Abstract
Previous studies have indicated that 6–30% of all newly synthesized proteins are rapidly degraded by the ubiquitin-proteasome system, however the relationship of ubiquitination to translation for these proteins has been unclear. We report that co-translational ubiquitination (CTU) is a robust process, with ~12–15% of nascent polypeptides being ubiquitinated in human cells. CTU products contained primarily K48-linked polyubiquitin chains, consistent with a proteasomal targeting function. While nascent chains have been shown previously to be ubiquitinated within stalled complexes (CTUS), the majority of nascent chain ubiquitination occurred within active translation complexes (CTUA). CTUA was increased in response to agents that induce protein misfolding, while CTUS was increased in response to agents that lead to translational errors or stalling. These results indicate that ubiquitination of nascent polypeptides occurs in two contexts, and define CTUA as a component of a quality control system that marks proteins for destruction while they are being synthesized.
Introduction
Newly synthesized proteins are prone to misfolding and aggregation (Ellis, 2001), and this is compounded by errors in processes affecting transcription, mRNA processing, translation, and protein localization (Levine et al., 2005; Ogle and Ramakrishnan, 2005; Pickrell et al., 2010). As a result, a significant fraction of newly synthesized proteins never attain their functional state. Timely and efficient clearance of misfolded proteins is crucial for maintaining the cellular functions, and numerous human diseases are associated with a deficiency in eliminating aberrant proteins, including neurodegenerative diseases, type 2 diabetes, cystic fibrosis, peripheral amyloidosis, cancer, and cardiovascular disease (Balch et al., 2008; Hartl et al., 2011; Levine et al., 2005; Morimoto, 2008). Understanding the mechanisms of protein folding, quality control, and disposal of misfolded proteins is therefore crucial for therapeutic intervention in these disease states.
In eukaryotic cells, the ubiquitin-proteasome system (UPS) is the major pathway for elimination of misfolded proteins (Qian et al., 2006; Wolf and Hilt, 2004). Substrates of the UPS are marked with ubiquitin via E1-E2-E3 enzyme cascades, and subsequently delivered to the 26S proteasome for degradation (Welchman et al., 2005). Surprisingly, between 6% and 30% of all eukaryotic newly synthesized proteins are very rapidly degraded by the UPS (Qian et al., 2006; Schubert et al., 2000), suggesting that the UPS plays an important role in quality control of newly synthesized proteins. The “DRiP” (Defective Ribosomal Products) hypothesis proposed that these degradation products serve an important biological function as a source of MHC class I peptides (Reits et al., 2000; Yewdell et al., 1996). While this hypothesis has been extensively debated (Yewdell and Nicchitta, 2006), there is little doubt that, for many proteins, synthesis and degradation are closely coupled, in a seemingly energetically wasteful process. Consistent with a role for ubiquitin in the process, it was recently reported that a large fraction of the total human ubiquitin-modified proteome is derived from newly synthesized proteins (Kim et al., 2011). Importantly, the relationship between protein translation, ubiquitination, and degradation has not been established. The simplest model is that newly translated proteins are targeted for ubiquitination after their release from the ribosome, perhaps after failing a quality control surveillance test or after unsuccessful attempts at chaperone-assisted folding (McClellan et al., 2005). Alternatively, certain protein chaperones engage nascent polypeptides as they emerge from the ribosome (Hartl et al., 2011; Preissler and Deuerling, 2012), so it is conceivable that protein fate decisions might be made while translation is in progress. Consistent with this, Turner and Varshavsky showed that an engineered protein bearing an amino-terminal (N-end) degradation signal could be degraded co-translationally in S. cerevisiae (Turner and Varshavsky, 2000). While this implied that the protein was ubiquitinated co-translationally, N-end rule ligases have not been shown to target their natural substrates co-translationally. The cystic fibrosis transmembrane conductance regulator (CFTR), which is a very large protein prone to misfolding, was shown to be subject to co-translational ubiquitination in an in vitro rabbit reticulocyte lysate translation system (Sato et al., 1998), although this may have been related to the very slow translation rate in that system.
An additional aspect of initial protein quality control is the recognition and disposal of translation products produced from defective mRNAs (Shoemaker and Green, 2012). For example, poly-lysine containing sequences generated from the poly-A tails of non-stop mRNAs triggers stalling of the nascent chain within the exit tunnel, and two ubiquitin ligases – Ltn1 and the CCR4/NOT complex – have been implicated in the ubiquitination and degradation of these protein products (Bengtson and Joazeiro, 2010; Brandman et al., 2012; Dimitrova et al., 2009). In addition to nonstop decay (NSD), other mRNA surveillance mechanisms associated with translational stalling include no-go decay (NGD) and nonsense-mediated decay (NMD). The protein products of these stalled ribosomes, as well as those of ribosomes backed up behind stall sites, are likely to also be specifically targeted for degradation, but the mechanisms remain largely uncharacterized.
We previously demonstrated that ISG15, an interferon-induced ubiquitin-like modifier, is stochastically conjugated to newly synthesized proteins by Herc5, a ribosome-associated HECT domain ligase (Durfee et al., 2010). These results suggested that ISG15 is co-translationally conjugated to nascent polypeptides, and we proposed that this is an attempt to interfere with the function of newly synthesized viral proteins (Durfee et al., 2010; Skaug and Chen, 2010). This, along with the reports on degradation of newly synthesized proteins, led us to address whether ubiquitin, perhaps similarly to ISG15, is co-translationally conjugated to ribosome-associated nascent polypeptides. We report that co-translational ubiquitination (CTU) is a surprisingly robust process in human cells and that CTU occurs in at least two contexts: within stalled complexes (CTUS) and within active translation complexes (CTUA). The latter is remarkable in that it implies that a protein can be marked for degradation before synthesis is completed.
Results
Co-translational ubiquitination is a robust process in mammalian cells
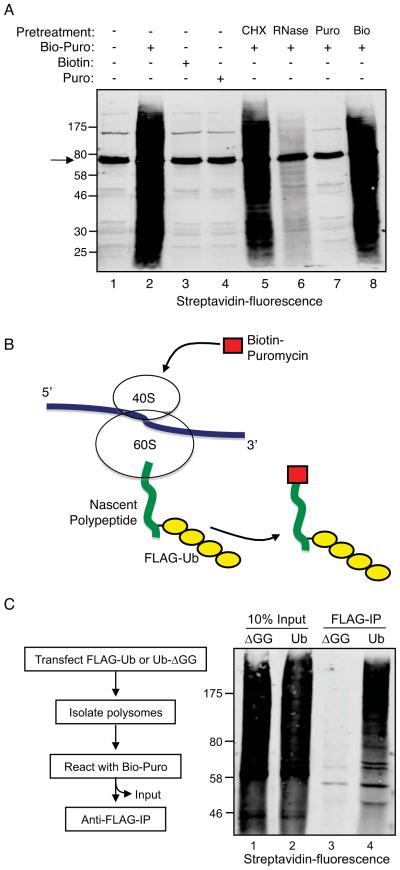
Puromycin is a structural analog of aminoacyl-tRNA that blocks translation by forming a covalent bond with the carboxyl-terminus of nascent polypeptides (Pestka, 1971). We utilized biotin-conjugated puromycin (Bio-Puro) to label polysome-associated nascent polypeptides in vitro. To establish reaction parameters, polysomes were collected from HEK293T cell extracts by centrifugation through 35% sucrose and incubated in vitro with Bio-Puro. Total reaction products were resolved by SDS-PAGE, blotted to nitrocellulose, and probed with fluorescent streptavidin. Figure 1A (lane 2) shows the complete spectrum of reaction products. Generation of the products was dependent on addition of Bio-Puro, as opposed to either free biotin (lane 3) or unlabeled puromycin (lane 4), and products were almost completely eliminated when polysomes were pretreated with RNase or unlabeled puromycin (Figure 1A, lanes 6 and 7). The inhibitory effect of RNase on this reaction was likely due to cleavage of the peptidyl-tRNA ester linkage, which leads to release of nascent chains (Kelkar et al., 2012). The prior addition of cycloheximide to polysomes in vitro (CHX; lane 5) had no effect on the Bio-Puro reaction, consistent with the fact that CHX inhibits ribosome translocation without affecting peptidyl transferase activity (David et al., 2012).
Figure 1. Co-translational ubiquitination (CTU) in human cells.
