Abstract
Synthetic fragrances are persistent environmental pollutants that tend to bioaccumulate in animal tissues. They are widely used in personal care products and cleaning agents. Worldwide production of Galaxolide and Tonalide are in excess of 4500 tons annually. Because of their widespread production and use, they have been detected in surface waters and fish in the US and Europe. Consumption of contaminated water and fish from such sources leads to bioaccumulation and eventual toxicity. Since fragrances and flavors bear structural similarities to polyisoprenes, it was of interest to determine whether toxicity by Galaxolide and Tonalide may be linked with polyisoprenylated methylated protein methyl esterase (PMPMEase) inhibition. A concentration-dependent study of PMPMEase inhibition by Galaxolide and Tonalide as well as their effects on the degeneration of cultured cells were conducted. Galaxolide and Tonalide inhibited purified porcine liver PMPMEase with Ki values of 11 and 14 µM, respectively. Galaxolide and Tonalide also induced human cancer cell degeneration with EC50 values of 26 and 98 µM (neuroblastoma SH-SY5Y cells) and 58 and 14 µM (lung cancer A549 cells), respectively. The effects on cell viability correlate well with the inhibition of PMPMEase activity in the cultured cells. Molecular docking analysis revealed that the binding interactions are most likely between the fragrance molecules and hydrophobic amino acids in the active site of the enzyme. These results appear to suggest that the reported neurotoxicity of these compounds may be associated with their inhibition of PMPMEase. Exposure to fragrances may pose a significant risk to individuals predisposed to developing degenerative disorders.
Keywords: prenylation, isoprenylation, fragrances, tonalide, esterase, galaxolide, neurotoxicity, methylation, polycyclic musks, polyisoprenylation
INTRODUCTION
All trans-geranylgeranyl and trans, trans-farnesyl moieties are the two polyisoprenes used in the modification of some eukaryotic proteins (Nalivaeva and Turner, 2001). Polyisoprenylated protein-metabolizing enzymes such as polyisoprenylated methylated protein methyl transferase (PPMTase), which methylates the terminal carboxylate of the polyisoprenylated cysteine, display a preference for the polyisoprenyl groups of the substrates for high-affinity interactions (Gosser et al., 1997; Kloog and Cox, 2004). Likewise, polyisoprenylated methylated protein methyl esterase (PMPMEase) which hydrolyzes the methyl ester products of PPMTase has a high affinity for polyisoprenylated ester substrates (Lamango 2005); Lamango et al., 2009; Oboh and Lamango, 2008; Aguilar et al., 2011) and polyisoprenylated inhibitors (Aguilar et al., 2011). PMPMEase was recently purified from porcine liver, sequenced and identified as a highly homologous porcine version of human carboxylesterase 1 (hCE1) (Oboh and Lamango, 2008; Duverna et al., 2010). The crystal structure of hCE1 shows a large and hydrophobic active site with some flexible subsites (Bencharit et al., 2002, 2003). It has been suggested that these may be adaptive elements for interacting with the diversity of polyisoprenylated proteins in cells (Lamango et al., 2009; Duverna et al., 2010). These also imply that small hydrophobic molecules can enter the active site of the enzyme and competitively inhibit catalysis. Indeed, various crystal structures of hCE1 in complex with molecules such as cocaine, tacrine, and various organophosphorus nerve agents have been solved (Bencharit et al., 2002, 2003). The active site is large enough to accommodate molecules such as cholesteryl esters and fatty acyl coenzyme A (Mukherjee et al., 1993; Tsujita and Okuda, 1993). It appears that the “promiscuous” nature of the enzyme may cause it to be able to metabolize as well as be inhibited by a wide range of endogenous and exogenous compounds. Such inhibitions may extend beyond the limits required for normal physiology and thus result in toxicities.
