Abstract
The conditioned place preference (CPP) paradigm entails Pavlovian conditioning and allows evaluating the acquisition and extinction of drug-associated memory. Epigenetic regulation of chromatin structure by acetylation and deacetylation of histone proteins is critical for formation of long-term memory (LTM). We have recently shown that a single administration of the histone deacetylase (HDAC) inhibitor sodium butyrate (NaB) facilitated extinction of fear-associated memory in mice. Using the CPP paradigm, the present study investigated the effect of NaB on cocaine-associated memory. C57B/6 mice were conditioned by either fixed daily doses of cocaine (5mg/kg × 4 and 15mg/kg × 4 days) or an escalating schedule (3,6,12 and 24mg/kg). Acute administration of NaB (1.2g/kg) prior to conditioning by fixed doses of cocaine increased the expression and impaired the extinction of place preference compared to control subjects. Subjects that were conditioned by 15mg/kg × 4 cocaine and received a single injection of NaB following the first or the second CPP test showed impaired extinction compared to control mice that received saline instead of NaB. Subjects that were conditioned by escalating schedule of cocaine and subsequently received repeated injections of NaB during daily reexposure to nonreinforced context showed either enhancement or no effect on place preference. Acute administration of NaB (1.2g/kg) to naïve mice resulted in marked increase in acetylation of histone H3 lysine 14 (H3K14) and histone H4 lysine 8 (H4K8) in hippocampus but not amygdala. Results suggest that regardless of the scheduling of either cocaine or NaB administration, NaB-induced histone hyperacetylation in the hippocampus may strengthen cocaine-associated contextual memory.
Keywords: histone acetylation, cocaine, conditioned place preference, sodium butyrate
1. Introduction
Increasing evidence support the role of learning and memory in the development of drug addiction. Preclinical and clinical observations suggest that neural substrates and pathways associated with learning and memory are controlled by addictive drugs resulting in persistent drug-seeking behavior and failure to extinguish such maladaptive behavior (Hyman, 2005; Hyman, Malenka & Nestler, 2006; Torregrossa, Corlett & Taylor, 2011). Drug-paired stimuli converge into conditioned stimuli that can induce powerful cravings and precipitate relapse in abstinent drug users (Robinson & Berridge, 1993; Stewart, 2000).
Pavlovian conditioning entails reinforcement learning; pairing of an unconditioned stimulus (US) with a neutral context or cue confers conditioned stimulus (CS) properties to this stimulus. When the US is appetitive, reexposure to the CS elicits approach behavior (e.g., conditioned response). The conditioned place preference (CPP) paradigm has been used extensively to investigate the motivational effects of drugs of abuse. Drugs of abuse act as reinforcers because they influence learning and memory processes (White 1996; White & Milner 1992). Indeed, the face validity of the CPP paradigm lies in that it can model learning and memory processes pertinent to addictive behavior (White & Carr, 1985). These include acquisition, extinction, and reinstatement of drug-induced conditioned response (Itzhak & Martin, 2002; Mueller & Stewart, 2000; Sanchis-Segura & Spanagel, 2006). These behavioral phenotypes are relevant for a) the development of drug-seeking behavior, b) learning to extinguish drug-seeking, and c) the potential for relapse (reinstatement of conditioned behavior).
Both the acquisition and extinction of conditioned response involve associative learning; in the latter, repeated exposure to nonreinforced context elicits extinction learning and the formation of a new memory, e.g., the US does not follow the CS. Extinction learning is relevant to cue exposure therapy which could be useful to control maladaptive behaviors, such as obsessive compulsive disorders (OCD) (Franklin & Foa, 2011), and phobias (de Carvalho, Freire & Nardi, 2010). Pharmacotherapy in conjunction with cue exposure therapy may be particularly useful for extinction of drug-seeking behavior (Kiefer & Dinter, 2013; Myers & Carlezon, 2012). The ultimate goal, however, is not only to accelerate extinction learning but also to afford resistance to the reinstatement of conditioned response to drug and drug-associated cues.
Recent studies underscore the role of histone acetylation in learning and memory (Peixoto & Abel, 2013; Sweatt, 2009). Histones can undergo posttranslational covalent modifications at the N-terminal tails including acetylation, methylation, phosphorylation, ADP-ribosylation, sumoylation and ubiquitination (Shilatifard, 2006). Histone acetylation allows more accessible binding to co-activators and activation of the transcriptional machinery. Several studies have reported the role of histone acetylation in the consolidation of hippocampus-dependent (Guan et al., 2009; Levenson et al., 2004; Vecsey et al., 2007) and lateral amygdala-dependent (Monsey, Ota, Akingbade, Hong & Schafe, 2011) long-term memory (LTM). A recent study demonstrated the role of histone acetylation in the hippocampus-infralimbic structures in extinction of fear memory (Stafford, Raybuck, Raybinin & Lattal, 2012). Using the histone deacetylase (HDAC) inhibitor sodium butyrate (NaB), we recently found that a single/acute administration of the HDAC inhibitor to mice either prior to fear conditioning training or post-conditioning trials (during extinction training) accelerated the extinction of cued fear memory (Itzhak, Anderson, Kelley & Petkov, 2012). This finding suggests that NaB-induced acute histone hyperacetylation may have long-term effects on transcriptional regulation underlying the extinction of fear memory.
The present study was undertaken to investigate the effect of NaB on acquisition and extinction of cocaine-induced CPP in C57BL/6 mice. The major finding is that unlike the accelerating effect of NaB on extinction of fear memory, the HDAC inhibitor hindered the extinction of cocaine-associated contextual memory.
2. Materials and Methods
2.1. Subjects
Male C57BL/6 mice (8 weeks old) were purchased from Jackson Laboratories (Bar Harbor, Maine). Mice were housed in a group of 5 per cage with food and water ad-libitum and were acclimatized to the vivarium for one week before experiments began. Animal care was in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, National Academy Press, 1996) and was approved by the University of Miami Animal Care and Use Committee.
2.2. Conditioning apparatus
Custom-designed Plexiglas cages (42L × 20W × 20H cm; Opto-Max Activity Meter v2.16; Columbus Instruments, Columbus, OH) were used to monitor place preference. The training context consisted of two compartments separated by an opaque removable divider. One side of the divider was black/white striped and the other side was black. One compartment had black and white striped (2.0cm apart) walls and a white floor covered with stainless steel grid; the other compartment had black walls and smooth black floor, thus providing distinct visual and tactile cues. Each compartment was scanned by seven infrared beams at a rate of 10 Hz (2.54 cm intervals). The horizontal sensors were mounted alongside opposing lengths of each compartment. A null zone of 8 cm was assigned at the interface of the two compartments to ensure that only full entry into one compartment or the other was registered as distinct time spent on each side. Time spent in each compartment and locomotor activity were recorded and analyzed by the Opto-max interface and software.
