Abstract
The vaginal epithelium provides a barrier to pathogens and recruits immune defenses through the secretion of cytokines and chemokines. Several studies have shown that mucosal sites are innervated by norepinephrine-containing nerve fibers. Here we report that norepinephrine potentiates the proinflammatory response of human vaginal epithelial cells to products produced by Staphylococcus aureus, a pathogen that causes menstrual toxic shock syndrome. The cells exhibit immunoreactivity for catecholamine synthesis enzymes and the norepinephrine transporter. Moreover, the cells secrete norepinephrine and dopamine at low concentrations. These results indicate that norepinephrine may serve as an autocrine modulator of proinflammatory responses in the vaginal epithelium.
Keywords: Norepinephrine, catecholamine, epithelium, superantigens, toxic shock syndrome, Staphylococcus aureus
1. Introduction
Primary and secondary lymphoid tissues, including those constituting the mucosal immune system, are innervated by catecholamine-containing nerves that originate in paravertebral sympathetic chain ganglia (Elenkov et al., 2000). Catecholamines, and in particular norepinephrine (NE), can interact directly with leukocytes to regulate innate and adaptive immune responses (Nance and Sanders, 2007; Sanders, 2012). Within the mucosal immune system, epithelial cells expressing a variety of pathogen recognition receptors play a key role in first-line defense against pathogen invasion at mucosal surfaces (Ashida et al., 2011; Ryu et al., 2010; Wira et al., 2005). In addition to providing a physical barrier to pathogen entry, these cells secrete and control the thickness and viscosity of a mucus barrier, support a protective commensal microflora population at and above the mucosal surface, and secrete antimicrobial peptides and other effector molecules, including several cytokines and chemokines. As with leukocytes, epithelial cells at many mucosal sites express adrenergic receptors, and NE has been shown to alter epithelial defense functions. These functions include the vectorial secretion of secretory IgA and other defense molecules (Gross et al., 2010; Linden, 1996; Schmidt et al., 2007), interactions of epithelial cells with bacteria (Chen et al., 2006; Green et al., 2003), epithelial mucus, ion and fluid transport (Holmgren and Olsson, 2011), and vectorial cytokine and chemokine secretion (Chiu et al., 2007; Cox et al., 2007; Prause et al., 2003; Salathe, 2002).
The vaginal and ectocervical mucosae are important sites for the development of infections, including those associated with pathogenic bacteria, such as Staphylococcus aureus, and viruses, such as HIV. Compared to type I mucosal sites in the gut and endocervix that contain a single layer of columnar epithelium, the vaginal mucosa represents a type II mucosal site consisting of stratified, squamous epithelial layers that rely on intercellular lipids instead of tight junctions to create a defensive physical barrier (Blaskewicz et al., 2011; Iwasaki, 2010; Kumamoto and Iwasaki, 2012). Pathogenic microorganisms must traverse the multi-cellular epithelial barrier to reach the submucosa where immune cells, lymphatic vessels, and blood vessels reside. In some infections of the vaginal tract, pathogens or their exotoxins evoke the release of proinflammatory cytokines and chemokines from mucosal epithelial cells, which act to disrupt barrier function and recruit adaptive immune cells to the mucosa. Staphylococcal superantigens, including toxic shock syndrome toxin-1 (TSST-1), can induce inflammation from the epithelium through the binding of CD40 on epithelial cells (Brosnahan et al., 2008; Spaulding et al., 2012). This proinflammatory response is thought to recruit T lymphocytes and macrophages to the submucosa, where the superantigens can then activate these adaptive immune cells through the binding of the T cell receptor (TCR) and major histocompatibility complex II (MHC II) to produce a cytokine storm that leads to menstrual toxic shock syndrome (mTSS) (Brosnahan and Schlievert, 2011). The severe hypotension accompanying this life-threatening syndrome is conventionally offset by treatment with catecholamine-based vasopressor drugs, including NE and dopamine (Chuang et al., 2005; Povoa and Carneiro, 2010; Reiss, 2000).
There is anatomical evidence in animals and humans indicating that the cervicovaginal mucosa is innervated by nerves containing several peptidergic and non-peptidergic neurotransmitters, including NE, vasoactive intestinal peptide (VIP), and neuropeptide Y (NPY) (Hoyle et al., 1996; Jorgensen et al., 1989; Lakomy et al., 1995; Lakomy et al., 1987; Owman et al., 1967; Papka et al., 1985; Rosengren and Sjoberg, 1967). Vaginal epithelial cells could potentially interact with neurotransmitters present in these neural elements as well as in circulating blood. In addition, experiments by Pullar and her colleagues (Pullar et al., 2007) indicate that stratified squamous epithelial cells of the cornea appear to synthesize catecholamines. It is not known if epithelial cells of the vagina might similarly produce these substances. Indeed, the role of neurotransmitters in regulating vaginal epithelial cell functions remains unknown. Therefore in the present study, we addressed the hypotheses that (1) NE, VIP, and NPY modulate the release of immune mediators from these cells after stimulation by bacterial proinflammatory products and (2) human vaginal epithelial cells are capable of synthesizing and secreting catecholamines.
2. Materials and Methods
Cell lines and general reagents
Two immortalized human vaginal epithelial cell (HVEC) lines were employed in this study. One line (HVEC-1) was obtained from ATCC (CRL-2616), while the other (HVEC-2) was kindly provided by Dr. Patrick Schlievert (University of Iowa). Both lines were transformed using HPV-16 E6/E7. Cells were cultured in keratinocyte serum-free medium (KSFM, Life Technologies, Grand Island, NY) with streptomycin, penicillin (Mediatech, Inc., Manassas, VA) and amphotericin B (Life Technologies), each at a 1% final concentration. Cells were grown at 37 °C with 5% CO2 in 96-well tissue culture plates. On experiment days, KSFM without antimicrobial drugs was used, as the latter have been shown to decrease cytokine production from the cells (unpublished observation). The bacterial superantigens toxin shock syndrome toxin-1 (TSST-1) and staphylococcal enterotoxin C (SEC) were provided by Dr. Schlievert and solubilized in KSFM at working concentrations of 100 μg TSST-1/ml (unless otherwise noted) and 10 μg SEC/ml. Purified peptidoglycan from S. aureus was obtained from Sigma-Aldrich (St. Louis, MO) and used at a concentration of 10 μg/ml. NE (Sigma-Aldrich) was used at a concentration of 10 μM, unless otherwise noted. IKK 16 (Tocris/R&D Systems, Minneapolis, MN) was used at a concentration of 1 μM. VIP (EMD Millipore, Billerica, MA) and NPY (Sigma-Aldrich) were both used at 0.1 μM.
Detection and quantification of cytokines and chemokines
HVECs grown to near confluency were incubated with TSST-1, SEC, peptidoglycan, or neurotransmitters/neuropeptides, alone or in combination, for 6 hours at 37 °C with 5% CO2. Supernates were then collected and frozen at −20 °C until later analysis.
Quantikine ELISA kits from R&D Systems (Minneapolis, MN) were used to detect IL-6 (D6050), IL-8 (D8000C), and MIP-3α (DM3A00) secreted into the tissue culture medium. For some assays, cytotoxicity was determined using the CellTiter 96® AQueous Assay (Promega, Madison, WI).
Adrenergic receptor determination
The following drugs were used in an aqueous concentration of 1 μM to block potential adrenergic receptors on HVECs: phentolamine (α-adrenergic antagonist, Sigma-Aldrich), propranolol (β-adrenergic antagonist, Sigma-Aldrich), atenolol (β1-selective adrenergic antagonist, Sigma-Aldrich), ICI 1118551 (β2-selective adrenergic antagonist, Sigma-Aldrich), and SR 59230A (β2/3-adrenergic antagonist, Tocris Bioscience/R&D Systems, Minneapolis, MN). Cells were incubated with TSST-1 with or without 10 μM NE and one of the adrenergic receptor antagonists for 6 hours. At the end of each experiment, culture supernatants were collected and assayed for IL-8 and/or IL-6 production by the cells (see above). cAMP assays. Intracellular cAMP levels were determined using a cAMP Parameter Assay Kit from R&D Systems (KGE002B). Cells were incubated with TSST-1 or peptidoglycan with or without NE for 15 minutes, 30 minutes, 1 hour, 3 hours, or 6 hours; cell culture supernates were subsequently collected and cells were lysed according to the manufacturer’s instructions. Supernates were analyzed by ELISA for IL-8 or IL-6, while lysates were analyzed by ELISA for intracellular cAMP. Additional experiments utilized NKH 477 (1 μM, Tocris/R&D Systems) to directly stimulate adenylate cyclase.
