Abstract
The Egr family of transcription factors plays a key role in long-term plasticity and memory in a number of vertebrate species. Here we identify and characterize ApEgr (GenBank: KC608221), an Egr homolog in the marine mollusk Aplysia californica. ApEgr codes for a predicted 593-amino acid protein with the highly conserved trio of zinc-fingered domains in the C-terminus that characterizes the Egr family of transcription factors. Promoter analysis shows that the ApEgr protein selectively recognizes the GSG motif recognized by vertebrate Egrs. Like mammalian Egrs, ApEgr is constitutively expressed in a range of tissues, including the CNS. Moreover, expression of ApEgr is bi-directionally regulated by changes in neural activity. Of most interest, the association between ApEgr function and memory may be conserved in Aplysia, as we observe rapid and long-lasting up-regulation of expression after long-term sensitization training. Taken together, our results suggest that Egrs may have memory functions that are conserved from mammals to mollusks.
1. Introduction
Exploration of the cellular mechanisms of long-term memory has focused attention on several families of transcription factors (Alberini, 2009; Korzus, 2003). Remarkably, most of these transcription factors have conserved functions in memory and plasticity across much of the animal kingdom. For example, the CREB family of transcription factors is important for long-term memory in mammals (Bourtchuladze et al., 1994), insects (Yin et al., 1994), mollusks (Bartsch, Casadio, Karl, Serodio, & Kandel, 1998; Guo, Senzel, Li, & Feng, 2010), and nematodes (Timbers & Rankin, 2011). Similarly C/EBPs are known to influence plasticity and memory in mammals (Arguello et al., 2013; Taubenfeld, Milekic, Monti, & Alberini, 2001) and mollusks (Alberini, Ghirardi, Metz, & Kandel, 1994).
One prominent gap in our knowledge relates to the Early Growth Response (Egr) family of transcription factors. This family plays critical roles in in long term plasticity and memory in mammals (Poirier et al., 2008) and has also been implicated in song-learning in avian species (Moorman, Mello, & Bolhuis, 2011). To date, however, relatively few invertebrate Egr homologs have been characterized, and none of these have been attributed functions in learning and memory.
In mammals, the Egr family consists of 4 immediate-early genes, all encoding transcription factors: Egr-1 (NGFI-A, krox-24, zif268, TIS8, zenk), Egr-2 (krox-20), Egr-3, and Egr-4 (NGFI-C) (for a review, see Beckmann & Wilce, 1997). Wilm’s tumour protein, WT-1 (Call et al., 1990), is also considered an Egr, but it is not expressed in the brain and has a fundamentally different genomic organization (each zinc-finger is encoded by a separate exon). We focus here on Egrs 1 through 4.
The Egr family is characterized by a distinctive triplet of C2H2 zinc finger domains that recognizes a consensus DNA sequence known as the GSG motif (5′ GCG(G/T)GGGCG-3′). Although details vary across the different family members, Egrs 1-4 are constitutively expressed in the mammalian brain (Crosby et al., 1992; Mack, Cortner, Mack, & Farnham, 1992; Milbrandt, 1987; Yamagata et al., 1994) as well as a range of other tissues. In addition, all Egrs exhibit rapid and protein-synthesis independent up-regulation of transcriptional expression in response to a variety of physiological manipulations (reviewed in Beckmann & Wilce, 1997).
With the exception of Egr-4, the Egrs are strongly implicated in memory and plasticity. Most attention has been focused on Egr-1 (Knapska & Kaczmarek, 2004). Egr-1 expression is up-regulated by a variety of learning paradigms (e.g. Guzowski, Setlow, Wagner, & McGaugh, 2001; Hall, Thomas, & Everitt, 2000; Malkani & Rosen, 2000). It is also up-regulated by the induction of LTP (Cole, Saffen, Baraban, & Worley, 1989) via activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) signaling cascade (Davis, Vanhoutte, Pages, Caboche, & Laroche, 2000). The level of up-regulation correlates with the persistence of LTP (Abraham et al., 1993). Moreover, disruption of Egr-1 function disrupts both the maintenance of plasticity as well as memory consolidation (Jones et al., 2001). There has been some controversy over the time course and specificity of Egr-1 activation (summarized in Alberini, 2009). This may be, however, due to the potential for Egr-1 to exhibit two distinct waves of expression following some forms of long-term memory training (Katche, Goldin, Gonzalez, Bekinschtein, & Medina, 2012). Moreover, the evidence from experimental manipulation of Egr-1 has become increasingly convincing. Specifically, a recent study (Yang et al., 2012) confirmed original reports that selective knockdown of Egr-1 impairs late-phase LTP as well as hippocampal-dependent long-term memory (the alias Zif268 was used in this report). Moreover, increasing Egr-1 expression in the hippocampus was sufficient to rescue memory and plasticity deficits that had been generated by the deletion of cAMP receptors.
Egr-3 has also been convincingly tied to memory function, but its roles are distinct from Egr-1. Specifically, Egr-3 knockout mice have normal development and Egr-1 levels, but are impaired at both early and late LTP as well as several short- and long-term memory tasks (Li et al., 2007). Egr-2 also has a distinctive role in memory. Egr-2 mRNA is up-regulated in the hippocampus by LTP (Williams et al., 1995), leading to initial speculation that it helps stabilize long-term plasticity. Recent evidence suggests, however, that Egr-2 may function as a memory repressor, as a conditional mouse knockout exhibited improved motor learning and recognition memory (Poirier, Cheval, & Mailhes, 2007). Egr-4 has not been linked to memory and plasticity, and has been specifically reported not to be regulated by fear conditioning (Malkani & Rosen, 2000). Thus, Egr 1, 2, and 3 serve important but distinct roles in long-term memory.
Despite the intense scrutiny in mammalian and avian species, surprisingly little is known about the learning and memory functions of Egrs in invertebrate species. In D. melanogaster the genes stripe (Lee, VijayRaghavan, Celniker, & Tanouye, 1995), huckabein (Brönner et al., 1994), and perhaps klumpfuss (Klein & Campos-ortega, 1997) have significant homology to mammalian Egrs. In C. elegans sequence analysis suggests 4 potential homologs (egrh-1, egrh-2, egrh-3, and ZK337.2; Clary & Okkema, 2010). To date, however, none of these putative homologs have been shown to bind to the GSG motif. Outside of these two species, numerous sequence similarities have been noted, but no other invertebrate Egr homologs have been convincingly established. Moreover, experimental analysis of the known Egr homologs has been limited to exploring roles in body patterning and sexual development—learning and memory functions seem so far unexplored. Suggestively, CNS injection of an antisense sequence designed for rat Egr-1 was able to disrupt the development of sensitization in the terrestrial land snail (Nikitin & Kozyrev, 2007). However, the probe design was in an area which shows little homology to the known invertebrate Egrs (e.g. Drosophila and C. elegans), making it difficult to interpret the significance of this finding.
To better understand the degree of conservation of Egr memory function, we screened the recently published draft of the Aplysia californica genome for candidate Egr homologs. We report here the characterization of ApEgr (GenBank: KC608221), a transcript which shares many of the key characteristics of mammalian Egrs: activation of the same GSG consensus sequence, constitutive expression in the CNS as well as a range of other tissues, and bi-directional regulation by neuronal activity. Most intriguingly, we observe that ApEgr expression is rapidly and persistently up-regulated by long-term sensitization. Taken together, our results suggest that Egrs have memory functions that are conserved from mammals to mollusks. Our work also provides an avenue to better understand the behavioral relevance of the Egr family in a model organism tractable for multiple levels of analysis.