2.3. General conditioning procedure
Conditioning and testing were carried out in a test room separate from the housing room. This room had dimmed lighting provided by a reading lamp, which was placed in one corner of the room facing a wall, and contained two 18-inch F15T8 white fluorescent bulbs, 15 Watts each. On the first day, between 12:00-14:00h, mice were habituated (20min) to the training context; time spent in each compartment was recorded to determine preconditioning compartment-preference/aversion. Using black and white striped compartment instead of a white compartment eliminated biased preference of one compartment over the other, which we previously observed (Itzhak & Anderson, 2012). Accordingly, mice were trained by cocaine in an unbiased design; in each group, half of the subjects were paired with cocaine in the black compartment and the other half were paired in the striped compartment. All experiments comprised of four training days (days 2-5; Fig. 1), two sessions a day: a morning (10:00-12:00h) saline session and an afternoon (14:00-16:00h) cocaine session (30min each). Saline and cocaine were administered intraperitoneally (IP). Because the last conditioning session was drug, and to ensure formation of LTM, mice were first tested for place preference on day 8 (see Fig. 1). Preference for the drug-paired and saline-paired compartments from day 8 and forward (extinction by free exploration sessions) were recorded daily, in the absence of the removable compartment-divider, for 20min, between 12:00 and 14:00h,
Fig. 1.
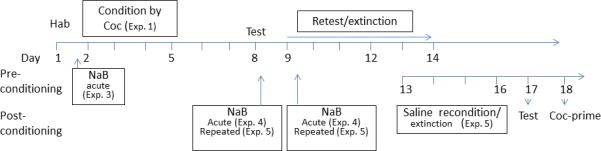
Timeline of the experimental design. Day 1 represents habituation (Hab). From day 2 through 5, mice were trained by various schedules of cocaine. The first conditioned place preference (CPP) test was done on day 8. On subsequent days, 9 through 12 or 14, mice were retested daily. Reexposure to the nonreinforced context was intended to induce extinction (Experiment 1). The effect of NaB on preconditioning by cocaine was determined by administration of the HADC inhibitor on day 2, 60min prior to administration of cocaine (Experiment 3). The effect of NaB on extinction was determined by administration of the HDAC inhibitor acutely either on day 8 or day 9 immediately following a CPP test (Experiment 4), or repeatedly from day 8 through 12 or day 9 through 12 immediately following each retest/extinction session (Experiment 5). Because repeated testing did not induce extinction, some groups underwent extinction by reconditioning with saline from day 13 through 16 mice (Experiment 5). Subsequently, mice were tested on day 17 and on day 18 the effect of priming dose of cocaine was investigated (Experiment 5).
2.4. Experiment 1: Conditioning by different schedules of cocaine
Mice (n=32) were habituated to the conditioning apparatus (day 1) and then conditioned for 4 days by cocaine a) 5mg/kg , b) 15mg/kg, c) 3,6,12 and 24mg/kg; one dose per day, and d) 0mg/kg, which corresponds to a control group. After the test of place preference (day 8), mice were reexposed to the CPP apparatus (20min) for 4 days (days 9-12) in an attempt to induce extinction learning (Fig. 1).
2.5. Experiment 2: Effect of NaB on total H3K14 and H4K8 histone acetylation levels in hippocampus and amygdala
The aim of this experiment was to investigate the effects of a single administration of NaB on H3 and H4 histone acetylation in the hippocampus and amygdala, two substrates that have a major role in acquisition of place preference (Ferbinteanu & McDonald, 2001; Hiroi & White, 1991; Meyers, Zavala & Neisewander, 2003). Mice (n=5/group) received a single intraperitoneal (IP) injection of either saline (0.9% NaCl) or NaB (1.2g/kg). The dose of NaB was based on studies by Levenson et al. (2004) and our previous studies (Itzhak et al. 2012); lower dose of NaB had no detectable effect on hippocampal H3/H4 histone acetylation as determined by ELISA kits (described below). Sixty minutes after the administration of NaB mice were sacrificed by cervical dislocation and hippocampus and amygdala were freshly dissected, snap-frozen on dry ice and stored at -80°C. Histone extractions were prepared as follows. Tissue was resuspended in 5 volumes of Triton extraction buffer (TEB) which comprised phosphate buffered saline (PBS) containing Triton X-100 (0.5% v/v), phenylmethylsulfonyl fluoride (2mM; PMSF), and sodium azide (0.02% w/v; NaN3). The suspension was rocked for 10min (4°C) and centrifuged (10,000 × g; 10 min; 4°C) to spin down the nuclei. The supernatant was discarded and the nuclei were washed with half of the volume of TEB as before and again centrifuged. The pellet was resuspended in 2 volumes of HCl (0.2N) and incubated overnight (4°C) to extract the histone fraction. On the following day, the mixture was centrifuged (14,000 × g; 10 min; 4°C); the supernatant was collected and neutralized by a balance buffer containing DTT (Epigentek). Aliquots were taken for determination of protein concentration and the rest was stored at -80°C.
H3K14 and H4K8 acetylation assays were performed according to directions of ELISA kits (Active Motif; CA; Epigentek, NY). Essentially, histone extracts were mixed with kit-provided sample buffer and loaded (amygdala, 120μg/ml; hippocampus, 60μg/ml) into a 96-well plate that had wells coated with capture antibody. The plate was incubated overnight at 4°C, with gentle rocking. On the following day, wells were washed 4 times with kit-provided wash buffer; the final wash was aspirated out. Histone detection antibody was added, and the plate was incubated for 1h at 37°C. Thereafter the plate was again washed 4 times, and a horseradish peroxidase (HRP) conjugated secondary antibody was added. Following a 30min incubation (37°C), the plate was washed as before, and TMB substrate (3,3′, 5,5′-tetramethylbenzidine) was added. The reaction was allowed to develop for 30min at room temperature, after which kit-provided stop solution was added. Sample absorbance was then determined via a spectrophotometer (450nm).
2.6. Experiment 3: Effect of pretraining NaB on acquisition and extinction of cocaine-induced CPP Conditioning by 5mg/kg cocaine
Following habituation (day 1), on day 2, mice received either saline (n=10) or NaB (1.2g/kg; n=8) 60min prior to administration of the first dose of cocaine (5mg/kg). Although we did not investigate the pharmacokinetics of NaB, the timing of administration was based on results of H3 and H4 histone acetylation (Experiment 2) and our previous studies on the effect of NaB on fear conditioning (Itzhak et al., 2012). On subsequent days (3-5) saline and NaB injections before cocaine injections were omitted. Following the test day (day 8) mice were reexposed to the training context (20min/day) for 6 days in order to determine the extinction rate (Fig. 1). We did not investigate the effects of multiple injections of NaB on conditioning by cocaine because results suggested that a single administration of NaB was sufficient to a) increase expression of cocaine CPP induced by 5 mg/kg cocaine and b) impair subsequent extinction of place preference (Fig. 4A). Likewise our previous study had shown that a single administration of NaB prior to fear conditioning had subsequent long-term effect on extinction of fear memory (Itzhak et al. 2012).
Fig. 4.