Immunohistochemistry
Clinically normal human vaginal biopsies (N = 2) were obtained through the Tissue Procurement Facility at the University of Minnesota and were immersion fixed in modified Zamboni’s fixative (4% paraformaldehyde and 0.2% picric acid in 0.1M phosphate buffer, pH 6.9) for 2 h at room temperature. Tissues were washed in PBS and stored in PBS containing 10% sucrose and 0.05% sodium azide until cut into 14 μm slide-mounted cryostat sections. HVECs were grown to near confluency on 8-well culture slides (BD Biosciences, San Jose, CA), then fixed with either modified Zamboni’s fixative or 4% formaldehyde. Blocking buffer (PBS containing 0.3% Triton-X, 1% BSA, 1% normal donkey serum, and 0.01% sodium azide) was added to tissue sections or cells for 1 hour at room temperature and then primary antibodies were added and allowed to incubate overnight at 4 °C. Unbound antibody was removed by a series of three washes with PBS and secondary antibody (diluted in blocking buffer) was allowed to incubate on the tissue sections or cells for 1 hour at room temperature. Unbound antibody was once again removed by three PBS washes and specimens were cover slipped using Vectashield mounting medium with or without 4‘,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA) for visualization. Images were taken using a FluoView 1000 confocal microscope and adjusted for brightness in Adobe Photoshop. Primary antibodies and titers were as follows: mouse anti-human norepinephrine transporter (NET; MAB 5620; EMD Millipore, Billerica, MA), 1:1000; mouse anti-human dopamine β-hydroxylase (DBH; catalog number 22806, ImmunoStar, Hudson, WI), 1:1000; rabbit anti-human tyrosine hydroxylase (TH; catalog number 22941, ImmunoStar), 1:1000; rabbit anti-human vasoactive intestinal peptide (VIP; catalog number 20077, ImmunoStar), 1:1000; rabbit anti-human neuropeptide Y (NPY; catalog number 22940, ImmunoStar), 1:1000; rabbit anti-human β2-adrenergic receptor (ab36956, Abcam, Cambridge, MA), 1:20. A blocking peptide (Abcam, AB38102, 10 μg/ml) was used to confirm the specificity of β2-adrenergic receptor-like immunoreactivity. Fluorescently labeled secondary antisera were used, and secondary antibody-only controls were used to verify all immunoreactive staining.
RT-PCR
Reactions were carried out to identify mRNA for NET, TH, and DBH in the two HVEC lines. Briefly, RNA from cells was harvested and reverse-transcribed using the RNEasy Mini and QuantiTech kits from Qiagen (Valencia, CA). Quantitative PCR was done using an ABI 7500 real-time PCR system (Applied Biosystems/Life Technologies, Carlsbad, CA). The following primer sets were used: NET (forward 5‘-ATGGAGTGGACAGGTTCAGC-3‘, reverse 5‘-TGGCTTGAAGTTGATGATGC-3‘) and TH (forward 5‘-ATTGCTGAGATCGCCTTCCA-3‘, reverse 5‘-AATCTCCTCGGCGGTGTACTC-3‘ (Stutterheim et al., 2009)). Numerous primers were designed in attempts to detect DBH, but none yielded positive results.
Detection of norepinephrine and dopamine synthesis by HVECs
The 2-CAT (N-D) Research ELISA kit from Immuno Biological Laboratories (Minneapolis, MN) was used to detect NE and dopamine synthesized by HVECs. Cells were grown in T75 tissue culture flasks for 24 hours with 1 mM tyrosine (Sigma-Aldrich), to provide adequate amounts of precursor for catecholamine synthesis. EDTA (1 mM, Sigma-Aldrich) and sodium metabisulfite (4 mM, Sigma-Aldrich) were added at the conclusion of the incubation per manufacturer’s instructions to prevent catecholamine degradation. Cell culture media were frozen and lyophilized and then reconstituted in a small volume of water prior to analysis. Cells were treated with 3 μM ionomycin (Sigma-Aldrich) for 20 minutes to release any intracellular catecholamines and then supernates were collected and either concentrated by lyophilization or tested directly. Assays were performed according to the manufacturer’s instructions.
3. Results
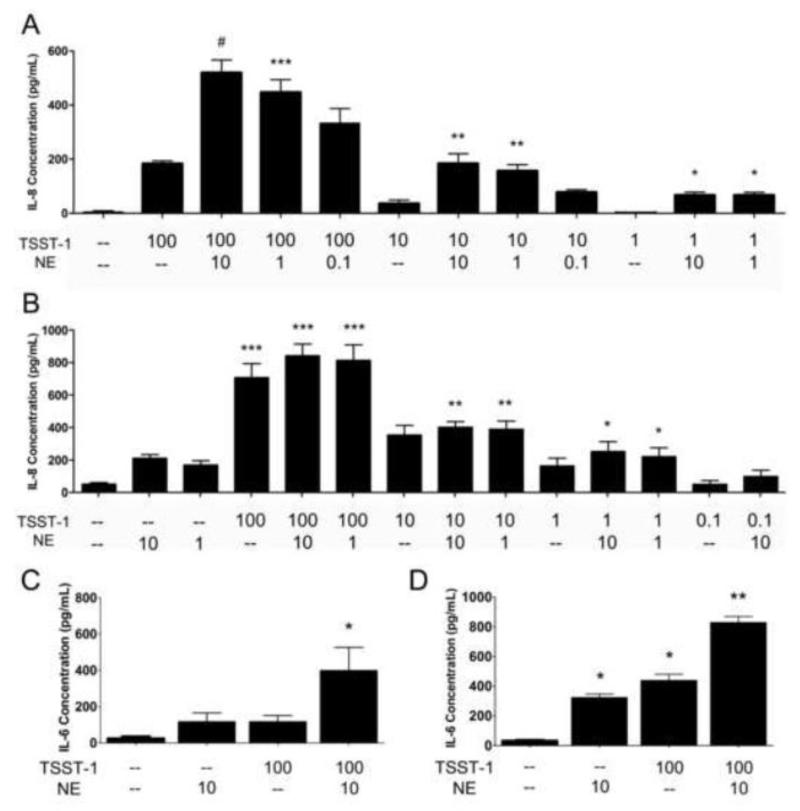
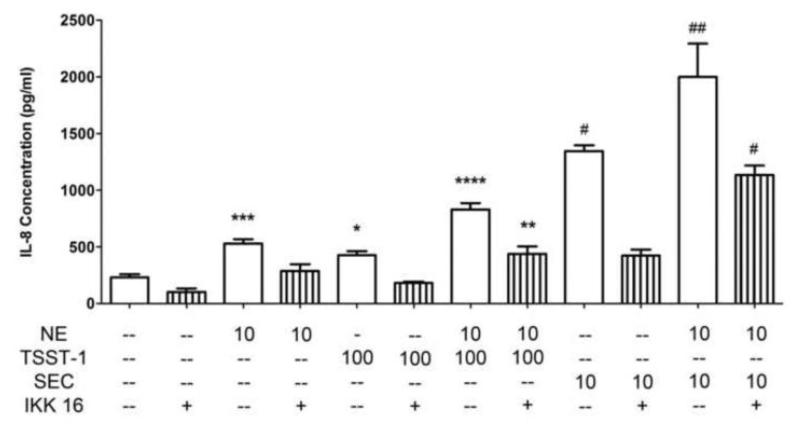
To determine the function of NE in the vaginal epithelium, we examined the ability of NE to alter immune responses of HVECs to proinflammatory stimuli. NE has been shown to act as a catecholate-type siderophore and increase the virulence of some pathogenic bacteria, including S. aureus (Beasley et al., 2011; Freestone et al., 2008). Therefore we chose to assess the well-characterized proinflammatory response of HVECs to the S. aureus superantigen TSST-1 rather than to live bacterial cells (Brosnahan et al., 2008; Peterson et al., 2005). Although it had little effect on the release of cytokines per se, NE significantly enhanced TSST-1 evoked IL-8 secretion in both HVEC lines in a concentration-related manner (Figure 1 a,b). NE also enhanced IL-6 responses to TSST-1 in both cell lines (Figure 1 c,d). Using another proinflammatory stimulus, the TLR-2 agonist peptidoglycan, we were able to show that NE also augmented the IL-6 and IL-8 response of HVECs to this stimulus (Figure 2). In contrast, dopamine did not affect IL-8 secretion from toxin-treated cells (data not shown). IKK 16, an inhibitor of IκB kinase and thus of NF-κB activation (Waelchli et al., 2006), reduced IL-8 production in HVECs treated with peptidoglycan or the superantigens TSST-1 and SEC. However, IKK 16 did not alter the ability of NE to enhance IL-8 secretion from these cells (Figure 3).
Figure 1.