A. Validation of in vitro biotin-puromycin (Bio-Puro) conjugation reaction. Polysomes from HEK293T cells were used in all reactions. Reaction with Bio-Puro is shown in lane 2; Bio-Puro was deleted in lane 1, and replaced by free biotin (Bio) in lane 3 or by untagged puromycin (Puro) in lane 4. Polysomes were pretreated with cycloheximide (CHX), RNase, untagged puromycin or biotin in lane 5, 6, 7, and 8 respectively. Bio-Puro was detected with fluorescently-labeled streptavidin. Background bands seen, for example, in lane 1, represent endogenous biotin-conjugated proteins (e.g., arrow at 74 kD represents priopionyl coA carboxylase). B. Scheme for detection of CTU. If CTU occurs in vivo, polysomes should contain nascent polypeptides modified with both FLAG-ubiquitin and Bio-Puro. C. Polysome-associated nascent polypeptides are conjugated to FLAG-Ub. Cells were transfected with plasmids expressing wild type FLAG-Ub (Ub) or FLAG-Ub-ΔGG (ΔGG). Nascent chains were labeled with Bio-Puro in vitro, then immunoprecipitated with anti-FLAG antibody, and blotted with fluorescent streptavidin (lanes 3 and 4). Lanes 1 and 2 represent 10% of input levels of Bio-Puro labeled nascent chains (prior to FLAG immunoprecipitation). See also Figure S1.
In vitro Bio-Puro labeling was used to determine whether nascent polypeptides were ubiquitinated on polysomes isolated from cells expressing FLAG-Ub. As illustrated in Figure 1B, if nascent polypeptides were ubiquitinated in cells, then the in vitro Bio-Puro reaction would generate polypeptides modified with both FLAG-Ub and Bio-Puro. In contrast, if ubiquitination of newly synthesized proteins was strictly post-translational (i.e., after release of peptides from ribosomes), then Bio-Puro-conjugated polypeptides would not contain FLAG-Ub. Polysomes were isolated from HEK293T cells expressing FLAG-Ub or FLAG-Ub-ΔGG (a non-conjugatable form of ubiquitin) and nascent chains were labeled in vitro with Bio-Puro. Figure 1C (lanes 1 and 2) shows that the distribution of total biotin-labeled nascent polypeptides was similar in both reactions. An anti-FLAG immunoprecipitation (IP) was used to isolate ubiquitinated proteins, which were then analyzed by SDS-PAGE and immunoblotting with fluorescent streptavidin. Figure 1C (lanes 3 and 4) shows that the FLAG-Ub IP pulled down a broad distribution of proteins conjugated to Bio-Puro, while the FLAG-Ub-ΔGG IP did not. This result indicated that polysome-associated nascent polypeptides were ubiquitinated in cells. Importantly, NEM was included in the cell lysis buffer to block any potential post-lysis ubiquitination activity, and control reactions confirmed that there was no ubiquitination activity in NEM-containing lysis buffer (Figure S1).
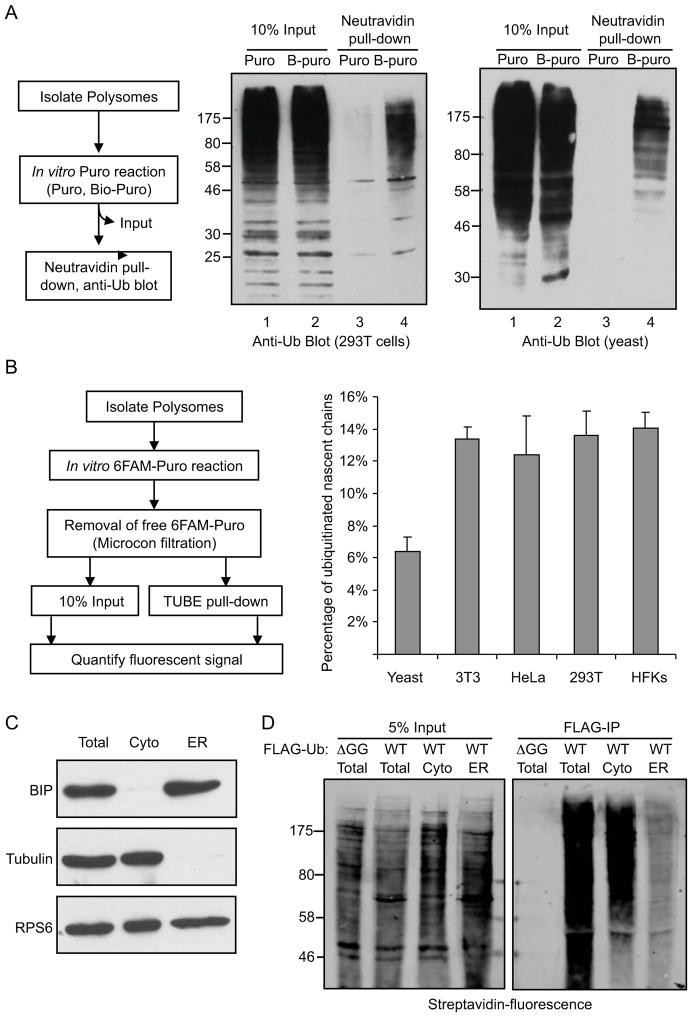
To eliminate the possibility that CTU was a result of overexpression of FLAG-Ub, similar experiments were done with untransfected cells, examining conjugates to endogenous ubiquitin. Polysomes were collected from HEK293T cells and incubated in vitro with Bio-Puro (illustrated in Figure 2A, left). Biotin-labeled nascent chains were isolated on neutravidin-agarose and immunoblotted with anti-ubiquitin antibody. Consistent with the results above, Figure 2A (middle) shows that a broad set of nascent polypeptides was modified with ubiquitin. Similar results were obtained using polysomes isolated from Saccharomyces cerevisiase cells expressing only endogenous ubiquitin (Figure 2A, right).
Figure 2. Polysome-associated nascent polypeptides are conjugated to endogenous ubiquitin in human and yeast cells.
A. Polysomes from untransfected HEK293T or S. cerevisiae cells were incubated with either Bio-Puro or untagged Puro to label associated nascent chains. Biotin labeled polypeptides were isolated by neutravidin pull down and analyzed by anti-ubiquitin immunoblotting. B. Quantitation of CTU in different cell types. Polysomes were isolated from the indicated cell types, and polysome-associated nascent chains were labeled with fluorescently-tagged puromycin (6-FAM-dC-Puro). 10% of the total reactions were used to estimate the amount of total nascent chains (input); the remainder was subject to TUBE pull down to isolate ubiquitinated proteins, and fluorescence intensity was measured. Error bars indicate standard error of the mean (SEM) of three independent experiments. C. CTU occurs predominantly on cytosolic rather than ER-associated polysomes. Fractionation of cytosolic and ER-associated polysomes was validated by immunoblotting with antibodies that recognize tubulin (cytosolic protein), Bip (ER luman protein) and RPS6 (small ribosomal protein). D. Cytosolic and ER-associated polysomes were isolated from HEK293T cells expressing FLAG-Ub. Polysome-associated nascent chains were labeled with Bio-Puro and then immunoprecipitated with anti-FLAG antibody. Left panel is 5% input of total Bio-Puro conjugation products, and right panel represents immunoprecipitation of nascent proteins that were modified with both FLAG-Ub and Bio-Puro. See also Figure S2.
The percentage of nascent chains that were ubiquitinated in cells was estimated by using fluorescently labeled puromycin (6-FAM-dC-Puro; Figure 2B, left). As above, polysomes were isolated from untransfected cells and total nascent polypeptides were labeled in vitro with 6-FAM-dC-Puro. Ubiquitinated polypeptides were then purified from the reaction on a ubiquitin-binding protein matrix (TUBE-Agarose, LifeSensors). The percentage of ubiquitinated nascent chains was determined by measuring the fluorescent signal of the TUBE pull-down compared to the fluorescent signal from the input nascent polypeptides (correcting for the efficiency of the TUBE pull-down, determined separately to be approximately 73%; Figure S2). In HEK293T cells, 12–15% of the total nascent polypeptides were co-translationally ubiquitinated (Figure 2B, right). Similar values were obtained in other human (HeLa) and mouse (NIH3T3) cell lines, as well as primary human foreskin keratinocytes (HFKs). These results indicate that CTU is a robust process across many mammalian cell types. The fraction of ubiquitinated nascent chains was reproducibly lower in budding yeast cells, with approximately 6% of total nascent chains being ubiquitinated (Figure 2B).
