paige et al., 1995; Gallinat et al., 2000), though decreased LDAEP slopes with chronic SSRI administration existed in healthy adults (Simmons et al., 2011). As such, it is unclear if chronic (weeks/months) administration of 5-HT-targeting drugs, in particular, alters LDAEP slopes or whether they are unlikely to be radically influenced by antidepressants (i.e., are trait-like). LDAEP slope changes during the course of treatment, particularly during the early stages, could potentially index whether a drug alters brain activity in a manner associated with eventual therapeutic outcome.
This study aimed to verify and compare the utility of baseline scalp N1, P2, N1/P2 LDAEP and source-localized N1, P2 LDAEP slopes in characterizing and predicting response to chronic treatment (12 weeks) with the SSRI escitalopram (ESC), bupropion (BUP) or ESC+BUP in MDD. We also probed if early LDAEP changes (by 1 week) were associated with treatment response. Scalp- and standardized low-resolution brain electromagnetic tomography (sLORETA)-derived LDAEP slopes were assessed, as evidence suggests that these indices may yield somewhat distinct results and exhibit different sensitivity (Mulert et al., 2002; Hagenmuller et al., 2011). The stability of scalp and sLORETA LDAEP slopes during treatment was also examined; to our knowledge, sLORETA-derived LDAEP slope stability during antidepressant treatment has not yet been probed. Finally, we assessed which baseline LDAEP measure(s) best discriminated antidepressant treatment responders from non-responders. Given that precedent research has noted a main effect of sex on the LDAEP (Hensch et al., 2008; Oliva et al., 2011; Jaworska et al., 2012), sex was used as a covariate in our analyses. We hypothesized that treatment responders (≥50% decrease in baseline Montgomery-Åsberg Depression Rating Scale scores) would be characterized by steeper baseline LDAEPs. Given the putative synergistic effects of drug combinations, we predicted normalization (LDAEP slope decreases) to emerge by 1 week with ESC+BUP; we did not expect slope changes for the monotherapies at this time. Though response evaluation to the three regimens was not our focus, we nevertheless expected hastened and more pronounced responses with ESC+BUP. Treatment-specific effects were exploratory as samples were small when groups were subdivided by treatment regimens.
Methods
Patients
Fifty-three adults (N=53) with a primary diagnosis of MDD, SCID-IV-I/P-assessed by psychiatrists, were initially recruited; most had previous major depressive episodes (mean duration since illness onset 13.3 years). The 17-item Hamilton Rating Scale for Depression (HAMD17; Hamilton, 1960) and Montgomery-Åsberg Depression Rating Scale (MADRS; Montgomery & Åsberg, 1979) were administered. All patients had MADRS scores ≥22 at time of drug randomization (outlined below). Exclusion criteria included: Bipolar disorder (BP-I/II/NOS), psychosis history, current (<6 months) drug/alcohol abuse/dependence, seizure history, unstable (≥3 months) medical condition, history of anorexia/bulimia and significant suicide risk. Participants with hearing loss (using hearing aids and/or unable to hear 60 dB SPL, 1000 Hz, as assessed by an audiometric test) were also excluded. Patients with a secondary diagnosis of some anxiety disorder were included (N=33: no co-morbidity; N=12: sub-threshold anxiety; N=8: secondary diagnosis of some anxiety disorder). At randomization, patients were not taking psychoactive drugs; appropriate drug washout periods were applied for previously medicated patients. Participants were tested pre-, 1 and 12 weeks post-treatment. This study was approved by the Royal Ottawa Health Care Group and University of Ottawa Social Sciences and Humanities Research Ethics Boards; informed consent was obtained from all participants who were compensated $30.00 CDN/session.
Antidepressant Regimens
Patients were recruited from a clinical trial wherein they were randomized to one of three antidepressant regimens (double-blind): escitalopram (ESC) + placebo, bupropion (BUP) + placebo or ESC+BUP. Patients were assessed weekly for the first four weeks and then bi-weekly. Dosing was raised only if tolerated and remission (HAMD17 ≤ 7 over at least two consecutive visits) not reached. Clinical measures of interest were: 1. MDD severity: Assessed by HAMD17 and MADRS pre-, 1 and 12 weeks post-treatment and rating changes. 2. Response: ≥50% MADRS reduction from baseline to week 12 (or last session carried forward; ratings were not carried forward if dropout occurred before 6 weeks).
LDAEP Paradigm
Before testing, participants abstained for >3 hr from caffeine and/or smoking/nicotine, as well as from alcohol/non-prescription drugs beginning at midnight. They were seated in a sound- and light-attenuated chamber with their eyes fixated ~1 m in front. Auditory stimuli (1000 Hz, 30 ms, 10 ms rise/fall) were presented (Neurobehavioral Systems Inc, Albany, CA, USA) binaurally through headphones (TDH-49, Northeastern Technologies, Glen Cove, NY, USA). Stimuli at five intensities (60, 70, 80, 90, 100 dB SPL) were presented 80 times each (no identical tones presented back-to-back; inter-stimulus interval: 1200-1800 ms; similar to the paradigm used by Linka et al., 2004; Juckel et al., 2007).
Electrophysiological Recordings & Data Reduction
EEG was recorded (500 Hz) with a cap embedded with 32 Ag/AgCl electrodes (EasyCap, Inning a. Ammersee, Germany) positioned according to the 10-10 system (Chatrian et al., 1985); an AFz electrode was the ground and averaged mastoids (TP9/10) were the reference. Electrooculographic activity was also monitored and impedance maintained at ≤5 KΩ (BrainVision Recorder, Gilching, Germany). Signals were filtered (0.1-30 Hz) and ocular-corrected (Gratton et al., 1983). Data was segmented into 600 ms epochs/intensity (−100-500 ms post-stimulus). Artifact rejection followed, which excluded epochs of +/−75 μV and with faulty channels/drift. Epochs were baseline corrected (mean activity 100 ms pre-stimulus) and averaged for each participant/intensity. >40 epochs/intensity were included in analyses (BrainVision Analyzer, Gilching, Germany).
Auditory Evoked Potentials (AEPs) & LDAEP Extraction
Similarly to procedures outlined elsewhere (Jaworska et al., 2012), N1 and P2 peak time windows were established from grand-averages at Cz (as in Linka et al., 2005; Juckel et al., 2007; Simmons et al., 2011). N1 was the most negative peak at 70-140 ms and P2 was the most positive peak at 110-260 ms post-stimulus. The peak-to-peak amplitude between N1 and P2 (N1/P2) was also calculated at each intensity/participant. A mean slope was constructed for N1, P2 and N1/P2, with intensity being the independent and AEP amplitudes the dependent variables [three scalp loudness-dependence of the auditory potential (LDAEP) slopes were calculated: N1 LDAEP, P2 LDAEP, N1/P2 LDAEP]. Due to faulty channels (specifically Cz), insufficient epochs, patient dropout and/or hearing impairments data was available for N=51 at baseline, N=48 at week 1 and N=46 at week 12 for the scalp LDAEP analyses.
Source Localization
As previously outlined (Jaworska et al., 20120), sLORETA (Pascual-Marqui, 2002) was used to estimate neural activity (μA/mm2) in primary auditory cortices [region of interest (ROI); 1500 mm3 from left (12 voxels), 1375 mm3 from right (11 voxels) hemispheres] for scalp-derived N1 and P2 AEPs at each intensity/participant (artifact- and ocular-corrected averaged epochs from 28 electrodes) for each session (see Pizzagalli et al., 2001 for assumptions underlying LORETA – sLORETA’s precursor). Average current densities at 70-140 ms (N1) and 110-260 ms (P2) per intensity were obtained. These were used to generate mean sLORETA-LDAEP slopes (N1, P2 sLORETA-LDAEP) collapsed across hemispheres; the N1/P2 sLORETA-LDAEP slope was not assessed as preliminary analyses indicated it did not yield unique information (given that it is calculated from mean current densities of the N1 and P2 AEPs). Several more participants were excluded from sLORETA analyses than from the AEP analyses as sLORETA analyses can only be carried out when all subjects have the same channels included in the source-analyses algorithm (N=48 at baseline and week 1, and N=44 at week 12).
