Abstract
Objective
To determine the impact of surgical margin status on overall survival (OS) of patients undergoing hepatectomy for colorectal liver metastases (CLM) after modern preoperative chemotherapy.
Summary Background Data
In the era of effective chemotherapy for CLM, the association between surgical margin status and survival has become controversial.
Methods
Clinicopathologic data and outcomes for 378 patients treated with modern preoperative chemotherapy and hepatectomy were analyzed. The effect of positive margins on OS was analyzed in relation to pathologic and computed tomography-based morphologic response to chemotherapy.
Results
Fifty-two of 378 resections (14%) were R1 resections (tumor-free margin < 1 mm). The 5-year OS rates for patients with R0 resection (margin ≥ 1 mm) and R1 resection were 55% and 26%, respectively (P=0.017). Multivariate analysis identified R1 resection (P=0.03) and minor pathologic response to chemotherapy (P=0.002) as the 2 factors independently associated with worse survival. The survival benefit associated with negative margins (R0 vs. R1 resection) was greater in patients with suboptimal morphologic response (5-year OS rate: 62% vs. 11%, P=0.007) than in patients with optimal response (3-year OS rate: 92% vs. 88%, P=0.917) and greater in patients with minor pathologic response (5-year OS rate: 46% vs. 0%, P=0.002) than in patients with major response (5-year OS rate: 63% vs. 67%, P=0.587).
Conclusions
In the era of modern chemotherapy, negative margins remain an important determinant of survival and should be the primary goal of surgical therapy. The impact of positive margins is most pronounced in patients with suboptimal response to systemic therapy.
INTRODUCTION
Hepatic resection represents the basis for curative treatment of colorectal liver metastases (CLM), resulting in 5-year survival rates as high as 58% in selected patients.1 Recent improvements in survival after resection of CLM are due to multiple factors.2 Advances in operative techniques and strategies (i.e., portal vein embolization and staged hepatectomy), as well as surgical selection criteria based less on conventional clinicopathologic factors and more on the ability to clear disease while leaving behind an adequate future liver remnant, have contributed to an increasing number of patients being offered resection with curative intent.3–5 Importantly, these advances have been paralleled by the development of highly effective chemotherapeutic and biologic agents for patients with metastatic colorectal cancer.6–10
Contemporary systemic therapy for patients with CLM typically includes either oxaliplatin- or irinotecan-containing regimens, often combined with targeted agents such as bevacizumab or cetuximab. Preoperative administration of such systemic therapy in patients with CLM has been shown to result in high response rates and increased rates of resectability.7–9 Recent investigations have also demonstrated that response to preoperative chemotherapy as assessed by histologic evaluation of the surgical specimen after resection of CLM is a valuable predictor of survival in patients with CLM.11, 12 In addition, we recently found that chemotherapy-induced morphologic changes detected on preoperative computed tomography (CT) correlated with both pathologic response and survival and appeared to represent a useful clinical surrogate for disease biology.13
Traditionally, one of the criteria used to select patients for hepatectomy for CLM has been the predicted ability to achieve pathologically negative surgical margins. This paradigm is supported by several studies demonstrating that so-called R1 resection (tumor-free margin < 1 mm) is associated with worse overall survival than R0 resection (tumor-free margin ≥ 1 mm).14–18 However, other studies have found that R1 resection does not achieve independent significance as a predictor of survival in multivariate analysis.15, 19, 20 These include a study published in 2008 that found similar 5-year overall survival rates following R0 and R1 resection (61% and 57%, respectively) among 436 patients treated with perioperative chemotherapy and an aggressive surgical approach.19 These conflicting findings regarding the impact of positive margins raise the question of whether R1 resection negatively impacts survival because of the adverse effect of microscopic residual tumor left behind at the time of surgery or rather reflects a more aggressive tumor phenotype that makes complete resection with histologically negative margins harder to achieve. That question is difficult to answer on the basis of results of prior studies given the heterogeneity of the patients and treatment modalities in those studies.
The objective of the current study was to better understand the impact of surgical margin status on overall survival after surgical resection of CLM while accounting for tumor biology as assessed by response to preoperative chemotherapy. To do this, we analyzed a homogeneous group of patients with CLM who had been treated with modern chemotherapeutic agents followed by hepatic resection. Response to systemic therapy was evaluated according to previously established CT-based morphologic and pathologic criteria.
METHODS
Patient Inclusion Criteria
The prospectively maintained hepatobiliary surgical database at The University of Texas MD Anderson Cancer Center was queried to identify all consecutive patients with CLM treated with preoperative chemotherapy and subsequent hepatectomy with curative intent between September 1997 and January 2010. Patients treated with concomitant radiofrequency ablation were excluded. Patients with extrahepatic metastases were also excluded from the study. Clinicopathologic data (described in detail under Statistical Analysis below) were extracted from the patients’ medical records. Institutional Review Board approval was obtained prior to data retrieval and analysis.
Preoperative Assessment
Preoperative assessment included a medical history, physical examination, laboratory evaluation, and imaging studies. Helical CT with liver protocol or magnetic resonance imaging was used to define the extent and location of CLM. Beginning in 1998, fluorodeoxyglucose positron emission tomography was selectively used to rule out extensive extrahepatic disease and to confirm the metastatic nature of atypical lesions.21
Imaging studies were reviewed by an experienced hepatobiliary radiologist, and our previously described CT-related morphologic criteria were used to assess morphologic response to preoperative chemotherapy.13 In brief, CLM response was classified in group 3 if there was heterogeneous attenuation and a thick, poorly defined tumor-liver interface; in group 1 if there was homogeneous low attenuation and a thin, sharply defined tumor-liver interface; and in group 2 if there were mixed characteristics. Morphologic response to preoperative chemotherapy was defined as optimal if CLM changed from group 3 or 2 to group 1. Morphologic response was defined as suboptimal if CLM changed from group 3 to group 2, remained in the same category, or changed to a higher-numbered group. In patients with multiple CLM, morphologic response was scored on the basis of the response seen in the majority of lesions.