Given the functional diversity of polyisoprenylated proteins and the fact that PMPMEase and PPMTase constitute the only reversible step of the polyisoprenylation pathway, the consequences of the inhibition of polyisoprenylated protein function may be profound. Judging from the hydrophobic nature of the PMPMEase active site, it was opined that synthetic musks may bind to its active site and inhibit its enzymatic activity. The worldwide commercial production and use of polycyclic musks has reached an estimated 5600 tons per annum (Rimkus, 1999; Zhou et al., 2009). Since these are components of various consumer products such as detergents, perfumes, and lotions, human exposure includes direct application, inhalation, and consumption of foods such as fish that have been exposed to waste water discharges (Reiner and Kannan, 2006). The synthetic musks lack the functional groups that render molecules amenable to biodegradation. As such, they tend to bioaccumulate to very high concentrations in fatty tissues (Müller et al., 1996; Schnell et al., 2009; Schiavone et al., 2010) from where they emanate to interact with molecules in the body. Levels of Galaxolide and Tonalide as high as 0.44 µg have been detected per gram of human breast milk (Liebl and Ehrenstorfer, 1993; Rimkus and Wolf, 1996; Reiner et al., 2007). They have also been detected at 0.033 µg per gram of human adipose tissue and 159 µg Galaxolide and 58 µg Tonalide per gram of lipid, respectively, in fish filet (Rimkus, 1999; Rimkus and Wolf, 1996). Tonalide and Galaxolide have been detected in fish at 290 and 2100 ng/g levels (Ramirez et al., 2009). Assuming an approximate tissue density of 1 g/mL, these translate to 1.1 and 8.1 µM for Tonalide and Galaxolide, respectively. A higher physiological impact would be expected from the cumulative concentrations of these plus other musks and compounds with similar biochemical profiles acting on the same cellular target.
Despite the wide scale use of synthetic musks, little is known about their effects on human health. The degenerative properties of nitro- and polycyclic musks have been reported (Spencer et al., 1984; Salvito, 2005; Shubert, 2006; Schnell et al., 2009). Progressive neuronal degeneration and myelin bubbling was observed in rats following repeated treatments with the polycyclic musk, acetyl ethyl tetramethyl tetralin (Spencer et al., 1979). In this study, the inhibition of PMPMEase by Tonalide and Galaxolide was analyzed. The effects of the most inhibitory and/or environmentally persistent fragrances on cell viability were also studied. Micromolar concentrations of Tonalide and Galaxolide inhibited PMPMEase and induced apoptosis in human neuroblastoma SH-SY5Y and lung cancer A549 cells.
MATERIALS AND METHODS
Materials
Galaxolide, Tonalide, β-cyclocitral, γ-dodecalactone, ω-pentadecalactone, ε-decalactone, pentanal, dimethyl sulfoxide, methanol, acetonitrile, tris HCl, ethanolamine, Triton X-100, acridine orange (AO), and ethidium bromide (EB) were purchased from Sigma-Aldrich (St. Louis, MO). Dulbecco’s modified Eagle’s Medium (DMEM) and F-12 Medium (Ham’s F-12 Medium), heat-inactivated fetal bovine serum (FBS), streptomycin, penicillin, and trypsin-EDTA were obtained from Invitrogen Corporation (Carlsbad, CA). The substrate, RD-PNB (L-N-(4-Nitrobenzoyl)-S-(trans, trans-farnesyl) cysteine methyl ester [(R)-methyl 3-((2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienylthio)-2-(4-nitrobenzamido)propanoate]) was synthesized in our laboratory as previously described (Lamango et al., 2009). The cell culture plates and culture flasks (75 cm2) were purchased from BD Biosciences (San Jose, CA). SH-SY5Y and A549 cells were obtained from ATCC (Manassas, VA). Resazurin was obtained from Promega (Madison, WI).