CTU predominantly occurs on cytosolic rather than ER-associated polysomes
Secreted and membrane proteins comprise approximately 30% of the eukaryotic proteome (Stevens and Arkin, 2000), and these are synthesized primarily on ER-associated polysomes. We determined the relative contribution of cytosolic and ER-associated polysomes to total CTU by fractionating the two types of polysomes by sequential detergent extraction (Jagannathan et al., 2011). Digitonin was used to selectively solubilize the plasma membrane and release cytosolic polysomes from FLAG-Ub-transfected cells. The permeabilized cells were washed and ER-bound polysomes were then solubilized with Triton-X100. The fractionation efficiency was validated via western blot analysis with antibodies that recognize tubulin (a cytosolic protein) and BIP/GRP78 (an ER lumen protein; Figure 2C). Immunoblotting of a ribosomal protein (RPS6) showed that polysomes were present in both cytosolic and ER fractions. The cytosolic and ER-derived polysomes were collected by centrifugation through 35% sucrose and used in in vitro Bio-Puro conjugation reactions (Figure 2D). Nascent polypeptides on cytosolic polysomes were heavily ubiquitinated, while the signal from ER-associated polysomes was approximately 5-fold less (normalized for total nascent chains). This indicated that CTU occurs predominantly, but not exclusively, on cytosolic polysomes (Figure 2D, right).
CTU targets are polyubiquitinated with K48 and K11 chains
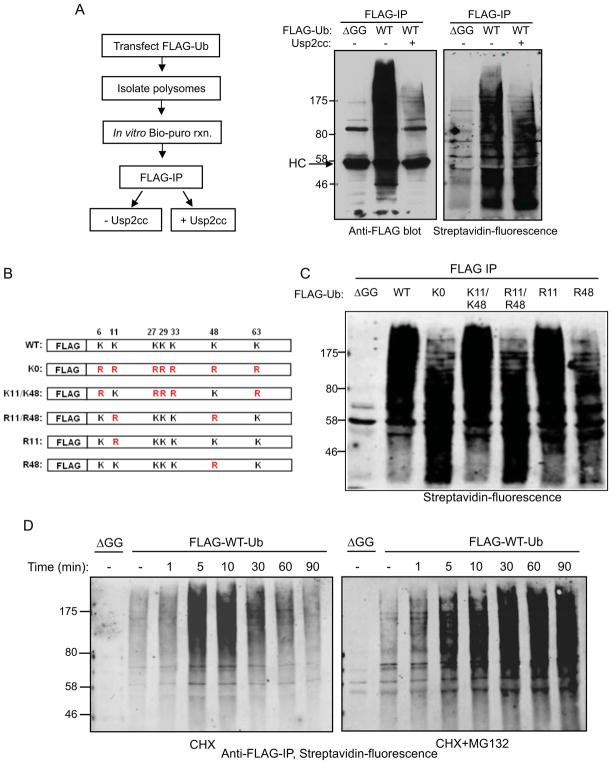
To determine the ubiquitin chain topology on CTU target proteins, FLAG-Ub-containing and Bio-Puro-labeled CTU products were incubated with the catalytic core of ubiquitin-specific protease 2 (USP2cc) to strip ubiquitin from target proteins. Addition of USP2cc to ubiquitinated nascent polypeptides led to a nearly complete loss of anti-FLAG-Ub immunoreactivity (Figure 3A, left). When the same reaction products were probed with fluorescent streptavidin, the average molecular weight of the CTU products was significantly decreased (Fig. 3A, right), suggesting that most puromycin-labeled CTU targets were modified with multiple ubiquitin molecules.
Figure 3. CTU products contain primarily K48-linked polyubiquitin chains.
A. CTU target proteins are multubiquitinated. Ubiquitinated nascent polypepetides were labeled with Bio-Puro, and immunoprecipitated with anti-FLAG antibody. The immunoprecipitated products were treated with the catalytic domain of ubiquitin-specific protease 2 (USP2cc) to strip ubiquitin (see schematic, left). Middle panel is the anti-FLAG-Ub blot analysis of samples before and after USP2cc treatment (HC is IgG heavy chain). Right panel shows the fluorescent-streptavidin blot of the FLAG IPs. The molecular weight change of CTU products after USP2cc treatment indicates that the majority of the CTU products contained multiple ubiquitin moieties. B. Schematic diagram of mutant forms of FLAG-ubiquitin expressed in HEK293T cells. C. Polysomes were isolated from cells expressing the indicated form of ubiquitin. Nascent chains were labeled with Bio-Puro in vitro, and then immunoprecipitated with anti-FLAG antibody. The immunoprecipitated products were subject to SDS-PAGE, and detection was with fluorescent streptavidin. All exogenously expressed ubiquitin mutants were expressed at similar levels (not shown). D. HEK293T cells were transfected with plasmids expressing FLAG-Ub or FLAG-Ub-ΔGG. Prior to lysis, cells were treated with CHX alone or simultaneously with CHX and MG132 for the indicated times. Polysomes were isolated from these cells and reacted with Bio-Puro. Nascent polypeptides were analyzed by anti-FLAG immunoprecipitation and detection with fluorescent streptavidin. See also Figure S3.
CTU products were purified and characterized by LC-MS/MS to determine chain type linkages and to identify a preliminary set of CTU targets. Figure S3A shows the Coomassie blue-stained SDS-PAGE gel of purified ubiquitinated nascent chains. Proteins greater than 30 kD were excised from the gel and tryptic peptides were identified by LC-MS/MS. The mass spectrometry analysis indicated the presence of K48- and K11-linked polyubiquitin chains within the purified CTU products, with no evidence for other chain types. Both of these chain types can target proteins for proteasomal degradation (Behrends and Harper, 2011; Xu et al., 2009). To confirm the chain topology observed by the LC-MS/MS analysis, a series of FLAG-Ub mutants (Figure 3B) were expressed in HEK293T cells. Polysomes were isolated, nascent chains were labeled with Bio-Puro, and FLAG-Ub-conjugates were immunoprecipitated and probed with fluorescent streptavidin. Expression of lysine-less ubiquitin (K0) led to a sharp decrease in the average apparent molecular weight distribution of CTU products (Figure 3C). Expression of ubiquitin that retained only K11 and K48 (K11/K48) supported production of CTU products that appeared identical to those produced in the presence of wild-type FLAG-Ub. In contrast, expression of R11/R48 ubiquitin (with all other lysines intact) resulted in CTU products that appeared similar to those produced in the presence of K0 ubiquitin. Expression of R48 ubiquitin had a similar effect as R48/R11 on the average length of CTU products, while R11 ubiquitin had no detectable effect on conjugates. These results suggest that K48-linked chains are the predominant type of chain formed on CTU products. In addition to the identification of K48- and K11-linked chains, the mass spectrometry analysis of CTU products identified approximately 130 putative CTU target proteins (Table S1), all of which were previously identified in a large-scale ubiquitin proteomics study that identified ~5000 ubiquitinated human proteins (Kim et al., 2011). These proteins are distributed in diverse intracellular compartments, with nuclear and cytosolic proteins predominating (Figure S3B).
To confirm that CTU products were subject to proteasomal degradation, cells were treated with cycloheximide prior to cell lysis for various periods of time to trap nascent polypeptides on polysomes. Polysomes were then isolated, nascent chains labeled with Bio-Puro, and FLAG-Ub-conjugates were immunoprecipitated and probed with fluorescent streptavidin. As shown in Figure 3D (left), the amount of ubiquitinated nascent chains increased after CHX treatment, reaching a peak between 5 and 10 minutes, then gradually decreased. A possible explanation for these observations was that CTU products transiently accumulated on cycloheximide-stalled polysomes and were then subject to proteasomal degradation over time. To test this, the experiment was repeated in the presence of both CHX and the proteasome inhibitor MG132. As shown in Figure 3D (right), the amount of CTU products continued to increase beyond the 10 minute time point (Figure 3D, right), indicating that ubiquitinated nascent chains are subject to proteasomal degradation.