Treatment plans were made during case presentations at a multidisciplinary liver tumor conference attended by hepatobiliary surgeons, diagnostic radiologists, interventional radiologists, and medical oncologists. Decision-making was based on the location and extent of CLM, the presence of extrahepatic disease, and radiographic response to preoperative chemotherapy. Hepatectomy was considered in patients with CT volumetry indicating that all CLM could be safely resected with preservation of a sufficient future liver remnant. In patients with an anticipated insufficient future liver remnant volume, preoperative portal vein embolization was used to induce hypertrophy of the future liver remnant.22
Surgical Procedure
During laparotomy, the peritoneal cavity was inspected to rule out previously unrecognized extrahepatic disease. Intraoperative ultrasonography of the liver was performed in each case to confirm and to better define the location of CLM and their relation to portal pedicles and hepatic veins. Parenchymal transection was performed under total or selective hepatic inflow vascular exclusion using the cavitron ultrasonic surgical aspirator (CUSA, Valleylab, Boulder, CO), and hemostasis was achieved using saline-linked cautery (dissecting sealer DS 3.0, Tissuelink Medical, Inc., Dover, NH).23 For this study, major hepatectomy was defined as resection of 3 or more contiguous liver segments according to Couinaud’s classification.24
Postoperative Evaluation
Postoperative mortality was defined as any death within 90 days following resection, and postoperative morbidity was defined as any complication within the same time period. Postoperative complications were graded according to a standard classification system.25 Major complications were classified as complications requiring surgical, endoscopic, or radiologic intervention (grade III); life-threatening complications requiring intensive-care management (grade IV); and death (grade V). Postoperative liver insufficiency was defined as a postoperative peak bilirubin level > 7 mg/dL.26
All specimens were subjected to histologic evaluation to confirm the diagnosis of metastatic colorectal cancer, the degree of pathologic response to preoperative chemotherapy, and the width of the tumor-cell-free surgical margin. Margin width was determined prospectively using the shortest distance from the edge of the tumor to the line of parenchymal transection. R1 resection was defined as the presence of tumor cells within the space up to 1 mm from the transection line, whereas R0 resection was defined as complete tumor resection with no tumor cells within 1 mm of the transection line.15 Pathologic response to preoperative chemotherapy was graded as previously described,12 with the area of residual viable tumor cells within each metastatic lesion estimated as a percentage of the total tumor surface area. CLM with 0–49% residual viable tumor cells were considered to have a major pathologic response, while CLM with ≥ 50% residual viable tumor cells were considered to have a minor pathologic response. In patients with multiple tumor nodules, the mean of the values for the various nodules was used to define overall pathologic response.
Statistical Analysis
Quantitative and qualitative variables were summarized in terms of median (range), mean (standard deviation), and frequency (percentage). Comparisons between groups were analyzed with the chi-square or Fisher exact test for categorical variables and the Mann-Whitney U test for continuous variables, as appropriate. Patients were stratified by pathologic response to preoperative chemotherapy, and the clinicopathologic characteristics of patients who had a major pathologic response were compared with the clinicopathologic characteristics of patients who had a minor response. Overall survival rates were calculated from the date of resection to the date of death or last follow-up using the Kaplan-Meier method and compared using log-rank tests. The effect of R1 resection on overall survival was analyzed in the entire patient cohort as well as in subgroups of patients stratified by pathologic and morphologic response to preoperative chemotherapy.
To identify risk factors associated with overall survival in groups of patients treated with preoperative chemotherapy and subsequent hepatectomy for CLM, we evaluated the following clinicopathologic variables in univariate analysis: sex (male versus female), age (≥ 60 versus < 60 years), body mass index (> 30 versus ≤ 30 kg/m2), timing of detection of CLM (synchronous versus metachronous), location of the primary tumor (rectum versus colon), status of the regional lymph nodes for the primary tumor (positive versus negative), preoperative chemotherapy for CLM (irinotecan versus oxaliplatin), preoperative systemic therapy with bevacizumab (administered versus not), preoperative systemic therapy with cetuximab (administered versus not), number of cycles of preoperative chemotherapy (≥ 6 versus < 6), pathologic response to preoperative chemotherapy (major versus minor), 2-stage hepatectomy (performed versus not), portal vein embolization (performed versus not), associated procedure (performed versus not), major hepatectomy (performed versus not), major postoperative complication (yes versus no), blood transfusion required (yes versus no), estimated blood loss (> 1000 mL versus ≤ 1000 mL), number of CLM (multiple versus solitary), diameter of the largest of the CLM (≥ 3 versus < 3 cm), carcinoembryonic antigen (CEA) level (≥ 10 ng/mL versus < 10 ng/mL), status of the resection margins on microscopic analysis (R1 versus R0), and postoperative chemotherapy for CLM (administered versus not).
All variables associated with survival with P ≤ 0.1 in the univariate proportional hazards models were subsequently entered into a Cox multivariate regression model with backward elimination. P values ≤ 0.05 were considered statistically significant. Statistical analyses were performed using the SPSS software package, version 19.2 (SPSS, Chicago, IL).
RESULTS
Patient Characteristics
Among 494 consecutive patients who underwent hepatectomy for CLM after preoperative chemotherapy during the study period, 116 patients were treated with concomitant radiofrequency ablation and were excluded from the study. The clinicopathologic data of the remaining 378 patients are summarized in Table 1. The median age was 58 years (range, 25–85 years), and 56% of the patients were male. The median number of CLM was 2 (range, 1–75), and 49% of patients had CLM with largest diameter ≥ 3 cm. Preoperative chemotherapy regimens included oxaliplatin in 62% of patients and irinotecan in 38% of patients, and the median number of cycles was 6 (range, 2–36). Bevacizumab and cetuximab were used in combination with chemotherapy in 61% and 6% of the patients, respectively. Postoperative chemotherapy was offered to medically fit patients with the intention of completing a combined preoperative plus postoperative total of 6 months of systemic treatment. A total of 217 patients (57%) had a major pathologic response to preoperative chemotherapy, while 161 patients (43%) had a minor pathologic response.
Table 1.
Clinicopathologic Characteristics and Outcomes of 378 Patients who Underwent Hepatectomy for CLM According to the Pathologic Tumor Response to Preoperative Chemotherapy
| Characteristic or Outcome | All Patients (N = 378) | Major Pathologic Response (N = 217) | Minor Pathologic Response (N = 161) | P* |
|---|---|---|---|---|
| Male sex, n (%) | 212 (56) | 122 (56) | 90 (56) | 0.950 |
| Median age (range), years | 58 (25–85) | 58 (25–85) | 57 (27–82) | 0.490 |
| Mean age (SD), years | 57 (11) | 58 (11) | 57 (12) | 0.490 |
| Median body mass index (range), kg/m2 | 27 (16–48) | 28 (18–48) | 27 (16–48) | 0.912 |
| Mean body mass index (SD), kg/m2 | 28 (6) | 28 (6) | 28 (6) | 0.912 |
| Synchronous CLM, n (%) | 246 (65) | 151 (70) | 95 (59) | 0.033 |
| Rectal primary tumor, n (%) | 105 (28) | 52 (24) | 53 (33) | 0.055 |
| Node-positive primary tumor, n (%) | 267 (71) | 156 (72) | 111 (69) | 0.534 |
| Preoperative chemotherapy for CLM | ||||
| Irinotecan, n (%) | 143 (38) | 59 (27) | 84 (52) | < 0.0001 |
| Oxaliplatin, n (%) | 235 (62) | 158 (73) | 77 (48) | < 0.0001 |
| Bevacizumab, n (%) | 230 (61) | 147 (68) | 83 (52) | 0.001 |
| Cetuximab, n (%) | 21 (6) | 13 (6) | 8 (5) | 0.668 |
| Median number of cycles (range) | 6 (2–36) | 6 (2–32) | 7 (2–36) | 0.103 |
| Mean number of cycles (SD) | 8 (6) | 8 (6) | 9 (7) | 0.103 |
| Two-stage hepatectomy, n (%) | 30 (8) | 19 (9) | 11 (7) | 0.494 |
| Portal vein embolization, n (%) | 47 (12) | 26 (12) | 21 (13) | 0.757 |
| Associated procedure, n (%) | 63 (17) | 31 (14) | 32 (20) | 0.149 |
| Major hepatectomy, n (%) | 259 (69) | 149 (69) | 110 (68) | 0.944 |
| Postoperative complication, n (%) | 107 (28) | 54 (25) | 53 (33) | 0.086 |
| Major postoperative complication, n (%) | 61 (16) | 32 (15) | 29 (18) | 0.390 |
| Median estimated blood loss (range), mL | 300 (0–6000) | 300 (10–6000) | 300 (0–3500) | 0.070 |
| Mean estimated blood loss (SD), mL | 422 (512) | 387 (502) | 468 (523) | 0.070 |
| Blood transfusion, n (%) | 68 (18) | 31 (14) | 37 (23) | 0.030 |
| Median number of CLM (range) | 2 (1–75) | 2 (1–75) | 2 (1–16) | 0.592 |
| Mean number of CLM (SD) | 3 (4) | 3 (5) | 3 (3) | 0.592 |
| Median largest diameter of CLM (range), cm | 2 (1–18) | 2 (1–16) | 3 (1–18) | < 0.0001 |
| Mean largest diameter of CLM (SD), cm | 3 (3) | 3 (2) | 4 (3) | < 0.0001 |
| Median preoperative CEA level (range), ng/mL | 3 (0–1392) | 2 (0–727) | 5 (1–1392) | < 0.0001 |
| Mean preoperative CEA level (SD), ng/mL | 27 (106) | 14 (63) | 44 (143) | < 0.0001 |
| Positive surgical margins, n (%) | 52 (14) | 23 (11) | 29 (18) | 0.033 |
| Postoperative chemotherapy for CLM, n (%) | 260 (69) | 153 (71) | 107 (67) | 0.330 |
Comparison of patients with major versus minor pathologic response.