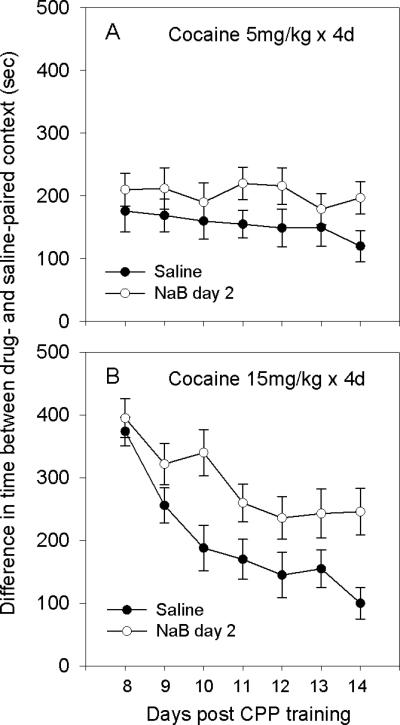
Acute administration of NaB prior to conditioning by cocaine inhibits subsequent extinction of place preference. A. Groups conditioned by 5mg/kg cocaine showed a significant group effect F=[1,14]=5.276; p<0.05, but no significant time effect (p=0.417). B. Groups conditioned by 15mg/kg cocaine showed a significant group effect F[1,18]=4.87; p<0.05, a significant time effect F=5.15; p<0.01 and a significant interaction F=3.29; p<0.05.
Conditioning by 15mg/kg cocaine
Following habituation (day 1), on day 2, mice received either saline (n=8) or NaB (1.2g/kg; n=10) 60min prior to administration of the first dose of cocaine (15mg/kg). Subsequently mice were tested as described for the 5mg/kg conditioning experiment.
2.7. Experiment 4: Effect of a single administration of NaB on extinction of place preference which was acquired by a fixed daily dose of cocaine
The goal of this experiment was to investigate whether a single administration of NaB, accelerates extinction learning. Mice were conditioned by cocaine (15mg/kg ×4 days; n=32). On day 8, mice were tested for CPP and randomly assigned to four groups. One group received saline (n=8) and a second group received NaB (1.2g/kg; n=8) immediately after the first CPP test on day 8. The other two groups received saline (n=8) or NaB (1.2g/kg, n=8) on day 9 immediately following the second CPP test (Fig. 1). NaB was administered immediately post-test day 8 or 9 in order to facilitate context-no drug memory consolidation. Mice were then repeatedly re-exposed to the context for a total of 7 days and time spent in each compartment was recorded.
2.8. Experiment 5: Effect of repeated administration of NaB on extinction of place preference which was acquired by escalating dose of cocaine
The goal of this experiment was two-fold: a) to determine whether place preference acquired by escalating doses of cocaine is modulated by NaB, and b) to investigate the effect of repeated administration of NaB (as opposed to a single administration). Mice (n=24) were conditioned by escalating doses of cocaine (3,6,12 and 24mg/kg), which induces stable long-term response to cocaine-associated context (Itzhak & Anderson, 2012). Following the first CPP test on day 8, mice were randomly assigned to 3 groups (n=8/group): groups 1 and 2 received saline or NaB (1.2g/kg) immediately after the first CPP test (day 8) and then again daily immediately after each test, through day 12; group 3, received NaB (1.2g/kg) from day 9 through 12. Because repeated exposure to the nonreinforced context did not result in extinction (Fig. 6), mice were subjected to a more robust extinction protocol where they were reconditioned by saline for 4 days (two daily sessions, 30min each); the latter is also known as extinction by confinement. One day after a CPP test (which showed extinction) reinstatement of place preference was tested after cocaine priming (11.25mg/kg; average of the daily dose during conditioning) (see Fig. 1 for the timeline).
Fig. 6.
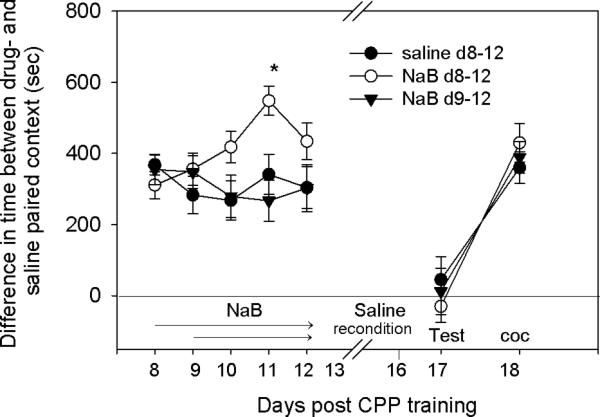
Effect of repeated administration of NaB on extinction of CPP that was acquired following conditioning by escalating dose of cocaine. Mice were conditioned by escalating doses of cocaine (3,6,12 and 24mg/kg). Two groups received saline and NaB from day 8 through 12, and an additional group received NaB from day 9 through 12 immediately following each test session. Because repeated exposure to the nonreinforced context did not cause inhibition of place preference, mice were reconditioned by saline for 4 days (days 13-16). A test on day 17 showed the loss of place preference. Reinstatement of place preference was determined by cocaine priming (11.25mg/kg) on day 18. CPP was equally reinstated in all groups. Two-way ANOVA, group (saline, NaB d8-12 and NaB d9-12) × training (context re-exposure, saline reconditioning, reinstatement) resulted in a significant group effect F[2,147]=4.97; p<0.01, a significant training effect F[6,147]=26.22; p<0.001. Bonferroni post hoc analysis showed significant difference between saline and the NaB d8-12 group (p=0.015) and between NaB d8-12 and NaB d9-12 (P=0.017); no significant difference was observed between saline and NaB d9-12 groups.
2.9. Statistical analyses
Results of conditioned place preference, including experiments 1, 3 and 4, were analyzed by two-way repeated measures ANOVA: group as between subjects effect and time as within subjects effect. Significance of within subjects effect (time), and interaction between group × time were analyzed by Greenhouse-Geisser test. When the group effect was significant multiple comparisons was done by post hoc Bonferroni test. Results of Experiment 5 were analyzed by two-way ANOVA: group (saline; NaB d8-12; NaB d9-12) × training (context reexposure; saline reconditioning; reinstatement), followed by Bonferroni multiple comparison test. Results of acetylation levels of H3K14 and H4K8 were analyzed by unpaired student's t-test. A p value less than 0.05 was considered significant.
3 Results
3.1. Experiment 1: Conditioning by different schedules of cocaine
The goal of this experiment was to investigate the magnitude and persistence of CPP induced by various schedules of cocaine. Four groups of mice were conditioned by a) 0 × 4; b) 5 × 4; c) 15 × 4; and d) 3,6,12,24 mg/kg cocaine. After 72h, mice were tested for place preference, daily from day 8 through 12. Results analyzed by two-way repeated measures ANOVA resulted in significant group effect F[3,28]=9.564; p<0.001, a significant time effect F=4.74; p=0.003, and a significant interaction F=2.876; p=0.004. Specifically, control group was significantly different from all drug groups (p<0.05) suggesting that the lowest dose of cocaine (5mg/kg × 4 days) resulted in significant CPP (Fig. 2). The latter group was significantly lower than the escalating dose group (p=0.014). The differences between 5mg/kg and 15mg/kg (× 4 days) were not statistically significant. In addition, although the differences between the escalating dose group and the 15mg/kg dose group were not statistically significant (Fig. 2), the former group showed relatively stable CPP over time which we considered “resistant to extinction” by free exploration (Itzhak & Anderson, 2012).