CTU occurs within both stalled and active translation complexes
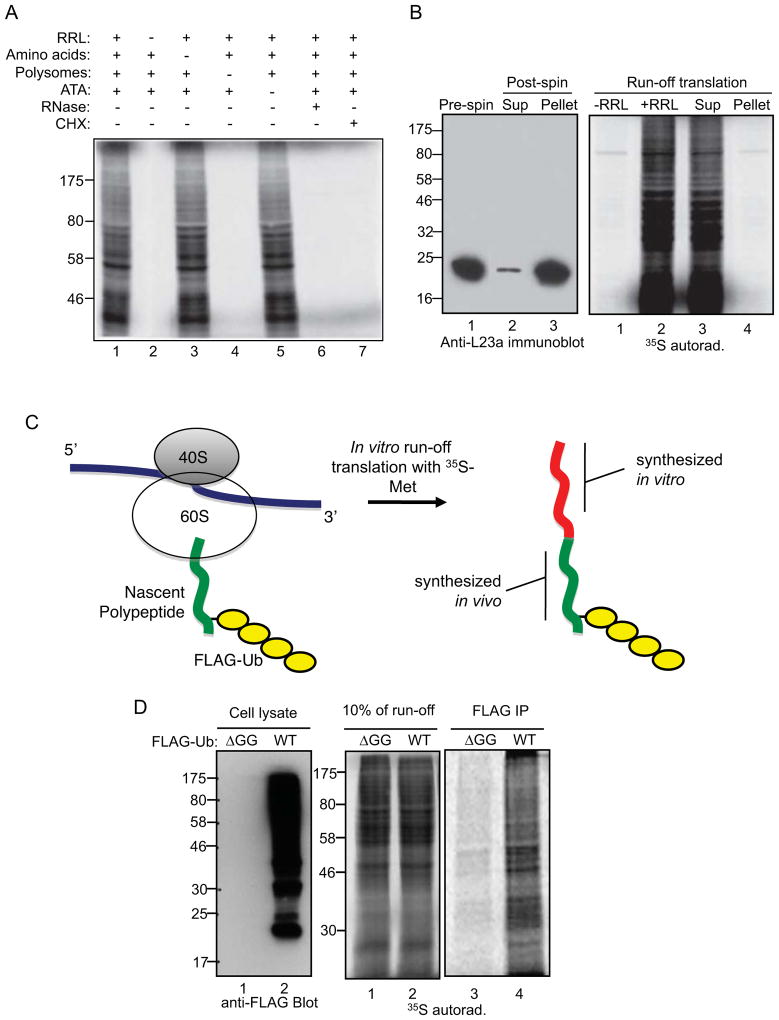
In vitro run-off translation reactions were used to address whether CTU occurs within active or stalled translation complexes. Polysomes were isolated from cell extracts by centrifugation through 35% sucrose, and translation of the associated nascent chains was completed in vitro in the presence of 35S-methionine, rabbit reticulocyte lysate (RRL), and aurintricarboxylic acid (ATA; an inhibitor of translation initiation) (Stewart et al., 1971). Radiolabeled proteins generated in the run-off reactions represent proteins that were initiated in vivo and completed in vitro. Figure 4A (lane 1) shows the spectrum of total radiolabeled products from a reaction programmed with polysomes from HEK293T cells, and lane 2 shows that this was dependent on addition of RRL. Generation of the products was eliminated when polysomes were pretreated with RNase or cycloheximide (lanes 6 and 7). Products were not significantly altered when reactions were performed without addition of exogenous unlabeled amino acids or ATA (lanes 3 and 5), indicating that there was a sufficient supply of endogenous amino acids in the RRL and that translation initiation from polysome-associated mRNAs was inefficient in the RRL system. Pre-clearing of ribosomes from the RRL did not diminish run-off translation products (Figure 4B), as expected since all products should have been generated from cell-derived ribosomes.
Figure 4. CTU occurs within active translation complexes.
A. Requirements for in vitro run-off translation reactions. A complete run-off reaction is shown in lane 1. A single component was deleted in lane 2 (rabbit reticulocyte lysate; RRL), lane 3 (exogenous amino acids), lane 4 (polysomes), and lane 5 (aurintricarboxylic acid; ATA). RNase or CHX was added to the reactions in lanes 6 and 7. B. Ribosomes present in RRL are not required for the run-off reaction. Ribosomes were depleted from RRL by ultracentrifugation, and untreated RRL or ribosome-depleted supernatant (Sup) was used in run-off reactions. Left panel shows an immunoblot for the L23a ribosomal protein in RRL (pre-spin), the supernatant (Sup), and the pellet. C. The CTU model predicts that if ubiquitin is conjugated to nascent chains on actively translating polysomes and translation can proceed following CTU, then in vitro run-off products will contain both ubiquitin and 35S-methionine. Green represents the portion of the polypeptide chain translated in vivo, and red represents the 35S-methionine-containing polypeptide translated in vitro. D. Cells were transfected with plasmids expressing FLAG-Ub (WT) or FLAG-Ub-ΔGG (ΔGG), and polysomes from these cells were used in run-off reactions. Left panel shows an anti-FLAG immunoblot of total cell extracts, center panel shows 10% of the run-off reactions, and right panel shows the anti-FLAG immunoprecipitates of the run-off reactions.
Polysomes from cells transfected with FLAG-Ub or FLAG-Ub-ΔGG were collected and used in run-off translation reactions. As illustrated in Figure 4C, if CTU occurred in cells within translation-competent complexes, then 35S-labeled run-off products should also contain FLAG-Ub. In contrast, if CTU occurred post-translationally or if CTU products were derived exclusively from irreversibly stalled polysomes, then in vitro run-off translation products should not yield products that contained both 35S-Met and FLAG-Ub. Figure 5D shows 35S-Met was robustly incorporated into FLAG-Ub-containing polypeptides, indicating that at least a portion of CTU products were generated within active, elongation-competent translation complexes.
Figure 5. CTUA accounts for the majority of the total ubiquitination activity against nascent chains.
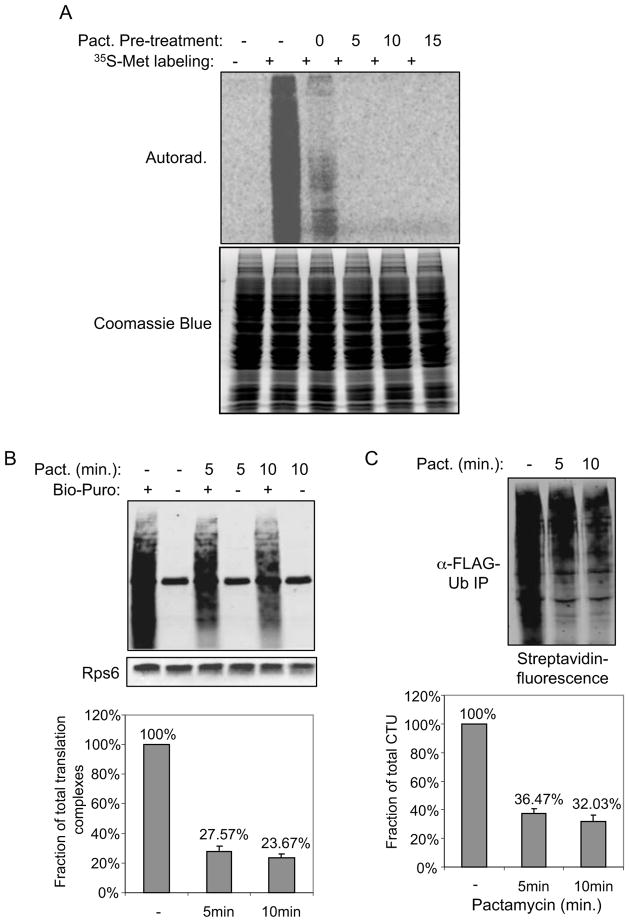
A. A brief treatment of cells with pactamycin was sufficient to allow run off of actively translating nascent chains. Cells were treated with pactamycin for the indicated times, followed by a 5 min. labeling with 35S-methionine. In lane 3, pactamycin was added simultaneously with the 35S-Met. Total cell lysate was subject to SDS-PAGE, and analyzed by autoradiography. Coomassie Blue staining shows proteins were loaded equally in each lane. B. A significant amount of nascent chains are in stalled ribosomes. Cells were either untreated or pretreated with pactamycin for 5 or 10 min. to run off of actively translating ribosomes. Polysomes were isolated from these cells and incubated with Bio-Puro in vitro to label associated nascent chains. Biotin-labeled nascent chains were subject to SDS-PAGE, and blotted with fluorescent streptavidin. A blot for a ribosomal protein, Rps6, is shown as a loading control. Error bars indicate SEM of three independent replicates. C. CTUS accounts for approximately one-third of CTUT. Cells expressing FLAG-Ub were treated with pactamycin for 10 min. and polysome-associated nascent chains were labeled with Bio-Puro in vitro and then immunoprecipitated with anti-FLAG antibody. The immunoprecipitated products were subject to SDS-PAGE analysis, and the fluorescent streptavidin signal was quantitated relative to cells that were not treated with pactamycin. Error bars represent SEM of three independent experiments.