2. Materials and methods
2.1 Animals
Animals (75–125g) were obtained from the RSMAS National Resource for Aplysia (Miami, FL) and maintained at 16° C in one of two 90-gallon aquariums with continuously circulating artificial sea water (Instant Ocean, Aquarium Systems Inc.). Animals were separately housed in floating colanders, fed dried seaweed twice a week, and maintained on a 12 hr light-dark cycle. 2 days prior to any experimental testing, animals were fed to satiation and then food deprived for the remainder of the experiment. To eliminate the possibility of batch/shipment effects, animals from at least 2 different shipments were used for each experiment.
2.2 5′RACE and 3′RACE
To obtain full length ApERG cDNA sequence, we performed both 5′RACE and 3′RACE with SMARTer RACE cDNA amplification kit (Clontech, Mountain View, CA). 5_RACE PCR was performed using the adaptor primers provided in the kit and a gene specific primer: TGCGGCTGAAAGAGCGGCTGCAGAT. The 3′RACE PCR also used provided adaptor primers and a gene specific primer: CAGCTCCGCCCAGTCCAGCCCAGAG. PCR bands obtained from RACE reactions were subcloned into pGEM®-T Easy Vector (Promega, Madison, WI). Cloned fragments were then sequenced.
2.3 DNA constructs
To measure transcriptional activity of ApEgr, a luciferase construct was created by inserting two copies of the GSG motif recognized by Egrs (Yoshino et al., 2002) into the XhoI and HindIII sites of the pNL3.1[Nluc/minP] Vector (Promega, Madison, WI), producing GSG (5′TGCGGGGGCGTGGGGCTGCGTGGGCGTGGGGC3′ )-luciferase. As a control, a CRE luciferase construct was created in the same way, but with the insertion of CRE motifs (based on McDonnell, Latif, Rees, Bevan, & Hill, 1998) recognized by the CREB family of transcription factors (5′CTCCTTGGCTGACGTCAGTAGAGAGATCCCATTGACGTCATACT3′).
GFP fusion protein constructs were made using pNEX3-GFP (kind gift from Dr. Wayne Sossin). Gene specific primers were used to amplify the coding regions of ApCREB (817bp; from the ApCREB reference sequence, GenBank: NM_001256437, which is based on the CDS for isoform D, GenBank: FJ210651.1), MsEGR1 (1602 bp; GenBank: NM_007913) and ApEGR (1779 bp predicted ORF; GenBank: KC608221). Amplified sequences were subcloned into XhoI and SacII sites of pNEX3-GFP. All sequences were confirmed to be correct by DNA sequencing.
2.4 Cell culture of S2 cells and Reporter Gene Assays
S2 Drosophila cells were obtained from DGRC (Indiana University, Bloomington, IN) and maintained in M3+BPYE+10% FBS media and grown in 48 well plates at a density of 150K cells/well. Cells were transfected according to manufacturer’s protocol using Cellfectin reagent (Invitrogen, Carlsbad, CA) using ~300 ng of total DNA (luciferase construct+GFP fusion protein construct) and 2 ul of Cellfectin reagent. Cells were incubated for 48 hours prior to lysis. Transfection success was monitored by the presence of GFP positive cells. GloLysis reagent (Promega, Madison, WI) was used to lyse cells. To assess cell viability, ~12.5% of the lysate was combined with the appropriate reagent in the CellTiter-Glo® Luminescent Cell Viability Assay kit (Promega, Madison, WI). Transcriptional activity was measured in Relative Light Units (RLUs) using a GloMax20/20 Luminometer (Promega, Madison, WI). Luciferase activity was measured with 12.5% of the lysate reagents from the the Nano-Glo® Luciferase Assay System kit (Promega, Madison, WI). This system provides accurate reporter measurement via normalization to cell viability readings (as opposed to a dual-luciferase approach). All assays were performed in triplicate with appropriate controls.
2.5 Activity-Dependent Regulation
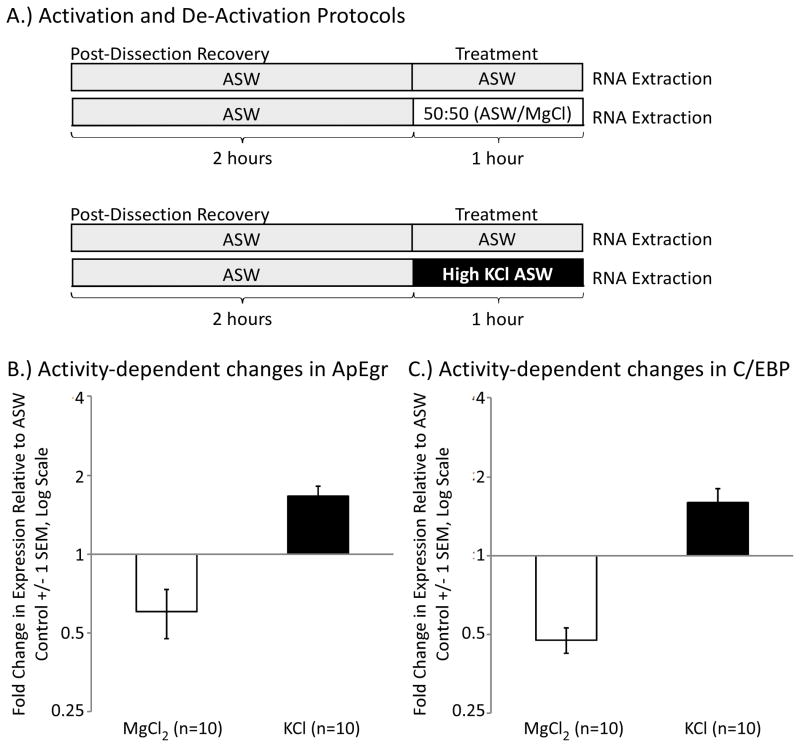
We analyzed activity-dependent transcription in isolated pedal/pleural ganglia (Kim et al., 2006; Ye, Shobe, Sharma, Marina, & Carew, 2008). Animals were anesthetized with an injection of isotonic 333mM MgCl2 (50% of body weight). An incision was then made along the ventral midline, and the left and right pedal/pleural ganglia were separately harvested and loosely pinned to a Sylgard-coated petri dish filled with artificial sea water. To facilitate penetration of different solutions, the sheath covering each ganglion was lightly nicked with dissecting scissors. Dishes were then covered and maintained at 15° C in a tissue culture incubator.
After 2 hours of recovery, each left/right pair of ganglia was randomly assigned to either a control or treatment condition (Figure 6A). Control ganglia received a bath exchange of Artificial Sea Water (ASW: 460 mM NaCl, 55mM MgCl2, 11mM CaCl2, 10mM KCl, 10mM Tris pH 7.6). Treatment ganglia received a bath exchange of either a) a 50:50 mix of ASW with isotoninc 333mM MgCl2, which suppresses most synaptic transmission in Aplysia (Liao & Walters, 2002) or b) a high KCl solution of ASW (High-KCl ASW: 370mM NaCl, 55mM MgCl2, 11mM CaCl2, 100mM KCl, 10mM Tris pH 7.6) which causes prolonged elevation of resting membrane potentials (Berry & Arch, 1981). 1 hour after treatment, ganglia were rapidly harvested for qPCR analysis.