Fig. 2.
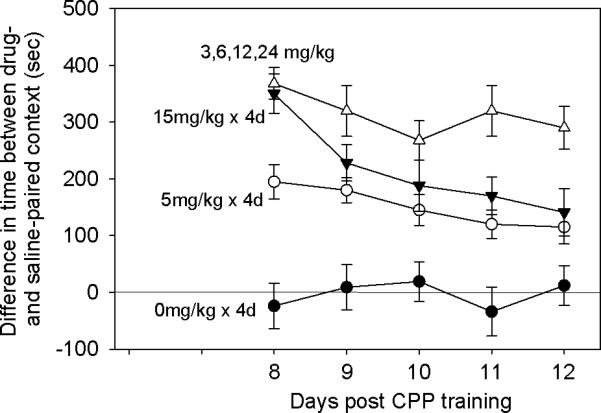
Different dose schedules of cocaine result in distinct profile of CPP expression over time. Mice were conditioned by different schedules of cocaine or saline (control). Data represent the magnitude of CPP from day 8 (first test) through 12. Two-way repeated measures ANOVA resulted in significant group effect F[3,28]=9.564; p<0.001, a significant time effect F=4.74; p=0.003, and a significant interaction F=2.876; p=0.004. Control group (0mg/kg) was significantly different from all drug groups (p<0.05). The 5mg/kg group was significantly lower than the escalating dose (3,6,12, 24mg/kg) group (p=0.014). The differences between 5mg/kg and 15mg/kg (× 4 days) and the difference between15mg/kg × 4 days group and the escalating dose group were not statistically significant.
3.2. Experiment 2: Effect of NaB on total H3K14 and H4K8 histone acetylation levels in hippocampus and amygdala
This experiment investigated the effects of a single administration of NaB on H3 and H4 histone acetylation in the hippocampus and amygdala. H3K14 acetylation in the hippocampus was increased by 31% of control (saline; p<0.05); no effect was observed in the amygdala (Fig. 3A). H4K8 acetylation in the hippocampus was increased by 180% of control (saline; p<0.001); no effect was observed in the amygdala (Fig. 3B).
Fig. 3.
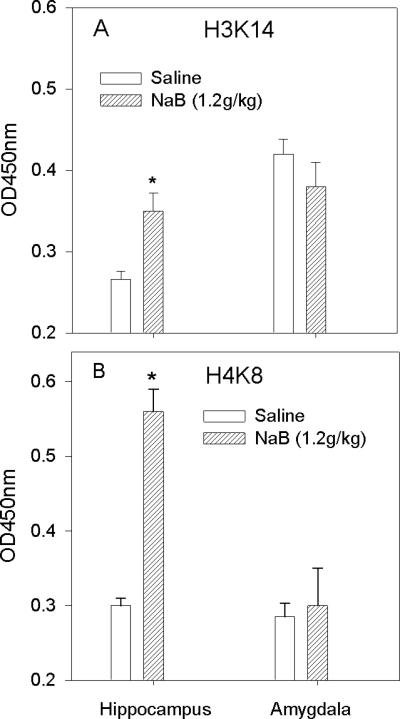
Histone acetylation following acute administration of NaB to naïve mice. Acetylation of H3 lysine 14 and H4 lysine 8 were determined 60min after the administration of NaB (1.2g/kg). A. H3K14 acetylation in the hippocampus was increased by 31% of control (saline; p<0.05); no effect was observed in the amygdala. B. H4K8 acetylation in the hippocampus was increased by 180% of control (saline; p<0.001); no effect was observed in the amygdala.
3.3. Experiment 3: Effect of pretraining NaB on acquisition and extinction of cocaine-induced CPP
The goal of this experiment was to investigate the effect of NaB on acquisition and subsequent extinction of place preference induced by 5 and 15mg/kg cocaine. Fig. 4A depicts the effect of a single administration of NaB prior to conditioning by 5mg/kg cocaine. A two-way repeated measures ANOVA showed a significant group effect F[1,14]=5.276; p<0.05 but no significant time effect (p=0.417) and no significant interaction (group × time; p=0.88), suggesting that NaB increased expression of CPP, and there was no significant extinction. Fig. 4B depicts the effect of NaB on acquisition and persistence of CPP induced by 15mg/kg cocaine. There was a significant group effect F[1,18]=4.87; p<0.05, a significant time effect F=5.15; p<0.01 and a significant interaction F=3.29; p<0.05. Results suggest that acute administration of NaB had long-lasting effect on strengthening expression of CPP, or inhibiting the decay (extinction) of place preference following repeated exposure to nonreinforced context. Locomotor activity was recorded throughout the experiments. Results showed no significant differences between locomotor activity of the NaB/cocaine group and saline/cocaine group, either during conditioning or extinction sessions. These findings suggest that CPP was not influenced by motor behavior.
3.4. Experiment 4: Effect of a single administration of NaB on extinction of place preference which was acquired by a fixed daily dose of cocaine
The goal of this experiment was to investigate whether a single administration of NaB accelerates extinction of CPP which was acquired by a fixed daily dose of cocaine (15mg/kg × 4). Administration of NaB on day 8, immediately after the first CPP test, resulted in subsequent impairment in the decay of CPP compared to control (saline) group (Fig. 5A). There was a significant group effect F[1,14]=5.61; p<0.05, a significant time effect F=10.64; p<0.001 and no significant interaction.
Fig. 5.
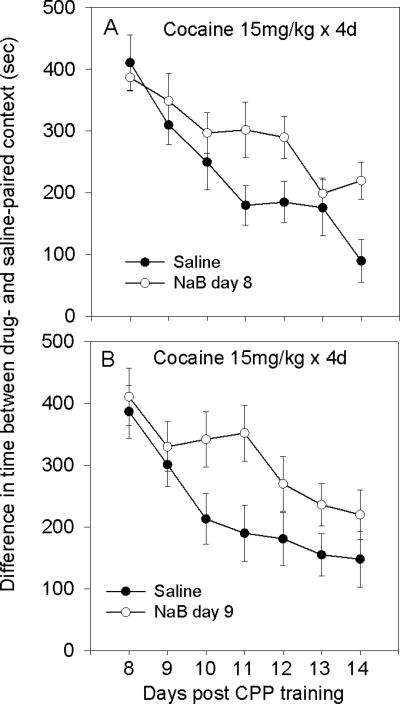
Acute administration of NaB following the first or the second CPP tests inhibits subsequent extinction of place preference that was acquired by a fixed daily dose of cocaine. Mice were conditioned by 15mg/kg cocaine. A. There was a significant difference in the expression of CPP over time between groups that received saline and NaB on day 8 immediately after the first CPP test: group effect F[1,14]=5.61; p<0.05, and time effect F=10.64; p<0.001. B. Results of NaB and saline administration on day 9 showed a similar trend. There was a significant group effect F[1,14]=5.26; p<0.05 and a significant time effect F=5.189; p<0.01.