We next sought to determine the relative proportion of total CTU products (CTUT) that were generated from active (CTUA) versus stalled (CTUS) translation complexes. Before addressing this, we first determined the proportion of total nascent chains that are in stalled translation complexes in cells, regardless of their ubiquitination state. We utilized a brief treatment of cells with pactamycin (5 or 10 minutes), an inhibitor of translation initiation, to allow run-off of all nascent chains present within active translation complexes. At an estimated translation rate of 5–9 amino acids per second (Orlowski and Ross, 1981; Ross and Orlowski, 1982), 5 minutes of pactamycin treatment is sufficient to allow completion of translation of all but the longest proteins. Figure 5A shows that a 5 minute treatment of 293T cells with pactacmycin was sufficient to block any detectable incorporation of 35S-methionine into cellular proteins in a subsequent 5 minute metabolic pulse. We therefore labeled nascent chains from untreated cells and from pactamycin treated cells (5 or 10 minutes) with fluorescently-labeled puromycin and quantitated the total fluorescent signal. The fluorescent-puromycin signal from pactamycin-treated cells was approximately 27% and 22% of the untreated cells at the 5 and 10 minute time points, respectively (Figure 5B), indicating that nearly a quarter of all nascent chains are in stalled complexes. The slight decrease in stalled nascent chains between the 5 and 10 minute time points is likely to represent the clearing of stalled complexes over time, either by degradation mechanisms or the resumption of transiently stalled complexes.
We next determined the fraction of total CTU products (CTUT) that were derived from stalled translation complexes (CTUS) by quantitating the amount of doubly-tagged (ubiquitinated and puromycylated) nascent chains that remained on polysomes after 5 or 10 minutes of pactamycin treatment. As shown in Figure 5C, CTUS was approximately 36% of CTUT at the 5 minute time point, and slightly less (32%) at the 10 minute time point. Therefore, CTUS accounts for approximately a third of CTUT, and CTUA (the fraction of CTU products that ran-off during pactamycin treatment) accounts for the remaining two-thirds. Considering that ~25% of nascent chains are in stalled complexes, we infer that approximately 15–18% of stalled nascent chains are ubiquitinated, while 11–14% of nascent chains in active translation complexes are ubiquitinated.
CTU is enhanced under conditions that promote protein misfolding or translational errors
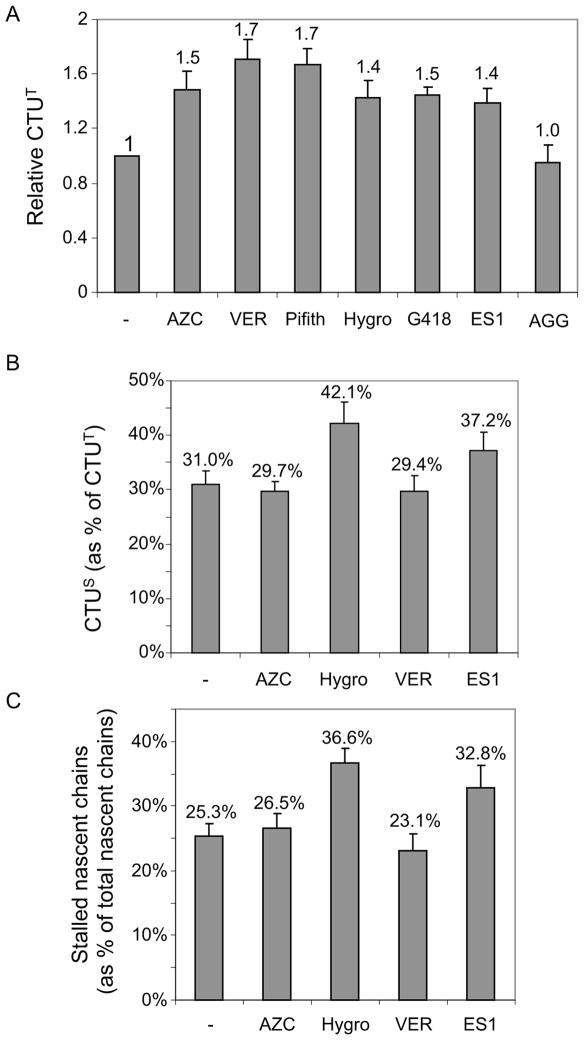
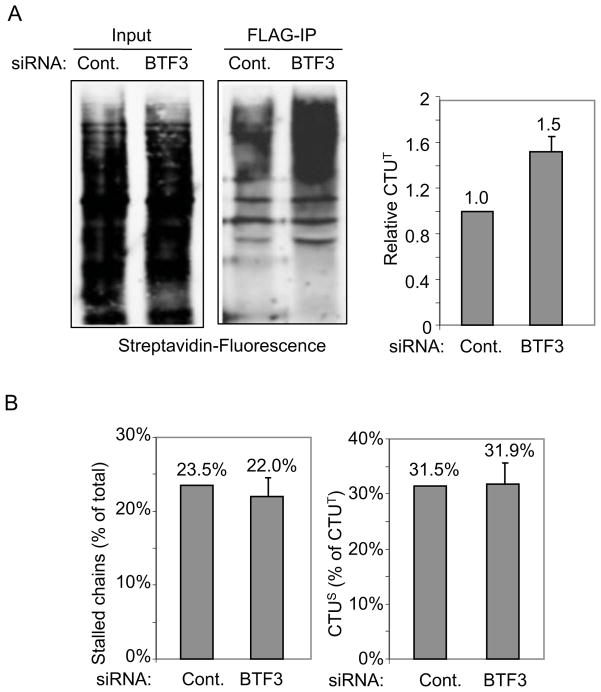
We next determined how CTUT was affected by agents that cause protein misfolding or translational stalling. Treatment with L-Azetidine-2-carboxylic acid (AZC), a proline analog that induces protein misfolding, enhanced CTUT by approximately 1.5-fold (Figure 6A). The role of Hsp70 family chaperones in CTU was tested using Hsp70 inhibitors. VER155008 is an adenosine-derived inhibitor (Williamson et al., 2009) and 2-Phenylethynesulfonamide (Pifithrin-μ) disrupts the association of Hsp70 with several co-chaperones (e.g, Hsp40, BAG-1L/1M) and substrate proteins (Leu et al., 2009). Both inhibitors led to an approximately 1.7-fold increase in CTUT. While Hsp70 is linked to co-translational protein folding, Hsp90 functions post-translationally (Hartl et al., 2011). Consistent with this, an inhibitor of Hsp90, 17-AGG, had no effect on CTUT. Aminoglycoside antibiotics, such as hygromycin B and G418, affect translational fidelity and read-through of stop codons (Brodersen et al., 2000), and both agents increased CTUT products to a similar degree (~1.4-fold). Eeyarestatin 1 (ES1), an inhibitor of Sec61-dependent translocation that prevents translation into the ER (Cross et al., 2009; McKibbin et al., 2012), led to an approximately 1.4-fold increase in CTUT. To discern whether the effects on CTUT were due increased CTUS or CTUA, we pre-treated cells with pactamycin (10 minutes) to run-off active translation complexes. Both hygromycin B and ES1 led to a substantial increase in CTUS (approximately 42% and 37% of CTUT, respectively, compared to 31% for untreated cells; Figure 6B) and an increase in total stalled nascent chains (Figure 6C), consistent with a model where translational stalling triggers CTUS. A short treatment with cycloheximide (10 minutes) resulted in a 4–5-fold increase in stalled complexes and 3-fold increase in CTUS (i.e., nearly all CTU was CTUS; Figure S4). AZC and Hsp70 inhibition did not lead to increased CTUS and did not affect the fraction of stalled nascent chains, indicating that enhanced CTUA accounted for the increased CTUT induced by these agents. As the primary effects of AZC and Hsp70 are on protein folding, these results suggest that CTUA represents a quality control pathway that ubiquitinates nascent chains in response to folding errors. Consistent with this, siRNA knockdown of the BTF3 subunit of the NAC (Nascent Polypeptide-Associated Complex) (Preissler and Deuerling, 2012) resulted in a 1.5-fold enhancement of CTUT (Figure 7A and Figure S5) but did not affect accumulation of stalled ribosomes or CTUS (Figure 7B). Together, these results indicate that CTUA is enhanced under conditions where folding of nascent polypeptides is impaired.
Figure 6. CTU was enhanced under conditions that promoted translational errors or protein misfolding.
A. CTUT was analyzed after treating cells for 60 min. with either AZC, the Hsp70 chaperone inhibitors VER155008 (VER) or Pifithrin (Pfith), hygromycin B (Hygro), G418, eeyarestatin 1 (ES1) or the Hsp90 inhibitor 17-AGG (AAG). CTU products were quantified by measuring fluorescent-streptavidin signal on blots, and relative signals were normalized in all panels to signal in the absence of inhibitor. Error bars indicate SEM of three independent replicates. B. Cells expressing FLAG-Ub were treated with the indicated agents for 60 min., and CTUS was quantitated as in Figure 6. Error bars indicate SEM of three independent experiments. C. Cells were treated with the indicated agents for 60 min., and the stalled nascent chains were quantitated relative to total nascent chains. Error bars indicate SEM of three independent experiments. See also Figure S4.