Norepinephrine (NE) enhances IL-8 and IL-6 secretion by HVECs in response to toxic shock syndrome toxin-1 (TSST-1). Cells were incubated with decreasing amounts of TSST-1 and NE to examine the effect of concentration on the IL-8 responses. A) HVEC-1 cells. #Significantly higher than medium only control, TSST-1 (100 μg/ml), and TSST-1 (100 μg/ml) + NE (0.1 μM) and ***significantly higher than medium only control and TSST-1 (100 μg/ml) by one-way ANOVA [F(4,25)=28.43, p<0.0001] and Tukey’s post-hoc test. **Significantly higher than medium only control and TSST-1 (10 μg/ml) by one-way ANOVA [F(4,25)=13.80, p<0.0001] and Tukey’s post-hoc test. *Significantly higher than medium only control and TSST-1 (1 μg/ml) by one-way ANOVA [F(3,17)=15.43, p<0.0001] and Tukey’s post-hoc test. N=6 (except TSST-1 1 μg/ml experiments, N=3). B) HVEC-2 cells. ***Significantly higher than medium only and NE only controls by one-way ANOVA [F(5,69)=28.94, p<0.0001] and Tukey’s post-hoc test. **Significantly higher than medium only and NE only controls by one-way ANOVA [F(5,63)=16.56, p<0.0001] and Tukey’s post-hoc test. *Significantly higher than medium only control by one-way ANOVA [F(5,54)=4.591, p=0.0015] and Tukey’s post-hoc test. N=6-15. Cells were incubated with 100 μg/ml TSST-1 and/or 10 μM NE and assessed for IL-6 production by ELISA. C) HVEC-1 cells. *Significantly higher than all other conditions by one-way ANOVA [F(3,32)=5.268, p<0.01] and Tukey’s post-hoc test. N=9. D) HVEC-2 cells. **Significantly higher than all other conditions and *significantly higher than medium only control by one-way ANOVA [F(3,41)=109.9, p<0.0001] and Tukey’s post-hoc test. N=15. TSST-1 concentrations in μg/ml and NE concentrations in μM. Error bars represent the standard error of the mean.
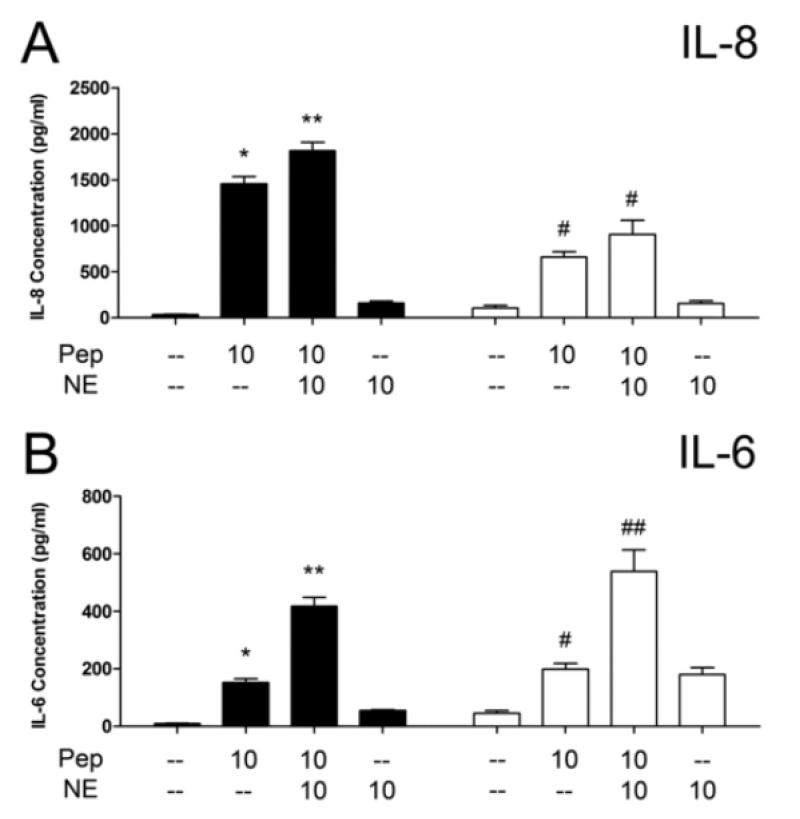
Figure 2.
Norepinephrine (NE) enhances IL-8 and IL-6 secretion by HVECs in response to peptidoglycan. A) IL-8 production by two lines of HVECs in the presence of peptidoglycan +/− NE. *Significantly higher than medium only and NE only controls and **significantly higher than all other conditions by one-way ANOVA [F(3,31)=212.7, p<0.0001] and Tukey’s post-hoc test. #Significantly higher than medium only and NE only controls by one-way ANOVA [F(3,31)=23.51, p<0.0001] and Tukey’s post-hoc test. B) IL-6 production by HVECs. *Significantly higher than medium only and NE only controls and **significantly higher than all other conditions by one-way ANOVA [F(3,32)=110.1, p<0.0001] and Tukey’s post-hoc test. #Significantly higher than medium only control and ##significantly higher than all other conditions by one-way ANOVA [F(3,31)=29.25, p<0.0001] and Tukey’s post-hoc test. HVEC-1: black bars, HVEC-2: white bars. Peptidoglycan (Pep) concentrations in μg/ml and NE in μM. N=9, three independent experiments. Error bars represent the standard error of the mean.
Figure 3.
Norepinephrine (NE)-based enhancement of IL-8 responses in HVECs is not NF-κB-dependent. The selective inhibitor of IκB kinase (IKK), IKK 16, decreases IL-8 levels from HVEC-1 cells through the indirect inhibition of NF-κB. The ability of NE to cause increased IL-8 production in response to two bacterial exotoxins, however, is not altered in the presence of 1 μM IKK 16. ##Significantly higher than all other conditions and #significantly higher than all other conditions except SEC+NE by one-way ANOVA [F(6,35)=29.53, p<0.0001] and Tukey’s post-hoc test. ****Significantly higher than all other conditions and ***significantly higher than medium only, TSST-1+IKK, and NE+IKK and **significantly higher than medium only and TSST-1+IKK, and *significantly higher than TSST-1+IKK by one-way ANOVA [F(6,35)=23.12, p<0.0001] and Tukey’s post-hoc test. TSST-1 (toxic shock syndrome toxin-1) and SEC (staphylococcal enterotoxin C) concentrations in μg/ml and NE concentration in μM. N=6, two independent experiments. Similar results were obtained with the HVEC-2 line. Error bars represent the standard error of the mean.
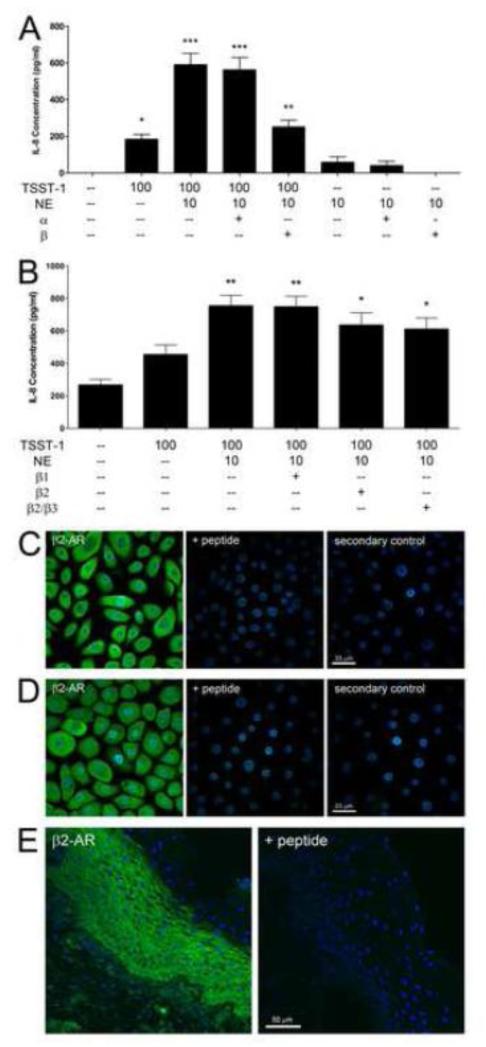
The β-adrenergic antagonist propranolol, the β2-selective adrenergic receptor antagonist ICI 118551 and the mixed β3/β2-adrenergic receptor antagonist SR 59230A (MacDonald and Watt, 1999; Yamanishi et al., 2002) prevented the ability of NE to enhance IL-8 secretion from HVECs treated with TSST-1. Neither the α-adrenergic receptor antagonist phentolamine nor the β1-adrenergic receptor antagonist atenolol altered NE action in HVECs (Figure 4 a,b). To confirm that β2-adrenergic receptors were expressed by HVECs, fluorescence immunocytochemistry was performed using an antiserum directed against residues 1-100 of the human β2-adrenergic receptor. Receptor-like immunoreactivity was detected in association with the plasma membrane in some cells, but was also detected in the cytoplasm (Figure 4 c,d). Additionally, β2-adrenergic receptor immunoreactivity was detected in human vaginal epithelium (Figure 4 e).
Figure 4.