Figure 6.
ApEgr expression is bidirectionally regulated by neural activity. A) Experimental protocol. Left and right pleural ganglia were isolated, allowed to recover for 2 hours, and then randomly assigned to a 1-hour treatment. To determine the effects of decreased activity, ganglia were treated with Artificial Sea Water (ASW) or a mix of 50:50 ASW to 333 mM MgCl2, which blocks synaptic transmission in Aplysia. For increased activity, conditions were ASW or a high-KCl formulation of ASW. Ganglia were immediately processed after treatment. B) Changes in ApEgr following MgCl2 and KCl treatment (n = 10 per condition) expressed as the ratio of treated to control ganglia from the same animal. C) Changes in C/EBP in the same ganglia.
2.6 Long-Term Sensitization
To assess learning-related changes in ApEgr expression, we drew upon a collection of CNS tissue harvested from animals 1 and 24 hours after long-term sensitization training (initial results with these samples reported in Bonnick et al., 2012). Animals were given unilateral long-term sensitization training following a standard protocol (Wainwright, Zhang, Byrne, & Cleary, 2002) but with a higher stimulus amplitude (90 mA AC) to increase the reliability of behavioral and transcriptional responses. Specifically, training consisted of 4 rounds of noxious shock applied at 30 minute intervals to one side of the body, with each round consisting of 10 pulses of 500ms duration at a rate of 1hz. Behavioral sensitization was confirmed in the 24-hour group by measuring the tail-elicited siphon withdrawal reflex before and 24 hours after training. We selected the pleural ganglia for analysis, as they contain the cell bodies of the tail-sensory neurons which are thought to serve as an important site for the neural plasticity underlying behavioral sensitization (Guan et al., 2002). Specifically, we compared the expression of ApEgr in the pleural ganglia from the trained side to expression in the matched pleural ganglia on the untrained side. For additional details on the collection and validation of these samples, please see Bonnick et al. (2012).
2.7 PCR
Because dissection can alter the expression of some Aplysia immediate-early genes (Alberini et al., 1994) we harvested tissue samples rapidly, usually within less than 5 minutes from anesthetization. Tissue was then rapidly homogenized and RNA extracted using Trizol (Invitrogen, Carlsbad CA) and Direct-Zol (Zymo, Irvine, CA) or RNeasy Mini Kit (Qiagen, Gaithersburg, MD). Samples were homogenized using the Bullet Blender (NextAdvance, Averill Park, NY). Quantity and quality of RNA was assessed using the NanoDrop 2000 (Thermo Scientific, Wilmington, DE). RNA was reverse transcribed using oligo(dT) primes with the RevertAid First Strand cDNA Synthesis Kit (Fermentas, Glen Burnie MD).
Quantitative PCR was conducted using Sybr Green and the MyIQ real time PCR system (Bio-Rad, Los Angeles CA). Primers were validated for correct PCR efficiency (Bonnick et al., 2012). For ApEgr we used a forward primer of AAGCTGTGACCGCAGATTTT and a backward primer of CATGGGTTGTCAAATGGTCA. qPCR samples were analyzed in triplicate and the relative amounts of each transcript were determined using the ddCT method and the Bio-rad IQ5 gene expression analysis (Bio-Rad, Los Angeles CA). For standard PCR to evaluate expression in different cells and ganglia, GoTaq® Green Master Mix was used according to the manufacturer’s instructions (Promega, Madison, WI).
All qPCR expression levels were normalized to levels of histone H4, a transcript which is stable during long-term sensitization training (Bonnick et al., 2012). For experimental manipulations of ApEgr expression, a fold-change score was calculated for each pair of ganglia as the ratio of expression from treated to untreated. Statistical analyses were conducted on fold-change scores using a one-sample t-test against the expected value of 1 for the null hypothesis. Mean fold-change scores are reported with 95% confidence intervals in brackets and Cohen’s d is reported as an estimate of effect size ( [Observed –Expected] / SD).
2.8 Bioinformatics
For protein sequence alignment, Geneious 6.0.5 was utilized (http://www.geneious.com/). Our goal for alignment was simply to place ApEgr within the context of known and confirmed Egrs. Therefore, we selected only exemplars from mammals (Egrs 1-4 from human and mouse) as well as the confirmed Egr homologs from Drosophila melanogaster and C. elegans (see introduction). After an initial attempt at alignment, however, poor alignment led us to remove three sequences from the analysis: klumpfuss, egrh-2, and ZK337.2
The phylogenetic tree was generated based on the alignment using the neighbor-joining method with the Jukes-Cantor model of genetic distance (both defaults within Geneious). Distance values are expressed in units of substitutions per site for each sequence within the alignment.
To predict the protein sequence and motifs for ApEgr, we used the NCBI ORF predictor: (http://www.ncbi.nlm.nih.gov/gorf/gorf.html).
3. Results
3.1 ApEgr encodes an egr-like protein
We screened the draft Aplysia genome for DNA sequences matching the region encoding the conserved zinc-finger domain from the mammalian Egrs. We identified a contig (GenBank: AASC02058700.1) with high similarity in this region and then used 5′ and 3′ RACE to confirm expression and delineate the full 3340 bp mRNA for this transcript, which we have termed ApEgr (GenBank: KC608221). Comparing the mRNA sequence from RACE to available genomic contigs suggests the presence of 2 exons separated by a 1075 bp intron (Figure 1). The predicted 593-amino-acid sequence contains the distinctive triplet of C2H2 zinc-finger domains which characterizes the Egr family of transcription factors.
Figure 1.
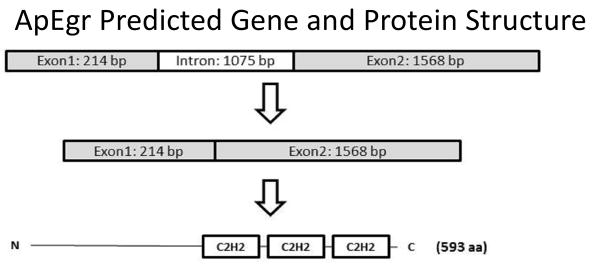
Predicted ApEgr gene structure. Comparison of mRNA determined via RACE with available genomic contigs suggests 2 exons separated by a 1075 bp intron. The NCBI open-reading frame predictor predicts an expressed protein containing the distinctive triplet of C2H2 zinc-finger domains near the C terminus that characterizes the Egr family.