Enzyme Assays
PMPMEase used for the assays was obtained as previously described (Oboh and Lamango, 2008; Lamango et al., 2009). The RD-PNB substrate and fragrances were dissolved in DMSO. The enzyme assays and analysis were conducted as previously described (Oboh and Lamango, 2008; Lamango et al., 2009). Briefly, the fragrances were pre-incubated with the assay mixture for 15 min before the addition of RD-PNB substrate (1 mM) followed by further incubation at 37°C. The assay mixture contained the enzyme in the presence of the fragrances in 100 mM Tris–HCl, pH 7.4 in a total incubation volume of 100 µL. Reactions were stopped by adding 200 µL of methanol and placing them on ice for at least 5 min before centrifugation at 5000 × g for 5 min. The supernatants were analyzed by reversed phase HPLC as previously described (Oboh and Lamango, 2008; Lamango et al., 2009).
Effects of Fragrances on Cell Viability
Human neuroblastoma SH-SY5Y and human lung cancer A549 cells were initially cultured in a 1:1 mixture of Dulbecco’s modified Eagle’s Medium (DMEM) and F12 Medium (Invitrogen, Carlsbad, CA), supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum (Invitrogen, Carlsbad, CA), 100 U/mL penicillin and 100 µg/mL streptomycin (Invitrogen, Carlsbad, CA) in 75 cm2 vented culture flasks. The neuroblastoma cells were chosen based on the reported neurotoxicity of some musk fragrances while the lung cancer cells were selected because the lungs constitute a principal route of exposure. The cultures were incubated at 37°C in 5% CO2/95% humidified air. When cells had reached 80–90% confluence, they were trypsinized and seeded onto 96-well plates at a density of 105 for human neuroblastoma SH-SY5Y or 2 × 104 for human lung cancer A549 cells and incubated at 37°C in 5% CO2/95% humidified air.
Cell Viability Assays
Cell Titer-Blue Cell Viability Assay kit (CTB) with fluorescence readout was used to measure resazurin reduction as a marker of metabolic activity following treatment with fragrances. Fragrances were dissolved in acetone (final concentration of the acetone in each well was 1%). The human neuroblastoma SH-SY5Y cells in 96-well plates were exposed to varying concentrations of fragrances in serum-free DMEM/F12 for 24, 48, and 72 h. The concentrations of Galaxolide and Tonalide used were within the range of concentrations that have been detected in animal tissues (Ramirez et al., 2009). Resazurin (Promega, Madison, WI) was used to measure the cell viability according to the vendor instructions. Identical amounts of the fragrances were added to the samples at 24 h for the 48-h exposures and 24 and 48 h for the 72-h exposures. Resazurin (0.05%, 20 µL) was added to each well containing 100 µL of culture medium and the contents gently mixed and incubated in the dark for 2 h at room temperature before measurement of the fluorescence with excitation at 560 nm and detection at 590 nm using FLx 800 Microplate Fluorescence Reader (Bio-Tek Instruments, Winooski, VM). Cell viability was expressed as the percentage of the fluorescence in the treated cells relative to that of the controls.
PMPMEase Activity in Fragrance-Treated Cells
Human neuroblastoma SH-SY5Y cells were seeded in 24-well plates at the density of 5 × 105 with each well containing 500 µL of culture medium as described above. The cells were either treated with Galaxolide or Tonalide (0–200 µM) and incubated for 24 h. Some of the medium (320 µL) in each well was removed and Triton–X 100 was then added to the remaining 180 µL of medium to a final concentration of 0.1%. These were thoroughly mixed and aliquots of the resulting lysate were assayed for PMPMEase activity using the RD-PNB substrate.
Apoptosis Assay
The modified acridine orange and ethidium bromide (AO/EB) staining method (Ribble et al., 2005) was employed in the studies. Briefly, cells were seeded onto 24-well plates at a density of 2 × 105 for human lung cancer A549 or 3 × 105 for human neuroblastoma SH-SY5Y cells and treated with 0–10 µM fragrances followed by incubation for 24 h at 37°C in 5% CO2/95% humidified air. Staining was carried out in the dark with a combined solution of EB/AO at 10 µg/mL for 15 min. Cells were then viewed under the microscope at 400× magnification using a 480/30 nm excitation filter. Images of the cells were then captured with an Olympus DP70 Camera.