Mediation of NE action by HVEC β2-adrenergic receptors. A) Phentolamine and propranolol (1 μM each) block α- and β-adrenergic receptors, respectively, on HVEC-1 cells. ***Significantly higher than all other conditions and **significantly higher than medium only, NE only, NE+propranolol only, and NE+phentolamine only controls and *significantly higher than medium only and NE+propranolol only controls by one-way ANOVA [F(7,64) = 40.46, p<0.0001] and Tukey’s post-hoc test. N=9, three independent experiments. Similar results were obtained with the HVEC-2 cell line. B) Selective β-adrenergic antagonists, atenolol (β1), ICI 118551 (β2), and SR 59230A (β3 > β2) at 1 μM each, were used to determine which receptor subtype was targeted by NE on HVECs. ICI 118551 and SR 59230A, both antagonists at the β2-adrenergic receptor, decreased the ability of NE to enhance the IL-8 response of HVEC-1 cells to TSST-1. **Significantly higher than all other conditions and *significantly higher than medium only control by one-way ANOVA [F(5,48)=8.986, p<0.0001] and Tukey’s post-hoc test. N=9, three independent experiments. Similar results were obtained with the HVEC-2 cell line. TSST-1 concentration in μg/ml and NE concentration in μM. Error bars represent the standard error of the mean. Immunoreactivity for β2-adrenergic receptor on C) HVEC-1, D) HVEC-2 cells, and E) human vaginal epithelium (Abcam rabbit anti-human β2-AR, ab36956, 1:20). A blocking peptide (ab38102, 10 μg/ml) was used to confirm the specificity of the reactivities, along with secondary only controls.
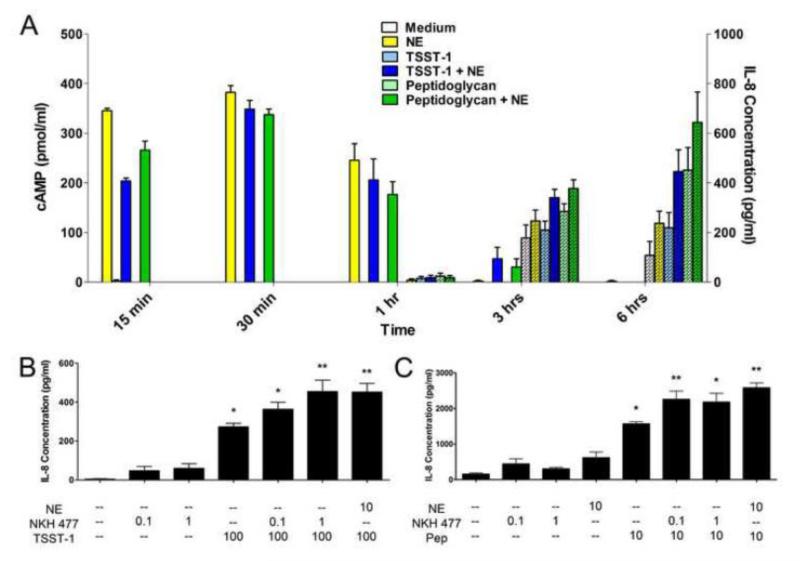
Because all β-adrenergic receptor subtypes are positively coupled to adenylate cyclase, we compared the relationship between the time course of intracellular cAMP elevations and the IL-8 potentiating effect produced by NE in HVECs. Cultured cells were treated with TSST-1 or peptidoglycan in the absence and presence of NE for time intervals ranging from 15 min – 6 hrs. At each time point, the cell culture medium was collected for measurement of IL-8 content, and HVECs were lysed to determine intracellular cAMP concentrations. After treatment with NE, intracellular cAMP concentrations in HVEC-2 cells peaked at 30 min and declined over the remaining time period. In contrast, IL-8 secretion exhibited a slower onset (Figure 5 a). Similar results were obtained in HVEC-1 cells, although they manifested higher resting cAMP levels than the HVEC-2 cells (data not shown). The forskolin analog NKH 477, which increases adenylate cyclase activity through a receptor-independent mechanism, also augmented IL-8 secretion by HVECs in response to TSST-1 and peptidoglycan (Figure 5 b,c).
Figure 5.
Norepinephrine (NE)-based enhancement of IL-8 responses in HVECs is dependent on cAMP signaling. A) HVEC-2 cells were incubated with toxic shock syndrome toxin-1 (TSST-1, 100 μg/ml), peptidoglycan (10 μg/ml), or NE (10 μM) over a total period of 6 hours. At various time points, supernates were collected to determine IL-8 concentrations secreted into the medium and cells were lysed to look at intracellular levels of cAMP. Plain bars indicate cAMP levels, while hatched bars indicate IL-8 levels. N=6, two independent experiments. Similar results were obtained with HVEC-1 cells. B) HVEC-2 cells were incubated with TSST-1 (100 μg/ml), NE (10 μM), or the forskolin analog NKH 477 (1 μM). **Significantly higher than all other conditions and *significantly higher than medium only and both NKH 477 only controls by one-way ANOVA [F(6,35)=32.66, p<0.0001] and Tukey’s post-hoc test. N=6, two independent experiments. C) HVEC-1 cells were incubated with peptidoglycan (10 μg/ml), NE (10 μM), or NKH 477 (1 μM). **Significantly higher than all other conditions and *significantly higher than medium only, NE only, and both NKH 477 only controls by one-way ANOVA [F(7,34)=43.58, p<0.0001] and Tukey’s post-hoc test. N=6, two independent experiments. Error bars represent the standard error of the mean.
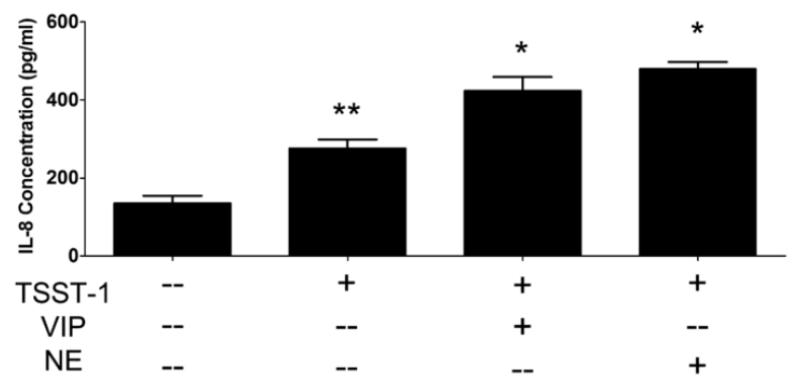
VIP also increases adenylate cyclase activity through interactions with G-protein coupled receptors (Dickson and Finlayson, 2009), and VIP-containing nerve fibers have been previously identified in vaginal tissue (Hoyle et al., 1996). Therefore, we also examined the possibility that VIP could potentiate the proinflammatory response of HVECs. VIP significantly augmented the IL-8 response of HVEC-2 cells to TSST-1 (Figure 6) and peptidoglycan (data not shown), similar to that seen with NE. Interestingly, VIP had no effect on HVEC-1 cells (data not shown). Immunoreactivity for VIP-containing nerve fibers was also confirmed in the submucosa of human vaginal tissue (data not shown).
Figure 6.
Vasoactive intestinal peptide (VIP) also potentiates IL-8 response of HVECs to TSST-1. A) HVEC-2 cells were incubated with TSST-1 (100 μg/ml), NE (10 μM), and/or VIP (0.1 μM) for 6 hours and cell supernates were analyzed by ELISA for IL-8. *Significantly higher than all other conditions and *significantly higher than medium only control by one-way ANOVA [F(3,35)=45.08, p<0.0001] and Tukey’s post-hoc test. N=9, three independent experiments. Error bars represent the standard error of the mean.
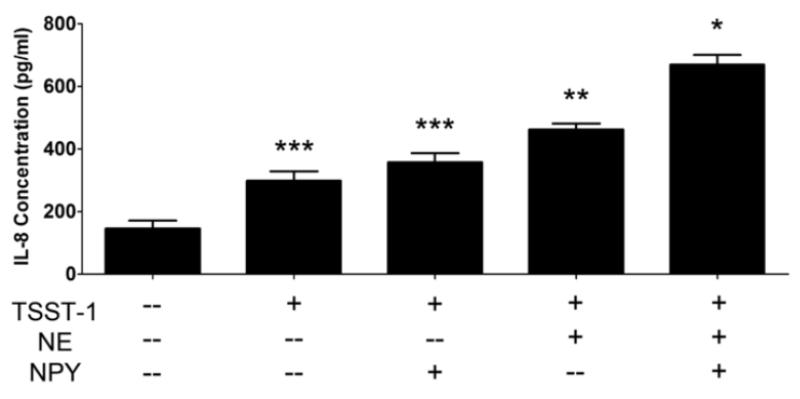
In an additional set of experiments, we examined the possibility that NPY could act to enhance the effects of NE on the proinflammatory response of HVECs. Nerve fibers immunoreactive for NPY have been demonstrated in the human vagina (Hoyle et al., 1996; Jorgensen et al., 1989), and under some circumstances, NPY is co-released with NE from adrenergic nerves to augment vasoconstriction and other effects of NE (Lundberg et al., 1990). Indeed, NPY augmented the effects of NE on IL-8 release from HVECs in response to TSST-1 (Figure 7) or peptidoglycan (data not shown), when added in combination with NE. NPY-immunoreactive nerve fibers were detected in the subepithelial plexus of human vaginal tissue, confirming previous reports (data not shown) (Hoyle et al., 1996; Jorgensen et al., 1989).
Figure 7.