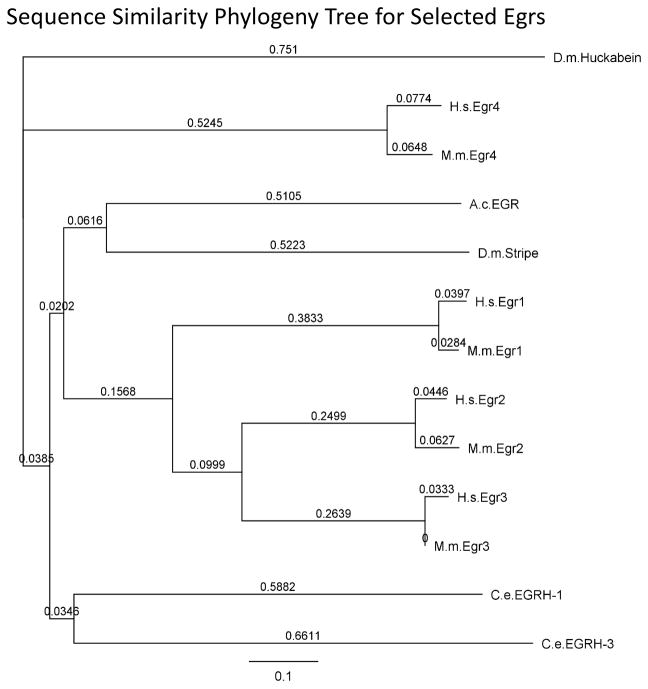
We compared the predicted protein sequence of ApEgr to representative mammalian Egrs (human and mouse Egrs1-4) as well as putative homologs from Drosophila (stripe and huckabein) and C. elegans (Egrh 1 and 3) (Figure 2). As expected, ApEgr is highly similar to other Egrs in the zinc-finger domain (up to 91%), but has a low overall similarity across species (18–28%; Supplemental Figure 1). The resulting gene phylogeny (Figure 3) suggests that ApEgr is most similar to stripe and almost equally similar to mammalian Egrs 1-3 (e.g. 25–28% similarity to mouse Egrs 1-3). This pattern is consistent with previous the possibility that ApEgr is homologous to some ancestral Egr prior to a set of duplication events, a pattern previously observed in zinc-finger proteins (Knight & Shimeld, 2001).
Figure 2.
Alignment of predicted ApEgr protein sequence with other selected Egrs. To place ApEgr within the context of known Egr homologs, it was aligned with exemplars from mammals (Egrs 1-4 from human and mouse) as well as the confirmed Egr homologs from Drosophila melanogaster (D.m.stripe, GenBank: NP_01163643; D.m.huckabein, GenBank: 2013347A)and C. elegans (C.e.Egrh-1, GenBank: NP_510462.2; C.e.Egrh-3, Genbank: CCD74212.1). Due to very poor alignment of their C2H2 domains, we excluded the proposed C. elegans homolog Egrh-2 and the Drosophila gene klumpfuss. C2H2 domains are noted as boxes.
Figure 3.
Gene phylogeny tree based on predicted sequence similarity from Figure 2. The figure was constructed using the neighbor-joining method with a Jukes-Cantor model of genetic distance. The values on the tree represent genetic distance between nodes expressed in units of substitution per site.
3.2 ApEgr selectively activates the GSG consensus sequence
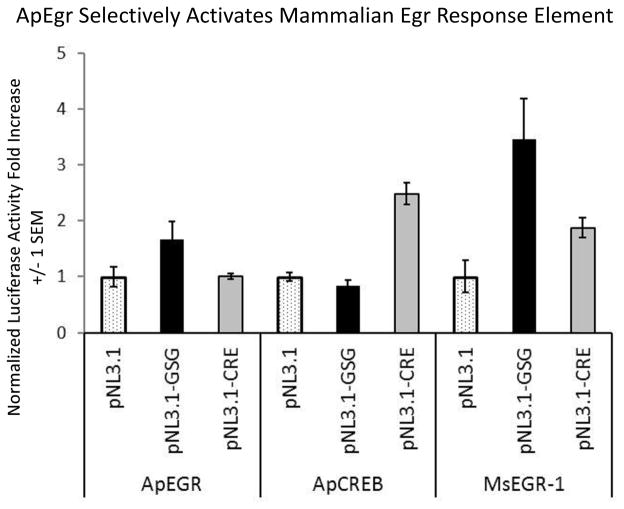
To determine if ApEgr encodes a functional transcription factor capable of activating the GSG motif recognized by mammalian Egrs, we utilized a luciferase assay. We created an ApEgr-GFP fusion construct (ApEgr in the pNEX3-GFP vector). This was co-transfected into S2 Drosophila cells with a luciferase construct containing either the GSG motif (pNL3.1-GSG), a CRE element (pNL3.1-CRE), or no additional promoter (pNL3.1) in front of the luciferase.
Cells transfected with both ApEgr and pNL3.1-GSG had a 1.7-fold increase in luciferase activity relative to controls transfected with ApEgr and a pNL3.1 vector without the GSG motif (pNL3.1). This activation was specific, as co-transfection of ApEgr with a CRE motif (pNL3.1-CRE) did not lead to elevated luciferase activity (left side of Figure 4).
Figure 4.
ApEgr selectively activates the GSG motif recognized by mammalian Egrs. Luciferase activity was measured in transfected S2 cells and normalized to cell viability. Bars represent luciferase activity normalized to the control co-transfection with an empty pNL3.1 vector (without an added response element) and are the average of 3 wells (+/− 1 SEM). Along with a vector encoding a transcription factor (ApEgr, ApCREB-1, or Mouse Egr-1) in pNEX3-GFP, cells were co-transfected with either an empty pNL3.1 luciferase (pNL3.1[Nluc/minP] ) vector (hashed bars), pNL3.1-GSG containing two GSG consensus motifs (black bars), or pNL3.1-CRE containing CRE recognition sites (grey bars).
As a positive control, we constructed a mouse Egr-GFP fusion construct (msEgr-1 in the pNEX3-GFP vector). Co-transfection with the different reporter vectors produced the same pattern of results, with luciferase activity enhanced by the combination of the msEgr-1 with the GSG domain (right side of Figure 4). Note, however, because each transcription factor is normalized to its own level of basal luciferase activity, results are not comparable across these constructs.
We also confirmed the selectivity of the GSG motif by showing that co-transfection with ApCREB-1 (ApCREB-1 in pNEX3-GFP) does not increase luciferase activity over control (middle of Figure 4). Taken together, these data indicate that ApEgr encodes a functional transcription factor which can recognize the consensus GSG motif recognized by the mammalian Egrs.
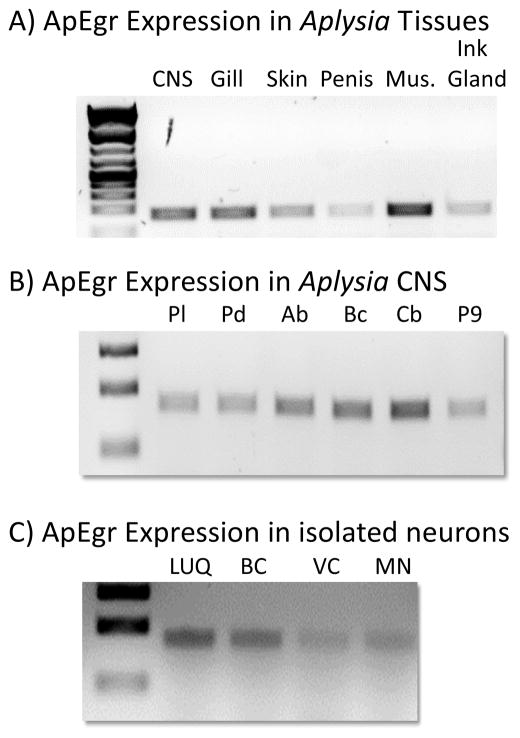
3.3 ApEgr is constituitively expressed throughout the body and CNS of Aplysia
To characterize the expression of ApEgr we conducted qualitative PCR on samples taken from Aplysia CNS, gill, skin, penis, muscle, and ink gland. We observed robust expression in each tissue (Figure 5A), and confirmed this pattern in triplicate (data not shown). To corroborate and extend this result, we consulted the recently published Illumina HiSeq data from the Broad Institute’s Aplysia transcriptome project (BioProject PRJNA77701). We found extensive fragments of ApEgr mRNA in all of the Broad samples: digestive system, gill, heart, hematopancreas, hermaphroditic ducts, muscle, ovotestis, salivary glands, and CNS. Similarly, the assemblages from each of these tissues contained near full-length fragments of apEgr (see http://aplysiagenetools.org)
Figure 5.
