Abstract
It is difficult to predict prognosis in patients with locoregional well-differentiated (WD) pancreatic neuroendocrine tumors (PanNET). We aimed to examine commonly used stratification systems (WHO 2004 and 2010 classifications, AJCC and ENETS staging, and the Hochwald grading system) for their power in predicting recurrence-free survival (RFS) in these patients. Seventy-five such patients (mean age 56 years, mean follow-up 79 months) that underwent resection with sufficient tissue material and follow up data were studied. RFS was correlated with variable clinicopathologic features and stratified with above-mentioned systems. Concordance-index (CI) was then calculated. With the WHO 2004 classification, 16, 35, and 24 PanNETs were classified as benign behavior, uncertain behavior, and well-differentiated endocrine carcinoma, respectively. By the WHO 2010 classification, 26, 41, and 8 tumors were grade 1, 2, and 3, respectively. Using the Hochwald system, 47 were low grade and 28 were intermediate grade. The AJCC staging information was complete for 62 patients (13 had lymph node status as Nx) and included: stage IA (19/62), IB (10/62), IIA (10/62), and IIB (23/62). The ENETS staging information was stage I (16/62), IIa (8/62), IIb (14/62), IIIa (0/62), and IIIb (24/62). The average Ki-67-proliferation index (PI) was 8.1%. Factors that predicted RFS included tumor size, nodal metastasis, vascular invasion, perineural invasion, necrosis, mitosis, and Ki-67 PI (all p<0.01). The CI for each system was: 0.6361 for WHO 2004, 0.6735 for WHO 2010, 0.6495 for AJCC staging, 0.6642 for ENETS staging, and 0.6851 for Hochwald grading system. When these systems were analyzed in conjunction with various additional important pathologic features, combination of Hochwald grading system and Ki-67 PI achieved the highest CI (0.7946). Therefore, while all these systems predict RFS well in locoregional WD PanNETs, the Hochwald grading systems achieves the highest predictive ability. Further predictive power can be achieved by combining the Hochwald grading system and Ki-67 PI.
Keywords: pancreatic neuroendocrine tumor, WHO classification, AJCC staging, ENETS staging, Hochwald grading system, Ki-67 proliferation index
Introduction
Well-differentiated (WD) pancreatic neuroendocrine tumors (PanNET) are uncommon neoplasm, with an annual incidence < 1/ 100,000 person-years in the general population 1,2. One of the challenges in managing patients with WD PanNET lies in predicting tumor behavior in patients with regional disease (no distant metastasis) at the time of presentation. The pathologic grading systems for PanNET have evolved dramatically in the past decade. In 2002, Hochwald et al proposed a system to stratify WD PanNET into low grade and intermediate grade based on mitotic counts and necrosis 3. In 2004, WHO proposed a scheme to classify WD PanNET (then pancreatic endocrine neoplasm, or PENs) as PEN-benign behavior (BB), PEN-uncertain behavior (UB), and WD endocrine carcinoma based on tumor size, mitotic count, proliferation index (PI), vascular invasion, perineural invasion, gross localization, and metastasis 3. In 2010, WHO simplified the classification scheme by applying only mitotic count and PI; PanNET is reclassified into WD neuroendocrine tumor (grade 1 and grade 2) and poorly-differentiated endocrine carcinoma (grade 3) 4. Besides the histopathologic classification schemes for PanNET, in 2010 the American Joint Committee on Cancer (AJCC) introduced its first TNM staging system for PanNET 5, which is derived from the staging classification for exocrine pancreatic adenocarcinomas. The European Neuroendocrine Tumor Society (ENETS) also proposed its TNM staging system 6. However, direct head-to-head comparison of these different systems in outcome prediction has not been performed.
Investigation on the expression of Ki-67 proliferation antigen in pancreatic neuroendocrine neoplasms has been carried out for over two decades, first as an attempt to explain difference in responses to chemotherapy 7 and later as a prognostic marker 8. MIB-1, the protein product for Ki-67, is a marker for cellular proliferation 9. MIB-1 protein is present during all active phases of the cell cycle (G1, S, G2, and mitosis), but is absent in resting cells (G0). Ki-67-based PI has been shown to be a significant prognostic factor in WD PanNET 10.
The objectives of this study were to compare the WHO 2004 and 2010 classifications, the Hochwald grading system, and the AJCC and ENETS staging systems for their predictive power for recurrence-free survival (RFS) in patients with locoregional WD PanNET. We also explored the possibility of combining each system with additional important pathologic features to improve predictive power in such patients.