CEA indicates carcinoembryonic antigen; CLM, colorectal liver metastases; SD, standard deviation.
The majority of the patients (259/378, 69%) underwent major hepatectomy, including 104 (28%) who underwent extended hepatectomy. Thirty patients (8%) underwent staged hepatectomy, consisting of a limited resection of CLM located in the left liver followed by right (± segment 4) portal vein embolization and subsequent right (or extended right) hepatectomy, to achieve complete tumor resection. At histologic evaluation, 52 patients (14%) had an R1 resection, while 326 patients (86%) had an R0 resection. Postoperative chemotherapy for CLM was administered to 260 patients (69%), including 71% of patients with minor pathologic response to preoperative therapy and 67% with major pathologic response. In patients with minor pathologic response to preoperative chemotherapy, the postoperative chemotherapy regimen was either enriched with bevacizumab or cetuximab (depending on prior exposure and k-ras mutation status), or the entire regimen was changed to a second line of modern chemotherapy.
Morphologic response to preoperative chemotherapy was evaluable for 202 of the 378 patients studied. In the remaining176 patients, morphologic response could not be assessed because of small tumor size, lack of high-quality prechemotherapy CT imaging, or the use of magnetic resonance imaging as the preoperative imaging modality. Sixty-five patients (32%) had an optimal morphologic response, and 137 patients (68%) had a suboptimal morphologic response.
Patient Characteristics by Response to Preoperative Chemotherapy
Patient characteristics by pathologic response to preoperative chemotherapy (major or minor) are summarized in Table 1. Synchronous presentation of CLM was more frequent in patients with a major pathologic response (70%) than in patients with a minor response (59%) (P = 0.033). The median diameter of the largest of the CLM was larger in patients with a minor pathologic response (3 [1–18] cm) than in patient with a major response (2 [1–16] cm) (P < 0.0001). Although within the normal range, the median preoperative CEA level was higher in patients with a minor pathologic response (5 [1–1392] ng/mL) than in patients with a major response (2 [0–727] ng/mL) (P < 0.0001). Fewer patients with a major pathologic response than patients with a minor response required perioperative blood transfusion (14% versus 23%) (P = 0.030). Finally, positive surgical margins were more common in patients with minor response than in patients with major response (18% versus 11%; P = 0.033). There was no association between the number of CLM and the response to preoperative chemotherapy (P = 0.592) or between necessity for major hepatectomy and the response to preoperative chemotherapy (P = 0.944).
When analyzing the association between morphologic response and pathologic response to preoperative chemotherapy, a close correlation between these factors was observed. In patients with type 1, type 2 and type 3 morphologic response, the percentage of patients with minor pathologic response was 7%, 13% and 81%, respectively. Surgical margin status was not, however, associated with the type of morphologic response to preoperative chemotherapy (P = 0.333).
Postoperative Mortality and Morbidity
The postoperative 90-day mortality rate was 3% (11 patients died). Three deaths were related to postoperative liver insufficiency in patients who underwent extended hepatectomy following prolonged preoperative chemotherapy (> 6 cycles). Four deaths were related to pulmonary or intra-abdominal infections, and 4 patients died of thromboembolic complications (myocardial infarction or pulmonary embolism). The postoperative 90-day morbidity rate was 28% (107/378 patients). Perioperative complications recorded using a standard grading system included 32 grade I, 12 grade II, 37 grade III, 15 grade IV and 11 grade V complications.25 Sixteen percent of study patients experienced a major complication necessitating operative, endoscopic, or radiologic intervention.
Long-Term Survival
At a median follow-up time of 32 months (range, 1–118 months), the median survival for the entire cohort (n = 378) was 62 months. The 3-, 5-, and 10-year overall survival rates were 70%, 53%, and 21%, respectively. Patients who underwent R0 resection (n = 326) had a significantly better 5-year overall survival rate than those who underwent R1 resection (n = 52) (55% versus 26%, P = 0.017) (Figure 1).
FIGURE 1.
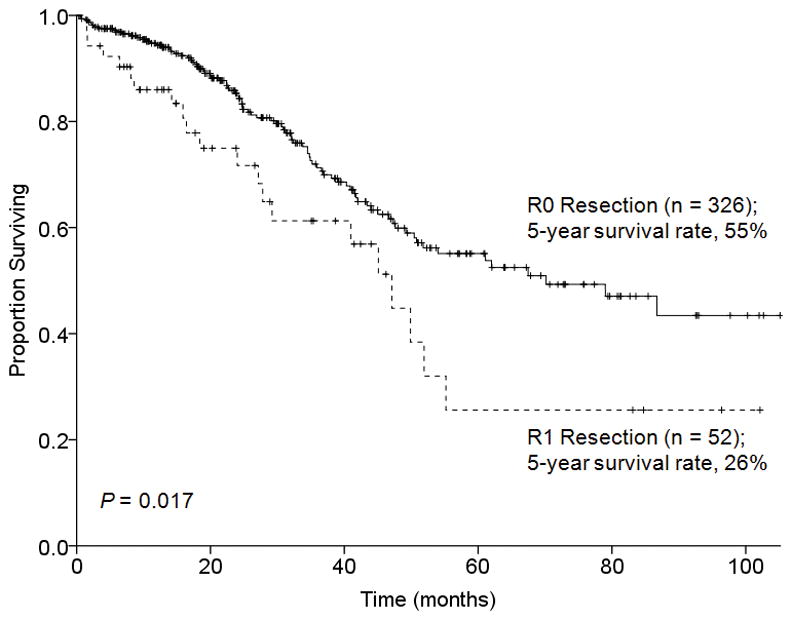
Overall survival by surgical margin status in 378 patients who underwent hepatectomy for CLM after preoperative chemotherapy.