Figure 7. Depletion of NAC activity enhances CTUT.
A. 293T cells were transfected with siRNAs targeting the mRNA for BTF3 (the β subunit of the NAC) or a control siRNA, and the FLAG-Ub plasmid DNA was transfected 15 hours later. Cells were harvested 48 hours post-siRNA transfection, and CTU products were analyzed by anti-FLAG immunoprecipitation followed by quantitation of Bio-Puro incorporation with fluorescent-streptavidin. Signal was normalized in all panels to that of the control siRNA. Error bar indicates SEM of three independent experiments. BTF3 siRNA knockdown was confirmed by immunoblotting (Figure S5). B. Total stalled nascent chains and CTUS was analyzed in BTF3 or control siRNA-treated cells, using a 10 min. pactamycin treatment (as in Figure 6). See also Figure S5. Error bars indicate SEM of three independent replicates.
Discussion
We have shown here that co-translational ubiquitination is a robust process in human cells, with 12–15% of all nascent polypeptides being ubiquitinated in a variety of cell lines and primary cells. We have also shown that CTU occurs in two contexts: in stalled complexes (CTUS) and in active translation complexes (CTUA). CTUS has been described previously in the ubiquitination of stalled translation products arising from non-stop mRNAs (Bengtson and Joazeiro, 2010; Brandman et al., 2012; Dimitrova et al., 2009). Stalling in this case is due to translation of the poly-A tail, generating a poly-lysine sequence that interacts strongly with the acidic ribosome exit tunnel. There are additional circumstances in which CTUS is likely to be activated, such as in clearing of translation products at internal stop codons (associated with nonsense-mediated mRNA decay) or other types of damaged mRNAs (Shoemaker and Green, 2012). While we found that ~22–27% of all ribosomes were in stalled complexes, only a fraction of these stalled complexes (~15–18%) contained ubiquitinated nascent chains, suggesting that there are multiple types of stalled complexes and possibly multiple responses to stalled complexes.
CTUA couples synthesis to protein degradation, suggesting that protein fate decisions are made in the narrow window of time between emergence of the nascent polypeptide from the exit tunnel and the release of the full-length protein from the ribosome. CTUA was demonstrated in two ways. First, nascent chains that were ubiquitinated in vivo could be translated to completion in vitro when added to a reticulocyte lysate system. Second, a short treatment with pactamycin was used to run-off active translation complexes in vivo, and this resulted in a loss of approximately two-thirds of all ubiquitinated nascent chains from polysomes. A link between CTUA and protein folding quality control is suggested by the fact that Hsc/Hsp70 inhibitors, siRNA depletion of NAC subsunits, and AZC, led to an increase in CTUA. Both Hsc/Hsp70 and the NAC engage nascent polypeptides to initiate correct folding pathways before translation is complete (Hartl et al., 2011). In yeast, Hsp70 SSB associates with approximately 45% of nascent polypeptides (Willmund et al., 2013), consistent with the possibility that the relationship between nascent chain misfolding and ubiquitination may affect an incredibly broad range of cellular proteins.
Two ubiquitin ligases have been implicated in ubiquitination of stalled nascent chains – Ltn1 and CCR4/Not complex (Bengtson and Joazeiro, 2010; Brandman et al., 2012; Dimitrova et al., 2009). Additional factors - Tae2, Rqc1, and Cdc48 and its cofactors- have also recently been shown to function with Ltn1 in degradation of stalled nascent peptides (Bengtson and Joazeiro, 2010; Brandman et al., 2012; Verma et al., 2013). As determined by siRNA knockdown, Ltn1 and CCR4/Not each accounted for less than 10% of total CTU activity in our assays (Figure S5). The ligases for CTUA have not yet been identified, but several reasonable candidates have been ruled out. CHIP is an Hsp70-associated RING E3 (Rosser et al., 2007), and the Ubr1 and Ubr2 ligases, have been shown to be involved in the co-translational degradation of artificial fusion proteins bearing destabilizing N-terminal residues (Turner and Varshavsky, 2000). siRNA knockdown of CHIP had no effect on overall CTU, and Ubr1/Ubr2 double-knock out mouse embryo fibroblasts did not display diminished overall CTU (Figure S5). Yeast Hul5 is a ubiquitin ligase that targets misfolded cytosolic proteins post-translationally (Fang et al., 2011), and siRNA knockdown of an apparent human ortholog (Ube3C) had no effect in our assays (Figure S5). While the identity of the CTUA ligase(s) remains to be determined, we envision that these ligases may in one of two basic ways: either as ribosome-associated ligase(s) that monitor nascent polypeptides as they emerge from the ribosome, or as free cytosolic ligase(s) that monitor exposed nascent chains, either with or without the cooperation of chaperones. The first model is akin to the modification of newly translated proteins by the ubiquitin-like modifier ISG15, an interferon-induced Ubl (Durfee et al., 2010). In this case, the ISG15 ligase, Herc5, co-fractionates with polysomes and continues to co-fractionate with ribosomes after release of nascent chains. In the second model, features of defective or misfolded nascent chains might be recognized by the E3, independent of interaction with the ribosome, itself.
Our analyses of CTU products indicated that they are modified primarily with K48-linked polyubiquitin chains, and proteasome inhibition led to the accumulation of ubiquitinated nascent chains on cycloheximide-stalled polysomes in cells. Whether the proteasome can begin degrading CTUA products before synthesis is complete (in the absence of cycloheximide-induced stalling) is not yet known. Our proteomic analysis preliminarily identified a set of ~130 CTU target proteins. These were mainly cytosolic or nuclear proteins, as opposed to membrane-associated proteins, consistent with our observation that less than 20% of CTU activity is associated with ribosomes at the ER. Further experimentation is required to distinguish between CTUS and CTUA targets in these analyses, and to determine whether subsets of CTU targets are ubiquitinated under different stress conditions. It should also be noted that CTU products accumulated on polysomes after cycloheximide treatment; this effect should be considered in experimental designs that include polysome isolation, as pre-treatment of cells with cycloheximide is a feature of most polysome isolation protocols.
Estimates of the fraction of newly synthesized proteins subject to rapid degradation in mammalian cells have varied from 6% to 30% (Princiotta et al., 2003; Qian et al., 2005; Schubert et al., 2000; Vabulas and Hartl, 2005; Wheatley et al., 1980). While this range illustrates the need for better techniques for assessing the turnover of newly synthesized proteins (Yewdell and Nicchitta, 2006), these reports are generally consistent with our finding that CTU is a robust process in mammalian cells. While results presented here suggest that CTUA is a component of a protein folding quality control system, the characteristics and recognition of nascent polypeptides with CTUS and CTUA complexes clearly require further definition. Key questions to be addressed concern the identification of the enzymes and degrons involved in CTUS and CTUA, how the enzymes are localized to the translational machinery or nascent peptides, and, in the case of CTUA, the basis for protein fate decisions being made in the course of translation.
Experimental Procedures
Cell culture, transfections, and antibodies
Saccharomyces cerevisiae strain BY4741 was grown at 30°C in rich medium (yeast extract peptone). HeLa, HEK293T, and NIH 3T3 cells (ATCC), and UBR1/2−/− and control MEFs (kindly provided by the Yong Tae Kwon, University of Pittsburgh) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum and 1% antibiotics mixture. Primary human keratinocytes (Lonza) were maintained in KGM-Gold Keratinocyte Growth Medium (Lonza). Plasmid DNA and siRNA transfections were performed with cells at 60%–80% confluence using Lipofectamine 2000 (Invitrogen). All plasmids, antibodies and siRNAs used in this study are listed in Table S2. IRDye 680RD Streptavidin was purchased from LI-COR Bioscience.
Polysome isolation
To isolate total polysomes, cells were lysed in high-salt polysome lysis buffer (additional details in the Supplemental Experimental Procedures). Lysates were clarified by centrifugation at 16,300 x g for 10 min. at 4°C, and supernatant was loaded on a 2 ml 35% sucrose cushion (with 10mM Tris pH 7.4, 85mM KCl, 5mM MgCl2) and centrifuged for 90 min. at 316,000 x g in a Beckman NVT 65.2 rotor at 4°C. Polysome-containing pellets were resuspended in 200 μl polysome buffer (10 mM Tris pH7.4, 10 mM NaCl, 3 mM MgCl2, and 0.2 mM DTT) and stored at −80°C. For drug treatments prior to polysome isolation, cells were treated with 2 μM pactamycin (Sigma-Aldrich) for the indicated times (Figures 6–8) or cells were treated for 60 min. (Figure 7) with either 0.5 mg/mL AZC (Sigma-Aldrich), 20 μM VER155008 (Tocris Bioscience), 50 μM Pifithrin-μ (Tocris Bioscience), 200 μg/ml hygromycin B (Cellgro), 1000 μg/ml geneticin (Gemini Bio Products), 20 μM Eeyarestatin 1 (Tocris Bioscience) or 20 nM 17-AAG (Selleck Chemicals). Cytosolic and ER-associated polysome isolation was performed as described (Jagannathan et al., 2011) with modifications detailed in the Supplemental Experimental Procedures.