Materials and Methods
Patients
The study was approved by the Institutional Review Board of the Washington University School of Medicine. Surgical pathology files at the Barnes-Jewish Hospital Department of Pathology were queried for PanNET between 1993 and 2009. The following patients were excluded: patients with multiple endocrine neoplasia (MEN) 1 syndrome, distant metastasis at the time of diagnosis, tumors arising from duodenum, bile duct, or ampulla of Vater, and insufficient tissue material for study. A total of 75 patients who met the above criteria were enrolled. Pertinent demographics and clinical information, including presenting symptoms (to indicate functionality), were recorded. All patients received regular follow up and the follow up information was retrieved from medical records.
Histopathology Examination
All routine hematoxylin and eosin (H&E) stained slides were re-reviewed. The following parameters were documented for each case: age, gender, tumor size, lymph node involvement status, margin status, perineural invasion, lymphovascular invasion, necrosis, and mitotic counts (recorded as both per 50 high power fields and per 10 HPFs; recorded by a pathologist [TCL]).
WHO 2004 and WHO 2010 Classification Systems
The WHO 2004 classification divided WD PEN into PEN with BB (confined to the pancreas, non-angioinvasive, no perineural invasion, <2cm in diameter, <2 mitoses/10 HPF, and <2% MIB-1-positive cells), PEN with UB (confined to the pancreas and one or more of the following features: ≥ 2 cm in diameter, 2–10 mitoses/10 HPF, >2% MIB-1-positive cells, angioinvasion, perineural invasion) and WD neuroendocrine carcinoma (gross invasion, metastasis) 11. The WHO 2010 classification divided PanNETs into WD NETs and high grade NECs (grade 3; G3) based on the mitotic count (using 20/10 HPFs as the cutoff) or Ki-67 PI (20%). The former was further stratified into grade 1 (G1; 0–1 mitosis/10 HPFs and Ki-67 PI <3%) and grade 2 (G3; 2–20 mitosis/10 HPFs or Ki-67 PI 3–20%).
Hochwald Grading System
The Hochwald grading system, first proposed in 2002 3, classifies tumors into low grade (no necrosis and < 2 mitoses/50 HPFs) and intermediate grade (necrosis or 2 – 50 mitoses/50 HPFs).
Tumor Staging by the AJCC-TNM System
The 2010 AJCC-TNM staging system defines T1 stage as tumor dimensions ≤ 2cm and limited to pancreas, T2 as > 2cm and limited to pancreas, T3 as tumor extending beyond the pancreas but without involvement of the celiac axis or the superior mesenteric artery, and T4 as tumor involving the celiac axis or the superior mesenteric artery (unresectable). Nodal status is defined as Nx: regional lymph nodes cannot be assessed; N0: no regional lymph node metastasis; N1: regional lymph node metastasis. Distant metastasis status is defined as M0: no distant metastasis, and M1: distant metastasis. Stage IA includes T1N0M0; IB: T2N0M0; IIA: T3N0M0; IIB includes T1-3N1M0. Stage III includes T4N0-1M0. M1 defines stage IV disease 5.
Tumor Staging by the ENETS-TNM System
The ENETS-TNM staging system defines T1 stage as tumor dimensions < 2cm and limited to pancreas, T2 as 2 – 4 cm and limited to pancreas, T3 as > 4 cm but limited to the pancreas or invading duodenum or bile duct, and T4 as tumor invading adjacent organs (stomach, spleen, colon, adrenal gland) or the wall of large vessels (celiac axis or the superior mesenteric artery). Nodal status is defined as NX: regional lymph nodes cannot be assessed; N0: no regional lymph node metastasis; N1: regional lymph node metastasis. Distant metastasis status is defined as M0: no distant metastasis, and M1: distant metastasis. Stage I includes T1N0M0; IIa: T2N0M0; IIb: T3N0M0; IIIa: T4N0M0; IIIb: Any TN1M0. M1 defines stage IV disease 12.
Proliferation index determination by Ki-67 (MIB-1) immunohistochemistry
One representative tumor paraffin block from each case was used to generate 4 µm unstained slides for Ki-67 (MIB-1) immunohistochemical staining with a prediluted antibody (Ventana Medical Systems, Arizona). The immunostains were performed on a Ventana Benchmark-XT automated stainer using the Ventana ultraView DAB detection kit. The antigen retrieval was performed using Ventana Tris-based buffer solution CC1, at 37 °C for 16 minutes. One thousand nuclei in the area with the highest density of staining were counted ("hotspot") manually by a pathologist blinded to the diagnosis (TCL), and percentage of Ki-67-expressing cells was recorded for each case.