Statistical Analyses
A. Antidepressant Response
Chi-square tests were used to assess responder/non-responder proportions (i.e., those with/without a >50% MADRS decrease from baseline to week 12, or last session carried forward) per treatment (ESC+BUP, BUP, ESC). Clinical rating changes [HAMD17 and MADRS response rates from baseline to weeks 1 and 12 ((week 1 − baseline)*100/basline; (week 12 − baseline)*100/baseline), reflecting early and late changes, respectively] were assessed between treatments with t-tests.
B. N1, P2, N1/P2 LDAEP & N1, P2 sLORETA-LDAEP Slopes
Chi-square tests were carried out on baseline scalp N1, P2, N1/P2 LDAEPs and N1, P2 sLORETA-LDAEPs divided into steep/shallow slopes (median split) to probe if responder/non-responder proportions differed on baseline slope when treatment groups were collapsed. Chi-square tests were also carried out with treatment groups not collapsed (significance set at p<.01 for these analyses). Multivariate analyses of covariance (MANCOVAs; sex as covariate) were carried out to assess if steep/shallow baseline scalp N1, P2, N1/P2 LDAEP and N1, P2 sLORETA-LDAEP slopes (entered as separate independent variables per MANCOVA; treatment was the second independent variable) were associated with early and late clinical symptom changes (i.e., early and late HAMD17 and MADRS changes; four dependent variables). Univariate analyses of covariance (ANCOVAs) assessed if early changes in scalp N1, P2, N1/P2 LDAEP and N1, P2 sLORETA-LDAEP slopes [((week 1 − baseline)/baseline)*100] were associated with response status (responder/non-responder) and altered by treatment (response status, treatment as fixed factors; sex as covariate).
Pearson’s correlations were carried out between scalp and sLORETA-derived LDAEP slopes at baseline and respective slopes at weeks 1 and 12; this was further assessed with single-measure intra-class correlations (ICC) using a one-way random effects model (as in Allen et al., 2004) to probe slope stability.
Finally, stepwise discriminant analyses were conducted to probe whether baseline N1, P2 sLORETA- and scalp-derived N1, P2, N1/P2 LDAEP slopes (i.e., 5 predictor variables) differentiated responders/non-responders (treatments collapsed).
All main effects and interactions were Greenhouse-Geisser corrected (for the univariate ANCOVAs); Bonferroni corrections were applied (incorporated within the SPSS syntax) to account for multiple comparisons in all the ANCOVAs. Unless stated otherwise, p<.05 was the significance level, means and standard error of the mean (SEMs) are presented.
Results
Patients
Two patients dropped out before week 6 and could not be classified as a responder/non-responder (N=51; Tables 1 and 2). Average ESC or BUP doses at week 12 (assessed by collapsing ESC or BUP doses from the monotherapy and combination regimens) did not differ between responders/non-responders in the current study (responders: BUP=375 mg, ESC=33 mg; non-responders: BUP=418 mg, ESC=35 mg). Eventual treatment responders and non-responders also did not differ on baseline HAMD17 and MADRS scores.
Table 1.
Baseline characteristics of individuals with major depressive disorder (MDD; means ± S.D.)
| MDD (Total N=51) |
|
|---|---|
| Sex (Females/Males) | 28/23 |
| Age (years) | 30.4 ± 11.8 |
| Education (years) | 16.0 ± 2.4 |
| Smoker (N) | 10 |
| Ethnicity (N) | 45 Caucasian; 3 Asian; 1 South Asian; 1 African |
| Depression Type (N) | 23 melancholic; 18 atypical; 11 neither/not catatonic |
Table 2.
Number of responders/non-responders per treatment group
| ESC+BUP | BUP | ESC | |
|---|---|---|---|
| N = Total | 17 | 16 | 18 |
| N = Responders/ Non-Responders |
12/5 | 7/9 | 7/11 |
ESC: escitalopram; BUP: bupropion
Treatment Response & Clinical Ratings
Chi-squared tests revealed no difference in responder/non-responder proportions per treatment (Table 1). T-tests indicated no treatment differences in MADRS and HAMD17-indexed late symptom changes (baseline to week 12). A difference between ESC+BUP versus BUP existed for HAMD17-indexed early symptom changes (baseline to week 1) [t(32)=2.26, p=.031; ESC+BUP: −26% ± 34; BUP: −2% ± 27]. Clinical scores over the course of treatment (collapsed across treatment regimens) are presented in Table 3.
Table 3.
Clinical ratings in individuals with Major Depressive Disorder (MDD) at baseline, weeks 1 and 12 post-treatment, collapsed across treatments (means ± S.D.)
| Clinical Measures | Baseline | Week 1 | Week 12* |
|---|---|---|---|
| Overall (N=51) | |||
|
| |||
| HAMD17 | 20.6 ± 4.9 | 17.7 ± 7.1 | 11.0 ± 8.4 |
| MADRS | 30.5 ± 5.2 | 26.2 ± 8.7 | 15.9 ± 12.5 |
|
| |||
| Responder (N=26) | |||
|
| |||
| HAMD17 | 19.6 ± 5.9 | 14.7 ± 5.6 | 4.4 ± 3.2 |
| MADRS | 29.4 ± 4.5 | 22.9 ± 8.0 | 5.8 ± 4.9 |
|
| |||
| Non-Responder (N=24) | |||
|
| |||
| HAMD17 | 21.6 ± 3.4 | 20.8 ± 7.3 | 17.8 ± 6.3 |
| MADRS | 31.7 ± 5.7 | 29.8 ± 8.0 | 26.4 ± 8.7 |
HAMD17: Hamilton Rating Scale for Depression, 17 item version
MADRS: Montgomery- Åsberg Depression Rating Scale
Scores are obtained at week 12 or last session carried forward (sessions <6 weeks were not carried forward)
Scalp N1, P2 & N1/P2 LDAEP
No differences in responder/non-responder proportions emerged when baseline scalp N1, P2 or N1/P2 LDAEPs were split into shallow/steep slopes (treatments collapsed/not collapsed).
MANCOVAs indicated no differences in HAMD17 or MADRS early or late response rates (% change from baseline to weeks 1 and 12) when baseline slope (shallow/steep for each of N1, P2 and N1/P2 LDAEP slopes) and treatment were fixed factors.