ApEgr is constitutively expressed in Aplysia tissue and CNS. A) Representative example of qualitative PCR for ApEgr from CNS, gill, skin, penis, muscle, and ink gland. The same pattern was confirmed in triplicate. B) Representative examples of qualitative PCR from the pleural (Pl), pedal (Pd), abdominal (Ab), buccal (Bc), and cerebral (Cb) ganglia as well as the P9 nerve. C) Representative examples of qualitative PCR from the LUQ neurons from the abdominal ganglia (LUQ), bag cells of the abdominal ganglia (BC), mechanosensory neurons of the VC cluster in the plueral ganglia (VC), and motor neurons of the pedal ganglia (MN).
To better understand CNS distribution, we conducted PCR on isolated CNS ganglia. We consistently observed ApEgr expression in all ganglia tested: pleural, pedal, abdominal, buccal, and cerebral (Figure 5B). In addition, we detected weak expression in the P9 nerve, which most likely stems from neuronal cell bodies located along the nerve (Bailey, Castellucci, Koester, & Kandel, 1979). Finally, we were also able to regularly detect ApEgr in samples of identified cell types: the LUQ neurons from the dorsal surface of the abdominal ganglia (Frazier, Kandel, Kupfermann, Waziri, & Coggeshall, 1967), bag cells of the abdominal ganglia, the VC cluster of mechanoreceptors of the pleural ganglia, and motor neurons of the pedal ganglia (Figure 5C). We did not test other cell types. The time taken after dissection to desheath ganglia and harvest individual cell types may have allowed some post-dissection changes in expression, so the results from isolated cell types may not reflect basal expression. Taken together, however, our results suggest that ApEgr is ubiquitously expressed in the CNS and tissues of late juvenile and adult Aplysia.
3.4 CNS ApEgr expression is bi-directionally regulated by neural activity
One of the hallmarks of the Egr family is transcriptional regulation by neural activity (Beckmann, Davidson, Goodenough, & Wilce, 1997; Honkaniemi & Sharp, 1999). We thus tested the effects of neural activity on transcriptional expression of ApEgr. First, we tested the effects of increased activity by exposing isolated pedal/plueral ganglia to either regular ASW or a high-KCl formulation of ASW for 1 hour (Figure 6A). We confirmed via extracellular nerve recordings that the treatment produces a prolonged increase in neural activity (data not shown). We found that high-KCl ASW produces a robust increase in ApEgr mRNA (Figure 6B). In 10 out of 10 animals tested, the pedal/plueral ganglia exposed to high KCl had higher ApEgr expression than the untreated ganglia from the same animal. The mean fold change was 1.66, a significant increase (95% CI [1.32, 2.01], Cohen’s d = 1.40 (see methods), t(8) = 4.43, p = 0.002). Similar to a prior report (Kim et al., 2006), we found that this treatment also increased expression of the immediate-early gene C/EBP (Figure 6C, Mean = 1.60, 95% CI [1.11, 2.07], d = 0.90, t(8) =2.86, p = 0.02).
Next we tested the effects of decreased neural activity by exposing isolated pedal/pleural ganglia to either ASW or a 50:50 mix of ASW and MgCl2, a solution commonly used as an anesthetic for Aplysia (Figure 6A). Again, we confirmed via extracellular nerve recordings that the treatment completely and persistently suppresses neural activity (data not shown). We found that decreased activity produces a modest reduction in ApEgr mRNA (Figure 6B). In 8 of 10 animals tested, the treated ganglia had lower expression than the untreated ganglia from the same animal (Mean = 0.60, 95% CI [0.31, 0.90], d = 0.98, t(8) = 3.10, p = 0.01). The treatment produced a similar but stronger decrease in C/EBP expression (Figure 6C, Mean = 0.48, 95% CI [0.36, 0.60], d = −3.2, t(8) = 10.06, p = 0.000008). Taken together, our results indicate that the expression of ApEgr is bi-directionally regulated by neural activity, similar to mammalian Egrs and to other Aplysia immediate-early genes.
3.5 CNS ApEgr expression is rapidly and persistently up-regulated by long-term sensitization training
Finally, we examined if ApEgr is regulated by long-term sensitization training. We drew on a collection of CNS tissues harvested from animals 1 and 24 hours after unilateral long-term sensitization training (initial results from these samples reported in Bonnick et al., 2012). All animals in the 24-hour group were confirmed to express significant long-term sensitization of the tail-elicited siphon withdrawal reflex on the trained side (post-test scores > 1.25 pre-test scores), and all samples passed a transcriptional control. Specifically, the 1 hour samples were all confirmed to have increased levels of C/EBP on the trained side. Similarly, 24-hour samples were confirmed to have training-induced increases in BiP, a chaperon protein which shows delayed but prolonged up-regulation after long-term sensitization training (Kuhl, Kennedy, Barzilai, & Kandel, 1992).
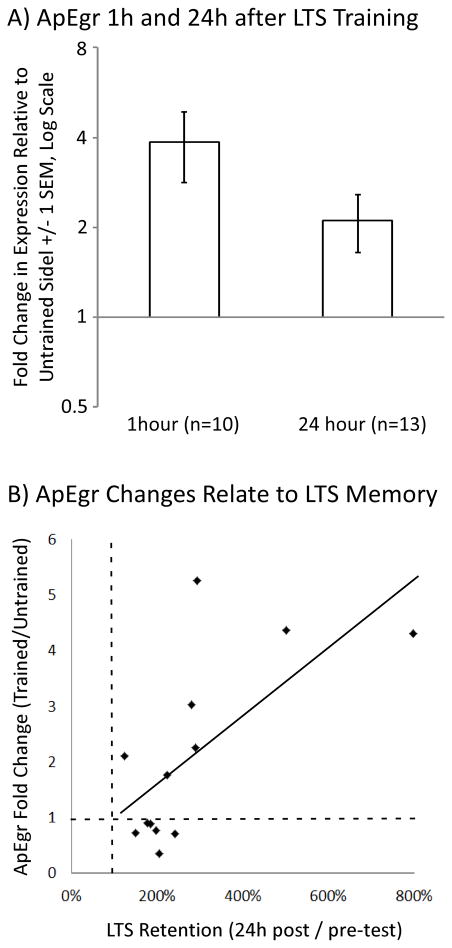
We found that long-term sensitization training produces a rapid and persistent up-regulation of ApEgr (Figure 7A). In the animals harvested 1 hour after training, ApEgr expression was higher in the trained pleural ganglion in 9 out of 10 samples, a significant increase (Mean = 3.85, 95% CI [1.52, 6.19], d = 0.87, t(8) = 2.76, p = 0.02). This effect was smaller but still evident 24 hours after training, with increased expression on the trained side in 7 of 13 animals, a significant increase (Mean = 2.10, 95% CI [1.11, 3.10], d = 0.67, t(11) = 2.42, p = 0.03). Moreover, the degree of change in ApEgr (ratio of expression trained to untrained side) was significantly correlated with the degree of long-term memory retention (ratio of 24-hour post-test to pre-test on the trained side) in trained animals (Figure 7B, r = 0.67, 95% CI [0.19, 0.89], p < 0.05). This correlation was not general to learning-related transcripts, as expression of BiP, which was also significantly up-regulated after training, was not correlated with memory (r = 0.09, 95% CI [−0.49, 0.61]).