The effect of surgical margin status on overall survival was analyzed in relation to morphologic tumor response to preoperative chemotherapy. Among the 65 patients with an optimal morphologic response, there was no difference in 3-year overall survival rates between patients with R0 and R1 resection (92% and 88%, respectively, P = 0.917) (Figure 2). In contrast, among the 137 patients with a suboptimal morphologic tumor response, the 5-year overall survival rate was significantly better following R0 resection (62% versus 11%, P = 0.007) (Figure 3).
FIGURE 2.
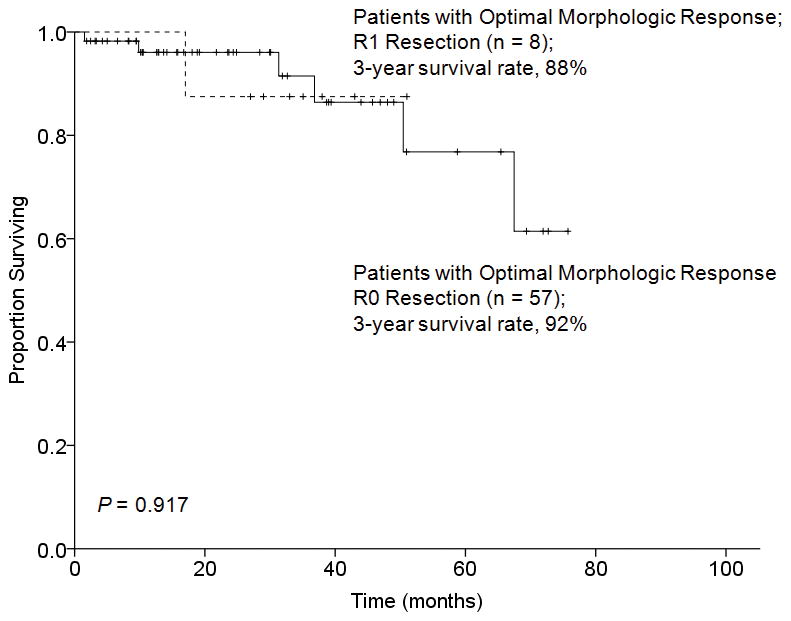
Overall survival by surgical margin status in 65 patients who underwent hepatectomy for CLM and had an optimal morphologic response to preoperative chemotherapy.
FIGURE 3.
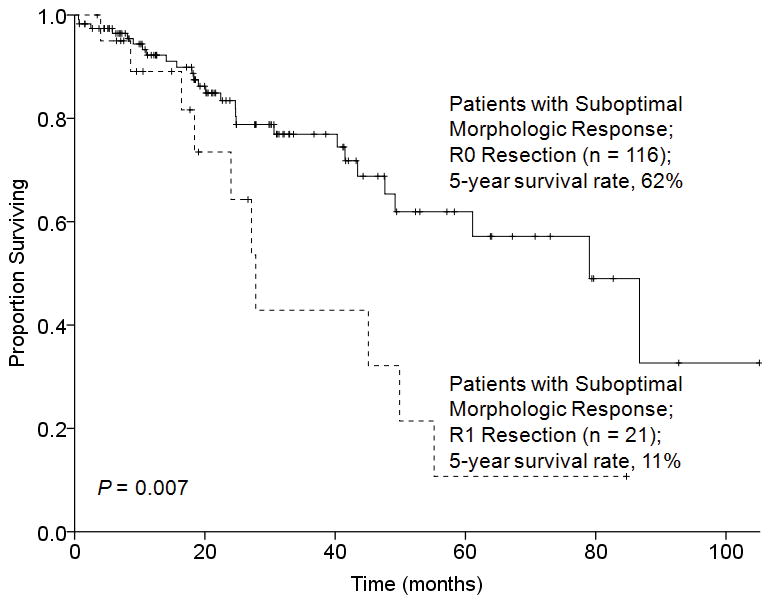
Overall survival by surgical margin status in 137 patients who underwent hepatectomy for CLM and had a suboptimal morphologic response to preoperative chemotherapy.
Likewise, in patients with a major pathologic response to preoperative chemotherapy, 5-year overall survival rates were similar following R0 and R1 resection (63% and 67%, respectively, P = 0.587) (Figure 4). However, in patients with a minor pathologic response to preoperative chemotherapy, the 5-year overall survival rate was significantly better following R0 resection (46% versus 0%, P = 0.002) (Figure 5).
FIGURE 4.
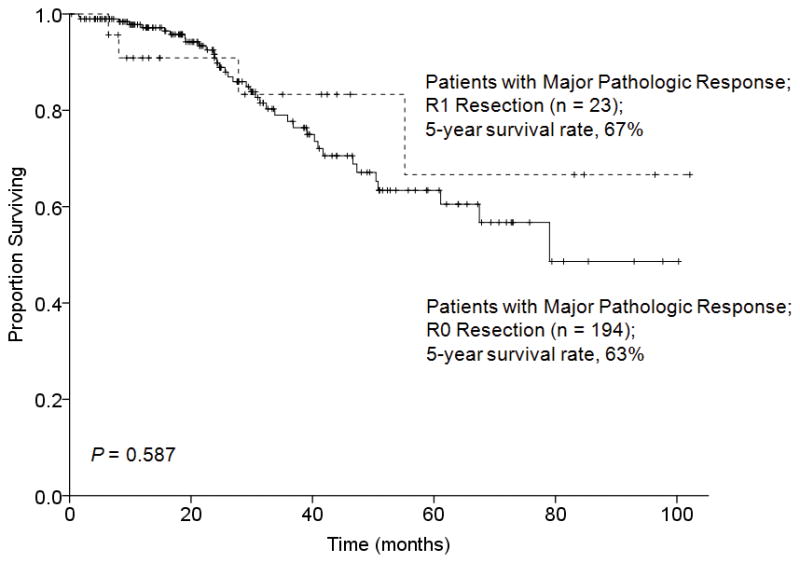
Overall survival by surgical margin status in 217 patients who underwent hepatectomy for CLM and had a major pathologic response to preoperative chemotherapy.
FIGURE 5.
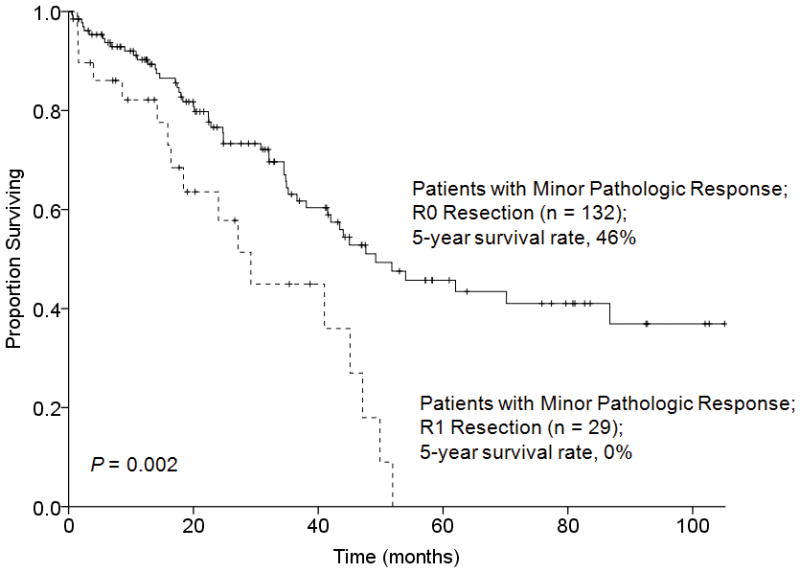
Overall survival by surgical margin status in 161 patients who underwent hepatectomy for CLM and had a minor pathologic response to preoperative chemotherapy.