Statistic analysis
The primary outcome of this study was recurrence-free survival (RFS), defined as the time from surgery to death due to disease, or to disease recurrence at local, regional or distant sites, whichever occurred first. Those patients alive and free of disease were censored at the date of the last contact. RFS was calculated using the Kaplan-Meier method, and comparisons were made using the log-rank tests. Univariate and multivariate Cox proportional hazard models were fit to assess the effects of different grading/staging systems and proliferation index on RFS. The overall predictive ability of each model was then assessed by calculating the concordance index (CI). The value of CI ranges from 0.5 to 1; a value of 1 is indicative of a model with perfect predictive power. All analyses were two-sided and significance was set at a p-value of 0.05. Statistical analyses were performed using SAS (SAS Institute, Cary, NC).
Results
There were 36 male and 39 female patients. The mean age at diagnosis was 56 years (range, 17 – 83). While some patients had more than one symptom, the most common presenting symptom was abdominal pain (n=28), followed by hypoglycermia (n=11), elevated serum gastrin (n=4), steatorrhea (n=4), jaundice (n=4), diarrhea (n=2). Four patients presented with nonspecific symptoms such as nausea, heartburn, weight loss and dyspnea. Twenty-five patients presented with no symptoms and were found on screening test or following up for other diseases. The mean tumor size was 3.0 cm (range, 0.2 – 14.0 cm). The tumor size distribution was: ≤ 2cm: 31, 2.1 – 4 cm: 26, and > 4cm: 18. Lymph node involvement, lymphovascular invasion, perineural invasion, positive resection margin were seen in 24 (24/62, lymph node dissection was not performed in 13), 25, 19, and 11 patients, respectively (Table 1). Areas of tumor necrosis were seen in 15 cases. The mean follow-up was 79 months (median 69 months, range 1 – 212 months). During the follow up, 20 developed recurrence (median time to recurrence 30 months).
Table 1.
Demographics and main histopathologic findings.
| Gender (M: F) | 36:39 |
| Age at diagnosis (y/o) | Mean 55.7 (range, 17 – 83) |
| Angiolymphatic invasion | 25 |
| Perineural invasion | 19 |
| Surgical margin(s) positive for tumor | 11 |
| Tumor necrosis | 15 |
| AJCC Stage (TNM) | |
| Stage IA (T1N0M0) | 19 |
| Stage IB (T2N0M0) | 10 |
| Stage IIA (T3N0M0) | 10 |
| Stage IIB (T1-3N1M0) | 23 |
| AJCC T stage | |
| T1 | 30 |
| T2 | 21 |
| T3 | 24 |
| N stage (applicable to both AJCC and ENETS) | |
| N0 | 38 |
| N1 | 24 |
| Nx | 13 |
| ENETS Stage (TNM) | |
| Stage I (T1N0M0) | 16 |
| Stage IIa (T2N0M0) | 8 |
| Stage IIb (T3N0M0) | 14 |
| Stage IIIa (T4N0M0) | 0 |
| Stage IIIb (T1-4N1M0) | 24 |
| ENETS T stage | |
| T1 | 25 |
| T2 | 19 |
| T3 | 29 |
| T4 | 2 |
| WHO classification (2004) | |
| Well-differentiated endocrine tumor with benign behavior | 16 |
| Well-differentiated endocrine tumor with uncertain behavior | 35 |
| Well-differentiated endocrine carcinoma | 24 |
| WHO classification (2010) | |
| Low grade neuroendocrine tumor (G1) | 26 |
| Intermediate grade neuroendocrine tumor (G2) | 41 |
| Neuroendocrine carcinoma (G3) | 8 |
| Hochwald system | |
| Low grade | 47 |
| Intermediate grade | 28 |
| Proliferation index by IHC (Ki-67) | |
| ≤ 5% | 44 |
| 5– 10% | 16 |
| >10% | 15 |
Pathologic features predictive of RFS
Among these parameters, tumor size (p=0.0008), lymph node involvement (p=0.0073), lymphovascular invasion (p=0.0222), perineural invasion (p=0.0002) and tumor necrosis (p<0.0001) were significantly predictive of RFS, whereas age, gender, and positive surgical margin were not (p=0.6036, 0.2813, and 0.1944, respectively). In this subset of patients with localized disease, functional status of the tumor did not correlate with RFS (p = 0.5189 between functional versus non-functional tumors).