Figure 7.
ApEgr mRNA is rapidly and persistently up-regulated by long-term sensitization training. A) Changes in ApEgr expression in the pleural ganglia 1 hour (n = 10) and 24 hours (n = 13) after unilateral long-term sensitization training. Data are expressed as the ratio of expression from the trained to the untrained side. B) Changes in ApEgr expression relate to degree of memory retention at 24 hours. Changes in ApEgr expression are plotted as the ratio of expression from the trained to untrained side (r = 0.67, 95% CI [0.19, 0.89], p < 0.05). The dotted line at 1 on the ApEgr axis represents no difference in expression. Memory expression is expressed as the ratio of tail-elicited siphon withdrawal duration 24 hours after training relative to duration prior to training. The dotted line at 1 on this axis represents no retention. ApEgr is an Aplysia homolog of the Early-Growth Response transcription factors. ApEgr recognizes the GSG motif recognized by Egrs. ApEgr is bidirectionally regulated by neural activity. ApEgr is rapidly and persistenly up-regulated during long-term sensitization.
4. Discussion
We identified a transcript, ApEgr, with strong homology to mammalian members of the Egr family: sequence homology in the critical zinc-finger domain that enables transcription-factor function, selective activation of the GSG motif recognized by mammalian Egrs, ubiquitous constitutive expression in CNS and other tissues, bidirectional regulation of expression by neural activity, and regulation correlated with long-term memory.
At this time, it does not seem possible to identify homology between ApEgr and a specific member of the mammalian Egr family. Outside of the region coding for the zinc-finger domain, we observe little similarity between ApEgr and the mammalian Egrs. Moreover, the expression pattern and functional characteristics of ApEgr we have documented are common to all the mammalian Egrs (with the exception of Egr-4 which is not yet known to be regulated by learning). Still, it seems that ApEgr clusters somewhat better with mammalian Egrs 1-3 (27–28% similarity) than to Egr-4 (22% similarity). One possibility is that ApEgr represents a basal form of Egr prior to a set of duplication events leading to vertebrate Egrs, a pattern which seems common amongst zinc-fingered proteins (Knight & Shimeld, 2001). Consistent with this possibility, we did not encounter any other putative Egr homologs in our screening of the draft Aplysia genome, nor in the collection of ESTs in GenBank, nor in the assemblage of Illumina reads produced by the Broad Institute. On the other hand, coverage in these sources is still rather poor, so we cannot rule out the possibility of additional Egr homologs within Aplysia.
In mammals, Egrs serve critical roles in long-term plasticity and memory. Our data is not conclusive, but it does demonstrate a) that long-term sensitization training persistently up-regulates ApEgr expression and b) that the level of up-regulation is correlated with the degree of memory retention 24 hours after training. This is strikingly similar to the pattern observed for Egr-1, which correlates with the expression of LTP (Abraham et al., 1993) and is essential for long-term memory maintenance (Jones et al., 2001; Yang et al., 2012). It seems possible, then, that ApEgr may also play a role in maintaining long-term sensitization memory, perhaps by sustaining the long-term facilitation at sensory-motor neuron synapses thought to underlie the expression of behavioral sensitization. It is worth considering, however, that Egr-2 is also up-regulated during LTP (Williams et al., 1995) but actually seems to serve as a memory repressor (Poirier et al., 2007). A key goal for future studies of ApEgr will be understanding what role, if any, it plays in long-term plasticity and memory.
Despite its relatively high basal level of expression, Egr-1 has proven useful for high-resolution mapping of neural circuitry in the mammalian and avian brain (Filipkowski, Knapska, & Kacz, 2006). To date, no IEGs in Aplysia have proven suitable for this purpose. It will be interesting to determine if in situ hybridization can resolve single-cell activation of ApEgr with sufficient fidelity to enable rapid characterization of functional circuits.
Egrs can influence memory by regulating the expression of a wide variety of genes involved in neural function. For example, Egr-1 is known to regulate the expression of genes involved in in synaptic function (synapsin I and II; Thiel, Schoch, & Petersohn, 1994), plasticity (Arg3.1/Arc; Li, Carter, Gao, Whitehead, & Tourtellotte, 2005), and excitability (NMDA receptor subunit 1; Bai & Kusiak, 1997) among many other transcripts. Currently available genomic data from Aplysia suggest a number of possible targets with upstream GSG motifs, including Ap-Creb1.
Finally, it will be interesting to explore the regulation of ApEgr. In mammals, ApEgr is upregulated by BDNF signaling via a C/EBP isoform (Calella et al., 2007). Supporting the possibility of similar regulation in Aplysia, we have so far always found Aplysia C/EBP to be co-regulated with ApEgr (following high KCl, MgCl2, and long-term sensitization training). To begin understanding its transcriptional regulation, we are currently working on the ApEgr promoter analysis.
One of the most important ideas to emerge from studying the neurobiology of Aplysia is the ‘molecular alphabet’ hypothesis proposed by Kandel and colleagues: that complex forms of learning are built from the same molecular building blocks as more elementary forms of learning (e.g. Hawkins & Kandel, 1984). This hypothesis has already found abundant support in the many homologies found between the molecular mechanisms of simple forms of learning in invertebrates and those in more complex forms of learning in vertebrates. The characterization of ApEgr helps close one of the notable gaps in this account, suggesting again a striking molecular continuity in the biological mechanisms of learning and memory.
Supplementary Material
ApEgr is an Aplysia homolog of the Early-Growth Response transcription factors.
ApEgr recognizes the GSG motif recognized by Egrs.
ApEgr is bidirectionally regulated by neural activity.
ApEgr is rapidly and persistenly up-regulated during long-term sensitization.