Predictors of Overall Survival
Results of analysis of predictors of overall survival in the entire cohort are shown in Table 2. On univariate analysis, predictors of worse overall survival were minor pathologic response (P < 0.0001), major postoperative complication (P = 0.021), need for blood transfusion (P = 0.004), multiple CLM (P = 0.025), diameter of the largest of the CLM ≥ 3 cm (P = 0.002), and R1 resection (P = 0.017). On multivariate analysis, only minor pathologic response (hazard ratio [HR] 1.91, 95% CI 1.27–2.86, P = 0.002) and R1 resection (HR 1.69, 95% CI 1.05–2.74, P = 0.03) were independently associated with overall survival (Table 2).
Table 2.
Univariate and Multivariate Analysis of Clinicopathologic Variables Associated with Overall Survival in 378 Patients Who Underwent Hepatectomy for CLM after Preoperative Chemotherapy
| Variable | N (%) | 5-year Overall Survival Rate (%) | Univariate Analysis P | Multivariate Analysis*
|
|
|---|---|---|---|---|---|
| P | Hazard Ratio (95% CI) | ||||
| Sex | 0.085 | NS | |||
| Male | 212 (56) | 45 | |||
| Female | 166 (44) | 59 | |||
| Age | 0.381 | ||||
| ≥ 60 years | 164 (43) | 48 | |||
| < 60 years | 214 (57) | 53 | |||
| Body mass index | 0.417 | ||||
| > 30 kg/m2 | 126 (34) | 48 | |||
| ≤ 30 kg/m2 | 250 (66) | 54 | |||
| Timing of detection of CLM | 0.205 | ||||
| Synchronous | 246 (65) | 48 | |||
| Metachronous | 132 (35) | 57 | |||
| Primary tumor site | 0.085 | NS | |||
| Rectum | 105 (28) | 39 | |||
| Colon | 273 (72) | 55 | |||
| Regional lymph nodes for primary tumor | 0.340 | ||||
| Positive | 267 (71) | 48 | |||
| Negative | 111 (29) | 57 | |||
| Preoperative chemotherapy | 0.771 | ||||
| Irinotecan | 143 (38) | 50 | |||
| Oxaliplatin | 235 (62) | 52 | |||
| Preoperative cetuximab | 0.352 | ||||
| Yes | 21 (6) | 0 | |||
| No | 357 (94) | 52 | |||
| Preoperative bevacizumab | 0.873 | ||||
| Yes | 230 (61) | 39 | |||
| No | 148 (39) | 53 | |||
| No. of cycles of chemotherapy | 0.321 | ||||
| ≥ 6 | 151 (40) | 48 | |||
| < 6 | 227 (60) | 57 | |||
| Pathologic response to chemotherapy | < 0.0001 | 0.002 | 1.91 (1.27–2.86) | ||
| Major | 217 (57) | 64 | |||
| Minor | 161 (43) | 39 | |||
| Two-stage hepatectomy | 0.685 | ||||
| Yes | 30 (8) | 56 | |||
| No | 348 (92) | 51 | |||
| Portal vein embolization | 0.193 | ||||
| Yes | 47 (12) | 39 | |||
| No | 331 (88) | 53 | |||
| Associated procedure | 0.320 | ||||
| Yes | 63 (17) | 47 | |||
| No | 315 (83) | 52 | |||
| Major hepatectomy | 0.337 | ||||
| Yes | 259 (69) | 49 | |||
| No | 119 (31) | 58 | |||
| Major postoperative complication | 0.021 | NS | |||
| Yes | 61 (16) | 40 | |||
| No | 317 (84) | 54 | |||
| Blood transfusion | 0.004 | NS | |||
| Yes | 68 (18) | 37 | |||
| No | 310 (82) | 56 | |||
| Estimated blood loss | 0.385 | ||||
| > 1000 mL | 24 (31) | 45 | |||
| ≤ 1000 mL | 354 (69) | 52 | |||
| Number of CLM | 0.025 | NS | |||
| Multiple | 233 (62) | 45 | |||
| Single | 145 (38) | 61 | |||
| Maximum largest diameter of CLM | 0.002 | NS | |||
| ≥ 3 cm | 186 (49) | 44 | |||
| < 3 cm | 192 (51) | 61 | |||
| Preoperative CEA level | 0.057 | NS | |||
| ≥ 10 ng/mL | 92 (24) | 43 | |||
| < 10 ng/mL | 286 (76) | 54 | |||
| Margin status for resection of CLM | 0.017 | 0.03 | 1.69 (1.05–2.74) | ||
| R1 | 52 (14) | 26 | |||
| R0 | 326 (86) | 55 | |||
| Postoperative chemotherapy for CLM | 0.464 | ||||
| Yes | 260 (69) | 49 | |||
| No | 116 (31) | 53 | |||
Cox regression multivariate analysis included all variables with P < 0.1 in univariate analysis.
CEA indicates carcinoembryonic antigen; CLM, colorectal liver metastases; NS, not significant; R0, surgical margin ≥ 1 mm; R1, no margin or surgical margin < 1 mm.
In patients with major pathologic response to preoperative chemotherapy (Table 3), univariate analysis demonstrated that predictors of worse overall survival were primary rectal carcinoma (P = 0.035), regional lymph node metastases of the primary tumor (P = 0.036), and major postoperative complication (P = 0.005). On multivariate analysis, only major postoperative complication (HR 2.40, 95% CI 1.21–4.76, P = 0.012) remained as a significant predictor of survival (Table 3).
Table 3.