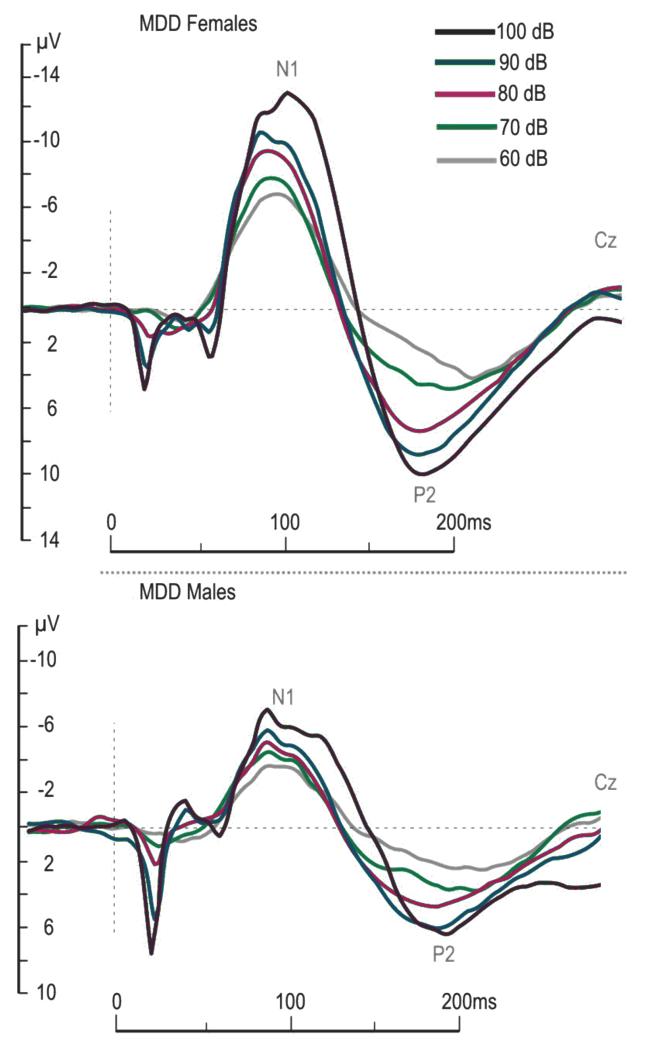
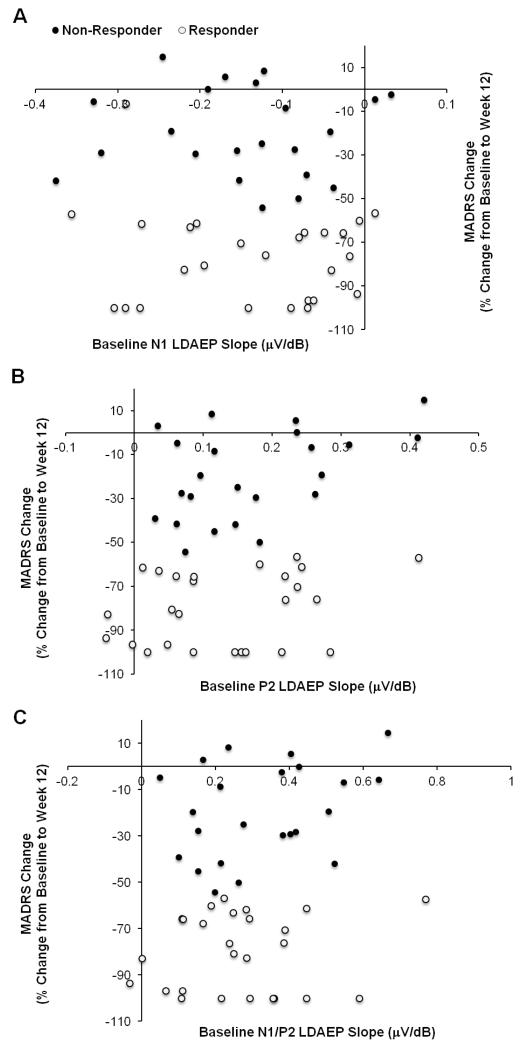
ANCOVAs indicated no differences in early N1 or N1/P2 LDAEP slope changes (from baseline to week 1) when treatment and response status (responder/non-responder) were the independent variables. A main effect of treatment was noted for P2 LDAEP early slope changes [F(1,39)=3.96, p=.027], with follow-up comparisons indicating a difference between ESC+BUP versus ESC. ESC+BUP induced early increases in P2 LDAEP slope (135.9% ± 38.6) while ESC induced small P2 LDAEP slope decreases (−6.0% ± 36.2). Representative baseline LDAEP waveforms (MDD males and females, not collapsed) are presented in Figure 1. The relationship between baseline N1, P2 and N1/P2 LDAEP slopes and late MADRS responses for both responders and non-responders is denoted in Figure 2. Exploratory correlations (p<.01) indicated no relationship between baseline scalp LDAEPs and late clinical response (data not shown).
Figure 1.
Mean auditory evoked potentials (at Cz) in males and females with Major Depressive Disorder (MDD) at baseline to increasing sound intensities
Figure 2.
Relationship between baseline scalp N1 (A), P2 (B) and N1/P2 (C) LDAEP slopes and MADRS response rates (%) from baseline to week 12; a greater decrease indicates a more pronounced antidepressant response. Treatment responders and non-responders are presented.
N1, P2 sLORETA-LDAEP Results
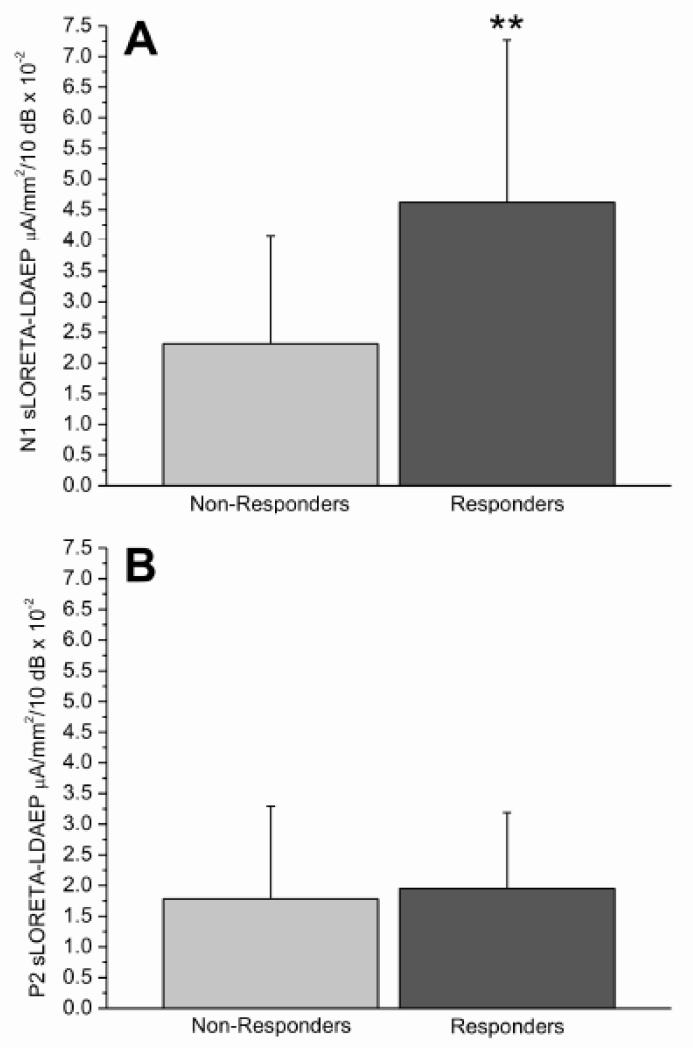
When baseline N1 sLORETA-LDAEP was split into shallow/steep slopes (treatments collapsed), a difference in responder/non-responder proportions existed [χ2(1,46)=12.55, p=.001], with more non-responders (77.3%; N=19) than responders (22.7%; N=5) with shallow slopes. More responders (75%; N=18) than non-responders (25%; N=6) had steep N1 sLORETA-LDAEP baseline slopes (Figure 2). When Chi-square analyses were carried out per treatment, more BUP non-responders tended to exhibit shallow baseline N1 sLORETA-LDAEP slopes (80%, N=4) than responders [20%, N=1; χ2(1,12)=5.18, p=.02]; more non-responders had steep baseline slopes (14.3%, N=1) than responders (85.7%, N=6). A similar trend existed for ESC [χ2(1,17)=5.13, p=.02], with more non-responders with shallow baseline slopes (87.5%, N=7) than responders (12.5%, N=1); fewer non-responders had steep baseline slopes (33.3%, N=3) versus responders (66.7%, N=6). No differences in responder/non-responder proportions based on baseline P2 sLORETA-LDAEP slopes (shallow/steep) existed (Figure 3).
Figure 3.