No mitotic activity was seen in 43 (57%) tumors. The mitotic figures in the remaining 32 tumors ranged from 1 to 19 per 50 HPFs (mean 3.8, median 2). The mean Ki-67 PI was 8.1% (median 3.9%, ranged from 0.2% to 65.6%, > 10% in 15 tumors). The Ki-67 PI had a better predictive ability on RFS when it was modeled as a continuous variable (CI = 0.7045) than as a categorical variable using 5% and 10% as the cutoff (≤ 5% vs 5–10% vs >10%, CI=0.6641).
Comparison of the WHO 2004 and 2010 classifications, the Hochwald grading system, AJCC and ENETS staging systems in predicting RFS
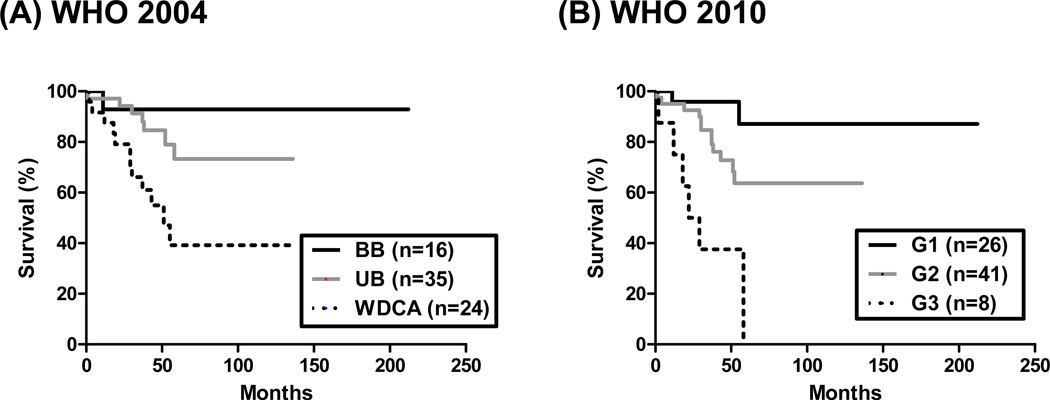
According to the WHO 2004 classification, 16 of 75 patients had “WD PEN with BB”, 35 of 75 had “WD PEN with UB”, and 24 had “WD endocrine carcinomas (WDCA)”. WDCAs had a significantly shorter RFS compared to both BB and UB groups (p = 0.0057). The median RFS for WDCA patients was 51 months, and the median RFS for both the BB and UB groups were undefined. There was, however, no significant difference in RFS between the BB and UB groups (p=0.3639; Figure 1A). The CI for the WHO 2004 classification in predicting RFS was 0.6361.
Figure 1.
Recurrence-free survival analysis stratified by the (A) WHO 2004 and (B) WHO 2010 classification schemes. (A) Patients with WD endocrine carcinoma (WDCA) showed significantly inferior RFS than patients with WD endocrine tumor with benign behavior (BB) or uncertain behavior (UB; p =0.0057). (B) Using the WHO 2010 classification, grade 3 tumors (neuroendocrine carcinoma) had significantly shortened RFS than grade 1 or 2 PanNETs (p<0.0001).
Analyzing the same dataset using the WHO 2010 classification, 26 of 75 patients had G1 tumors, 41 of 75 patients had G2 tumors, and 8 patients had G3 tumors (neuroendocrine carcinoma). The median RFS for G1 and G2 groups were undefined, whereas that of G3 was 25.5 months, (Figure 1B; p<0.0001). The CI for the WHO 2010 classification in predicting RFS was 0.6735.