The effect of administration of NaB after the second CPP test (day 9) was investigated in two additional groups that were conditioned by cocaine (15mg/kg × 4). Administration of NaB immediately after the second CPP test on day 9 also impaired subsequent decline of CPP compared to control group that received saline instead of NaB (Fig. 5B). There was a significant group effect F[1,14]=5.26; p<0.05 and a significant time effect F=5.189; p<0.01 and no significant interaction. Results suggest that a single administration of NaB following either the first (day 8) or the second (day 9) CPP test inhibited the extinction of place preference compared to control.
3.5. Experiment 5: Effect of repeated administration of NaB on extinction of place preference which was acquired by escalating dose of cocaine
As depicted in Fig. 2, conditioning by escalating doses of cocaine (3,6,12,24mg/kg), followed by daily reexposure to the CPP context, resulted in a relatively extinction-resistant place preference compared to conditioning by a fixed daily dose (15mg/kg). Note that the fixed daily dose was higher than the average daily dose of the escalating schedule (11.25mg/kg). The goals of this experiment were to investigate a) whether NaB influences extinction of CPP induced by escalating doses of cocaine and b) whether repeated administration of NaB influences extinction. After training by the escalating dose of cocaine (3,6,12 and 24mg/kg) mice were randomly assigned to three groups. Groups I and II received saline or NaB (1.2g/kg) daily, from day 8 through 12, immediately after each CPP test. Group III received NaB (1.2g/kg) from day 9 through 12, immediately after each CPP test. Because repeated exposure to the nonreinforced context did not cause inhibition of place preference (Fig. 6), mice were subjected to extinction by reconditioning with saline for four days (two daily sessions). As depicted in Fig. 6, a test on day 17 showed the loss of place preference, suggesting that reconditioning by saline induced extinction. To determine susceptibility to reinstatement of place preference, cocaine priming (11.25mg/kg; average of the daily dose during conditioning) was administered. Place preference was reinstated in all three groups, regardless of NaB administration (Fig. 6). Results of this experiments were analyzed by two-way ANOVA, group (saline, NaB d8-12 and NaB d9-12) × training (context re-exposure, saline reconditioning, reinstatement). There was a significant group effect F[2,147]=4.97; p<0.01, and a significant training effect F[6,147]=26.22; p<0.001. Bonferroni post hoc analysis showed significant differences between saline and the NaB d8-12 group (p=0.015) and between NaB d8-12 and NaB d9-12 (P=0.017); no significant difference was observed between saline and NaB d9-12 groups (Fig. 6). The differences between the groups suggest that administration of NaB from day 8 through 12, but not day 9 through 12, increased expression of CPP (Fig. 6).
4. Discussion
Recent studies suggest that regulation of chromatin structure is one of the essential molecular mechanisms that contribute to the formation of synaptic plasticity and LTM (Peixoto & Abel, 2013; Sweatt, 2009). One of the regulatory processes of chromatin structure is the acetylation and deacetylation of histone proteins. Histone acetyltransferases (HAT) acetylate conserved lysine amino acids on histone proteins by transferring an acetyl group from acetyl CoA to form ε-N-acetyl lysine.
Acetylation brings in a negative charge, which neutralizes the positive charge on the histones, and decreases the interaction of the N termini of histones with the negatively charged phosphate groups of DNA. As a result, the condensed chromatin is transformed into a more relaxed structure which is associated with greater levels of gene transcription (Jenuwein & Allis, 2001). HDACs are classes of enzymes that remove acetyl groups, increasing the positive charge of histone tails and the binding between histones and DNA. The increased DNA binding condenses DNA structure and prevents transcription.
Several HDAC inhibitors such as NaB, valproic acid, and trichostatin have been shown to increase acquisition and extinction of LTM (Bredy et al., 2007; Lattal, Barrett & Wood, 2007; Levenson et al., 2004; Vecsey et al., 2007; Wood, Attner, Oliveira, Brindle & Abel, 2006; Yeh, Lin & Gean, 2004). Our recent studies have shown that a single administration of NaB (1.2g/kg) a) rescued nitric oxide (NO)-dependent deficits in contextual fear memory and b) afforded a long-term accelerating effect on extinction of fear memory of wild type mice (Itzhak et al., 2012). Thus, the major goal of the present study was to investigate whether NaB would facilitate the extinction of cocaine-associated contextual memory. Our findings suggest that regardless of the scheduling of either cocaine or NaB administration, the HDAC inhibitor strengthens cocaine-associated contextual memory. Thus, unlike the facilitatory effect of NaB on the extinction of cued fear memory, the HDAC inhibitor did not accelerate extinction of cocaine-associated contextual memory.
The role of histone acetylation in the effects of cocaine-induced plasticity has been recently reviewed (Rogge & Wood, 2013). Chronic cocaine administration to rats resulted in increase in H3 histone acetylation in gene promoters of FosB, Cdk5 and BDNF in the striatum (Kumar et al. 2005). The authors also reported that NaB increased cocaine-induced CPP and locomotor sensitization (Kumar et al. 2005). Relatively low dose of NaB (150mg/kg) administered daily along with morphine or ethanol potentiated both CPP and locomotor sensitization induced by these drugs (Sanchis-Segura, Lopez-Atalaya & Barco, 2009). Consistent with the hypothesis that increased histone acetylation potentiates the effects of psychostimulants is the finding that mice deficient in CREB binding protein (CBP), which is a HAT, showed reduced histone acetylation on the FosB promoter and reduced sensitivity to cocaine (Levin et al. 2005; Malvaez, Mhillaj, Matheo, Palmery & Wood, 2011). It is thought that an increase in histone acetylation is associated with sensitization to various emotional and environmental stimuli such as chronic social defeat, cardiac stress, and pain (Renthal & Nestler, 2008).
The findings of the present study that administration of NaB prior to conditioning by cocaine a) increased expression of CPP induced by a low dose of cocaine (Fig. 4A) and b) impaired extinction of CPP induced by 15mg/kg cocaine support the observations that an increase in hippocampal histone acetylation (Fig. 3) may potentiate cocaine-induced behaviors. It should be noted, however, that we measured NaB-induced histone acetylation in naïve mice, and therefore we cannot make predictions about the status of histone acetylation in combination with cocaine administration (Experiment 3). Nevertheless, the finding that NaB had similar effects on cocaine CPP whether it was administered before training by cocaine (Experiment 3) or during extinction sessions (Experiments 4 and 5), suggests strengthening of drug-context association (spatial memory). Also, given the role of the hippocampus in CPP (Hiroi & White, 1991; Meyers et al., 2003), we assume that NaB-induced histone acetylation in the hippocampus is relevant to the modulation of cocaine CPP.
The novelty of the present findings also lies in the outcome that a single administration of the HDAC inhibitor had long-term effects on the expression of CPP. This discovery suggests that acute hyperacetylation resulted in long-term transcriptional effects which strengthen cocaine-associated contextual memory. It is unlikely that a single administration of NaB before the cocaine training session had a motivational effect that enhanced cocaine reward because administration of NaB in nonreinforced context (test and extinction sessions) also enhanced CPP.