Mean (+/− S.D) baseline N1 (A) and P2 (B) sLORETA-LDAEP slope values in eventual treatment responders (N=23) and non-responders (N=25) (treatment groups and sex collapsed; **p<0.01)
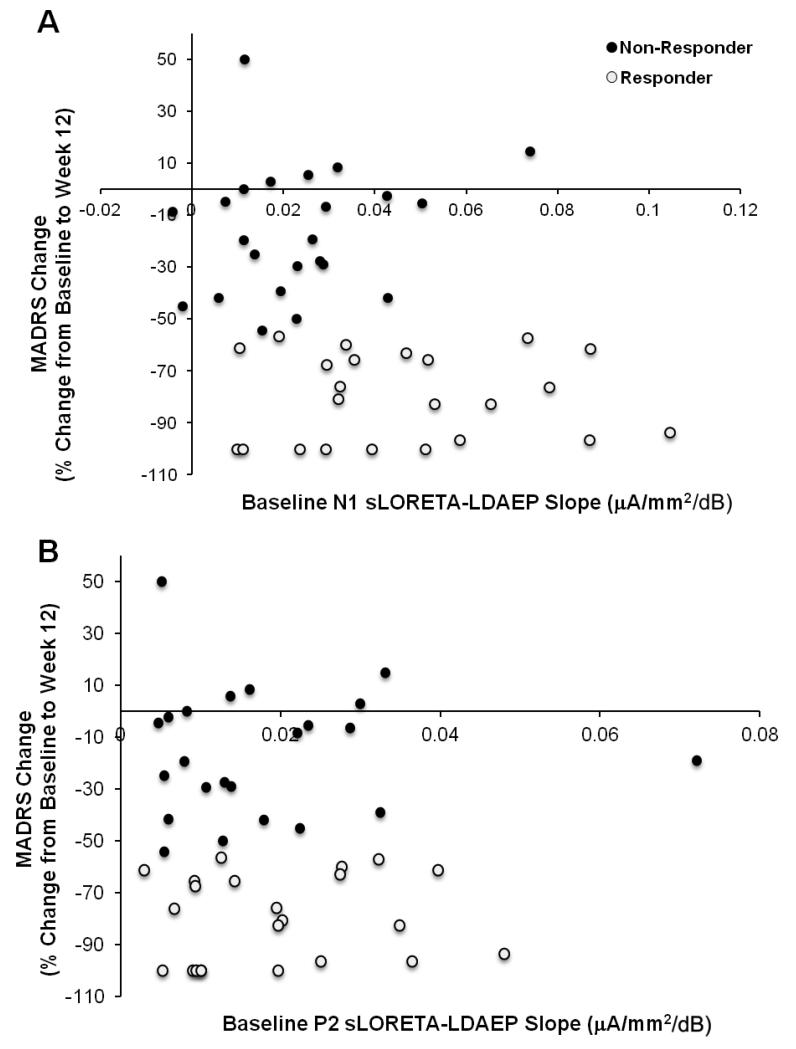
MANCOVAs indicated no differences in HAMD17 or MADRS early or late response rates (% change from baseline to weeks 1 and 12) when P2 sLORETA-LDAEP baseline slope (shallow/steep) and treatment were fixed factors. MANCOVAs indicated a trend for a main effect of N1 sLORETA-LDAEP baseline slope type (steep/shallow) [Wilks’ λ=.80, F(4,35)=2.25, p=.08]. Follow-up analyses indicated a main effect of slope type on late HAMD17 [F(1,38)=6.54, p=.015] and MADRS [F(1,38)=7.98, p=.007] changes, with greater decreases in those with steep (HAMD17: −62.1% ± 8.6; MADRS: −65.6 ± 8.6) versus shallow (HAMD17: −30.7% ± 8.9; MADRS: −29.8% ± 8.9) baseline N1 sLORETA-LDAEP slopes. The relationship between baseline N1 and P2 sLORETA-LDAEP slopes and late MADRS responses for both responders and non-responders is denoted in Figure 4; no correlations were noted between baseline sLORETA-LDAEPs and late clinical response (exploratory analyses; data not shown).
Figure 4.
Relationship between N1 (A) and P2 (B) sLORETA-LDAEP slopes and MADRS response rates (%) from baseline to week 12; a greater decrease indicates a more pronounced antidepressant response. Treatment responders and non-responders are presented.
ANCOVAs indicated no differences in early N1 sLORETA-LDAEP slope changes (% change from baseline to week 1) when treatment and response status (responder/non-responder) were the independent variables. A main effect of responder status was noted for P2 sLORETA-LDAEP early slope changes [F(1,36)=4.32, p=.045], with follow-up comparisons indicating slope decreases from baseline to week 1 in non-responders (−23.4% ± 16.9) and an slope increase in responders (25.1%, ± 16.11).
Discriminant Analyses
Only N1 sLORETA-LDAEP slopes discriminated responders/non-responders (λ=.78, F(1,43)=11.83, p=.001; canonical correlation=.54). Eighty-six percent (86%) of non-responders were correctly classified, while 65% of responders were correctly classified; 76% of cross-validated grouped cases were correctly classified.
LDAEP Stability
Baseline scalp N1 LDAEP correlated with week 1 (r=.80, p<.001, N=47) and 12 (r=.61, p<.001, N=45) slopes (ICC=.63). Baseline scalp P2 LDAEP correlated with slopes at weeks 1 (r=.72, p<.001, N=47) and 12 (r=.58, p<.001, N=45; ICC=.67). Finally, baseline scalp N1/P2 LDAEP correlated with slopes at weeks 1 (r=.80, p<.001, N=47) and 12 (r=.65, p<.001, N=45; ICC=.69). Baseline N1 sLORETA-LDAEP correlated with slopes at weeks 1 (r=.71, p<.001, N=44) and 12 (r=.46, p=.003, N=40; ICC=.46). Finally, baseline P2 sLORETA-LDAEP correlated only with the week 1 slope (r=.65, p<.001; ICC=.35).
Discussion
This study assessed the relationship between baseline scalp N1, P2, N1/P2 and sLORETA-localized N1, P2 LDAEP slopes, as well as early changes in these slopes, and treatment response to one of three antidepressant pharmacotherapy regimens. The stability of these LDAEP slopes over a 12-week treatment period was also examined. Finally, we probed which baseline scalp and sLORETA-derived LDAEP slopes best discriminated eventual antidepressant treatment responders/non-responders. To the best of our knowledge, this is the first clinical study to probe such issues within one relatively homogenous sample of depressed individuals. No clinical differences existed between treatments at week 12 in our patients, though clinical ratings decreased with treatment. Steeper baseline N1 sLORETA-LDAEP slopes existed in responders while non-responders had shallow slopes. P2 sLORETA-LDAEP slope decreases by week 1 were noted in non-responders, while increases existed in responders. Results were less robust for treatment-specific LDAEP effects, which was likely related to limited power; these analyses were exploratory. Both scalp and sLORETA-LDAEP slopes were stable over a week, but stability decreased over 12 weeks. The interpretation and implications of these results are discussed below.
We evaluated treatment response in order to probe the specificity of the association between baseline and early changes in LDAEPs and treatment outcome. We found comparable regimen efficacy by 12 weeks. However, early HAMD17 response rates were greater for ESC+BUP versus ESC (a similar trend was noted versus BUP, data not shown), suggesting that dual treatment may hasten initial response. Though this is consistent with research using other drug combinations (Nelson et al., 2004, Segrave & Nathan, 2005), a recent study did not indicate superior BUP+ESC efficacy to BUP or ESC alone (Rush et al., 2011), contrasting with preliminary work (Leuchter et al., 2008). The results of the clinical trial in which this study was couched within, probing the clinical utility of ESC+BUP versus monotherapy, await publication.