Univariate and Multivariate Analysis of Clinicopathologic Variables Associated with Overall Survival in 217 Patients with Major Pathologic Tumor Response to Preoperative Chemotherapy for CLM
| Variable | N (%) | 5-year Overall Survival Rate (%) | Univariate Analysis P | Multivariate Analysis*
|
|
|---|---|---|---|---|---|
| P | Hazard Ratio (95% CI) | ||||
| Sex | 0.134 | ||||
| Male | 122 (56) | 55 | |||
| Female | 95 (44) | 73 | |||
| Age | |||||
| ≥ 60 years | 95 (44) | 54 | 0.255 | ||
| < 60 years | 122 (56) | 72 | |||
| Body mass index | |||||
| > 30 kg/m2 | 73 (34) | 58 | 0.842 | ||
| ≤ 30 kg/m2 | 144 (66) | 66 | |||
| Timing of detection of CLM | |||||
| Synchronous | 151 (70) | 59 | 0.245 | ||
| Metachronous | 66 (30) | 72 | |||
| Primary tumor site | 0.035 | NS | |||
| Rectum | 52 (24) | 43 | |||
| Colon | 165 (76) | 68 | |||
| Regional lymph nodes for primary tumor | 0.036 | NS | |||
| Positive | 156 (72) | 57 | |||
| Negative | 61 (28) | 77 | |||
| Preoperative chemotherapy | 0.174 | ||||
| Irinotecan | 59 (27) | 68 | |||
| Oxaliplatin | 158 (73) | 61 | |||
| Preoperative cetuximab | 0.240 | ||||
| Yes | 13 (6) | 0 | |||
| No | 204 (94) | 66 | |||
| Preoperative bevacizumab | 0.310 | ||||
| Yes | 147 (68) | 57 | |||
| No | 70 (32) | 67 | |||
| No. of cycles of chemotherapy | 0.116 | ||||
| ≥ 6 | 130 (60) | 57 | |||
| < 6 | 87 (40) | 75 | |||
| Two-stage hepatectomy | 0.700 | ||||
| Yes | 19 (9) | 64 | |||
| No | 198 (91) | 64 | |||
| Portal vein embolization | 0.220 | ||||
| Yes | 26 (12) | 43 | |||
| No | 191 (88) | 67 | |||
| Associated procedure | 0.965 | ||||
| Yes | 31 (14) | 78 | |||
| No | 186 (86) | 63 | |||
| Major hepatectomy | 0.108 | ||||
| Yes | 149 | 59 | |||
| No | 68 | 76 | |||
| Major postoperative complications | 0.005 | 0.012 | 2.40 (1.21–4.76) | ||
| Yes | 32 (15) | 42 | |||
| No | 185 (85) | 68 | |||
| Blood transfusion | 0.093 | NS | |||
| Yes | 31 (14) | 43 | |||
| No | 186 (86) | 68 | |||
| Estimated blood loss | 0.850 | ||||
| > 1000 mL | 11 (5) | 48 | |||
| ≤ 1000 mL | 206 (95) | 65 | |||
| Number of CLM | 0.850 | ||||
| Multiple | 127 (58) | 60 | |||
| Single | 90 (42) | 69 | |||
| Maximum largest diameter of CLM | 0.312 | ||||
| ≥ 3 cm | 85 (39) | 62 | |||
| < 3 cm | 132 (61) | 64 | |||
| Preoperative CEA level | 0.939 | ||||
| ≥ 10 ng/mL | 34 (16) | 69 | |||
| < 10 ng/mL | 183 (84) | 63 | |||
| Margin status for resection of CLM | 0.587 | ||||
| R1 | 23 (11) | 67 | |||
| R0 | 194 (89) | 63 | |||
| Postoperative chemotherapy for CLM | 0.261 | ||||
| Yes | 153 (71) | 63 | |||
| No | 62 (29) | 62 | |||
Cox regression multivariate analysis included all variables with P < 0.1 in univariate analysis.
CEA indicates carcinoembryonic antigen; CLM, colorectal liver metastases; NS, not significant; R0, surgical margin ≥ 1 mm; R1, no margin or surgical margin < 1 mm.
In patients with minor pathologic response to preoperative chemotherapy (Table 4), univariate analysis demonstrated that predictors of worse overall survival were multiple CLM (P = 0.014), diameter of the largest of the CLM ≥ 3 cm (P = 0.039), and R1 resection (P = 0.002). On multivariate analysis, R1 resection (HR 2.04, 95% CI 1.15–3.60, P = 0.014) was the only factor found to predict worse overall survival.
Table 4.
Univariate and Multivariate Analysis of Clinicopathologic Variables Associated with Overall Survival in 161 Patients with Minor Pathologic Tumor response to Preoperative Chemotherapy for CLM
| Variable | N (%) | 5-year Overall Survival Rate (%) | Univariate Analysis P | Multivariate Analysis* | |
|---|---|---|---|---|---|
| P | Hazard Ratio (95% CI) | ||||
| Sex | 0.458 | ||||
| Male | 90 (56) | 36 | |||
| Female | 71 (44) | 42 | |||
| Age | 0.689 | ||||
| ≥ 60 years | 69 (43) | 40 | |||
| < 60 years | 92 (67) | 37 | |||
| Body mass index | 0.355 | ||||
| > 30 kg/m2 | 53 (33) | 37 | |||
| ≤ 30 kg/m2 | 106 (67) | 40 | |||
| Timing of detection of CLM | 0.202 | ||||
| Synchronous | 95 (59) | 35 | |||
| Metachronous | 66 (41) | 44 | |||
| Primary tumor site | 0.945 | ||||
| Rectum | 53 (33) | 37 | |||
| Colon | 108 (67) | 39 | |||
| Regional lymph nodes for primary tumor | 0.594 | ||||
| Positive | 111 (73) | 39 | |||
| Negative | 50 (29) | 37 | |||
| Preoperative chemotherapy | 0.705 | ||||
| Irinotecan | 84 (52) | 38 | |||
| Oxaliplatin | 77 (48) | 37 | |||
| Preoperative cetuximab | 0.656 | ||||
| Yes | 8 (5) | 63 | |||
| No | 153 (95) | 38 | |||
| Preoperative bevacizumab | 0.938 | ||||
| Yes | 83 (52) | 15 | |||
| No | 78 (48) | 41 | |||
| No. of cycles of chemotherapy | 0.994 | ||||
| ≥ 6 | 97 (60) | 39 | |||
| < 6 | 64 (40) | 38 | |||
| Two-stage hepatectomy | 0.505 | ||||
| Yes | 11 (4) | 44 | |||
| No | 150 (96) | 39 | |||
| Portal vein embolization | 0.438 | ||||
| Yes | 21 (13) | 34 | |||
| No | 140 (87) | 39 | |||
| Associated procedure | 0.442 | ||||
| Yes | 32 (20) | 31 | |||
| No | 129 (80) | 40 | |||
| Major hepatectomy | 0.990 | ||||
| Yes | 110 (68) | 39 | |||
| No | 51 (32) | 40 | |||
| Major postoperative complications | 0.552 | ||||
| Yes | 29 (18) | 38 | |||
| No | 132 (82) | 38 | |||
| Blood transfusion | 0.096 | NS | |||
| Yes | 37 (23) | 33 | |||
| No | 124 (77) | 41 | |||
| Estimated blood loss | 0.569 | ||||
| > 1000 mL | 13 (8) | 39 | |||
| ≤ 1000 mL | 148 (92) | 38 | |||
| Number of CLM | 0.014 | NS | |||
| Multiple | 55 (34) | 32 | |||
| Single | 106 (66) | 52 | |||
| Maximum largest diameter of CLM | 0.039 | NS | |||
| ≥ 3 cm | 101 (63) | 32 | |||
| < 3 cm | 60 (37) | 55 | |||
| Preoperative CEA level | 0.348 | ||||
| ≥ 10 ng/mL | 103 (64) | 33 | |||
| < 10 ng/mL | 58 (36) | 41 | |||
| Margin status for resection of CLM | 0.002 | 0.014 | 2.04 (1.15–3.60) | ||
| R1 | 29 (18) | 0 | |||
| R0 | 132 (82) | 46 | |||
| Postoperative chemotherapy for CLM | 0.859 | ||||
| Yes | 107 (66) | 34 | |||
| No | 54 (34) | 45 | |||
Cox regression multivariate analysis included all variables with P < 0.1 in univariate analysis.