Acknowledgments
This work was supported by a SOMAS award to RCJ and BM and by NIH Grant R15MH090998-01 to RCJ and ICJ. Thanks to Wayne Sossin for providing the PNEX vector, Tom Abrams for making the very helpful aplysiagenetools.org database available, and Scott Kreher for feedback and advice on this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. Works Cited
- Abraham WC, Mason SE, Demmer J, Williams JM, Richardson CL, Tate WP, Dragunow M. Correlations between immediate early gene induction and the persistence of long-term potentiation. Neuroscience. 1993;56(3):717–27. doi: 10.1016/0306-4522(93)90369-q. [DOI] [PubMed] [Google Scholar]
- Alberini CM. Transcription factors in long-term memory and synaptic plasticity. Physiological reviews. 2009;89(1):121–45. doi: 10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberini CM, Ghirardi M, Metz R, Kandel ER. C/EBP is an immediate-early gene required for the consolidation of long-term facilitation in Aplysia. Cell. 1994;76(6):1099–114. doi: 10.1016/0092-8674(94)90386-7. [DOI] [PubMed] [Google Scholar]
- Arguello aa, Ye X, Bozdagi O, Pollonini G, Tronel S, Bambah-Mukku D, Alberini CM. CCAAT Enhancer Binding Protein Plays an Essential Role in Memory Consolidation and Reconsolidation. Journal of Neuroscience. 2013;33(8):3646–3658. doi: 10.1523/JNEUROSCI.1635-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai G, Kusiak JW. Nerve growth factor up-regulates the N-methyl-D-aspartate receptor subunit 1 promoter in PC12 cells. The Journal of biological chemistry. 1997;272(9):5936–42. doi: 10.1074/jbc.272.9.5936. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Castellucci VF, Koester J, Kandel ER. Cellular studies of peripheral neurons in siphon skin of Aplysia californica. Journal of neurophysiology. 1979;42(2):530–57. doi: 10.1152/jn.1979.42.2.530. [DOI] [PubMed] [Google Scholar]
- Bartsch D, Casadio A, Karl KA, Serodio P, Kandel ER. CREB1 encodes a nuclear activator, a repressor, and a cytoplasmic modulator that form a regulatory unit critical for long-term facilitation. Cell. 1998;95(2):211–23. doi: 10.1016/s0092-8674(00)81752-3. [DOI] [PubMed] [Google Scholar]
- Beckmann AM, Davidson MS, Goodenough S, Wilce Pa. Differential expression of Egr-1-like DNA-binding activities in the naive rat brain and after excitatory stimulation. Journal of neurochemistry. 1997;69(6):2227–37. doi: 10.1046/j.1471-4159.1997.69062227.x. [DOI] [PubMed] [Google Scholar]
- Beckmann AM, Wilce PA. Egr transcription factors in the nervous system. Neurochemistry international. 1997;31(4):477–510. doi: 10.1016/s0197-0186(96)00136-2. discussion 517–6. [DOI] [PubMed] [Google Scholar]
- Berry RW, Arch S. Activation of neurosecretory cells enhances their synthesis of secretory protein. Brain research. 1981;215(1–2):115–23. doi: 10.1016/0006-8993(81)90495-9. [DOI] [PubMed] [Google Scholar]
- Bonnick K, Bayas K, Belchenko D, Cyriac A, Dove M, Lass J, Calin-Jageman RJ. Transcriptional Changes following Long-Term Sensitization Training and In Vivo Serotonin Exposure in Aplysia californica. (Z.-P. Feng, Ed.) PLoS ONE. 2012;7(10):e47378. doi: 10.1371/journal.pone.0047378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva aJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79(1):59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Brönner G, Chu-LaGraff Q, Doe CQ, Cohen B, Weigel D, Taubert H, Jäckle H. Sp1/egr-like zinc-finger protein required for endoderm specification and germ-layer formation in Drosophila. Nature. 1994;369(6482):664–8. doi: 10.1038/369664a0. [DOI] [PubMed] [Google Scholar]
- Calella AM, Nerlov C, Lopez RG, Sciarretta C, Von Bohlen und Halbach O, Bereshchenko O, Minichiello L. Neurotrophin/Trk receptor signaling mediates C/EBPalpha, -beta and NeuroD recruitment to immediate-early gene promoters in neuronal cells and requires C/EBPs to induce immediate-early gene transcription. Neural development. 2007 Jan;2:4. doi: 10.1186/1749-8104-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Call KM, Glaser T, Ito CY, Buckler aJ, Pelletier J, Haber Da, Lewis WH. Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms’ tumor locus. Cell. 1990;60(3):509–20. doi: 10.1016/0092-8674(90)90601-a. [DOI] [PubMed] [Google Scholar]
- Clary LM, Okkema PG. The EGR family gene egrh-1 functions non-autonomously in the control of oocyte meiotic maturation and ovulation in C. elegans. Development (Cambridge, England) 2010;137(18):3129–37. doi: 10.1242/dev.041616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole AJ, Saffen DW, Baraban JM, Worley PF. Rapid increase of an immediate early gene messenger RNA in hippocampal neurons by synaptic NMDA receptor activation. Nature. 1989;340(6233):474–6. doi: 10.1038/340474a0. [DOI] [PubMed] [Google Scholar]
- Crosby SD, Veile Ra, Donis-Keller H, Baraban JM, Bhat RV, Simburger KS, Milbrandt J. Neural-specific expression, genomic structure, and chromosomal localization of the gene encoding the zinc-finger transcription factor NGFI-C. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(14):6663. doi: 10.1073/pnas.89.14.6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S, Vanhoutte P, Pages C, Caboche J, Laroche S. The MAPK/ERK cascade targets both Elk-1 and cAMP response element-binding protein to control long-term potentiation-dependent gene expression in the dentate gyrus in vivo. The Journal of neuroscience. 2000;20(12):4563–72. doi: 10.1523/JNEUROSCI.20-12-04563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipkowski RK, Knapska E, Kacz c-Fos and Zif268 in Learning and Memory — Studies on Expression and Function. In. Immediate Early Genes in Sensory Processing, Cognitive Performance and Neurological Disorders. 2006:137–158. [Google Scholar]
- Frazier W, Kandel ER, Kupfermann I, Waziri R, Coggeshall RE. Morphological and functional properties of identified neurons in the abdominal ganglion of Aplysia californica. Journal of neurophysiology. 1967;30(6):1288–1351. [Google Scholar]
- Guan Z, Giustetto M, Lomvardas S, Kim JH, Miniaci MC, Schwartz JH, Kandel ER. Integration of long-term-memory-related synaptic plasticity involves bidirectional regulation of gene expression and chromatin structure. Cell. 2002;111(4):483–93. doi: 10.1016/s0092-8674(02)01074-7. [DOI] [PubMed] [Google Scholar]
- Guo C-H, Senzel A, Li K, Feng Z-P. De novo protein synthesis of syntaxin-1 and dynamin-1 in long-term memory formation requires CREB1 gene transcription in Lymnaea stagnalis. Behavior genetics. 2010;40(5):680–93. doi: 10.1007/s10519-010-9374-9. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Setlow B, Wagner EK, McGaugh JL. Experience-dependent gene expression in the rat hippocampus after spatial learning: a comparison of the immediate-early genes Arc, c-fos, and zif268. The Journal of neuroscience_: the official journal of the Society for Neuroscience. 2001;21(14):5089–98. doi: 10.1523/JNEUROSCI.21-14-05089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nature neuroscience. 2000;3(6):533–5. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- Hawkins RD, Kandel ER. Is there a cell-biological alphabet for simple forms of learning? Psychological review. 1984;91(3):375–91. [PubMed] [Google Scholar]
- Honkaniemi J, Sharp FR. Prolonged expression of zinc finger immediate-early gene mRNAs and decreased protein synthesis following kainic acid induced seizures. The European journal of neuroscience. 1999;11(1):10–7. doi: 10.1046/j.1460-9568.1999.00401.x. [DOI] [PubMed] [Google Scholar]
- Jones MW, Errington ML, French PJ, Finea Bliss TV, Garel S, Davis S. A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nature neuroscience. 2001;4(3):289–96. doi: 10.1038/85138. [DOI] [PubMed] [Google Scholar]