When treatments were collapsed, baseline scalp LDAEP slopes were not related to response, inconsistent with evidence that SSRI (Linka et al., 2004; Lee et al., 2005; Juckel et al., 2007; Mulert et al., 2007) and BUP (Paige et al., 1995) responders exhibit steeper baseline scalp LDAEP slopes than non-responders. While methodological differences may have contributed to this, our protocols were comparable with previous work. We also examined N1 and P2 LDAEP slopes, which may be more sensitive response predictors than the N1/P2 slope (Paige et al., 1994; Linka et al., 2004, 2005). Exploratory analyses indicated that baseline scalp LDAEP slope type (shallow/steep) also did not differentiate responders/non-responders to specific drug regimens.
Steeper baseline N1 sLORETA-LDAEP slopes were noted in treatment responders and were associated with more pronounced late depression rating decreases. Conversely, treatment non-responders exhibited shallow baseline N1 sLORETA-LDAEP slopes, which were also associated with smaller depression rating decreases. Similarly to others’ work (Mulert et al., 2002; Park et al., 2011), our results indicate that sLORETA-derived LDAEPs yield comparable results as other source localization methods (Galliant et al., 2000; Juckel et al., 2007; Mulert et al., 2007) in predicting treatment response. Only baseline N1 sLORETA-LDAEP slope was found to associate with antidepressant response, somewhat consistent with previous work indicating superior predictive utility of scalp-derived N1 LDAEPs (Linka et al., 2004; 2005; 2009; but see Paige et al., 1994; Gallinat et al., 2000; Mulert et al., 2007). Limited rationale exists as to which of the N1, P2 or N1/P2 LDAEPs (scalp or source localized) is most sensitive in predicting eventual response, as predictive utility using all three has been found. Source analysis has localized the N1 to the primary auditory cortex and planum temporale, while P2 sources appear more diffuse (Godey et al., 2001). Thus, the putatively more diverse P2 generators and its more likely influence by cognitive factors may have increased P2 and N1/P2 LDAEP variability and influenced their predictive utility. The specificity regarding the utility of baseline N1 sLORETA-LDAEP slopes in predicting/associating with response was confirmed by the discriminant analyses, which revealed that only baseline N1 sLORETA-LDAEP significantly discriminated antidepressant treatment responders from non-responders.
More BUP and ESC responders tended to exhibit steep baseline N1 sLORETA-LDAEPs while shallow slopes characterized non-responders. The ESC results are consistent with previous work assessing predictive utility of source-localized LDAEPs (Mulert et al, 2007) and scalp-derived N1 LDAEP slopes (Linka et al., 2004; 2005; 2009) to SSRI response. One known study has assessed the predictive utility of scalp LDAEPs to BUP response, and noted findings similar to ours (Paige et al., 1995). If we espouse an inverse relationship between the LDAEP and 5-HT activity, it seems reasonable that steep baseline LDAEPs would predict a positive BUP response, as the drug increases 5-HT neurotransmission (Ghanbari et al., 2010). However, steep baseline N1 sLORETA-LDAEP slopes may index a neurochemical environment responsive to several antidepressant classes that directly or indirectly modulate 5-HT activity, rather than serving solely as an index of 5-HT hypofunction. Although N1 sLORETA-LDAEP baseline slopes did not differentiate ESC+BUP responders/non-responders, it is premature to rule out that they do not predict dual treatment outcome; power issues may have contributed to these null results and treatment-specific results should be treated as preliminary and exploratory findings.
A main effect of response status existed on early (by week 1) P2 sLORETA-LDAEP slopes, with responders exhibiting slope increases and non-responders decreases. This finding is among the first to indicate that early LDAEP slope changes differentiate responders/non-responders and that LDAEPs may be modulated with weeklong treatment, which may subsequently stabilize with chronic treatment. Previous studies on scalp LDAEP changes over the course of antidepressant administration noted no changes with chronic SSRI (Gallinat et al, 2000) and BUP treatment in MDD (Paige et al, 1995), though one group found decreased slopes with chronic SSRI administration in healthy adults (Simmons et al, 2011).
A main effect of treatment was noted for early scalp P2 LDAEP slope changes, with ESC+BUP inducing early slope increases while ESC induced slight slope decreases. Short-term/acute SSRI treatment does not enhance 5-HT neurotransmission, as compensatory/homeostatic mechanisms buffer against 5-HT surges (i.e., 5-HT1A autoreceptor desensitization; Blier et al., 1987). Thus, the relatively modest P2 LDAEP slope decrease with ESC may reflect this initial homeostatic response to increased 5-HT availability. ESC+BUP treatment has been shown to induce greater 5-HT increases than SSRI monotherapy (Ghanbari et al., 2010). Thus, early P2 LDAEP slope increases may reflect more robust homeostatic/compensatory mechanisms following combination treatment. Furthermore, ESC+BUP induces more robust central neurochemical modulations, affecting DA, 5-HT and NA systems (Blier et al., 2010), and likely others, than the monotherapy regimens, as such, ESC+BUP treatment may induce more pronounced early LDAEP modulations (i.e., large P2 LDAEP slope increase). However, these inferences must be verified with larger sample sizes.
Only a handful of studies have examined if LDAEPs change with antidepressants and none, to our knowledge, have assessed sLORETA-LDAEP stability. Baseline scalp LDAEP slopes correlated with their respective slopes at later time points and moderate scalp LDAEP stability existed; the same was true for the N1 sLORETA-LDAEP. Baseline P2 sLORETA-LDAEP slopes only correlated with its respective slopes at week 1 and relatively weak slope stability existed. Given that sLORETA-derived LDAEP slopes were calculated from average current source density value in the primary auditory cortex over a time window corresponding to a particular AEP (versus a discrete time point), increased variability may have contributed to the diminished P2 sLORETA-LDAEP slope stability. It is difficult to speculate if chronic antidepressant treatment destabilized LDAEP slopes or whether LDAEPs, particularly sLORETA-derived slopes, are somewhat unstable electrophysiological measures. LDAEP stability assessments in healthy, unmedicated controls are required to resolve this.
Several study limitations must be acknowledged. First, once divided into responders/non-responders per treatment, samples were reduced and analyses became problematic. Thus, the treatment-specific results are preliminary, and interpretations/conclusions should be treated with caution. Second, sLORETA source-localization was used rather than dipole source analyses, which may limit our findings’ generalizability. Future sLORETA work should also employ larger electrode montages, which would increase source-localization accuracy. Finally, factors such as MDD subtype (though our sample was relatively homogenous) and psychiatric co-morbidity should be better controlled for in similar work.
Future work should replicate this study using a larger sample size of responders/non-responders following single and combination antidepressant pharmacotherapy. Additionally, the contribution of individual AEPs in predicting response should be explored. Previous work suggests that the auditory N1 is associated with treatment/intervention outcome (Danos et al., 1994; Spronk et al., 2011). Future research should also investigate mid-latency AEPs (MAEPs) in the context of depression and antidepressant response. As evident in Figure 1, MAEPs (i.e., Na, Pa, Nb, and Pb/P1, peaking at ~15-25, 25-40, 40-50 and 50-80 ms, respectively) were evoked using our LDAEP paradigm. To out knowledge, no one has yet inspected whether these pre-conscious AEPs are associated with MDD and/or treatment response. Other methodological aspects that should be considered in comparable future work include presenting standard deviations (SD; versus SEMs) to more accurately reflect a sample’s LDAEP slope value distributions, as well as indicating outlier presence. In the current study, three outliers in baseline LDAEP slopes measures were identified (2.5 SDs above the mean); their exclusion did not alter the results and they were thus included in the analyses (data not shown).
Finally, future work should further explore the putative utility of several electrophysiological markers in predicting treatment response within the same study, as certain markers may be better suited in predicting response to a particular class of antidepressants. The construction of a composite electrophysiological index may ultimately prove to be most sensitive in predicating response, however, large, methods-focused studies are required before this is possible.
Research Highlights.
-
-
Antidepressant responders had steep baseline N1 source localized-LDAEP (loudness dependence of the auditory evoked potential) slopes; non-responders had shallow ones.