As shown in Fig. 3, NaB-induced increases in H3K14 and H3K8 acetylation were observed in the hippocampus but not amygdala. The reason for that is unclear, but it could be that the method of detection we used (ELISA kit) was not sensitive enough to identify changes in acetylation in the amygdala. Alternatively, in the amygdala, NaB may acetylate other lysine residues on histone tails. In Aplysia, LTM synaptic plasticity was associated with increased binding of CBP to the promoter region of C/EBP (downstream of CREB) with concurrent acetylation of H3K14 and H4K8 and increased transcription of C/EBP (Guan et al. 2002). H4K8 acetylation mediates the recruitment of the SWI/SNF remodeling complex (Agalioti, Chen, & Thanos, 2002). This complex possesses a DNA-stimulated ATPase activity that destabilizes DNA-histones interactions, which increases transcription. H3K14 acetylation recruits transcription factor II D (TFIID) (Agalioti et al. 2002), which is one of several general transcription factors. Both H4K8 and H3K14 acetylation are required for activation of inteferon (IFN)-β gene (Agalioti et al.2002). IFN-β-1b and -1a caused cognitive improvement in multiple sclerosis patients (Amato et al. 2012), suggesting their role in learning and memory. H3K14 acetylation and H3 histone phosphorylation are regulated by the ERK/MAPK pathway in the hippocampus following fear conditioning (Chwang, Arthur, Schumacher, & Sweatt 2007) and object and space learning (Koshibu et al. 2009), suggesting their role in LTM. Together, these findings suggest that NaB-induced hyperacetylation of H3K14 and H4K8 we observed in the hippocampus could be relevant to strengthening drug-context associative learning.
Results of the present study further show that acute administration of NaB on day 8 or 9 (after the first and second CPP tests) also impaired the extinction of CPP induced by 15mg/kg cocaine. The effect of NaB after the first CPP test (day 8) may be explained as strengthening the reconsolidation of cocaine-associate memory because the first retrieval/reactivation of drug-memory occurred on day 8. However, on day 9, when the second exposure to the context occurred, extinction learning should have ensued, and NaB should have facilitated extinction learning. However, as depicted in Fig. 5B, extinction was impaired. It could be that after the second retrieval of cocaine-associated memory (day 9) reconsolidation rather than extinction transpired. We have previously found that cocaine-associated contextual memory can be manipulated by various drugs when administered during the second CPP test, i.e., second retrieval/reactivation of drug-memory (Kelley, Anderson & Itzhak, 2007). Also, results of that study suggested there is a time window where competition between reconsolidation and extinction learning may occur (Kelley et al. 2007).
Previous studies in mice, which acquired cocaine CPP, have shown that repeated administration of NaB (1.2g/kg) following the first CPP test and then daily thereafter for several days accelerated extinction of place preference (Malvaez, Sanchis-Segura, Vo, Lattal & Wood, 2010). Similar results were observed in the extinction of morphine-induced CPP (Wang, Zhang, Qing, Liu & Yang, 2010). In our studies described above (using 15mg/kg cocaine) we did not investigate multiple NaB injections because a) tests after the single administration of NaB showed increase in CPP expression (Fig. 5) and b) in our fear conditioning studies a single administration of NaB was sufficient to accelerate fear extinction (Itzhak et al. 2012). Nevertheless, we sought to determine whether repeated injections of NaB may accelerate the extinction of CPP induced by escalating doses of cocaine.
Conditioning by escalating doses of cocaine (3,6,12 & 24mg/kg) resulted in relatively stable expression of place preference over time (Fig. 2 and Itzhak & Anderson, 2012). We proposed (Itzhak & Anderson, 2012) that strengthening of drug-associated memory following conditioning by escalating dose of cocaine may be related to the theory that differences between predication and outcome (e.g., “surprise element”) (Rescola & Wagner, 1972) and prediction error (Schultz, 2007) are necessary for strengthening formation of long-term memory. We have tested several phosphodiesterase (PDE) inhibitors in an attempt to extinguish CPP induced by an escalating regimen of cocaine and found that only the PDE9 inhibitor BAY-73-6691 which selectively increases cGMP levels in hippocampus and amygdala accelerated extinction of cocaine CPP (Liddie, Anderson, Paz & Itzhak, 2012). In the present study mice conditioned by an escalating schedule of cocaine, began receiving NaB either after the first CPP test (day 8) or after the second test (day 9) and subsequently, daily for three additional days. The daily tests revealed that administration of the HDAC inhibitor from day 8-12 markedly increased CPP expression, and the administration of NaB from day 9-12 had no effect on CPP expression compared to control (Fig. 6). Hence, repeated administration of NaB did not accelerate extinction. The lack of extinction following repeated injections of NaB cannot be explained by a) inappropriate temporal administration of the HDAC inhibitor or b) conditioning by escalating schedule of cocaine, which is resistant to extinction. In a previous study when mice were conditioned by the same escalating schedule of cocaine (3,6,12 & 24mg/kg) and then began to receive the PDE9 inhibitor after the second CPP test (as described for NaB in Fig. 6), extinction of CPP was achieved, and reinstatement by cocaine priming was attenuated (Liddie et al., 2012).
The marked increase in CPP in the group that received NaB from day 8 through 12 may be due, again, to strengthening of reconsolidation of cocaine-associated memory following its first retrieval. The lack of effect of delayed administration of NaB, from day 9 through 12, may be due the formation of an already stable memory, which is insensitive to NaB-induced histone acetylation. This latter finding, however, seems to be in contrast to the increase in CPP expression observed in mice that have been conditioned by 15mg/kg cocaine and received NaB on day 9 (Fig. 5B). The difference, however, could be due to the strength of cocaine-associated memory; conditioning by 15mg/kg cocaine, may result in more labile drug-associated memory, which remains sensitive to NaB-induced histone acetylation longer than that acquired by the escalating schedule of cocaine. This hypothesis is supported by the differences in the persistence of CPP in the 15mg/kg and escalating-dose groups; the decay of CPP in the 15mg/kg group was faster than the escalating-dose group (Fig. 2).
The finding of increased CPP expression following repeated administration of NaB from day 8 through 12 is different from that reported by Malvaez et al. (2010) who described acceleration of extinction. The difference could be due to the schedule of cocaine administration; Malvaez et al. conditioned C57BL/6 mice by 20mg/kg cocaine, while we conditioned the C57BL/6 mice by escalating schedule of cocaine. Nevertheless, as described above, we observed an increase in the expression of CPP, rather than extinction, even when mice were conditioned by 15mg/kg cocaine.
A very recent study, however, showed that a selective HDAC3 inhibitor (RGFP966) enhanced extinction of cocaine-induced CPP (Malvaez et al. 2013). NaB has similar high affinity to HDAC1, HDAC2, HDAC3 and HDAC8, which belong to Class I HDAC (Kilgore et al., 2010). Thus, it is possible that the nonselective inhibition of HDACs by NaB, as opposed to selective inhibition of HDAC3, results in different transcription regulation activity and consequently different behavioral phenotypes.