Docking Analysis
Docking was employed to estimate the binding affinities of fragrances to the enzyme. Such in silico analysis is normally used to prescreen compounds for potential effectiveness prior to synthesis during pharmaceutical development. Having biochemically analyzed the commercially available fragrances, docking analysis was then employed to evaluate the likely potencies of the fragrances whose sales have been discontinued and were therefore not available for testing in our assay. The crystal structure of PMPMEase has not been solved, but it shares 79% sequence identity and 88% sequence similarity to human carboxylesterase 1 (hCE1). Therefore, the crystal structure of hCE1 was used as a template to construct a homology model of PMPMEase/porcine liver esterase (PLE) using the MODELLER program. The X-ray crystal structure of hCE1 [EC 3.1.1.1] used was 1YAH. The amino acid sequence of PLE (Accession: Q29550) was retrieved from SWISS PROT database and aligned with the sequence of hCE1. Docking analysis was performed with ArgusLab Version 4.01 which reports free energy calculation (AScore) based on a comprehensive search method which includes identifying nonrotatable bonds (nodes) of the molecule and placing it in the active site of the enzyme to calculate favorable rotations. Procedures for the docking of fragrances were essentially as previously described (Duverna et al., 2010).
Data Analysis
The concentration–response curves were obtained by plotting the percentage inhibition against the log of the inhibitor concentrations. Nonlinear regression plots were generated using Graphpad Prism version 4.0 for Windows (San Diego, CA). From these, the concentrations that inhibit 50% of the activity (IC50) were calculated. Statistical differences between control and treated groups were analyzed by one-way ANOVA followed by Dunnett’s post-test comparisons. p-values of less than 0.05 were considered statistically significant. All results were expressed as the means ± S.E.M.
RESULTS
Inhibition of Purified Porcine PMPMEase by Synthetic Musks
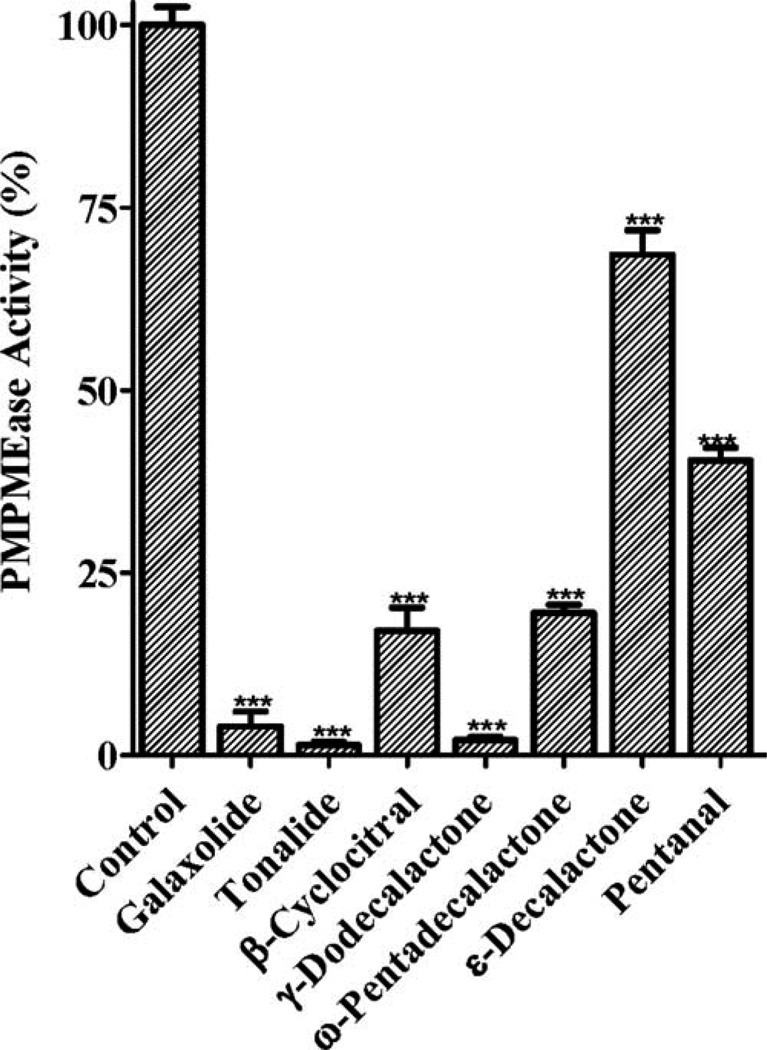
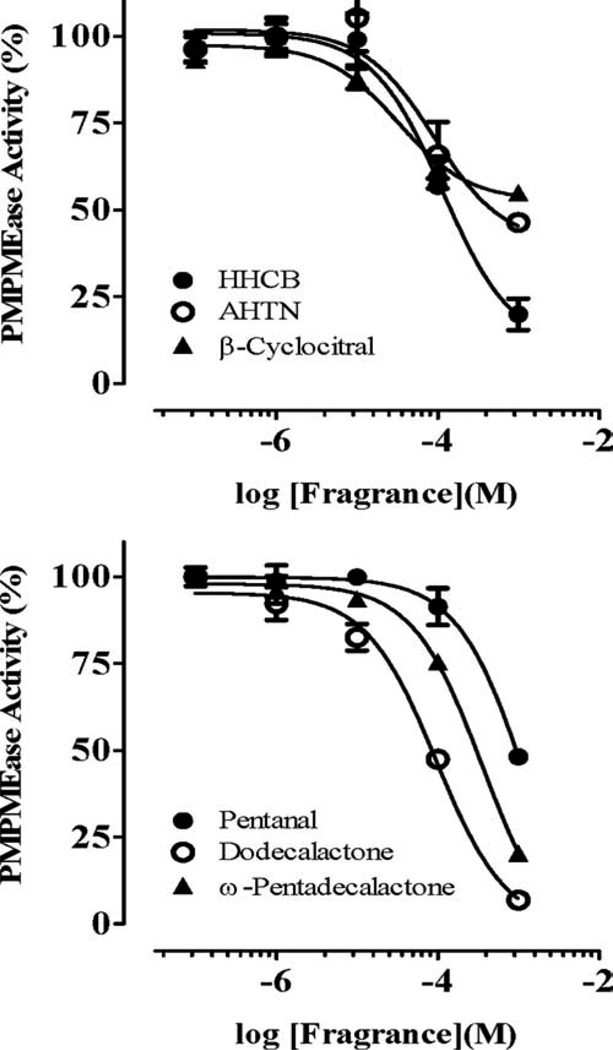
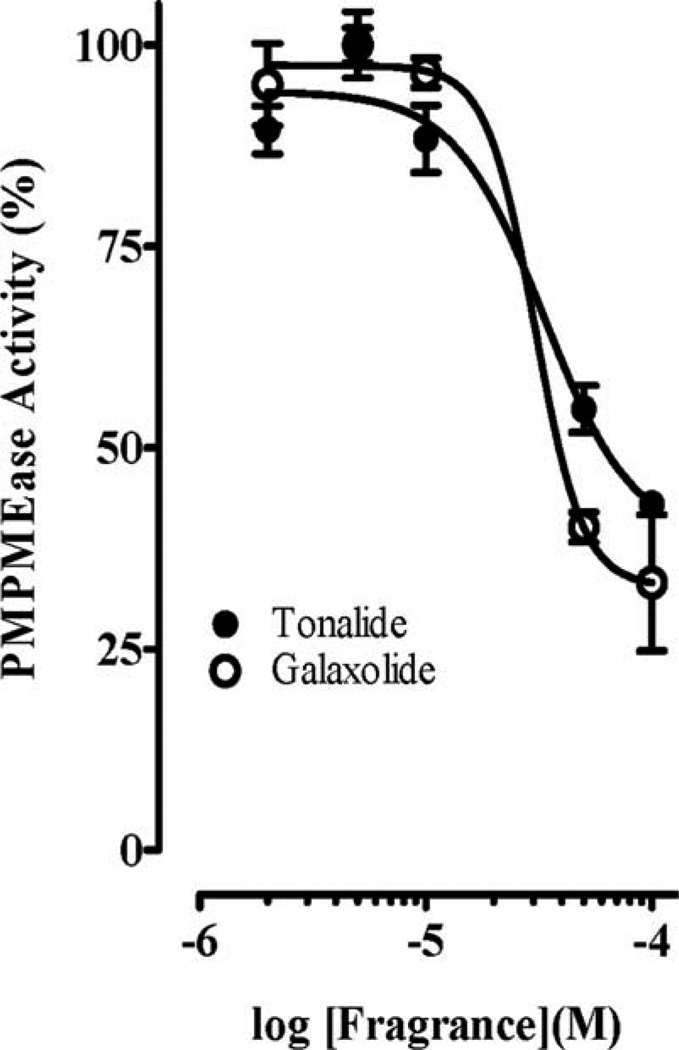
Polyisoprenylated proteins play diverse and vital roles in normal cell function as well as in health and disease. PMPMEase and PPMTase catalyze the only reversible and possibly regulatory step of the polyisoprenylation pathway. Given the fragrances’ neurotoxicities, the objective of this study was to determine their effects on PMPMEase activity. As shown in Fig. 1, all the fragrances tested had significant inhibition of PMPMEase compared with the control. The most inhibitory fragrances were Tonalide, γ-dodecalactone, β-cyclocitral, ω-pentadecalactone, Galaxolide, pentanal, and ε-decalactone which all suppressed the enzyme activity to less than 25%. Decalactone and pentanal were relatively less effective, inhibiting only 30 and 60%, respectively). A concentration-dependent analysis of the inhibitory fragrances (Fig. 2) revealed IC50 values