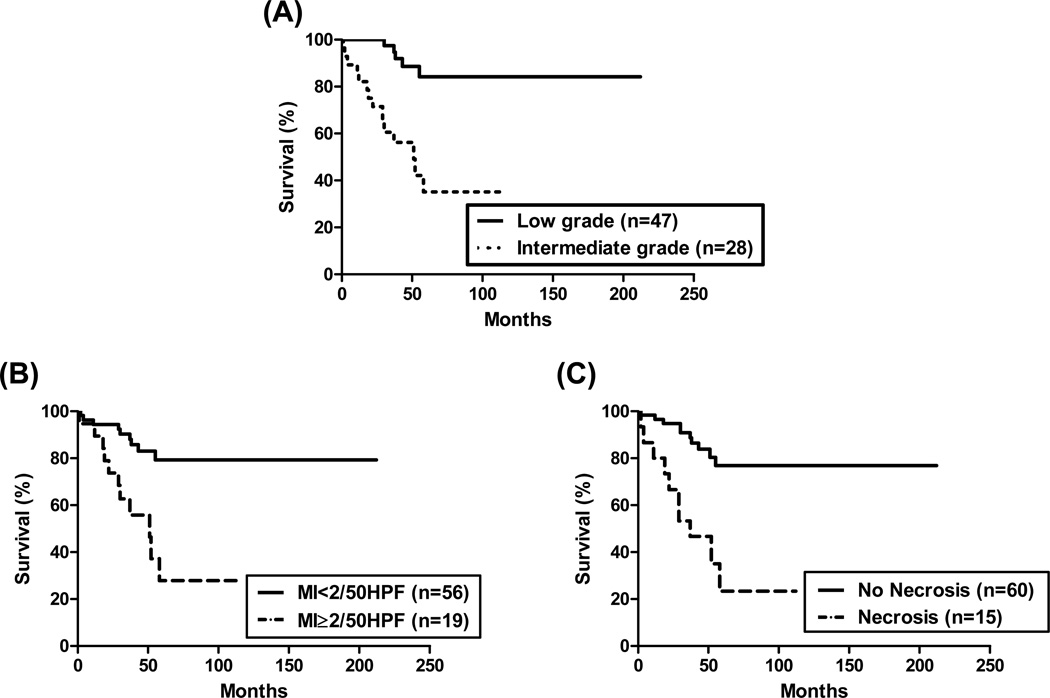
Using the Hochwald grading system, 47 (63%) PanNETs were categorized as low grade and 28 (37%) were categorized as intermediate grade. Recurrence was observed in 5 (11%) patients in the former and in 15 (54%) patients in the latter. Patients with low grade tumors had a significantly prolonged RFS than those with intermediate grade tumors (p<0.0001; Figure 2). The median RFS was 51 months in the former group and it was not defined in the latter. The CI for the Hochwald grading system to predict RFS was 0.6851.
Figure 2.
Recurrence-free survival analysis stratified by the Hochwald grading system. (A) Patients with low grade tumors showed significantly prolonged RFS compared to those with intermediate grade tumors (p<0.0001). Further analysis of the end points included in the Hochwald grading system: the presence of (B) mitotic index (MI) ≥ 2/50HPF and (C) necrosis were correlated with worse reduced RFS (p=0.0003 and < 0.0001, respectively).
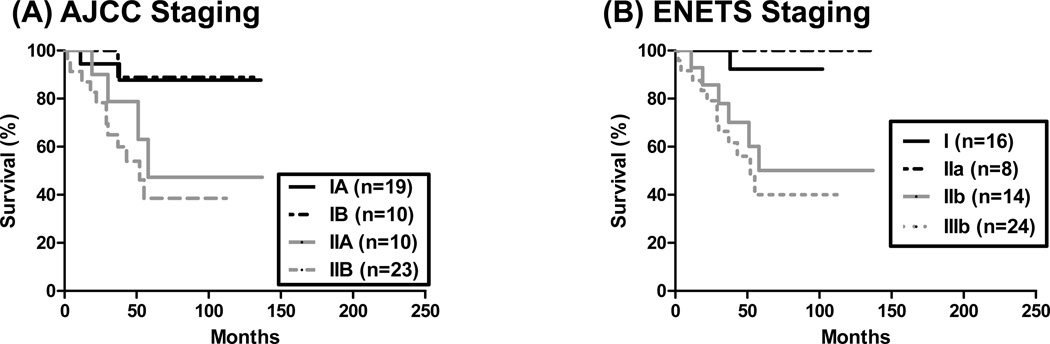
Thirteen patients did not undergo lymph node dissection in the resected specimen and were excluded from the AJCC and ENETS TNM analysis. Of the remaining 62 patients, by AJCC staging, 19 had stage IA, 10 had stage IB, 10 had stage IIA, and 23 had stage IIB disease, respectively. No patient had stage III disease. As shown in Figure 3A, patients with stage IIA disease had RFS of 58 months, stage IIB disease had RFS of 52 months, whereas those of stage IA and IB were not defined (p=0.0188). Of note, there was no statistical significance in RFS between stage IA and IB diseases (p=0.9155). The CI for AJCC stage in predicting RFS was 0.6495. By ENETS staging system, 16 had stage I, 8 had stage IIa, 14 had stage IIb, none had stage IIIa, and 24 had stage IIIb disease, respectively. As shown in Figure 3B, patients with stage IIIb disease had RFS of 52 months, whereas those of stage I and II were not defined (p=0.0110). The CI for ENETS stage in predicting RFS was 0.6642.
Figure 3.
Recurrence-free survival analysis stratified by the AJCC and ENETS TNM staging systems. (A) Using the AJCC staging system, patients with stage II disease were associated with reduced RFS compared to patients with stage I disease (p=0.0188). (B) By the ENETS staging system, patients with stage III disease were associated with reduced RFS compared with patients with stage I or II disease (p=0.0110).