-
-
Responders had P2 source localized-LDAEP slope increases by week 1 post-treatment; decreases existed in non-responders.
-
-
Slight decreases in scalp P2 LDAEP slopes existed with escitalopram treatment by week 1; increases existed with escitalopram+bupropion treatment.
-
-
Only baseline N1 sLORETA-LDAEP discriminated treatment responders/non-responders.
Acknowledgements
We would like to thank Chantel Hebert for assisting with recruitment and screening, as well as Dhrasti Shah, Joelle Choueiry and Danielle Impey for their help in testing.
Relevant Abbreviations
- LDAEP
loudness dependence of the auditory evoked potentials
- MDD
major depressive disorder (MDD)
- AEP
auditory evoked potentials
- 5-HT
5-hydroxytryptamine/serotonin
- HAMD17
Hamilton rating scale for depression
- MADRS
Montgomery-Åsberg Depression Rating Scale
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allen JJ, Urry HL, Hitt SK, Coan JA. The stability of resting frontal electroencephalographic asymmetry in depression. Psychophysiology. 2004;41(2):269–80. doi: 10.1111/j.1469-8986.2003.00149.x. [DOI] [PubMed] [Google Scholar]
- 2.Beucke JC, Uhl I, Plotkin M, Winter C, Assion HJ, Endrass T, Amthauer H, Kupsch A, Juckel G. Serotonergic neurotransmission in early Parkinson’s disease: a pilot study to assess implications for depression in this disorder. World J Biol Psychiatry. 2010;11(6):781–7. doi: 10.3109/15622975.2010.491127. [DOI] [PubMed] [Google Scholar]
- 3.Blier P, de Montigny C, Chaput Y. Modifications of the serotonin system by antidepressant treatments: implications for the therapeutic response in major depression. J Clin Psychopharmacol. 1987;7(6 Suppl):24S–35S. [PubMed] [Google Scholar]
- 4.Blier P, Ward HE, Tremblay P, Laberge L, Hébert C, Bergeron R. Combination of antidepressant medications from treatment initiation for major depressive disorder: a double-blind randomized study. Am J Psychiatry. 2010;167(3):281–8. doi: 10.1176/appi.ajp.2009.09020186. [DOI] [PubMed] [Google Scholar]
- 5.Chatrian GE, Lettich E, Nelson PL. Ten percent electrode system for topographic studies of spontaneous and evoked EEG activity. Am J EEG Technol. 1985;25:83–92. [Google Scholar]
- 6.Chen TJ, Yu YW, Chen MC, Wang SY, Tsai SJ, Lee TW. Serotonin dysfunction and suicide attempts in major depressives: an auditory event-related potential study. Neuropsychobiology. 2005;52(1):28–36. doi: 10.1159/000086175. [DOI] [PubMed] [Google Scholar]
- 7.Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007;62:847–55. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danos P, Kasper S, Scholl HP, Kaiser J, Ruhrmann S, Höflich G, Möller HJ. Clinical response to sleep deprivation and auditory-evoked potentials--preliminary results. Pharmacopsychiatry. 1994;27(2):70–1. doi: 10.1055/s-2007-1014281. [DOI] [PubMed] [Google Scholar]
- 9.Debener S, Strobel A, Kürschner K, Kranczioch C, Hebenstreit J, Maercker A, Beauducel A, Brocke B. Is auditory evoked potential augmenting/reducing affected by acute tryptophan depletion? Biol Psychol. 2002;59(2):121–33. doi: 10.1016/s0301-0511(01)00132-6. [DOI] [PubMed] [Google Scholar]
- 10.Dierks T, Barta S, Demisch L, Schmeck K, Englert E, Kewitz A, Maurer K, Poustka F. Intensity-dependence of auditory evoked potentials (AEPs) as biological marker for cerebral serotonin levels: effects of tryptophan depletion in healthy subjects. Psychopharmacology (Berl) 1999;146(1):101–7. doi: 10.1007/s002130051094. [DOI] [PubMed] [Google Scholar]
- 11.Fink G, Sumner BE, McQueen JK, Wilson H, Rosie R. Sex steroid control of mood, mental state and memory. Clin Exp Pharmacol Physiol. 1998;25:764–75. doi: 10.1111/j.1440-1681.1998.tb02151.x. [DOI] [PubMed] [Google Scholar]
- 12.Fitzgerald PB, Mellow TB, Hoy KE, Segrave R, Cooper NR, Upton DJ, Croft RJ. A study of intensity dependence of the auditory evoked potential (IDAEP) in medicated melancholic and non-melancholic depression. J Affect Disord. 2009;117(3):212–6. doi: 10.1016/j.jad.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Gallinat J, Bottlender R, Juckel G, Munke-Puchner A, Stotz G, Kuss HJ, Mavrogiorgou P, Hegerl U. The loudness dependency of the auditory evoked N1/P2-component as a predictor of the acute SSRI response in depression. Psychopharmacology (Berl) 2000;148(4):404–11. doi: 10.1007/s002130050070. [DOI] [PubMed] [Google Scholar]
- 14.Ghanbari R, El Mansari M, Blier P. Electrophysiological effects of the co-administration of escitalopram and bupropion on rat serotonin and norepinephrine neurons. J Psychopharmacol. 2010;24(1):39–50. doi: 10.1177/0269881108095714. [DOI] [PubMed] [Google Scholar]
- 15.Godey B, Schwartz D, de Graaf JB, Chauvel P, Liégeois-Chauvel C. Neuromagnetic source localization of auditory evoked fields and intracerebral evoked potentials: a comparison of data in the same patients. Clin Neurophysiol. 2001;112(10):1850–9. doi: 10.1016/s1388-2457(01)00636-8. [DOI] [PubMed] [Google Scholar]
- 16.Gopal KV, Bishop CE, Carney L. Auditory measures in clinically depressed individuals. II. Auditory evoked potentials and behavioral speech tests. Int J Audiol. 2004;43:499–505. doi: 10.1080/14992020400050064. [DOI] [PubMed] [Google Scholar]
- 17.Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55(4):468–84. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- 18.Guille V, Croft RJ, O’Neill BV, Illic S, Phan KL, Nathan PJ. An examination of acute changes in serotonergic neurotransmission using the loudness dependence measure of auditory cortex evoked activity: effects of citalopram, escitalopram and sertraline. Hum Psychopharmacol. 2008;23(3):231–41. doi: 10.1002/hup.922. [DOI] [PubMed] [Google Scholar]
- 19.Hagenmuller F, Hitz K, Darvas F, Kawohl W. Determination of the loudness dependence of auditory evoked potentials: single-electrode estimation versus dipole source analysis. Hum Psychopharmacol. 2011;26(2):147–54. doi: 10.1002/hup.1186. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hegerl U, Gallinat J, Juckel G. Event-related potentials. Do they reflect central serotonergic neurotransmission and do they predict clinical response to serotonin agonists? J Affect Disord. 2001;62(1-2):93–100. doi: 10.1016/s0165-0327(00)00353-0. [DOI] [PubMed] [Google Scholar]
- 22.Hegerl U, Juckel G. Auditory evoked dipole source activity: indicator of central serotonergic dysfunction in psychiatric patients? Pharmacopsychiatry. 1994;27(2):75–8. doi: 10.1055/s-2007-1014283. [DOI] [PubMed] [Google Scholar]
- 23.Hensch T, Wargelius HL, Herold U, Lesch KP, Oreland L, Brocke B. Further evidence for an association of 5-HTTLPR with intensity dependence of auditory-evoked potentials. Neuropsychopharmacology. 2006;31:2047–54. doi: 10.1038/sj.npp.1301020. [DOI] [PubMed] [Google Scholar]
- 24.Hensch T, Wargelius HL, Herold U, Strobel A, Oreland L, Brocke B. Electrophysiological and behavioral correlates of polymorphisms in the transcription factor AP-2beta coding gene. Neurosci Lett. 2008;436:67–71. doi: 10.1016/j.neulet.2008.02.062. [DOI] [PubMed] [Google Scholar]