Our findings thus far suggest that administration of NaB pre- or post-training by cocaine resulted in enhancement in place preference expression (days 8 through 12 or 14). It appears that the conditioning schedule may influence the mechanisms involved in the observed enhancement in place preference during extinction sessions. When conditioned by a fixed dose schedule, NaB seems to impair extinction (Fig. 4 & 5). Previous data from our lab has shown that following conditioning by fixed doses, the magnitude of place preference is maintained for >7 days if mice are not subject to extinction training (unpublished observations). This lends credence to the observation that NaB impaired extinction since control mice that underwent extinction training showed reduced magnitude of place preference over time. However, this impairment should not be viewed as impairment of new learning, e.g., extinction learning, but rather as the formation of a stable LTM which is more resistant to extinction. Acute NaB administration prior to conditioning by fixed dose of cocaine (day 2) resulted in the same effect (Fig. 4) as NaB administration post-conditioning (days 8 or 9; Fig. 5); it is unlikely that acute administration of NaB on day 2 (pre-conditioning) impaired extinction learning on subsequent days 8-14. Also, following conditioning by escalating dose of cocaine, repeated administration of NaB appears to enhance drug memory reconsolidation because the magnitude of place preference was higher compared to controls following subsequent extinction tests (Fig. 6). Because conditioning by escalating dose of cocaine did not result in extinction following free exploration of the conditioning apparatus, we performed extinction by confinement following saline administration. The two NaB groups as well as the control group equally extinguished place preference (Fig. 6), suggesting that the preceding NaB treatment had no effect on extinction learning by confinement.
In addition, cocaine priming following extinction by confinement resulted in similar level of place preference reinstatement, regardless of the previous treatments with NaB (Fig. 6), suggesting that NaB had no protective effect against reinstatement.
In summary, our results show that NaB-induced acute histone acetylation had long-term effect on strengthening cocaine-associated contextual memory. The temporal requirement for the solidification of drug-associated memory appears to be either shortly before (1h) training or 72 and 96h after the 4-day training period. The inhibition of extinction as a result of administration of NaB before CPP training may be due to strengthening LTM of drug-context association. On the other hand, inhibition of extinction as a result of administration of NaB in a nonreinforced context, following the CPP tests, may be due to strengthening of memory reconsolidation. The lack of effect of NaB on a group that was preconditioned by an escalating regimen of cocaine may be due to the formation of a non-labile (stable) LTM that is resistant to NaB effect. Overall, the amplification of cocaine-induced CPP by NaB is in agreement with several studies suggesting synergistic result of histone acetylation and the effects of drug of abuse. While NaB did not accelerate extinction of conditioned response to contextual appetitive CS, the HDAC inhibitor successfully accelerated extinction of conditioned freezing response to auditory-cued aversive CS (Itzhak et al. 2012). These findings suggest that different mechanisms may underlie the extinction of a) appetitive and aversive memory and b) contextual (place preference) and auditory-cued memory; further studies are necessary to elucidate these differences.
Highlight.
Na-Butyrate (NaB) increased histone acetylation in mouse hippocampus but not amygdala
Acute NaB injection prior to cocaine-induced place learning suppressed extinction
Acute NaB suppressed extinction also when given during place preference testing
Repeated NaB administration increased cocaine place preference
The HDAC inhibitor persistently strengthened cocaine-associated contextual memory
Acknowledgments
This work was supported by grant RO1DA026878 and R21DA029404 from the National Institute on Drug Abuse, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agalioti T, Chen G, Thanos D. Deciphering the transcriptional histone acetylation code for a human gene. Cell. 2002;111:381–392. doi: 10.1016/s0092-8674(02)01077-2. [DOI] [PubMed] [Google Scholar]
- Amato MP, Langdon D, Montalban X, Benedict RH, Deluca J, Krupp LB, et al. Treatment of cognitive impairment in multiple sclerosis: position paper. Journal of Neurology. 2012 Nov 23; doi: 10.1007/s00415-012-6678-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Bredy TW, Wu H, Crego C, Zellhoefer J, Sun YE, Barad M. Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learning & Memory. 2007;4:268–276. doi: 10.1101/lm.500907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chwang WB, Arthur JS, Schumacher A, Sweatt JD. The nuclear kinase mitogen- and stress-activated protein kinase 1 regulates hippocampal chromatin remodeling in memory formation. The Journal of Neuroscience. 2007;27:12732–12742. doi: 10.1523/JNEUROSCI.2522-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho MR, Freire RC, Nardi AE. Virtual reality as a mechanism for exposure therapy. World Journal of Biological Psychiatry. 2010;11(2 pt 2):220–230. doi: 10.3109/15622970802575985. [DOI] [PubMed] [Google Scholar]
- Ferbinteanu J, McDonald RJ. Dorsal/ventral hippocampus, fornix, and conditioned place preference. Hippocampus. 2001;11:187–200. doi: 10.1002/hipo.1036. [DOI] [PubMed] [Google Scholar]
- Franklin ME, Foa EB. Treatment of obsessive compulsive disorderes. Annual Reviews in Clinical Psychology. 2011;7:229–243. doi: 10.1146/annurev-clinpsy-032210-104533. [DOI] [PubMed] [Google Scholar]
- Guan Z, Giustetto M, Lomvardas S, Kim JH, Miniaci MC, Schwartz JH, et al. Integration of long-term-memory-related synaptic plasticity involves bidirectional regulation of gene expression and chromatin structure. Cell. 2002;111:483–493. doi: 10.1016/s0092-8674(02)01074-7. [DOI] [PubMed] [Google Scholar]
- Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459:55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi N, White NM. The lateral nucleus of the amygdala mediates expression of the amphetamine-produced conditioned place preference. Journal of Neuroscience. 1991;11:2107–2016. doi: 10.1523/JNEUROSCI.11-07-02107.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE. Addiction: a disease of learning and memory. American Journal of Psychiatry. 2005;162:1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annual Reviews in Neuroscience. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Anderson KL. Changes in the magnitude of drug-unconditioned stimulus during conditioning modulate cocaine-induced place preference in mice. Addiction Biology. 2012;17:706–716. doi: 10.1111/j.1369-1600.2011.00334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhak Y, Martin JL. Cocaine-induced conditioned place preference in mice: induction, extinction and reinstatement by related psychostimulants. Neuropsychopharmacology. 2002;26:130–134. doi: 10.1016/S0893-133X(01)00303-7. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Anderson KL, Kelley JB, Petkov M. Histone acetylation rescues contextual fear conditioning in nNOS KO mice and accelerates extinction of cued fear conditioning in wild type mice. Neurobiology of Learning and Memory. 2012;97:409–417. doi: 10.1016/j.nlm.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- Kelley JB, Anderson KL, Itzhak Y. Long-term memory of cocaine-associated context: disruption and reinstatement. Neuroreport. 2007;18:777–780. doi: 10.1097/WNR.0b013e3280c1e2e7. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Dibter C. New Approaches to addiction treatment based on learning and memory. Current Topics in Behavioral Neuroscience. 2013;13:671–684. doi: 10.1007/7854_2011_147. [DOI] [PubMed] [Google Scholar]