Neuropeptide Y (NPY) further enhances the effects of NE on the HVEC IL-8 response. HVEC-2 cells were incubated with TSST-1 (100 μg/ml), NE (10 μM), and/or NPY (0.1 μM) for 6 hours and cell supernates were analyzed by ELISA for IL-8. *Significantly higher than all other conditions, **significantly higher than TSST-1 only and medium only controls, and ***significantly higher than medium only control by one-way ANOVA [F(4,28)=51.77, p<0.0001] and Tukey’s post-hoc test. Similar results were obtained with peptidoglycan and HVEC-1 cells. N=6, two independent experiments. Error bars represent the standard error of the mean.
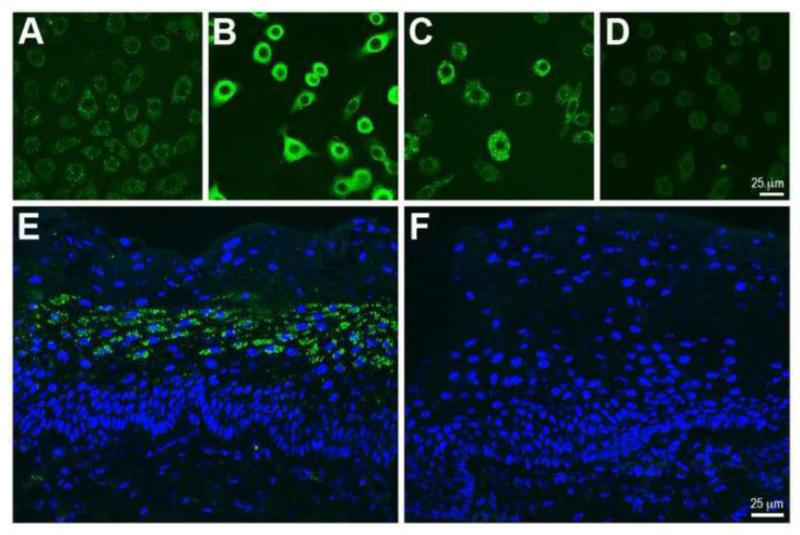
In order to address the hypothesis that HVECs produce catecholamines as do corneal epithelial cells (Pullar et al., 2007), we examined the presence of immunoreactivity to catecholamine synthesizing enzymes in these cell lines. Both HVEC lines exhibited immunoreactivities for TH and DBH (Figure 8 a,b), which appeared to be intracellularly localized. In addition, both cell lines exhibited immunoreactivity for NET (Figure 8c), a plasma membrane catecholamine transporter (Kristensen et al., 2011). NET immunoreactivity was also observed in stratified squamous epithelial cells of human vaginal mucosa (Figure 8 e,f). TH- and DBH-like immunoreactivities were not pronounced in the human vaginal epithelium (data not shown). Messenger RNAs for NET and TH, but not DBH, were detected in both HVEC lines by RT-PCR (data not shown).
Figure 8.
Localization of immunoreactivities for norepinephrine transporter (NET) and catecholamine synthetic enzymes in human vaginal epithelia. HVECs express both A) tyrosine hydroxylase (TH, mouse anti-TH, Immunostar, 1:1000, HVEC-1) and B) dopamine β-hydroxylase (DBH, rabbit anti-DBH, Immunostar, 1:1000, HVEC-1), important enzymes required for catecholamine synthesis. Additionally, HVECs demonstrate C) NET immunoreactivity (mouse anti-NET, Millipore, 1:1000, HVEC-1). (D Secondary only control for HVECs. E) Epithelial expression of NET is seen in human vaginal tissue. F) Secondary only control for vaginal tissue.
In a final set of experiments, HVECs grown in tyrosine-supplemented medium produced NE and dopamine at nanomolar concentrations (Table I). Catecholamine concentrations were measured in cell culture media (extracellular) and in cytosolic extracts (intracellular) by ELISA. Dopamine was secreted at respective mean concentrations of 0.022 pg/ml (± 0.001, N = 4 replicates) and 0.549 pg/ml (± 0.189, N = 5 replicates) in the media bathing HVEC-1 and HVEC-2 cells; cytosolic extracts from these respective cell lines manifested a mean dopamine concentration of 0.081 pg/ml (± 0.026, N = 5 replicates) and 0.291 pg/ml (± 0.103, N = 5 replicates). NE was secreted at a mean concentration of 0.544 pg/ml (N = 1 replicate) and 0.280 pg/ml (± 0.102, N = 5 replicates) in the media bathing HVEC-1 and HVEC-2 cells, respectively. Cytosolic extracts from HVEC-1 and HVEC-2 cells had a mean NE concentration of 0.050 pg/ml (± 0.019, N = 4 replicates) and 0.107 pg/ml (± 0.031, N = 7 replicates).
Table I.
Production of catecholamines by human vaginal epithelial cells.
| Dopamine (pg/ml) |
Norepinephrine (pg/ml) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Cell line | HVEC-1 | HVEC-2 | HVEC-1 | HVEC-2 | ||||
| Cellular compartment |
E | C | E | C | E | C | E | C |
| Lowest detected |
0.016 | 0.008 | 0.010 | 0.007 | 0.544 | 0.006 | 0.005 | 0.004 |
| Highest detected |
0.026 | 0.310 | 2.218 | 1.204 | 0.544 | 0.162 | 1.183 | 0.595 |
| Mean detected (± SE) |
0.022 (± 0.001) |
0.081 (± 0.026) |
0.549 (± 0.189) |
0.291 (± 0.103) |
0.544 (N/A) |
0.050 (± 0.019) |
0.280 (± 0.102) |
0.107 (± 0.031) |
| Number of replicates |
4 | 5 | 5 | 5 | 1 | 4 | 5 | 7 |
Production of dopamine and norepinephrine by two human vaginal epithelial cell (HVEC) lines as detected by ELISA. The catecholamines were detected intracellularly (cytoplasm) and in cell culture supernates (extracellular). The number of replicates indicates the number of conditions in which either catecholamine was above the limit of detection for the assay (12 possible replicates for each cellular compartment). E = extracellular concentration, C = cytosolic concentration, SE = standard error of the mean, N/A = not applicable.
4. Discussion
The results of this study indicate that NE, acting through β2-adrenergic receptors coupled to increased adenylate cyclase activity, potentiates epithelial cytokine secretion evoked by superantigens or TLR-2 stimulation. The β2-adrenergic receptor agonist albuterol has been found to increase vectorial secretion of an antibacterial protein (SPLUNC1) and reduce the intracellular load of the respiratory pathogen Mycoplasma pneumoniae in human bronchial epithelial cells (Gross et al., 2010). On the other hand, the β2-adrenergic receptor agonist procaterol or the permeable cAMP analog dibutyryl cAMP suppresses secretion of the chemokines CXCL10 and CCL5 from human bronchial epithelial cells stimulated by the TLR-3 agonist poly I:C (Lam et al., 2011). It is likely that this particular adrenergic receptor subtype similarly mediates host defense functions in vaginal epithelial cells. Cyclic AMP may represent one important component of NE action as cAMP elevations linked to β2-adrenergic receptor activation temporally precede cytokine release; additionally, the forskolin analog NKH 477 mimicked NE action. VIP, presumably by increasing adenylate cyclase activity, can also augment proinflammatory responses from one HVEC line similar to what was demonstrated with NE. The lack of effect of VIP on the HVEC-1 line may indicate the absence of a VIP receptor in this cell line, but this issue was not further explored. Cholera toxin and Escherichia coli heat-labile toxin have both been postulated to act as adjuvants through their ability to increase cAMP levels in various cell types (Lycke and Bemark, 2010). Our results indicate that agents that act to increase intracellular cAMP levels may generally augment immune responses in vaginal epithelial cells and perhaps epithelial cells in other locations as well.
Using immortalized cell lines allows for the characterization of specific cellular responses in the absence of other cell types; however they do come with limitations in that the cells are not in a natural environment and therefore responses may be altered. Although both lines used in this study are highly similar, differences in responses to the staphylococcal superantigens have been reported previously (Brosnahan et al., 2008), and here we further demonstrate that the cell lines have unique responses to peptidoglycan and VIP. We believe the use of two independently-generated vaginal epithelial cell lines allows us to gain a better understanding of how the cells would react in their native environment, and confirmation of an effect in both lines reinforces the potential for the effect to occur in vivo.
The ability of NPY to enhance the potentiation of IL-8 responses by NE adds another layer to the level of neuroimmunomodulation that may be occurring in the vaginal mucosa. NPY has been shown to cooperatively stimulate IL-6 production from macrophages in the murine spleen in the presence of NE (Straub et al., 2000), indicating a role for cotransmission of these molecules in neuroimmunomodulation in another peripheral organ. The combination of NPY and NE increased intracellular cAMP levels in tracheal gland cells to a level above that observed with NE alone (Merten and Figarella, 1994). As NPY-immunoreactive nerve fibers are clearly present directly beneath the vaginal epithelium, it is possible that NPY may play a role in further enhancing the vaginal inflammatory response to potential pathogens in the presence of NE.