In vitro puromycin and run-off translation assays
Each 100 μl Bio-Puro conjugation assay contained the following components: 10 mM Tris pH 7.4, 400 mM KCl, 3 mM MgCl2, 2 A260 units of polysomes, and 2 μM biotin-linked puromycin (Jena Bioscience). Puromycylation reactions were performed at 37°C for 90 min and post-reaction, 5% of each sample was analyzed to determine the total nascent chains labeled. For immunoprecipitation of FLAG-Ub modified nascent chains, two volumes RIPA buffer (50 mM Tris pH 7.4, 150 mM NaCl, 1% NP-40, 0.1% SDS) was added to equal amounts of total nascent chains, and the mixtures were incubated with anti-FLAG M2 Affinity Gel (Sigma-Aldrich) overnight at 4°C. The beads were washed four times with 0.1% NP-40 buffer (0.1% NP-40,100 mM Tris, pH 7.9, 100 mM NaCl). Proteins were eluted from beads by adding SDS-PAGE loading buffer and boiling at 90°C for 5 min., and the samples were analyzed by SDS-PAGE and probed with fluorescently tagged streptavidin. For the experiment shown in Figure 1A, polysomes were pretreated with either 200 μg/ml CHX (lane 5), 0.1 mg/ml RNase (lane 6), 10 μM unlabeled puromycin (lane 7), or 10 μg/ml biotin at 30°C for 15 min. In order to quantitate CTU (Figure 2B), the biotin-linked puromycin in the in vitro puromycin assay was replaced with fluorescent puromycin (2 μM 6-FAM-dc-Puro, Jena Bioscience). Two volumes of 0.1% NP40 buffer were added after reaction, and the mixtures were spun through Microcon centrifugal filters (YM-3, Millipore) to remove unreacted 6-FAM-dc-Puro. 10% of the total reaction products were saved as input to estimate the amount of total nascent chains, and the rest was subject to two rounds of Tandem Ubiquitin Binding Entity-Agarose (TUBE 2, LifeSensors) pull-downs to isolate ubiquitin conjugates. TUBE pull-down efficiency was evaluated by immunoblotting of pre- and post-down supernatants with anti-ubiquitin antibody. The fluorescent signal from the TUBE pull-down ubiquitin conjugates and from the input nascent polypeptides was measured with a SpectraMax-M3 Multi-mode plate reader, with the excitation wavelength at 485nm. For the experiment shown in Figure 4A, purified Bio-Puro labeled ubiquitinated nascent chains were incubated with 10nM USP2cc (Boston Biochem) at room temperature for 90 min. Run-off translations were performed as described previously (Vayda, 1995) with modifications detailed in the Supplemental Experimental Procedures.
Purification of CTU targets for LC-MS/MS
HEK293T (twenty 150-mm culture dishes) were transfected with 5 μg per dish of plasmid expressing FLAG-Ub. Two days post-transfection polysomes were isolated as described above and nascent polypeptides were labeled with Bio-Puro. Two volumes of RIPA buffer were added after the reaction, and the mixtures were incubated with anti-FLAG M2 Affinity Gel overnight at 4°C to isolate FLAG-Ub conjugates. The beads were washed four times with 0.1% NP-40 buffer and proteins were eluted from beads by adding PBS containing 2% SDS and boiling at 90°C for 5 min. Eluted proteins were diluted with 0.1% NP40 buffer to decrease the final concentration of SDS to 0.2%, and then incubated with Avidin Agarose Resin (Thermo Scientific) overnight at 4°C to isolate FLAG-Ub conjugated nascent polypeptides. The beads were washed four times with RIPA buffer, and proteins were eluted from beads by adding SDS-PAGE loading buffer and boiling at 90°C for 5 min. The samples were analyzed by SDS-PAGE and stained with Coomassie blue. Proteins greater than 30 kD were excised from the gel. LC-MS/MS was performed at the Taplin Mass Spectrometry Facility at Harvard Medical School.
Supplementary Material
Highlights.
Co-Translational Ubiquitination (CTU) of nascent polypeptides is a robust process
CTU occurs on both active (CTUA) and stalled (CTUS) translation complexes
Nascent polypeptides are polyubiquitinated, primarily with K48 chains
CTUA is enhanced in response to protein misfolding
Acknowledgments
We thank members of the Huibregtse lab and Arlen Johnson for critical comments on the manuscript. This work was supported by grants from the National Institutes of Health to J. M. H. (AI096090 and GM103619).
Footnotes
Supplemental information includes supplemental experimental procedures, five figures, and two tables, and can be found with this article on-line.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science (New York, NY. 2008;319:916–919. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- Behrends C, Harper JW. Constructing and decoding unconventional ubiquitin chains. Nat Struct Mol Biol. 2011;18:520–528. doi: 10.1038/nsmb.2066. [DOI] [PubMed] [Google Scholar]
- Bengtson MH, Joazeiro CA. Role of a ribosome-associated E3 ubiquitin ligase in protein quality control. Nature. 2010;467:470–473. doi: 10.1038/nature09371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandman O, Stewart-Ornstein J, Wong D, Larson A, Williams CC, Li GW, Zhou S, King D, Shen PS, Weibezahn J, et al. A ribosome-bound quality control complex triggers degradation of nascent peptides and signals translation stress. Cell. 2012;151:1042–1054. doi: 10.1016/j.cell.2012.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodersen DE, Clemons WM, Jr, Carter AP, Morgan-Warren RJ, Wimberly BT, Ramakrishnan V. The structural basis for the action of the antibiotics tetracycline, pactamycin, and hygromycin B on the 30S ribosomal subunit. Cell. 2000;103:1143–1154. doi: 10.1016/s0092-8674(00)00216-6. [DOI] [PubMed] [Google Scholar]
- Cross BC, McKibbin C, Callan AC, Roboti P, Piacenti M, Rabu C, Wilson CM, Whitehead R, Flitsch SL, Pool MR, et al. Eeyarestatin I inhibits Sec61-mediated protein translocation at the endoplasmic reticulum. J Cell Sci. 2009;122:4393–4400. doi: 10.1242/jcs.054494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David A, Dolan BP, Hickman HD, Knowlton JJ, Clavarino G, Pierre P, Bennink JR, Yewdell JW. Nuclear translation visualized by ribosome-bound nascent chain puromycylation. J Cell Biol. 2012;197:45–57. doi: 10.1083/jcb.201112145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrova LN, Kuroha K, Tatematsu T, Inada T. Nascent peptide-dependent translation arrest leads to Not4p-mediated protein degradation by the proteasome. J Biol Chem. 2009;284:10343–10352. doi: 10.1074/jbc.M808840200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durfee LA, Lyon N, Seo K, Huibregtse JM. The ISG15 conjugation system broadly targets newly synthesized proteins: implications for the antiviral function of ISG15. Molecular cell. 38:722–732. doi: 10.1016/j.molcel.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durfee LA, Lyon N, Seo K, Huibregtse JM. The ISG15 conjugation system broadly targets newly synthesized proteins: implications for the antiviral function of ISG15. Molecular cell. 2010;38:722–732. doi: 10.1016/j.molcel.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ. Macromolecular crowding: obvious but underappreciated. Trends in biochemical sciences. 2001;26:597–604. doi: 10.1016/s0968-0004(01)01938-7. [DOI] [PubMed] [Google Scholar]