- Katche C, Goldin A, Gonzalez C, Bekinschtein P, Medina JH. Maintenance of long-term memory storage is dependent on late posttraining Egr-1 expression. Neurobiology of learning and memory. 2012;98(3):220–227. doi: 10.1016/j.nlm.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Kim H, Lee S-H, Han J-H, Lee J-A, Cheang Y-H, Chang D-J, Kaang B-K. A nucleolar protein ApLLP induces ApC/EBP expression required for long-term synaptic facilitation in aplysia neurons. Neuron. 2006;49(5):707–18. doi: 10.1016/j.neuron.2006.01.035. [DOI] [PubMed] [Google Scholar]
- Klein T, Campos-ortega JA. klumpfuss, a Drosophila gene encoding a member of the EGR family of transcription factors, is involved in bristle and leg development. 1997;3134:3123–3134. doi: 10.1242/dev.124.16.3123. [DOI] [PubMed] [Google Scholar]
- Knapska E, Kaczmarek L. A gene for neuronal plasticity in the mammalian brain: Zif268/Egr-1/NGFI-A/Krox-24/TIS8/ZENK? Progress in neurobiology. 2004;74(4):183–211. doi: 10.1016/j.pneurobio.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Knight RD, Shimeld SM. Identification of conserved C2H2 zinc-finger gene families in the Bilateria. Genome biology. 2001;2(5):RESEARCH0016. doi: 10.1186/gb-2001-2-5-research0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzus E. The relation of transcription to memory formation. Acta biochimica Polonica. 2003;50(3):775–82. [PubMed] [Google Scholar]
- Kuhl D, Kennedy TE, Barzilai A, Kandel ER. Long-term sensitization training in Aplysia leads to an increase in the expression of BiP, the major protein chaperon of the ER. The Journal of cell biology. 1992;119(5):1069–76. doi: 10.1083/jcb.119.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC, VijayRaghavan K, Celniker SE, Tanouye Ma. Identification of a Drosophila muscle development gene with structural homology to mammalian early growth response transcription factors. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(22):10344–8. doi: 10.1073/pnas.92.22.10344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Carter J, Gao X, Whitehead J, Tourtellotte WG. The neuroplasticity-associated arc gene is a direct transcriptional target of early growth response (Egr) transcription factors. Molecular and cellular biology. 2005;25(23):10286–300. doi: 10.1128/MCB.25.23.10286-10300.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Yun SH, Keblesh J, Trommer BL, Xiong H, Radulovic J, Tourtellotte WG. Egr3, a synaptic activity regulated transcription factor that is essential for learning and memory. Molecular and cellular neurosciences. 2007;35(1):76–88. doi: 10.1016/j.mcn.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X, Walters ET. The use of elevated divalent cation solutions to isolate monosynaptic components of sensorimotor connections in Aplysia. Journal of neuroscience methods. 2002;120(1):45–54. doi: 10.1016/s0165-0270(02)00189-9. [DOI] [PubMed] [Google Scholar]
- Mack KJ, Cortner J, Mack P, Farnham PJ. krox 20 messenger RNA and protein expression in the adult central nervous system. Brain research Molecular brain research. 1992;14(1–2):117–23. doi: 10.1016/0169-328x(92)90018-7. [DOI] [PubMed] [Google Scholar]
- Malkani S, Rosen JB. Specific induction of early growth response gene 1 in the lateral nucleus of the amygdala following contextual fear conditioning in rats. Neuroscience. 2000;97(4):693–702. doi: 10.1016/s0306-4522(00)00058-0. [DOI] [PubMed] [Google Scholar]
- McDonnell J, Latif ML, Rees ES, Bevan NJ, Hill SJ. Influence of receptor number on the stimulation by salmeterol of gene transcription in CHO-K1 cells transfected with the human beta2-adrenoceptor. British journal of pharmacology. 1998;125(4):717–26. doi: 10.1038/sj.bjp.0702139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbrandt J. A nerve growth factor-induced gene encodes a possible transcriptional regulatory factor. Science (New York, NY) 1987;238(4828):797–9. doi: 10.1126/science.3672127. [DOI] [PubMed] [Google Scholar]
- Moorman S, Mello CV, Bolhuis JJ. From songs to synapses: molecular mechanisms of birdsong memory. Molecular mechanisms of auditory learning in songbirds involve immediate early genes, including zenk and arc, the ERK/MAPK pathway and synapsins. BioEssays_: news and reviews in molecular, cellular and developmental biology. 2011;33(5):377–85. doi: 10.1002/bies.201000150. [DOI] [PubMed] [Google Scholar]
- Nikitin VP, Kozyrev SA. Effects of Antisense Oligonucleotides to mRNA for the Early Gene zif268 on the Mechanisms of Synapse-Specific Plasticity. 2007;37(6) doi: 10.1007/s11055-007-0059-7. [DOI] [PubMed] [Google Scholar]
- Poirier R, Cheval H, Mailhes C. Paradoxical role of an Egr transcription factor family member, Egr2/Krox20, in learning and memory. Frontiers. 2007 Dec;1:1–12. doi: 10.3389/neuro.08/006.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier R, Cheval H, Mailhes C, Garel S, Charnay P, Davis S, Laroche S. Distinct functions of egr gene family members in cognitive processes. Frontiers in neuroscience. 2008;2(1):47–55. doi: 10.3389/neuro.01.002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubenfeld SM, Milekic MH, Monti B, Alberini CM. The consolidation of new but not reactivated memory requires hippocampal C/EBPbeta. Nature neuroscience. 2001;4(8):813–8. doi: 10.1038/90520. [DOI] [PubMed] [Google Scholar]
- Thiel G, Schoch S, Petersohn D. Regulation of synapsin I gene expression by the zinc finger transcription factor zif268/egr-1. The Journal of biological chemistry. 1994;269(21):15294–301. [PubMed] [Google Scholar]
- Timbers Ta, Rankin CH. Tap withdrawal circuit interneurons require CREB for long-term habituation in Caenorhabditis elegans. Behavioral neuroscience. 2011;125(4):560–6. doi: 10.1037/a0024370. [DOI] [PubMed] [Google Scholar]
- Wainwright ML, Zhang H, Byrne JH, Cleary LJ. Localized neuronal outgrowth induced by long-term sensitization training in aplysia. The Journal of neuroscience. 2002;22(10):4132–41. doi: 10.1523/JNEUROSCI.22-10-04132.2002. doi:20026347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J, Dragunow M, Lawlor P, Mason S, Abraham WC, Leah J, Tate W. Krox20 may play a key role in the stabilization of long-term potentiation. Molecular Brain Research. 1995;28(1):87–93. doi: 10.1016/0169-328X(94)00187-J. [DOI] [PubMed] [Google Scholar]
- Yamagata K, Kaufmann WE, Lanahan A, Papapavlou M, Barnes CA, Andreasson KI, Worley PF. Egr3/Pilot, a zinc finger transcription factor, is rapidly regulated by activity in brain neurons and colocalizes with Egr1/zif268. Learning & memory (Cold Spring Harbor, NY) 1994;1(2):140–52. doi: 10.1101/lm.1.2.140. [DOI] [PubMed] [Google Scholar]
- Yang Y, Shu X, Liu D, Shang Y, Wu Y, Pei L, Lu Y. EPAC Null Mutation Impairs Learning and Social Interactions via Aberrant Regulation of miR-124 and Zif268 Translation. Neuron. 2012;73(4):774–788. doi: 10.1016/j.neuron.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Shobe JL, Sharma SK, Marina A, Carew TJ. Small G proteins exhibit pattern sensitivity in MAPK activation during the induction of memory and synaptic facilitation in Aplysia. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(51):20511–6. doi: 10.1073/pnas.0808110105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin JC, Wallach JS, Del Vecchio M, Wilder EL, Zhou H, Quinn WG, Tully T. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell. 1994;79(1):49–58. doi: 10.1016/0092-8674(94)90399-9. [DOI] [PubMed] [Google Scholar]
- Yoshino M, Mizutani T, Yamada K, Tsuchiya M, Minegishi T, Yazawa T, Miyamoto K. Early growth response gene-1 regulates the expression of the rat luteinizing hormone receptor gene. Biology of reproduction. 2002;66(6):1813–9. doi: 10.1095/biolreprod66.6.1813. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.