TSST-1 and peptidoglycan interact respectively with CD40 and TLR-2 on the surface of vaginal epithelial cells and initiate NF-κB activation and nuclear translocation (Herbst-Kralovetz et al., 2008; Pivarcsi et al., 2005; Schaefers et al., 2012; Spaulding et al., 2012). Indeed, the IκB kinase inhibitor IKK 16 partially inhibited IL-8 secretion from HVECs exposed to either substance. Recent evidence suggests a link between β2-adrenergic receptor signaling and the activation state of NF-κB. Transgenic mice deficient in β2-adrenergic receptors manifest impairments in NF-κB activation (Ciccarelli et al., 2011). In the same vein, β2-adrenergic receptor antagonists have been found to reduce the activation (Liao et al., 2010) and transcriptional activity of NF-κB (Lin et al., 2010). In the present study however, the potentiating effect of NE appears to occur independently of NF-κB activation as NE augmented the diminished IL-8 responses of HVECs to TSST-1, SEC, and peptidoglycan in the presence of IKK 16. Indeed, in addition to their coupling to adenylate cyclase, β2-adrenergic receptors can potentially couple to β-arrestin, mitogen-associated protein kinase, PI-3-kinase and nitric oxide synthase signaling pathways in a tissue- and ligand-dependent manner (Evans et al., 2010). The signaling pathways that are linked to the immune potentiating actions of NE on HVECs are likely to be complex and remain to be fully determined.
In addition to the potential neural and hematic sources of NE in the vaginal mucosa, we have obtained evidence supporting the hypothesis that vaginal epithelial cells themselves are capable of synthesizing and secreting NE and dopamine. HVECs displayed immunoreactivities for two key catecholamine synthetic enzymes, TH and DBH. TH is the rate-limiting enzyme in the conversion of l-tyrosine to l-dopa, and DBH catalyzes the conversion of dopamine to NE. We did not investigate a third enzyme in the catecholamine synthetic pathway, phenylethanolamine N-methyltransferase (PNMT), which is expressed in a subset of catecholamine-producing cell types and catalyzes the conversion of NE to epinephrine. Corneal epithelial cells express both TH and PNMT immunoreactivities and contain detectable intracellular concentrations of epinephrine (Pullar et al., 2007). Immune cells such as polymorphonuclear leukocytes and macrophages also express TH and DBH and secrete catecholamines after exposure to the TLR-4 agonist lipopolysaccharide (Flierl et al., 2007). In addition, both HVECs and the human vaginal epithelium appear to express NET, an important plasma membrane uptake molecule that functions to regulate extracellular catecholamine concentrations. However, NET-like immunoreactivity appeared to be localized predominantly in the cell cytoplasm rather than at the cell surface. Many factors have been identified that influence the cellular trafficking of NET, such as intracellular phosphorylation pathways (Apparsundaram et al., 2001) and processing-associated alterations in the NET C-terminal sequence (Bauman and Blakely, 2002). The functional role of NET and the expression of ancillary termination mechanisms determining the half-life of extracellular catecholamines (e.g. degrading enzymes) remain to be fully investigated in HVECs.
In addition to their expression of synthetic and termination processes for catecholamines, both HVEC lines were found to produce and secrete NE and dopamine, albeit at low concentrations. Furthermore, these cells expressed β2-adrenergic receptors. It is therefore conceivable that NE may act in an autocrine fashion to regulate the proinflammatory responses of vaginal epithelial cells in situ. Under the present in vitro conditions however, the concentration range of NE (1-10 μM) necessary to significantly augment cytokine secretion by HVECs in response to TSST-1 or peptidoglycan was 3 – 5 orders of magnitude higher than the nanomolar concentrations of NE detected in HVEC culture medium. In comparison, macrophages reportedly secrete up to 30 pg/ml NE into culture medium after IL-4 exposure (Nguyen et al., 2011). HVECs were grown in monolayers in vitro, but are arranged in stratified layers in vivo; NE secreted by HVECs may be functionally relevant in the latter context due to the considerably smaller volume of extracellular fluid surrounding neighboring cells. Catecholamine production and secretion might also be augmented in the in situ epithelial environment.
Although the present investigation was conducted in vitro using two different HVEC lines, the results obtained offer some considerations of medical importance. In mTSS, it is thought that TSST-1 acts upon HVECs to recruit T lymphocytes and antigen-presenting cells to the vaginal mucosa, where interactions between these cells consequently lead to the overwhelming T cell proliferation and cytokine secretion that underlie this life-threatening syndrome (Brosnahan et al., 2008; Brosnahan and Schlievert, 2011). Based on our results, we hypothesize that NE would intensify the early events associated with mTSS (Figure 9). Endogenous NE derived from several potential sources (e.g. vaginal epithelial cells, blood, sympathetic nerve fibers) may promote a low level inflammatory state in the vaginal mucosa or influence the clinical course and severity of vaginal infections, such as those associated with S. aureus or HIV. These actions of NE could be subject to augmentation by physiological or pharmacological stimuli. Acute exposure to stressors could elevate NE and epinephrine concentrations in the circulation as well as stimulate NE and NPY outflow from peripheral sympathetic nerves innervating the female reproductive tract mucosa. Medications including catecholamine-derived vasopressors as well as antidepressant drugs and psychostimulants which block NE degradation or its NET-mediated uptake, may increase extracellular concentrations of NE in the vaginal mucosa. Selective blockade of β2-adrenergic receptors has been shown experimentally to enhance epidermal wound healing (Sivamani et al., 2009), inhibit the development of hypoxemic retinopathy (Martini et al., 2011), and decrease invasion of pancreatic adenocarcinomas (Zhang et al., 2010). Our results suggest that there may be an additional clinical indication for β2-adrenergic receptor antagonists in damping epithelial immune responses after their local application to the vaginal mucosa.
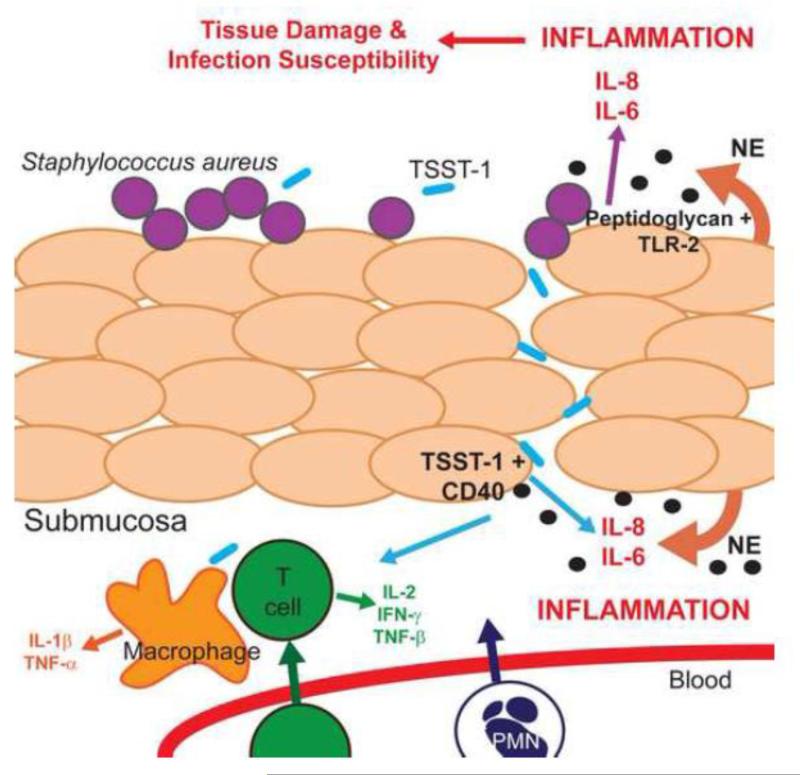
Figure 9.
Proposed autocrine action of NE in the vaginal mucosa. Stratified squamous epithelial cells with intercellular lipids constitute a physical barrier to infections in the vaginal tract. Potential pathogens, such as Staphylococcus aureus, can grow on the epithelial surface and directly stimulate TLRs to induce inflammation. In the case of peptidoglycan, IL-6 and IL-8 are produced by epithelial cells secondary to interactions of the bacterial cell wall with TLR-2. S. aureus also secretes exotoxins, such as toxic shock syndrome toxin-1 (TSST-1), which have been shown to interact with CD40 on vaginal epithelial cells to induce IL-6, IL-8, and MIP-3α. We hypothesize that the production of NE by vaginal epithelial cells contributes to an enhanced state of inflammation in the vaginal mucosa through the increased production of cytokines and chemokines in response to bacterial products. Enhanced inflammation can lead to degradation of barrier function and increased susceptibility to bacterial, fungal, and viral infections.
Highlights.
Norepinephrine enhances the IL-6 and IL-8 responses of vaginal epithelial cells.
The effects of NE are dependent on the presence of β2-adrenergic receptors.
VIP and NPY mimic and augment the actions of NE, respectively.
Human vaginal epithelial cells produce and secrete norepinephrine and dopamine.
Neurotransmitters in the vaginal mucosa may contribute to immunomodulation.