CEA indicates carcinoembryonic antigen; CLM, colorectal liver metastases; NS, not significant; R0, surgical margin ≥ 1 mm; R1, no margin or surgical margin < 1 mm.
DISCUSSION
The gold standard for the surgical management of CLM is complete resection with histologically negative margins.27 The current study confirms the importance of achieving an R0 resection in a homogeneous population treated with resection with curative intent after delivery of modern systemic chemotherapy consisting of oxaliplatin- or irinotecan-based regimens. Survival analysis of the entire patient cohort revealed a 5-year overall survival rate of 55% following R0 resection compared to 26% following R1 resection. These survival rates are similar to those reported in previous studies analyzing the impact of surgical margin status on outcome of patients with CLM.15, 20, 28 These results are in contrast to a recently published study by de Haas et al. in which no difference in overall survival was identified between patients with R0 versus R1 resection.19 Of note, however, only 73% of patients in that study were treated with preoperative systemic therapy and only 49% received contemporary chemotherapy regimens containing oxaliplatin or irinotecan. These differences in the delivery and composition of preoperative chemotherapy may explain the findings of each study.
In the current study, the magnitude of benefit associated with a negative margin was strongly influenced by the pathologic response to preoperative chemotherapy. For patients with a major pathologic response (0–49% residual viable tumor cells), surgical margin status did not impact survival: the 5-year overall survival rate was 63% following R0 resection and 67% following R1 resection. However, for patients with a minor pathologic response to preoperative chemotherapy (≥ 50% residual viable tumor cells), the 5-year overall survival rate was 46% following R0 resection and 0% following R1 resection although equivalent proportions of patients in the 2 groups received additional postoperative systemic therapy. In addition, multivariate analysis identified R1 margin as the only independent risk factor for poor survival in patients with a minor pathologic response to preoperative chemotherapy. These data indicate that postoperative systemic therapy cannot rescue patients from a combination of chemotherapy resistance and positive surgical margins and emphasize the dominant impact of tumor biology on outcomes.
Although pathologic response to preoperative chemotherapy was found to be a powerful predictor of long-term outcomes, it is obviously not available at the time of selection of patients for surgical resection of CLM. Given the frequent use of preoperative chemotherapy in patients with CLM,29 a noninvasive method for evaluating tumor response to cytotoxic chemotherapy and predicting long-term outcomes would facilitate informed consent, risk stratification, and the proper timing and sequence of tumor-directed therapy. In this regard, our group has published CT-based morphologic criteria that accurately predicted pathologic response to preoperative chemotherapy and correlated with overall survival in patients with CLM treated surgically and nonsurgically.13 The analysis of CT morphologic criteria in this study is concordant with our previous results, further supporting its reliability as a predictor of pathologic response.
Although we would not suggest that clearly resectable patients be denied surgical therapy based on morphologic response to preoperative chemotherapy, the study supports the value of morphologic (CT-based) response in selecting patients with extensive disease for resection of CLM. For patients with an optimal morphologic response, no survival difference was observed between patients who underwent R0 and those who underwent R1 resection (3-year overall survival rates were 92% and 88%, respectively), indicating that operative therapy should proceed in this group even when close margins are anticipated by proximity of tumors to vascular structures. However, for patients with a suboptimal morphologic response, the 5-year overall survival rate was 62% following R0 resection compared to only 11% following R1 resection, suggesting that patients in this group who would be anticipated to have close margins may be better served with second line systemic therapies. Thus, the influence of surgical margin status in relation to CT-based morphologic response to chemotherapy mirrors the influence of surgical margin status in relation to pathologic response identified in surgical specimens. For a variety of malignancies, response to chemotherapy is known to be a sensitive marker for tumor biology and is recognized as an important prognostic indicator.30–33 Previous data from our institution12 and others11 have demonstrated that degree of tumor cell killing in response to preoperative chemotherapy is a significant predictor of survival following hepatectomy for CLM. Similarly, in the current study, minor pathologic response was found to be an independent predictor of worse survival on multivariate analysis of the entire cohort. In light of these findings and the 0% 5-year survival rate in patients with a minor pathologic response to preoperative chemotherapy and R1 resection, aggressive surgical therapy should be pursued in patients with unfavorable tumor biology (as indicated by a suboptimal morphologic response to preoperative chemotherapy) only when a negative margin is clearly achievable. In contrast, in patients with an optimal morphologic response to preoperative chemotherapy, aggressive surgical therapy may be appropriate even if there is some concern that an R0 resection may not be achievable.
This study may be limited by its retrospective nature. It may also be limited by inclusion of patients treated with 2 different chemotherapy regimens, although the regimens were contemporary (oxaliplatin-based and irinotecan-based). Another potential limitation is that biologic agents that have been demonstrated to contribute to the effectiveness of cytotoxic therapy (i.e., bevacizumab)6, 34 were not used in all cases. Despite these potential limitations, this represents the first study to examine the impact of surgical margin status in a homogeneous patient population treated with modern cytotoxic chemotherapy. Another possible limitation of this study is that morphologic response to preoperative therapy could not be evaluated in all patients as the criteria for morphologic response are currently CT-based and some patients had undergone magnetic resonance imaging or had tumors too small to permit an accurate qualitative assessment of response. This represents the second study to confirm the association between morphologic response to preoperative therapy, pathologic response to preoperative therapy, and long-term outcomes. In the future, we plan to further investigate these associations and to validate the morphologic response criteria using alternative imaging modalities, including magnetic resonance imaging.
In most studies on CLM, prognostic factors associated with survival after resection of CLM have included number, size, and distribution of hepatic lesions, the disease status of the primary tumor lymph nodes, the disease-free interval before detection of CLM, and the preoperative CEA level.28, 35 These prognostic factors are recorded either at initial presentation or prior to resection but are unrelated to preoperative treatment and variably reflect tumor biology. In this context, the predicted likelihood of achieving a microscopically negative surgical margin has always been a concern but has continued to be a point of controversy.19 The current study, performed on a homogeneous cohort of patients who received preoperative chemotherapy with modern agents, serves to resolve some of this controversy. The findings indicate that patients with CLM and suboptimal response to preoperative chemotherapy are unlikely to benefit from surgery unless all tumors can be resected with microscopically negative margins.
In conclusion, this analysis supports a continued emphasis on achieving R0 resection in patients with CLM. The use of modern chemotherapy combined with aggressive surgical strategies has expanded the number of patients considered for resection of CLM and has resulted in improved long-term overall survival. For patients who demonstrate unfavorable tumor biology, as assessed by both traditional clinicopathologic criteria and more recently introduced imaging criteria, an aggressive surgical approach should be entertained only if an R0 resection is deemed feasible.
Acknowledgments
Source of Funding: This research was supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant, CA016672.
The authors thank Stephanie Deming and Ruth J. Haynes for their assistance with manuscript preparation.
Footnotes
Conflicts of Interest
The authors report no conflicts of interest relevant to this article.
Presented at the 97th Annual Clinical Congress of the American College of Surgeon, San Francisco, CA, October 23-27, 2011.