- Fang NN, Ng AH, Measday V, Mayor T. Hul5 HECT ubiquitin ligase plays a major role in the ubiquitylation and turnover of cytosolic misfolded proteins. Nat Cell Biol. 2011;13:1344–1352. doi: 10.1038/ncb2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 475:324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- Jagannathan S, Nwosu C, Nicchitta CV. Analyzing mRNA localization to the endoplasmic reticulum via cell fractionation. Methods in molecular biology (Clifton, NJ. 2011;714:301–321. doi: 10.1007/978-1-61779-005-8_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelkar DA, Khushoo A, Yang Z, Skach WR. Kinetic analysis of ribosome-bound fluorescent proteins reveals an early, stable, cotranslational folding intermediate. J Biol Chem. 2012;287:2568–2578. doi: 10.1074/jbc.M111.318766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W, Bennett EJ, Huttlin EL, Guo A, Li J, Possemato A, Sowa ME, Rad R, Rush J, Comb MJ, et al. Systematic and quantitative assessment of the ubiquitin-modified proteome. Molecular cell. 2011;44:325–340. doi: 10.1016/j.molcel.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leu JI, Pimkina J, Frank A, Murphy ME, George DL. A small molecule inhibitor of inducible heat shock protein 70. Molecular cell. 2009;36:15–27. doi: 10.1016/j.molcel.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine CG, Mitra D, Sharma A, Smith CL, Hegde RS. The efficiency of protein compartmentalization into the secretory pathway. Molecular biology of the cell. 2005;16:279–291. doi: 10.1091/mbc.E04-06-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan AJ, Tam S, Kaganovich D, Frydman J. Protein quality control: chaperones culling corrupt conformations. Nat Cell Biol. 2005;7:736–741. doi: 10.1038/ncb0805-736. [DOI] [PubMed] [Google Scholar]
- McKibbin C, Mares A, Piacenti M, Williams H, Roboti P, Puumalainen M, Callan AC, Lesiak-Mieczkowska K, Linder S, Harant H, et al. Inhibition of protein translocation at the endoplasmic reticulum promotes activation of the unfolded protein response. Biochem J. 2012;442:639–648. doi: 10.1042/BJ20111220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI. Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes & development. 2008;22:1427–1438. doi: 10.1101/gad.1657108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogle JM, Ramakrishnan V. Structural insights into translational fidelity. Annual review of biochemistry. 2005;74:129–177. doi: 10.1146/annurev.biochem.74.061903.155440. [DOI] [PubMed] [Google Scholar]
- Orlowski M, Ross JF. Relationship of internal cyclic AMP levels, rates of protein synthesis and mucor dimorphism. Arch Microbiol. 1981;129:353–356. doi: 10.1007/BF00406461. [DOI] [PubMed] [Google Scholar]
- Pestka S. Inhibitors of ribosome functions. Annu Rev Microbiol. 1971;25:487–562. doi: 10.1146/annurev.mi.25.100171.002415. [DOI] [PubMed] [Google Scholar]
- Pickrell JK, Pai AA, Gilad Y, Pritchard JK. Noisy splicing drives mRNA isoform diversity in human cells. PLoS genetics. 2010;6:e1001236. doi: 10.1371/journal.pgen.1001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preissler S, Deuerling E. Ribosome-associated chaperones as key players in proteostasis. Trends Biochem Sci. 2012;37:274–283. doi: 10.1016/j.tibs.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Princiotta MF, Finzi D, Qian SB, Gibbs J, Schuchmann S, Buttgereit F, Bennink JR, Yewdell JW. Quantitating protein synthesis, degradation, and endogenous antigen processing. Immunity. 2003;18:343–354. doi: 10.1016/s1074-7613(03)00051-7. [DOI] [PubMed] [Google Scholar]
- Qian SB, Bennink JR, Yewdell JW. Quantitating defective ribosome products. Methods Mol Biol. 2005;301:271–281. doi: 10.1385/1-59259-895-1:271. [DOI] [PubMed] [Google Scholar]
- Qian SB, Princiotta MF, Bennink JR, Yewdell JW. Characterization of rapidly degraded polypeptides in mammalian cells reveals a novel layer of nascent protein quality control. The Journal of biological chemistry. 2006;281:392–400. doi: 10.1074/jbc.M509126200. [DOI] [PubMed] [Google Scholar]
- Reits EA, Vos JC, Gromme M, Neefjes J. The major substrates for TAP in vivo are derived from newly synthesized proteins. Nature. 2000;404:774–778. doi: 10.1038/35008103. [DOI] [PubMed] [Google Scholar]
- Ross JF, Orlowski M. Growth-rate-dependent adjustment of ribosome function in chemostat-grown cells of the fungus Mucor racemosus. J Bacteriol. 1982;149:650–653. doi: 10.1128/jb.149.2.650-653.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosser MF, Washburn E, Muchowski PJ, Patterson C, Cyr DM. Chaperone functions of the E3 ubiquitin ligase CHIP. J Biol Chem. 2007;282:22267–22277. doi: 10.1074/jbc.M700513200. [DOI] [PubMed] [Google Scholar]
- Sato S, Ward CL, Kopito RR. Cotranslational ubiquitination of cystic fibrosis transmembrane conductance regulator in vitro. The Journal of biological chemistry. 1998;273:7189–7192. doi: 10.1074/jbc.273.13.7189. [DOI] [PubMed] [Google Scholar]
- Schubert U, Anton LC, Gibbs J, Norbury CC, Yewdell JW, Bennink JR. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 2000;404:770–774. doi: 10.1038/35008096. [DOI] [PubMed] [Google Scholar]
- Shoemaker CJ, Green R. Translation drives mRNA quality control. Nat Struct Mol Biol. 2012;19:594–601. doi: 10.1038/nsmb.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaug B, Chen ZJ. Emerging role of ISG15 in antiviral immunity. Cell. 2010;143:187–190. doi: 10.1016/j.cell.2010.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens TJ, Arkin IT. Do more complex organisms have a greater proportion of membrane proteins in their genomes? Proteins. 2000;39:417–420. doi: 10.1002/(sici)1097-0134(20000601)39:4<417::aid-prot140>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Stewart ML, Grollman AP, Huang MT. Aurintricarboxylic acid: inhibitor of initiation of protein synthesis. Proceedings of the National Academy of Sciences of the United States of America. 1971;68:97–101. doi: 10.1073/pnas.68.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner GC, Varshavsky A. Detecting and measuring cotranslational protein degradation in vivo. Science (New York, NY. 2000;289:2117–2120. doi: 10.1126/science.289.5487.2117. [DOI] [PubMed] [Google Scholar]
- Vabulas RM, Hartl FU. Protein synthesis upon acute nutrient restriction relies on proteasome function. Science. 2005;310:1960–1963. doi: 10.1126/science.1121925. [DOI] [PubMed] [Google Scholar]
- Vayda ME. Assessment of translational regulation by run-off translation of polysomes in vitro. Methods in cell biology. 1995;50:349–359. doi: 10.1016/s0091-679x(08)61042-0. [DOI] [PubMed] [Google Scholar]
- Verma R, Oania RS, Kolawa NJ, Deshaies RJ. Cdc48/p97 promotes degradation of aberrant nascent polypeptides bound to the ribosome. elife. 2013;2:e00308. doi: 10.7554/eLife.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welchman RL, Gordon C, Mayer RJ. Ubiquitin and ubiquitin-like proteins as multifunctional signals. Nat Rev Mol Cell Biol. 2005;6:599–609. doi: 10.1038/nrm1700. [DOI] [PubMed] [Google Scholar]
- Wheatley DN, Giddings MR, Inglis MS. Kinetics of degradation of “short-” and “long-lived” proteins in cultured mammalian cells. Cell Biol Int Rep. 1980;4:1081–1090. doi: 10.1016/0309-1651(80)90045-4. [DOI] [PubMed] [Google Scholar]
- Williamson DS, Borgognoni J, Clay A, Daniels Z, Dokurno P, Drysdale MJ, Foloppe N, Francis GL, Graham CJ, Howes R, et al. Novel adenosine-derived inhibitors of 70 kDa heat shock protein, discovered through structure-based design. Journal of medicinal chemistry. 2009;52:1510–1513. doi: 10.1021/jm801627a. [DOI] [PubMed] [Google Scholar]
- Willmund F, Del Alamo M, Pechmann S, Chen T, Albanese V, Dammer EB, Peng J, Frydman J. The cotranslational function of ribosome-associated hsp70 in eukaryotic protein homeostasis. Cell. 2013;152:196–209. doi: 10.1016/j.cell.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf DH, Hilt W. The proteasome: a proteolytic nanomachine of cell regulation and waste disposal. Biochimica et biophysica acta. 2004;1695:19–31. doi: 10.1016/j.bbamcr.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Xu P, Duong DM, Seyfried NT, Cheng D, Xie Y, Robert J, Rush J, Hochstrasser M, Finley D, Peng J. Quantitative proteomics reveals the function of unconventional ubiquitin chains in proteasomal degradation. Cell. 2009;137:133–145. doi: 10.1016/j.cell.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yewdell JW, Anton LC, Bennink JR. Defective ribosomal products (DRiPs): a major source of antigenic peptides for MHC class I molecules? J Immunol. 1996;157:1823–1826. [PubMed] [Google Scholar]
- Yewdell JW, Nicchitta CV. The DRiP hypothesis decennial: support, controversy, refinement and extension. Trends Immunol. 2006;27:368–373. doi: 10.1016/j.it.2006.06.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.