- Kilgore M, Miller CA, Fass DM, Hennig KM, Haggarty SJ, Sweatt JD, et al. Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of Alzheimer's disease. Neuropsychopharmacology. 2010;35:870–880. doi: 10.1038/npp.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshibu K, Gräff J, Beullens M, Heitz FD, Berchtold D, Russig H, et al. Protein phosphatase 1 regulates the histone code for long-term memory. The Journal of Neuroscience. 2009;29:13079–13089. doi: 10.1523/JNEUROSCI.3610-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;4:303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Lattal KM, Barrett RM, Wood MA. Systemic or intrahippocampal delivery of histone deacetylase inhibitors facilitates fear extinction. Behavioral Neuroscience. 2007;121:1125–1131. doi: 10.1037/0735-7044.121.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson JM, O'Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD. Regulation of histone acetylation during memory formation in the hippocampus. The Journal of Biological Chemistry. 2004;279:40545–40559. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- Levine AA, Guan Z, Barco A, Xu S, Kandel ER, Schwartz JH. CREB-binding protein controls response to cocaine by acetylating histones at the fosB promoter in the mouse striatum. Proceeding of the National Academy of Science USA. 2005;102:19186–19191. doi: 10.1073/pnas.0509735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddie S, Anderson KL, Paz A, Itzhak Y. The effect of phosphodiesterase inhibitors on the extinction of cocaine-induced conditioned place preference in mice. Journal of Psychopharmacology. 2012;26:1375–1382. doi: 10.1177/0269881112447991. [DOI] [PubMed] [Google Scholar]
- Malvaez M, McQuown SC, Rogge GA, Astarabadi M, Jacques V, Carreiro S, et al. HDAC3-selective inhibitor enhances extinction of cocaine-seeking behavior in a persistent manner. Proceedings of the National Academy of Science, USA. 2013 Jan 7; doi: 10.1073/pnas.1213364110. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvaez M, Mhillaj E, Matheos DP, Palmery M, Wood MA. CBP in the nucleus accumbens regulates cocaine-induced histone acetylation and is critical for cocaine-associated behaviors. The Journal of Neuroscience. 2011;31:16941–16948. doi: 10.1523/JNEUROSCI.2747-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvaez M, Sanchis-Segura C, Vo D, Lattal KM, Wood MA. Modulation of chromatin modification facilitates extinction of cocaine-induced conditioned place preference. Biological Psychiatry. 2010;67:36–43. doi: 10.1016/j.biopsych.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers RA, Zavala AR, Neisewander JL. Dorsal, but not ventral, hippocampal lesions disrupt cocaine place conditioning. Neuroreport. 2003;14:2127–2131. doi: 10.1097/00001756-200311140-00023. [DOI] [PubMed] [Google Scholar]
- Mueller D, Stewart J. Cocaine-induced conditioned place preference: reinstatement by priming injections of cocaine after extinction. Behavioral Brain Research. 2000;115:39–47. doi: 10.1016/s0166-4328(00)00239-4. [DOI] [PubMed] [Google Scholar]
- Myers KM, Carlezon WA., Jr. D-cycloserine effects on extinction of conditioned responses to drug-related cues. Biological Psychiatry. 2012;71:947–955. doi: 10.1016/j.biopsych.2012.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsey MS, Ota KT, Akingbade IF, Hong ES, Schafe GE. Epigenetic alterations are critical for fear memory consolidation and synaptic plasticity in the lateral amygdala. PLoS One. 2011;6:e19958. doi: 10.1371/journal.pone.0019958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto L, Abel T. The role of histone acetylation in memory formation and cognitive impairments. Neuropsychopharmacol. 2013;38:62–76. doi: 10.1038/npp.2012.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renthal W, Nestler EJ. Epigenetic mechanisms in drug addiction. Trends in Molecular Medicine. 2008;14:341–350. doi: 10.1016/j.molmed.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Research Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rogge GA, Wood MA. The role of histone acetylation in cocaine-induced neural plasticity and behavior. Neuropsychopharmacology. 2013;38:94–110. doi: 10.1038/npp.2012.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical conditioning II. Appleton-Century Crofts; New York: 1972. pp. 64–99. [Google Scholar]
- Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addiction Biology. 2006;11:2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- Sanchis-Segura C, Lopez-Atalaya JP, Barco A. Selective boosting of transcriptional and behavioral responses to drugs of abuse by histone deacetylase inhibition. Neuropsychopharmacology. 2009;34:2642–2654. doi: 10.1038/npp.2009.125. [DOI] [PubMed] [Google Scholar]
- Stewart J. Pathways to relapse: the neurobiology of drug- and stress-induced relapse to drug-taking. Journal of Psychiatry and Neuroscience. 2000;25:125–136. [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Multiple dopamine functions at different time courses. Annual Review of Neuroscience. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- Shilatifard A. Chromatin modificatios by methylation and ubiquitination: implications in the regulation of gene expression. Annual Reviews in Biochemistry. 2006;75:243–269. doi: 10.1146/annurev.biochem.75.103004.142422. [DOI] [PubMed] [Google Scholar]
- Stafford JM, Raybuck JD, Raybinin AE, Lattal KM. Increased histone acetylation in hippocampus-infralimbic network enhances fear extinction. Biological Psychiatry. 2012;72:25–33. doi: 10.1016/j.biopsych.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. Experience-dependent epigenetic modification in the central nervous system. Biological Psychiatry. 2009;65:191–197. doi: 10.1016/j.biopsych.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrossa MM, Corlett PR, Taylor JR. Neurobiology of Learning and Memory. 2011;96:609–923. doi: 10.1016/j.nlm.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecsey CG, Hawk JD, Lattal KM, Stein JM, Fabian SA, Attner MA, Cabrera SM, McDonough CB, Brindle PK, Abel T, Wood MA. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. The Journal of Neuroscience. 2007;27:6128–6140. doi: 10.1523/JNEUROSCI.0296-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Zhang Y, Qing H, Liu M, Yang P. The extinction of morphine-induced conditioned place preference by histone deacetylase inhibition. Neuroscience Letters. 2010;483:137–142. doi: 10.1016/j.neulet.2010.07.080. [DOI] [PubMed] [Google Scholar]
- White NM. Addictive drugs as reinforcers: multiple partial actions on memory systems. Addiction. 1996;91:921–949. [PubMed] [Google Scholar]
- White NM, Carr GD. The conditioned place preference is affected by two independent reinforcement processes. Pharmacology, Biochemistry and Behavior. 1985;23:37–42. doi: 10.1016/0091-3057(85)90127-3. [DOI] [PubMed] [Google Scholar]
- White NM, Milner PM. The psychobiology of reinforcers. Annual Reviews in Psychology. 1992;43:443–471. doi: 10.1146/annurev.ps.43.020192.002303. [DOI] [PubMed] [Google Scholar]
- Wood MA, Attner MA, Oliveira AM, Brindle PK, Abel T. A transcription factor-binding domain of the coactivator CBP is essential for long-term memory and the expression of specific target genes. Learning and Memory. 2006;13:609–617. doi: 10.1101/lm.213906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh SH, Lin CH, Gean PW. Acetylation of nuclear factor-kappaB in rat amygdala improves long-term but not short-term retention of fear memory. Molecular Pharmacology. 2004;65:1286–1292. doi: 10.1124/mol.65.5.1286. [DOI] [PubMed] [Google Scholar]