ranging from 33 µM for β-cyclocitral to over 1 mM for pentanal with the corresponding Ki values of 4 and 170 µM, respectively (Table I). Considering the relative worldwide production and use of Galaxolide and Tonalide, these were selected for further study.
Fig. 1.
Inhibition of purified pig liver PMPMEase by fragrances. Each fragrance (1 mM) was incubated with purified PMPMEase (5 µg) and 1 mM of RD-PNB substrate for 1 h. The reactions were stopped by the addition of methanol. These were then chilled to precipitate the proteins before centrifugation at 5000 × g for 5 min. The supernatants were then analyzed by reversed-phase HPLC for residual PMPMEase activity. The activities are expressed as percentages of the uninhibited controls (±SEM, N = 3). ***P < 0.001 versus untreated controls compared by one way ANOVA, followed by the Dunnett’s post-test.
Fig. 2.
Concentration-dependent inhibition of PMPMEase by fragrances. Purified porcine liver PMPMEase (0.2 µg/µL) was preincubated at 37°C for 15 min with the indicated concentrations of each inhibitor as described in the methods section. RD-PNB substrate (0.2 mM) was then added followed by further incubation for 1 h. Reactions were stopped by the addition of methanol. These were then chilled to precipitate the proteins before centrifugation at 5000 × g for 5 min. The supernatants were then analyzed by reversed-phase HPLC for residual PMPMEase activity. The activities are expressed as percentages of the uninhibited controls (± SEM, N = 3).
TABLE I.