Combining grading/staging systems with Ki-67 PI
We further explored whether incorporating grading or staging system with Ki-67 PI can improve the predictive accuracy of RFS. Since Ki-67 PI was part of the WHO 2004 and WHO 2010 classification criteria, analysis by combining Ki-67 PI and the WHO 2004 and 2010 classifications could not be performed. The CI was 0.7946, 0.7528, and 0.7774, respectively when Ki-67 PI was combined with the Hochwald grading system, the AJCC staging system, and the ENETS staging system, respectively. The CI of WHO 2010 classification was increased from 0.6735 to 0.7447 when the status of necrosis was also added to the analysis. When combined with necrosis, the CI of AJCC staging increased to 0.7522, and that of ENETS staging increased to 0.7645; however, none of these surpass the combination of Hochwald grading system or ENETS staging and KI-67 PI (Table 2).
Table 2.
Predictive power (CI) for combining various stratification systems and other variables.
| Grading system + variables | Concordance Index (CI) |
|---|---|
| WHO 2004 classification | 0.6361 |
| WHO 2010 classification | 0.6735 |
| Hochwald grading | 0.6851 |
| AJCC staging | 0.6495 |
| ENETS staging | 0.6642 |
| Ki-67 PI (continuous variable) | 0.7045 |
| Ki-67 PI (categorical, 0–5% vs 5–10 vs >10%) | 0.6641 |
| Necrosis | 0.6357 |
| Lymphovascular invasion | 0.55476 |
| Perineural invasion | 0.6013 |
| Ki-67 PI + Hochwald grading | 0.7946 |
| Ki-67 PI + AJCC staging | 0.7528 |
| Ki-67 PI + ENETS staging | 0.7774 |
| WHO 2004 classification + necrosis | 0.7340 |
| WHO 2010 classification + necrosis | 0.7447 |
| AJCC staging + necrosis | 0.7522 |
| ENETS staging + necrosis | 0.7645 |
Discussion
Stratification of PanNET has evolved over the last decade. In 2002, Hochwald et al. proposed a grading system based on mitotic count and necrosis 3. Subsequently, WHO adopted two classification systems in 2004 and 2010, respectively 3,4. In addition, in 2010 AJCC introduced its very first staging system for these tumors 3. In this study, we compared these grading systems, and the AJCC and ENETS staging systems for their ability to predict RFS in patients with WD locoregional PanNET. Our data showed that while all these various systems predicted RFS well, the Hochwald grading system showed the highest predictive power, which was further increased when it was combined with Ki-67 PI.
The Hochwald grading system incorporated both mitotic activity and tumor necrosis. In contrast, the WHO 2010 classification applied mitotic count and Ki-67 PI to stratify pancreatic neuroendocrine neoplasms into WD PanNETs and NECs 4. Our univariate analysis showed that mitotic count, Ki-67 PI, and tumor necrosis were all significant prognostic factors for regional WD PanNETs, and hence it is not surprising that the Hochwald grading system was superior in predicting RFS than the WHO 2010 classification, as the former combined two significant variables that are not highly correlated, whereas the latter incorporated two variables that are highly correlated. Our data also provides justification of the WHO 2010 classification over the WHO 2004 classification (CI 0.6735 vs 0.6361). It is worth noting that the WHO 2004 classification is neither a pure grading nor a pure staging system; it combines elements of both stage and grade into a single classification. However, one main issue with the WHO 2004 classification is that no significant difference in RFS was seen between the BB group and the UB group, and thus the clinical relevance of separating these two groups was uncertain. Similar results were also observed by other investigators 13. In many other organs, the AJCC staging remains the most significant prognostic indicator. In our patients with regional WD PanNET, neither the AJCC staging system (CI=0.6495) nor the ENETS staging system (CI=0.6642) had a higher CI then the Hochwald grading system (CI=0.6851).
The Hochwald grading system has many advantages for daily practice, including its simplicity for use and prognostic strength 3. Our study showed that it also possesses a higher prognostic prediction power than the WHO classification schemes. A recent study also demonstrates that its prediction power is further enhanced when combined with tumor size and metastasis status 13. However, the requirement for scoring 50 HPFs for mitotic figures can be limiting for smaller tumors. In our population of patients with regional WD PanNETs, the predictive power of the Hochwald grading system can be further improved by combing it with Ki-67.