References
- 1.Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–825. doi: 10.1097/01.sla.0000128305.90650.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27:3677–3683. doi: 10.1200/JCO.2008.20.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azoulay D, Castaing D, Krissat J, et al. Percutaneous portal vein embolization increases the feasibility and safety of major liver resection for hepatocellular carcinoma in injured liver. Ann Surg. 2000;232:665–672. doi: 10.1097/00000658-200011000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chun YS, Vauthey JN, Ribero D, et al. Systemic chemotherapy and two-stage hepatectomy for extensive bilateral colorectal liver metastases: perioperative safety and survival. J Gastrointest Surg. 2007;11:1498–1504. doi: 10.1007/s11605-007-0272-2. [DOI] [PubMed] [Google Scholar]
- 5.Jaeck D, Oussoultzoglou E, Rosso E, et al. A two-stage hepatectomy procedure combined with portal vein embolization to achieve curative resection for initially unresectable multiple and bilobar colorectal liver metastases. Ann Surg. 2004;240:1037–1049. doi: 10.1097/01.sla.0000145965.86383.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 7.Gruenberger B, Tamandl D, Schueller J, et al. Bevacizumab, capecitabine, and oxaliplatin as neoadjuvant therapy for patients with potentially curable metastatic colorectal cancer. J Clin Oncol. 2008;26:1830–1835. doi: 10.1200/JCO.2007.13.7679. [DOI] [PubMed] [Google Scholar]
- 8.Folprecht G, Gruenberger T, Bechstein WO, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 2009;11:38–47. doi: 10.1016/S1470-2045(09)70330-4. [DOI] [PubMed] [Google Scholar]
- 9.Wong R, Cunningham D, Barbachano Y, et al. A multicentre study of capecitabine, oxaliplatin plus bevacizumab as perioperative treatment of patients with poor-risk colorectal liver-only metastases not selected for upfront resection. Ann Oncol. 2011;22:2042–2048. doi: 10.1093/annonc/mdq714. [DOI] [PubMed] [Google Scholar]
- 10.Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007–1016. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubbia-Brandt L, Giostra E, Brezault C, et al. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol. 2007;18:299–304. doi: 10.1093/annonc/mdl386. [DOI] [PubMed] [Google Scholar]
- 12.Blazer DG, 3rd, Kishi Y, Maru DM, et al. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol. 2008;26:5344–5351. doi: 10.1200/JCO.2008.17.5299. [DOI] [PubMed] [Google Scholar]
- 13.Chun YS, Vauthey JN, Boonsirikamchai P, et al. Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA. 2009;302:2338–2344. doi: 10.1001/jama.2009.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altendorf-Hofmann A, Scheele J. A critical review of the major indicators of prognosis after resection of hepatic metastases from colorectal carcinoma. Surg Oncol Clin N Am. 2003;12:165–192. doi: 10.1016/s1055-3207(02)00091-1. [DOI] [PubMed] [Google Scholar]
- 15.Pawlik TM, Scoggins CR, Zorzi D, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715–724. doi: 10.1097/01.sla.0000160703.75808.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Figueras J, Burdio F, Ramos E, et al. Effect of subcentimeter nonpositive resection margin on hepatic recurrence in patients undergoing hepatectomy for colorectal liver metastases. Evidences from 663 liver resections. Ann Oncol. 2007;18:1190–1195. doi: 10.1093/annonc/mdm106. [DOI] [PubMed] [Google Scholar]
- 17.Are C, Gonen M, Zazzali K, et al. The impact of margins on outcome after hepatic resection for colorectal metastasis. Ann Surg. 2007;246:295–300. doi: 10.1097/SLA.0b013e31811ea962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elias D, Lasser P, Rougier P, et al. Frequency, technical aspects, results, and indications of major hepatectomy after prolonged intra-arterial hepatic chemotherapy for initially unresectable hepatic tumors. J Am Coll Surg. 1995;180:213–219. [PubMed] [Google Scholar]
- 19.de Haas RJ, Wicherts DA, Flores E, et al. R1 resection by necessity for colorectal liver metastases: is it still a contraindication to surgery? Ann Surg. 2008;248:626–637. doi: 10.1097/SLA.0b013e31818a07f1. [DOI] [PubMed] [Google Scholar]
- 20.Nuzzo G, Giuliante F, Ardito F, et al. Influence of surgical margin on type of recurrence after liver resection for colorectal metastases: a single-center experience. Surgery. 2008;143:384–393. doi: 10.1016/j.surg.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 21.Truant S, Huglo D, Hebbar M, et al. Prospective evaluation of the impact of [18F]fluoro-2-deoxy-D-glucose positron emission tomography of resectable colorectal liver metastases. Br J Surg. 2005;92:362–369. doi: 10.1002/bjs.4843. [DOI] [PubMed] [Google Scholar]
- 22.Ribero D, Abdalla EK, Madoff DC, et al. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg. 2007;94:1386–1394. doi: 10.1002/bjs.5836. [DOI] [PubMed] [Google Scholar]
- 23.Aloia TA, Zorzi D, Abdalla EK, et al. Two-surgeon technique for hepatic parenchymal transection of the noncirrhotic liver using saline-linked cautery and ultrasonic dissection. Ann Surg. 2005;242:172–177. doi: 10.1097/01.sla.0000171300.62318.f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg. 2005;12:351–355. doi: 10.1007/s00534-005-0999-7. [DOI] [PubMed] [Google Scholar]
- 25.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mullen JT, Ribero D, Reddy SK, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204:854–862. doi: 10.1016/j.jamcollsurg.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 27.Charnsangavej C, Clary B, Fong Y, et al. Selection of patients for resection of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol. 2006;13:1261–1268. doi: 10.1245/s10434-006-9023-y. [DOI] [PubMed] [Google Scholar]
- 28.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pozzo C, Basso M, Cassano A, et al. Neoadjuvant treatment of unresectable liver disease with irinotecan and 5-fluorouracil plus folinic acid in colorectal cancer patients. Ann Oncol. 2004;15:933–939. doi: 10.1093/annonc/mdh217. [DOI] [PubMed] [Google Scholar]
- 30.Kuerer HM, Newman LA, Smith TL, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17:460–469. doi: 10.1200/JCO.1999.17.2.460. [DOI] [PubMed] [Google Scholar]
- 31.Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680–2686. doi: 10.1002/1097-0142(19940601)73:11<2680::aid-cncr2820731105>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 32.Swisher SG, Hofstetter W, Wu TT, et al. Proposed revision of the esophageal cancer staging system to accommodate pathologic response (pP) following preoperative chemoradiation (CRT) Ann Surg. 2005;241:810–817. doi: 10.1097/01.sla.0000161983.82345.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ajani JA, Mansfield PF, Crane CH, et al. Paclitaxel-based chemoradiotherapy in localized gastric carcinoma: degree of pathologic response and not clinical parameters dictated patient outcome. J Clin Oncol. 2005;23:1237–1244. doi: 10.1200/JCO.2005.01.305. [DOI] [PubMed] [Google Scholar]
- 34.Ribero D, Wang H, Donadon M, et al. Bevacizumab improves pathologic response and protects against hepatic injury in patients treated with oxaliplatin-based chemotherapy for colorectal liver metastases. Cancer. 2007;110:2761–2767. doi: 10.1002/cncr.23099. [DOI] [PubMed] [Google Scholar]
- 35.Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]