Inhibition of PMPMEase by synthetic fragrances
| Fragrance | IC50(µM) | Ki(µM) |
|---|---|---|
| Tonalide | 114 | 14 |
| Galaxolide | 90 | 11 |
| β-Cyclocitral | 33 | 4 |
| Dodecalactone | 96 | 12 |
| ω-Pentadecalactone | 364 | 46 |
| Pentanal | 1367 | 173 |
Effect of Fragrances on Cancer Cell Viability
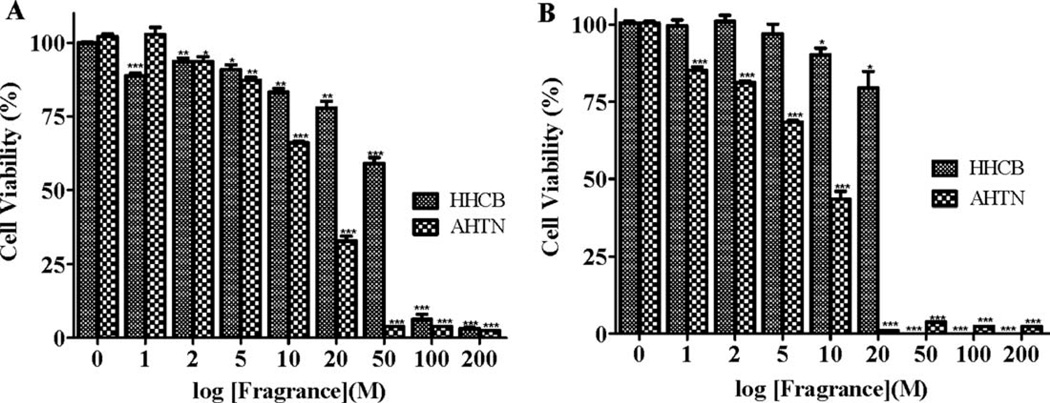
Polyisoprenylation has been implicated in several degenerative disorders such as Parkinson’s disease and choroideraemia. We therefore sought to understand what the effect of inhibition of PMPMEase may have on cells that are readily exposed to fragrances such as lung cells and those that may be affected in degenerative disorders such nerve cells. The more potent fragrances (Galaxolide and Tonalide) were used in the study on human neuroblastoma SH-SY5Y and human lung cancer A549 cells. Both Galaxolide and Tonalide caused significant degeneration of both cancer cell types as shown in Fig. 3.
Fig. 3.
Galaxolide and Tonalide induce the degeneration of (A) human lung cancer A549 cells and (B) human neuroblastoma SH-SY5Y cells in a concentration-dependent manner. A549 cells (2.5 × 106 cells/mL) were cultured and seeded in 96-well plates containing serum-free RPMI 1640/F12 medium as described in the methods. The cells were initially treated with Tonalide and Galaxolide (1–200 µM) followed by supplementation with the same amounts of compounds at 0, 24, and 48 h. At 48 h after treatment with the indicated concentrations of the respective compounds, cell viability was measured by fluorescence using the resazurin reduction assay. Both compounds induced the degeneration of cells in a concentration-dependent manner. The results are expressed as the means (±SEM, N = 4) relative to the control untreated cells. *P < 0.05, **P < 0.01, and ***P < 0.001 versus untreated control cells compared by ANOVA, followed by the Dunnett’s post-test.