Acknowledgements
The authors would like to thank Dr. Patrick Schlievert (Department of Microbiology, Carver College of Medicine, University of Iowa, Iowa City, IA) for his generous donation of HVEC-2 cells, TSST-1, and SEC. We would also like to thank Dr. Peter Southern (Department of Microbiology, University of Minnesota Medical School, Minneapolis, MN) for his donation of fixed tissue specimens.
This project was supported by NIDA/NIH DA-10200 (DRB). AJB was a postdoctoral trainee in the PharmacoNeuroImmunology Training Program at the University of Minnesota, supported by NIDA/NIH T32DA007097.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apparsundaram S, Sung U, Price RD, Blakely RD. Trafficking-dependent and -independent pathways of neurotransmitter transporter regulation differentially involving p38 mitogen-activated protein kinase revealed in studies of insulin modulation of norepinephrine transport in SK-N-SH cells. J Pharmacol Exp Ther. 2001;299:666–677. [PubMed] [Google Scholar]
- Ashida H, Ogawa M, Kim M, Mimuro H, Sasakawa C. Bacteria and host interactions in the gut epithelial barrier. Nat Chem Biol. 2011;8:36–45. doi: 10.1038/nchembio.741. [DOI] [PubMed] [Google Scholar]
- Bauman PA, Blakely RD. Determinants within the C-terminus of the human norepinephrine transporter dictate transporter trafficking, stability, and activity. Arch Biochem Biophys. 2002;404:80–91. doi: 10.1016/s0003-9861(02)00232-1. [DOI] [PubMed] [Google Scholar]
- Beasley FC, Marolda CL, Cheung J, Buac S, Heinrichs DE. Staphylococcus aureus transporters Hts, Sir, and Sst capture iron liberated from human transferrin by Staphyloferrin A, Staphyloferrin B, and catecholamine stress hormones, respectively, and contribute to virulence. Infect Immun. 2011;79:2345–2355. doi: 10.1128/IAI.00117-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaskewicz CD, Pudney J, Anderson DJ. Structure and function of intercellular junctions in human cervical and vaginal mucosal epithelia. Biol Reprod. 2011;85:97–104. doi: 10.1095/biolreprod.110.090423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnahan AJ, Schaefers MM, Amundson WH, Mantz MJ, Squier CA, Peterson ML, Schlievert PM. Novel toxic shock syndrome toxin-1 amino acids required for biological activity. Biochemistry. 2008;47:12995–13003. doi: 10.1021/bi801468w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnahan AJ, Schlievert PM. Gram-positive bacterial superantigen outside-in signaling causes toxic shock syndrome. FEBS J. 2011 doi: 10.1111/j.1742-4658.2011.08151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Lyte M, Stevens MP, Vulchanova L, Brown DR. Mucosally-directed adrenergic nerves and sympathomimetic drugs enhance non-intimate adherence of Escherichia coli O157:H7 to porcine cecum and colon. Eur J Pharmacol. 2006;539:116–124. doi: 10.1016/j.ejphar.2006.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu JC, Hsu JY, Fu LS, Chu JJ, Chi CS. Comparison of the effects of two long-acting beta2-agonists on cytokine secretion by human airway epithelial cells. J Microbiol Immunol Infect. 2007;40:388–394. [PubMed] [Google Scholar]
- Chuang YY, Huang YC, Lin TY. Toxic shock syndrome in children: epidemiology, pathogenesis, and management. Paediatr Drugs. 2005;7:11–25. doi: 10.2165/00148581-200507010-00002. [DOI] [PubMed] [Google Scholar]
- Ciccarelli M, Sorriento D, Cipolletta E, Santulli G, Fusco A, Zhou RH, Eckhart AD, Peppel K, Koch WJ, Trimarco B, Iaccarino G. Impaired neoangiogenesis in beta(2)-adrenoceptor gene-deficient mice: restoration by intravascular human beta(2)-adrenoceptor gene transfer and role of NFkappaB and CREB transcription factors. Br J Pharmacol. 2011;162:712–721. doi: 10.1111/j.1476-5381.2010.01078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox SW, Ebersole LE, Carpenter GH, Proctor GB. Effects of autonomic agonists and immunomodulatory cytokines on polymeric immunoglobulin receptor expression by cultured rat and human salivary and colonic cell lines. Arch Oral Biol. 2007;52:411–416. doi: 10.1016/j.archoralbio.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Dickson L, Finlayson K. VPAC and PAC receptors: From ligands to function. Pharmacol Ther. 2009;121:294–316. doi: 10.1016/j.pharmthera.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- Evans BA, Sato M, Sarwar M, Hutchinson DS, Summers RJ. Ligand-directed signalling at beta-adrenoceptors. Br J Pharmacol. 2010;159:1022–1038. doi: 10.1111/j.1476-5381.2009.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flierl MA, Rittirsch D, Nadeau BA, Chen AJ, Sarma JV, Zetoune FS, McGuire SR, List RP, Day DE, Hoesel LM, Gao H, Van Rooijen N, Huber-Lang MS, Neubig RR, Ward PA. Phagocyte-derived catecholamines enhance acute inflammatory injury. Nature. 2007;449:721–725. doi: 10.1038/nature06185. [DOI] [PubMed] [Google Scholar]
- Freestone PP, Sandrini SM, Haigh RD, Lyte M. Microbial endocrinology: how stress influences susceptibility to infection. Trends Microbiol. 2008;16:55–64. doi: 10.1016/j.tim.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Green BT, Lyte M, Kulkarni-Narla A, Brown DR. Neuromodulation of enteropathogen internalization in Peyer’s patches from porcine jejunum. J Neuroimmunol. 2003;141:74–82. doi: 10.1016/s0165-5728(03)00225-x. [DOI] [PubMed] [Google Scholar]
- Gross CA, Bowler RP, Green RM, Weinberger AR, Schnell C, Chu HW. beta2-agonists promote host defense against bacterial infection in primary human bronchial epithelial cells. BMC Pulm Med. 2010;10:30. doi: 10.1186/1471-2466-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst-Kralovetz MM, Quayle AJ, Ficarra M, Greene S, Rose WA, 2nd, Chesson R, Spagnuolo RA, Pyles RB. Quantification and comparison of toll-like receptor expression and responsiveness in primary and immortalized human female lower genital tract epithelia. Am J Reprod Immunol. 2008;59:212–224. doi: 10.1111/j.1600-0897.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- Holmgren S, Olsson C. Autonomic control of glands and secretion: a comparative view. Auton Neurosci. 2011;165:102–112. doi: 10.1016/j.autneu.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Hoyle CH, Stones RW, Robson T, Whitley K, Burnstock G. Innervation of vasculature and microvasculature of the human vagina by NOS and neuropeptide-containing nerves. J Anat. 1996;188(Pt 3):633–644. [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A. Antiviral immune responses in the genital tract: clues for vaccines. Nat Rev Immunol. 2010;10:699–711. doi: 10.1038/nri2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen JC, Sheikh SP, Forman A, Norgard M, Schwartz TW, Ottesen B. Neuropeptide Y in the human female genital tract: localization and biological action. Am J Physiol. 1989;257:E220–227. doi: 10.1152/ajpendo.1989.257.2.E220. [DOI] [PubMed] [Google Scholar]
- Kristensen AS, Andersen J, Jorgensen TN, Sorensen L, Eriksen J, Loland CJ, Stromgaard K, Gether U. SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol Rev. 2011;63:585–640. doi: 10.1124/pr.108.000869. [DOI] [PubMed] [Google Scholar]
- Kumamoto Y, Iwasaki A. Unique features of antiviral immune system of the vaginal mucosa. Curr Opin Immunol. 2012 doi: 10.1016/j.coi.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakomy M, Kaleczyc J, Majewski M, Sienkiewicz W. Peptidergic innervation of the bovine vagina and uterus. Acta Histochem. 1995;97:53–66. doi: 10.1016/S0065-1281(11)80206-0. [DOI] [PubMed] [Google Scholar]
- Lakomy M, Szatkowska C, Chmielewski S. The adrenergic and AChE-positive nerves in pig vagina. Anat Anz. 1987;164:39–46. [PubMed] [Google Scholar]
- Lam KP, Chu YT, Kuo CH, Wang WL, Tok TS, Chin YY, Chen SC, Hung CH. Suppressive effects of procaterol on expression of IP-10/CXCL 10 and RANTES/CCL 5 by bronchial epithelial cells. Inflammation. 2011;34:238–246. doi: 10.1007/s10753-010-9229-9. [DOI] [PubMed] [Google Scholar]