- 25.Jaworska N, Blier P, Fusee W, Knott V. Scalp- and sLORETA-derived loudness dependence of auditory evoked potentials (LDAEPs) in unmedicated depressed males and females and healthy controls. Clin Neurophysiol. 2012 doi: 10.1016/j.clinph.2012.02.076. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 26.Juckel G, Hegerl U, Giegling I, Mavrogiorgou P, Wutzler A, Schuhmacher C, Uhl I, Brüne M, Mulert C, Pogarell O, Rujescu D. Association of 5-HT1B receptor polymorphisms with the loudness dependence of auditory evoked potentials in a community-based sample of healthy volunteers. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(4):454–8. doi: 10.1002/ajmg.b.30628. [DOI] [PubMed] [Google Scholar]
- 27.Juckel G, Hegerl U, Molnár M, Csépe V, Karmos G. Auditory evoked potentials reflect serotonergic neuronal activity-a study in behaving cats administered drugs acting on 5-HT1A autoreceptors in the dorsal raphe nucleus. Neuropsychopharmacology. 1999;21(6):710–6. doi: 10.1016/S0893-133X(99)00074-3. [DOI] [PubMed] [Google Scholar]
- 28.Juckel G, Mavrogiorgou P, Bredemeier S, Gallinat J, Frodl T, Schulz C, Möller HJ, Hegerl U. Loudness dependence of primary auditory-cortex-evoked activity as predictor of therapeutic outcome to prophylactic lithium treatment in affective disorders--a retrospective study. Pharmacopsychiatry. 2004;37(2):46–51. doi: 10.1055/s-2004-815524. [DOI] [PubMed] [Google Scholar]
- 29.Juckel G, Molnár M, Hegerl U, Csépe V, Karmos G. Auditory-evoked potentials as indicator of brain serotonergic activity-first evidence in behaving cats. Biol Psychiatry. 1997;41(12):1181–95. doi: 10.1016/s0006-3223(96)00240-5. [DOI] [PubMed] [Google Scholar]
- 30.Juckel G, Pogarell O, Augustin H, Mulert C, Muller-Siecheneder F, Frodl T, Mavrogiorgou P, Hegerl U. Differential prediction of first clinical response to serotonergic and noradrenergic antidepressants using the loudness dependence of auditory evoked potentials in patients with major depressive disorder. J Clin Psychiatry. 2007;68(8):1206–1212. doi: 10.4088/jcp.v68n0806. [DOI] [PubMed] [Google Scholar]
- 31.Kähkönen S, Jääskeläinen IP, Pennanen S, Liesivuori J, Ahveninen J. Acute tryptophan depletion decreases intensity-dependence of auditory evoked magnetic N1/P2 dipole source activity. Psychopharmacology (Berl) 2002;164(2):221–7. doi: 10.1007/s00213-002-1194-z. [DOI] [PubMed] [Google Scholar]
- 32.Lam RW, Hossie H, Solomons K, Yatham LN. Citalopram and bupropion-SR: combining versus switching in patients with treatment-resistant depression. J Clin Psychiatry. 2004;65(3):337–40. [PubMed] [Google Scholar]
- 33.Lee IH, Yang YK, Chen PS, Huang HC, Yeh TL, Lu RB, Chiu NT, Yao WJ, Lin SH. Loudness dependence of auditory evoked potentials (LDAEP) correlates with the availability of dopamine transporters and serotonin transporters in healthy volunteers-a two isotopes SPECT study. Psychopharmacology (Berl) 2011;214(3):617–24. doi: 10.1007/s00213-010-2064-8. [DOI] [PubMed] [Google Scholar]
- 34.Lee TW, Yu YW, Chen TJ, Tsai SJ. Loudness dependence of the auditory evoked potential and response to antidepressants in Chinese patients with major depression. J Psychiatry Neurosci. 2005;30(3):202–5. [PMC free article] [PubMed] [Google Scholar]
- 35.Leuchter AF, Lesser IM, Trivedi MH, Rush AJ, Morris DW, Warden D, Fava M, Wisniewski SR, Luther JF, Perales M, Gaynes BN, Stewart JW. An open pilot study of the combination of escitalopram and bupropion-SR for outpatients with major depressive disorder. J Psychiatr Pract. 2008;14(5):271–80. doi: 10.1097/01.pra.0000336754.19566.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linka T, Müller BW, Bender S, Sartory G, Gastpar M. The intensity dependence of auditory evoked ERP components predicts responsiveness to reboxetine treatment in major depression. Pharmacopsychiatry. 2005;38(3):139–43. doi: 10.1055/s-2005-864126. [DOI] [PubMed] [Google Scholar]
- 37.Linka T, Müller BW, Bender S, Sartory G. The intensity dependence of the auditory evoked N1 component as a predictor of response to Citalopram treatment in patients with major depression. Neurosci Lett. 2004;367(3):375–8. doi: 10.1016/j.neulet.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 38.Linka T, Sartory G, Bender S, Gastpar M, Müller BW. The intensity dependence of auditory ERP components in unmedicated patients with major depression and healthy controls. An analysis of group differences. J Affect Disord. 2007;103(1-3):139–45. doi: 10.1016/j.jad.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 39.Linka T, Sartory G, Gastpar M, Scherbaum N, Müller BW. Clinical symptoms of major depression are associated with the intensity dependence of auditory event-related potential components. Psychiatry Res. 2009;169(2):139–43. doi: 10.1016/j.psychres.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 40.Manjarrez-Gutierrez G, Marquez RH, Mejenes-Alvarez SA, Godinez-Lopez T, Hernandez-R J. Functional change of the auditory cortex related to brain serotonergic neurotransmission in type 1 diabetic adolescents with and without depression. World J Biol Psychiatry. 2009;10:877–83. doi: 10.1080/15622970902717032. [DOI] [PubMed] [Google Scholar]
- 41.Marcus SC, Olfson M. National trends in the treatment for depression from 1998 to 2007. Arch Gen Psychiatry. 2010;67(12):1265–73. doi: 10.1001/archgenpsychiatry.2010.151. [DOI] [PubMed] [Google Scholar]
- 42.Massey AE, Marsh VR, McAllister-Williams RH. Lack of effect of tryptophan depletion on the loudness dependency of auditory event related potentials in healthy volunteers. Biol Psychol. 2004;65(2):137–45. doi: 10.1016/j.biopsycho.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Montgomery SA, Åsberg S. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 44.Mulert C, Jäger L, Propp S, Karch S, Störmann S, Pogarell O, Möller HJ, Juckel G, Hegerl U. Sound level dependence of the primary auditory cortex: Simultaneous measurement with 61-channel EEG and fMRI. NeuroImage. 2005;28(1):49–58. doi: 10.1016/j.neuroimage.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 45.Mulert C, Juckel G, Augustin H, Hegerl U. Comparison between the analysis of the loudness dependency of the auditory N1/P2 component with LORETA and dipole source analysis in the prediction of treatment response to the selective serotonin reuptake inhibitor citalopram in major depression. Clin Neurophysiol. 2002;113(10):1566–72. doi: 10.1016/s1388-2457(02)00252-3. [DOI] [PubMed] [Google Scholar]