The AJCC staging system incorporates pancreatic endocrine tumors along with other exocrine malignancies, and its correlation with prognosis was recently validated 14,15. Our study focused on patients with resectable WD PanNET, and thus, all stage IV and T4 (stage III) patients were excluded. Our study shows that stage II patients have worse prognosis than patients with stage I diseases. However, no statistical significance was seen between stage IA and IB patients, or between stage IIA and IIB patients. A recent large scale study (including patients with stage IV diseases) indicates that the ENETS staging system is superior to the AJCC system in predicting overall survival 6. Our data suggests that for locoregional PanNET, the ENETS staging system has a slightly higher predictive power for RFS compared to the AJCC staging system. As the current study focused on patients with regional diseases, the limited numbers of death events prevented meaningful analysis for overall survival. And thus, a larger cohort will be needed to study overall survival.
Our study also validates results from previous publications that PI assessed by Ki-67 immunohistochemistry is a powerful, independent prognostic predictor. However, there are limitations to the application of Ki-67 stain. First, the intensity threshold for positivity may be subjective, and it is known that the quality of the stain varies between laboratories. It remains to be established if interobserver/inter-institutional reproducibility can be established. Second, as the “hot spot” approach is being used, and areas with intensive Ki-67 signals do not always correlate with areas with high mitotic figures on H&E-stained slides, the selection of areas for immunostain is crucial. The discrepancy between Ki-67 PI and mitotic count has been recently explored 16, and more studies are under way to further clarify the issue. Comparison with antibodies recognizing mitotic figures (e.g. phosphohistone-H3) will be of particular interest. In addition, given the heterogeneity in PI within the tumor, it is likely that performing Ki-67 stains on tissue microarrays will be inaccurate in determining the PI.
Also noteworthy is that in routine pathology practice, a small proportion of the PanNETs that are resected are from patients with MEN 1 syndrome. These tumors show distinct morphology features and genetic alterations 19,20. While these patients were excluded in this analysis, it has been suggested that these patients have superior prognosis compared to patients with sporadic PanNETs 17,18. In addition, mTOR pathway and genes involved in telomerase maintenance (DAXX, ATRX) have been recently identified to play a significant role in up to 40% of PanNET 21,22. It remains to be seen how management of PanNET harboring mutations in mTOR pathway will benefit from small molecule-based targeted therapies 23, and how it correlates with our findings.
In summary, we have shown that while the Hochwald grading, WHO classifications, AJCC and ENETS staging systems all correlated with RFS in locoregional PanNET, the Hochwald grading system shows the highest CI. Its predictive power can be further improved by combing it with Ki-67 PI.
Acknowledgement
The authors wish to acknowledge the support of the Biostatistics Core, Siteman Comprehensive Cancer Center, Barnes-Jewish Hospital and Washington University in St. Louis.
Grant support: Department of Pathology and Immunology, Washington University in St. Louis, and NCI Cancer Center Support Grant P30 CA091842.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no conflicts of interest or funding to disclose.
References
- 1.Halfdanarson TR, Rubin J, Farnell MB, et al. Pancreatic endocrine neoplasms: epidemiology and prognosis of pancreatic endocrine tumors. Endocr Relat Cancer. 2008;15:409–427. doi: 10.1677/ERC-07-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klimstra DS, Modlin IR, Coppola D, et al. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707–712. doi: 10.1097/MPA.0b013e3181ec124e. [DOI] [PubMed] [Google Scholar]
- 3.Hochwald SN, Zee S, Conlon KC, et al. Prognostic factors in pancreatic endocrine neoplasms: an analysis of 136 cases with a proposal for low-grade and intermediate-grade groups. J Clin Oncol. 2002;20:2633–2642. doi: 10.1200/JCO.2002.10.030. [DOI] [PubMed] [Google Scholar]
- 4.Klimstra DS, Arnold R, Capella C, et al. WHO Classification of Tumours of the Digestive System. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. Tumours of the pancreas. Lyon: International Agency for Research on Cancer (IARC); 2010. pp. 279–337. [Google Scholar]