- Liao X, Che X, Zhao W, Zhang D, Bi T, Wang G. The beta-adrenoceptor antagonist, propranolol, induces human gastric cancer cell apoptosis and cell cycle arrest via inhibiting nuclear factor kappaB signaling. Oncol Rep. 2010;24:1669–1676. doi: 10.3892/or_00001032. [DOI] [PubMed] [Google Scholar]
- Lin PY, Shen HC, Chen CJ, Wu SE, Kao HL, Huang JH, Wang DL, Chen SC. The inhibition in tumor necrosis factor-alpha-induced attenuation in endothelial thrombomodulin expression by carvedilol is mediated by nuclear factor-kappaB and reactive oxygen species. J Thromb Thrombolysis. 2010;29:52–59. doi: 10.1007/s11239-009-0318-2. [DOI] [PubMed] [Google Scholar]
- Linden A. Increased interleukin-8 release by beta-adrenoceptor activation in human transformed bronchial epithelial cells. Br J Pharmacol. 1996;119:402–406. doi: 10.1111/j.1476-5381.1996.tb16000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg JM, Franco-Cereceda A, Lacroix JS, Pernow J. Neuropeptide Y and sympathetic neurotransmission. Ann N Y Acad Sci. 1990;611:166–174. doi: 10.1111/j.1749-6632.1990.tb48930.x. [DOI] [PubMed] [Google Scholar]
- Lycke N, Bemark M. Mucosal adjuvants and long-term memory development with special focus on CTA1-DD and other ADP-ribosylating toxins. Mucosal Immunol. 2010;3:556–566. doi: 10.1038/mi.2010.54. [DOI] [PubMed] [Google Scholar]
- MacDonald A, Watt K. Characterisation of the atypical beta-adrenoceptor in rabbit isolated jejunum using BRL 37344, cyanopindolol and SR 59230A. J Auton Pharmacol. 1999;19:91–95. doi: 10.1046/j.1365-2680.1999.00121.x. [DOI] [PubMed] [Google Scholar]
- Martini D, Monte MD, Ristori C, Cupisti E, Mei S, Fiorini P, Filippi L, Bagnoli P. Antiangiogenic effects of beta2-adrenergic receptor blockade in a mouse model of oxygen-induced retinopathy. J Neurochem. 2011;119:1317–1329. doi: 10.1111/j.1471-4159.2011.07530.x. [DOI] [PubMed] [Google Scholar]
- Merten MD, Figarella C. Neuropeptide Y and norepinephrine cooperatively inhibit human tracheal gland cell secretion. Am J Physiol. 1994;266:L513–518. doi: 10.1152/ajplung.1994.266.5.L513. [DOI] [PubMed] [Google Scholar]
- Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987-2007) Brain Behav Immun. 2007;21:736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owman C, Rosenbren E, Sjoberg NO. Adrenergic innervation of the human female reproductive organs: a histochemical and chemical investigation. Obstet Gynecol. 1967;30:763–773. [PubMed] [Google Scholar]
- Papka RE, Cotton JP, Traurig HH. Comparative distribution of neuropeptide tyrosine-, vasoactive intestinal polypeptide-, substance P-immunoreactive, acetylcholinesterase-positive and noradrenergic nerves in the reproductive tract of the female rat. Cell Tissue Res. 1985;242:475–490. doi: 10.1007/BF00225412. [DOI] [PubMed] [Google Scholar]
- Peterson ML, Ault K, Kremer MJ, Klingelhutz AJ, Davis CC, Squier CA, Schlievert PM. The innate immune system is activated by stimulation of vaginal epithelial cells with Staphylococcus aureus and toxic shock syndrome toxin 1. Infect Immun. 2005;73:2164–2174. doi: 10.1128/IAI.73.4.2164-2174.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivarcsi A, Nagy I, Koreck A, Kis K, Kenderessy-Szabo A, Szell M, Dobozy A, Kemeny L. Microbial compounds induce the expression of pro-inflammatory cytokines, chemokines and human beta-defensin-2 in vaginal epithelial cells. Microbes Infect. 2005;7:1117–1127. doi: 10.1016/j.micinf.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Povoa P, Carneiro AH. Adrenergic support in septic shock: a critical review. Hosp Pract (Minneap) 2010;38:62–73. doi: 10.3810/hp.2010.02.280. [DOI] [PubMed] [Google Scholar]
- Prause O, Laan M, Lotvall J, Linden A. Pharmacological modulation of interleukin-17-induced GCP-2-, GRO-alpha- and interleukin-8 release in human bronchial epithelial cells. Eur J Pharmacol. 2003;462:193–198. doi: 10.1016/s0014-2999(03)01341-4. [DOI] [PubMed] [Google Scholar]
- Pullar CE, Zhao M, Song B, Pu J, Reid B, Ghoghawala S, McCaig C, Isseroff RR. Beta-adrenergic receptor agonists delay while antagonists accelerate epithelial wound healing: evidence of an endogenous adrenergic network within the corneal epithelium. J Cell Physiol. 2007;211:261–272. doi: 10.1002/jcp.20934. [DOI] [PubMed] [Google Scholar]
- Reiss MA. Toxic shock syndrome. Prim Care Update Ob Gyns. 2000;7:85–90. doi: 10.1016/s1068-607x(00)00027-5. [DOI] [PubMed] [Google Scholar]
- Rosengren E, Sjoberg NO. The adrenergic nerve supply to the female reproductive tract of the cat. Am J Anat. 1967;121:271–283. doi: 10.1002/aja.1001210207. [DOI] [PubMed] [Google Scholar]
- Ryu JH, Kim CH, Yoon JH. Innate immune responses of the airway epithelium. Mol Cells. 2010;30:173–183. doi: 10.1007/s10059-010-0146-4. [DOI] [PubMed] [Google Scholar]
- Salathe M. Effects of beta-agonists on airway epithelial cells. J Allergy Clin Immunol. 2002;110:S275–281. doi: 10.1067/mai.2002.129412. [DOI] [PubMed] [Google Scholar]
- Sanders VM. The beta2-adrenergic receptor on T and B lymphocytes: do we understand it yet? Brain Behav Immun. 2012;26:195–200. doi: 10.1016/j.bbi.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefers MM, Breshears LM, Anderson MJ, Lin YC, Grill AE, Panyam J, Southern PJ, Schlievert PM, Peterson ML. Epithelial proinflammatory response and curcumin-mediated protection from staphylococcal toxic shock syndrome toxin-1. PLoS One. 2012;7:e32813. doi: 10.1371/journal.pone.0032813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt LD, Xie Y, Lyte M, Vulchanova L, Brown DR. Autonomic neurotransmitters modulate immunoglobulin A secretion in porcine colonic mucosa. J Neuroimmunol. 2007;185:20–28. doi: 10.1016/j.jneuroim.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivamani RK, Pullar CE, Manabat-Hidalgo CG, Rocke DM, Carlsen RC, Greenhalgh DG, Isseroff RR. Stress-mediated increases in systemic and local epinephrine impair skin wound healing: potential new indication for beta blockers. PLoS Med. 2009;6:e12. doi: 10.1371/journal.pmed.1000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaulding AR, Lin YC, Merriman JA, Brosnahan AJ, Peterson ML, Schlievert PM. Immunity to Staphylococcus aureus secreted proteins protects rabbits from serious illnesses. Vaccine. 2012;30:5099–5109. doi: 10.1016/j.vaccine.2012.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RH, Schaller T, Miller LE, von Horsten S, Jessop DS, Falk W, Scholmerich J. Neuropeptide Y cotransmission with norepinephrine in the sympathetic nerve-macrophage interplay. J Neurochem. 2000;75:2464–2471. doi: 10.1046/j.1471-4159.2000.0752464.x. [DOI] [PubMed] [Google Scholar]
- Stutterheim J, Gerritsen A, Zappeij-Kannegieter L, Yalcin B, Dee R, van Noesel MM, Berthold F, Versteeg R, Caron HN, van der Schoot CE, Tytgat GA. Detecting minimal residual disease in neuroblastoma: the superiority of a panel of real-time quantitative PCR markers. Clin Chem. 2009;55:1316–1326. doi: 10.1373/clinchem.2008.117945. [DOI] [PubMed] [Google Scholar]
- Waelchli R, Bollbuck B, Bruns C, Buhl T, Eder J, Feifel R, Hersperger R, Janser P, Revesz L, Zerwes HG, Schlapbach A. Design and preparation of 2-benzamido-pyrimidines as inhibitors of IKK. Bioorg Med Chem Lett. 2006;16:108–112. doi: 10.1016/j.bmcl.2005.09.035. [DOI] [PubMed] [Google Scholar]
- Wira CR, Grant-Tschudy KS, Crane-Godreau MA. Epithelial cells in the female reproductive tract: a central role as sentinels of immune protection. Am J Reprod Immunol. 2005;53:65–76. doi: 10.1111/j.1600-0897.2004.00248.x. [DOI] [PubMed] [Google Scholar]
- Yamanishi T, Chapple CR, Yasuda K, Yoshida K, Chess-Williams R. The role of beta(3)-adrenoceptors in mediating relaxation of porcine detrusor muscle. Br J Pharmacol. 2002;135:129–134. doi: 10.1038/sj.bjp.0704470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Ma QY, Hu HT, Zhang M. beta2-adrenergic antagonists suppress pancreatic cancer cell invasion by inhibiting CREB, NFkappaB and AP-1. Cancer Biol Ther. 2010;10:19–29. doi: 10.4161/cbt.10.1.11944. [DOI] [PubMed] [Google Scholar]