- 46.Mulert C, Juckel G, Brunnmeier M, Karch S, Leicht G, Mergl R, Möller HJ, Hegerl U, Pogarell O. Prediction of treatment response in major depression: integration of concepts. J Affect Disord. 2007;98(3):215–25. doi: 10.1016/j.jad.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 47.Nathan PJ, Segrave R, Phan KL, O’Neill B, Croft RJ. Direct evidence that acutely enhancing serotonin with the selective serotonin reuptake inhibitor citalopram modulates the loudness dependence of the auditory evoked potential (LDAEP) marker of central serotonin function. Hum Psychopharmacol. 2006;21(1):47–52. doi: 10.1002/hup.740. [DOI] [PubMed] [Google Scholar]
- 48.Nelson JC, Mazure CM, Jatlow PI, Bowers MB, Jr, Price LH. Combining norepinephrine and serotonin reuptake inhibition mechanisms for treatment of depression: a double-blind, randomized study. Biol Psychiatry. 2004;55(3):296–300. doi: 10.1016/j.biopsych.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 49.Norra C, Becker S, Bröcheler A, Kawohl W, Kunert HJ, Buchner H. Loudness dependence of evoked dipole source activity during acute serotonin challenge in females. Hum Psychopharmacol. 2008;23(1):31–42. doi: 10.1002/hup.880. [DOI] [PubMed] [Google Scholar]
- 50.Oliva J, Leung S, Croft RJ, O’Neill BV, O’Kane J, Stout J, Phan KL, Nathan PJ. The loudness dependence auditory evoked potential is insensitive to acute changes in serotonergic and noradrenergic neurotransmission. Hum Psychopharmacol. 2010;25(5):423–7. doi: 10.1002/hup.1133. [DOI] [PubMed] [Google Scholar]
- 51.Oliva JL, Leung S, Croft RJ, O’Neill BV, Stout JC, Nathan PJ. Evidence for sex differences in the loudness dependence of the auditory evoked potential in humans. Hum Psychopharmacol. 2011;26(2):172–6. doi: 10.1002/hup.1187. [DOI] [PubMed] [Google Scholar]
- 52.O’Neill BV, Croft RJ, Leung S, Guille V, Galloway M, Phan KL, Nathan PJ. Dopamine receptor stimulation does not modulate the loudness dependence of the auditory evoked potential in humans. Psychopharmacology (Berl) 2006;188(1):92–9. doi: 10.1007/s00213-006-0501-5. [DOI] [PubMed] [Google Scholar]
- 53.O’Neill BV, Guille V, Croft RJ, Leung S, Scholes KE, Phan KL, Nathan PJ. Effects of selective and combined serotonin and dopamine depletion on the loudness dependence of the auditory evoked potential (LDAEP) in humans. Hum Psychopharmacol. 2008;23(4):301–12. doi: 10.1002/hup.926. [DOI] [PubMed] [Google Scholar]
- 54.Paige SR, Fitzpatrick DF, Kline JP, Balogh SE, Hendricks SE. Event-related potential amplitude/intensity slopes predict response to antidepressants. Neuropsychobiology. 1994;30(4):197–201. doi: 10.1159/000119161. [DOI] [PubMed] [Google Scholar]
- 55.Paige SR, Hendricks SE, Fitzpatrick DF, Balogh S, Burke WJ. Amplitude/intensity functions of auditory event-related potentials predict responsiveness to bupropion in major depressive disorder. Psychopharmacol Bull. 1995;31(2):243–8. [PubMed] [Google Scholar]
- 56.Park YM, Kim DW, Kim S, Im CH, Lee SH. The loudness dependence of the auditory evoked potential (LDAEP) as a predictor of the response to escitalopram in patients with generalized anxiety disorder. Psychopharmacology (Berl) 2011;213(2-3):625–32. doi: 10.1007/s00213-010-2061-y. [DOI] [PubMed] [Google Scholar]
- 57.Park YM, Lee SH, Kim S, Bae SM. The loudness dependence of the auditory evoked potential (LDAEP) in schizophrenia, bipolar disorder, major depressive disorder, anxiety disorder, and healthy controls. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(2):313–6. doi: 10.1016/j.pnpbp.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 58.Pascual-Marqui RD. The sLORETA method: Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Method Find Exp Clin. 2002;24(Suppl D):5–12. [PubMed] [Google Scholar]
- 59.Pizzagalli D, Pascual-Marqui RD, Nitschke JB, Oakes TR, Larson CL, Abercrombie HC, Schaefer SM, Koger JV, Benca RM, Davidson RJ. Anterior cingulate activity as a predictor of degree of treatment response in major depression: evidence from brain electrical tomography analysis. Am J Psychiatry. 2001;158(3):405–15. doi: 10.1176/appi.ajp.158.3.405. [DOI] [PubMed] [Google Scholar]
- 60.Posternak MA, Zimmerman M. Therapeutic effect of follow-up assessments on antidepressant and placebo response rates in antidepressant efficacy trials: meta-analysis. Br J Psychiatry. 2007;190:287–92. doi: 10.1192/bjp.bp.106.028555. [DOI] [PubMed] [Google Scholar]
- 61.Rush AJ, Trivedi MH, Stewart JW, Nierenberg AA, Fava M, Kurian BT, Warden D, Morris DW, Luther JF, Husain MM, Cook IA, Shelton RC, Lesser IM, Kornstein SG, Wisniewski SR. Combining medications to enhance depression outcomes (CO-MED): acute and long-term outcomes of a single-blind randomized study. Am J Psychiatry. 2011;168(7):689–701. doi: 10.1176/appi.ajp.2011.10111645. [DOI] [PubMed] [Google Scholar]
- 62.Segrave R, Croft RJ, Illic S, Luan Phan K, Nathan PJ. Pindolol does not augment central serotonin function increases to citalopram in humans: an auditory evoked potential investigation. Pharmacol Biochem Behav. 2006;85(1):82–90. doi: 10.1016/j.pbb.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 63.Segrave R, Nathan P. Pindolol augmentation of selective serotonin reuptake inhibitors: accounting for the variability of results of placebo-controlled double-blind studies in patients with major depression. Hum Psychopharmacol. 2005;20(3):163–74. doi: 10.1002/hup.672. [DOI] [PubMed] [Google Scholar]
- 64.Simmons JG, Nathan PJ, Berger G, Allen NB. Chronic modulation of serotonergic neurotransmission with sertraline attenuates the loudness dependence of the auditory evoked potential in healthy participants. Psychopharmacology (Berl) 2011;217(1):101–10. doi: 10.1007/s00213-011-2265-9. [DOI] [PubMed] [Google Scholar]
- 65.Smith DJ, Kyle S, Forty L, Cooper C, Walters J, Russell E, Caesar S, Farmer A, McGuffin P, Jones I, Jones L, Craddock N. Differences in depressive symptom profile between males and females. J Affect Disord. 2008;108(3):279–84. doi: 10.1016/j.jad.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 66.Spier SA. Use of bupropion with SRIs and venlafaxine. Depress Anx. 1998;7(2):73–5. [PubMed] [Google Scholar]
- 67.Thase ME. Evaluating antidepressant therapies: remission as the optimal outcome. J Clin Psychiatry. 2003;13:18–25. [PubMed] [Google Scholar]
- 68.Uhl I, Gorynia I, Gallinat J, Mulert C, Wutzler A, Heinz A, Juckel G. Is the loudness dependence of auditory evoked potentials modulated by the selective serotonin reuptake inhibitor citalopram in healthy subjects? Hum Psychopharmacol. 2006;21(7):463–71. doi: 10.1002/hup.803. [DOI] [PubMed] [Google Scholar]
- 69.Wutzler A, Winter C, Kitzrow W, Uhl I, Wolf RJ, Heinz A, Juckel G. Loudness dependence of auditory evoked potentials as indicator of central serotonergic neurotransmission: simultaneous electrophysiological recordings and in vivo microdialysis in the rat primary auditory cortex. Neuropsychopharmacology. 2008;33(13):3176–81. doi: 10.1038/npp.2008.42. [DOI] [PubMed] [Google Scholar]
- 70.Yadav A, Tandon OP, Vaney N. Auditory evoked responses during different phases of menstrual cycle. Indian J Physiol Pharmacol. 2002;46(4):449–56. [PubMed] [Google Scholar]