- 5.Exocrine and endocrine pancreas. New York, NY: Springer; 2010. AJCC Cancer Staging Manual; pp. 241–249. [Google Scholar]
- 6.Rindi G, Falconi M, Klersy C, et al. TNM staging of neoplasms of the endocrine pancreas: results from a large international cohort study. J Natl Cancer Inst. 2012;104:764–777. doi: 10.1093/jnci/djs208. [DOI] [PubMed] [Google Scholar]
- 7.von Herbay A, Sieg B, Schurmann G, et al. Proliferative activity of neuroendocrine tumours of the gastroenteropancreatic endocrine system: DNA flow cytometric and immunohistological investigations. Gut. 1991;32:949–953. doi: 10.1136/gut.32.8.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pelosi G, Bresaola E, Bogina G, et al. Endocrine tumors of the pancreas: Ki-67 immunoreactivity on paraffin sections is an independent predictor for malignancy: a comparative study with proliferating-cell nuclear antigen and progesterone receptor protein immunostaining, mitotic index, and other clinicopathologic variables. Hum Pathol. 1996;27:1124–1134. doi: 10.1016/s0046-8177(96)90303-2. [DOI] [PubMed] [Google Scholar]
- 9.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 10.Jamali M, Chetty R. Predicting prognosis in gastroentero-pancreatic neuroendocrine tumors: an overview and the value of Ki-67 immunostaining. Endocr Pathol. 2008;19:282–288. doi: 10.1007/s12022-008-9044-0. [DOI] [PubMed] [Google Scholar]
- 11.Heitz P, Komminoth P, Perren A, et al. Pathology and genetics of endocrine organs. In: DeLellis RA, Lloyd RV, Heitz P, Eng C, editors. Tumours of the endocrine pancreas. Lyon: International Agency for Research on Cancer (IARC); 2004. pp. 177–182. [Google Scholar]
- 12.Rindi G, Kloppel G, Alhman H, et al. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2006;449:395–401. doi: 10.1007/s00428-006-0250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrone CR, Tang LH, Tomlinson J, et al. Determining prognosis in patients with pancreatic endocrine neoplasms: can the WHO classification system be simplified? J Clin Oncol. 2007;25:5609–5615. doi: 10.1200/JCO.2007.12.9809. [DOI] [PubMed] [Google Scholar]
- 14.Strosberg JR, Cheema A, Weber J, et al. Prognostic validity of a novel American Joint Committee on Cancer Staging Classification for pancreatic neuroendocrine tumors. J Clin Oncol. 2011;29:3044–3049. doi: 10.1200/JCO.2011.35.1817. [DOI] [PubMed] [Google Scholar]
- 15.Strosberg JR, Cheema A, Weber JM, et al. Relapse-Free Survival in Patients With Nonmetastatic, Surgically Resected Pancreatic Neuroendocrine Tumors: An Analysis of the AJCC and ENETS Staging Classifications. Ann Surg. 2012;256:321–325. doi: 10.1097/SLA.0b013e31824e6108. [DOI] [PubMed] [Google Scholar]
- 16.Goodell PP, Krasinskas AM, Davison JM, et al. Comparison of methods for proliferative index analysis for grading pancreatic well-differentiated neuroendocrine tumors. Am J Clin Pathol. 2012;137:576–582. doi: 10.1309/AJCP92UCXPJMMSDU. [DOI] [PubMed] [Google Scholar]
- 17.Tomassetti P, Campana D, Piscitelli L, et al. Endocrine pancreatic tumors: factors correlated with survival. Ann Oncol. 2005;16:1806–1810. doi: 10.1093/annonc/mdi358. [DOI] [PubMed] [Google Scholar]
- 18.Jensen RT, Berna MJ, Bingham DB, et al. Inherited pancreatic endocrine tumor syndromes: advances in molecular pathogenesis, diagnosis, management, and controversies. Cancer. 2008;113:1807–1843. doi: 10.1002/cncr.23648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anlauf M, Schlenger R, Perren A, et al. Microadenomatosis of the endocrine pancreas in patients with and without the multiple endocrine neoplasia type 1 syndrome. Am J Surg Pathol. 2006;30:560–574. doi: 10.1097/01.pas.0000194044.01104.25. [DOI] [PubMed] [Google Scholar]
- 20.Corbo V, Dalai I, Scardoni M, et al. MEN1 in pancreatic endocrine tumors: analysis of gene and protein status in 169 sporadic neoplasms reveals alterations in the vast majority of cases. Endocr Relat Cancer. 17:771–783. doi: 10.1677/ERC-10-0028. [DOI] [PubMed] [Google Scholar]
- 21.Jiao Y, Shi C, Edil BH, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331:1199–1203. doi: 10.1126/science.1200609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heaphy CM, de Wilde RF, Jiao Y, et al. Altered telomeres in tumors with ATRX and DAXX mutations. Science. 2011;333:425. doi: 10.1126/science.1207313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Wilde RF, Edil BH, Hruban RH, et al. Well-differentiated pancreatic neuroendocrine tumors: from genetics to therapy. Nat Rev Gastroenterol Hepatol. 2012;9:199–208. doi: 10.1038/nrgastro.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]