Apoptosis Assay
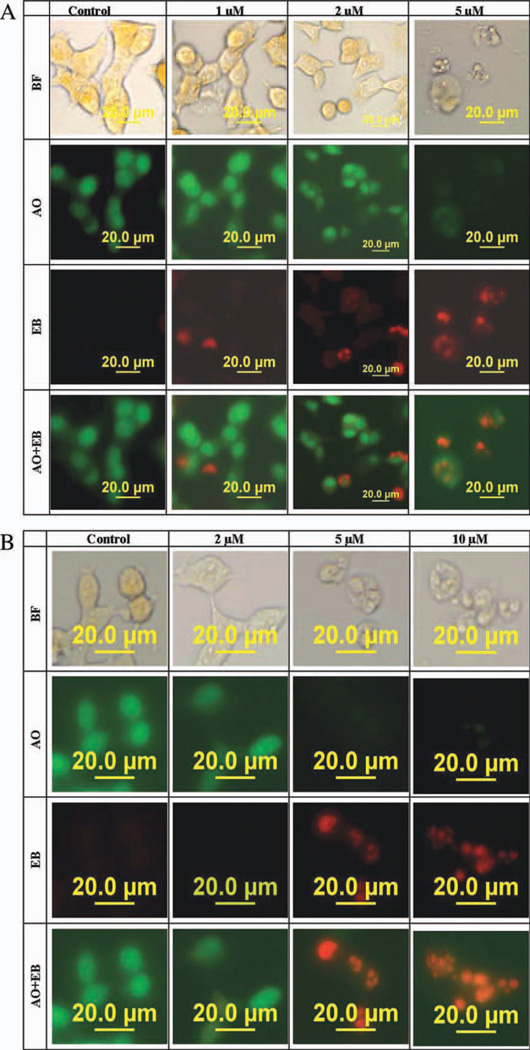
The dual staining of the cells with the fluorescent DNA binding dyes, ethidium bromide (EB), and acridine orange (AO) was to test whether the cytotoxic effect of the fragrances is due to necrosis or apoptosis. The nuclei of all cells appear green because AO easily permeates cells. EB permeates only the cells with lost cytoplasmic membrane integrity and stains their nuclei red. Since EB dominates over AO, live cells have a normal green nucleus whilst apoptotic cells display condensed and fragmented orange chromatin. Cells that have died from direct necrosis have structurally normal orange nuclei (Ribble et al., 2005). After 48-h exposure of human lung cancer A549 and human neuroblastoma SH-SY5Y cells to Tonalide [Fig. 4(A,B)], apoptosis was observed in a concentration-dependent manner. The nuclei of control cells were stained green by AO [Fig. 4(A,B)] signifying live cells. The nuclei of cells treated with Tonalide (2–10 µM) were condensed, fragmented stained orange indicating cells dying through apoptosis.
Fig. 4.
Morphological observation with acridine orange and ethidium bromide (AO/EB) staining of (A) human lung cancer A549 cells and (B) human neuroblastoma SH-SY5Y cells in serum-free medium after a 48-h exposure to Tonalide (0–10 µM). Each well was treated once with the respective final concentration. Cells were stained with EB/AO at a final concentration of 10 µg/mL. When stained with EB/AO, live cells with normal nuclei appear green; apoptotic cells with condensed or fragmented chromatin in the nuclei appear orange. Row one show pictures of cells taken with bright field (BF) focus. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
PMPMEase Activity in Galaxolide and Tonalide-Treated Human Lung Cancer A549 Cells
The PMPMEase activity in the treated cells was investigated to determine whether the observed effect on cell viability was indeed due to PMPMEase inhibition as suggested by the inhibition of the purified enzyme by the compounds. As shown in Fig. 5, a concentration-dependent inhibition of PMPMEase was observed when human lung cancer A549 cells were treated with fragrances followed by lysis and determination of the residual enzyme activity. Thus the effects on cellular PMPMEase correlated with the degenerative effects of the fragrances on cells. This further suggests that the cell degeneration caused by fragrances most likely results from PMPMEase inhibition.
Fig. 5.
PMPMEase activity in Galaxolide- and Tonalide-treated human lung cancer A549 cells. A549 cells were cultured and seeded in 24-well plates (106 cells/mL) containing serum-free RPMI 1640/F12 medium as described in the methods. Cells were treated with varying concentrations of Galaxolide and Tonalide (1–200 µM) for 24 h. They were then lysed for the determination of PMPMEase activity using RDPNB as the substrate. The results are relative to the controls and are the means (±SEM, N = 4).
Docking Analysis
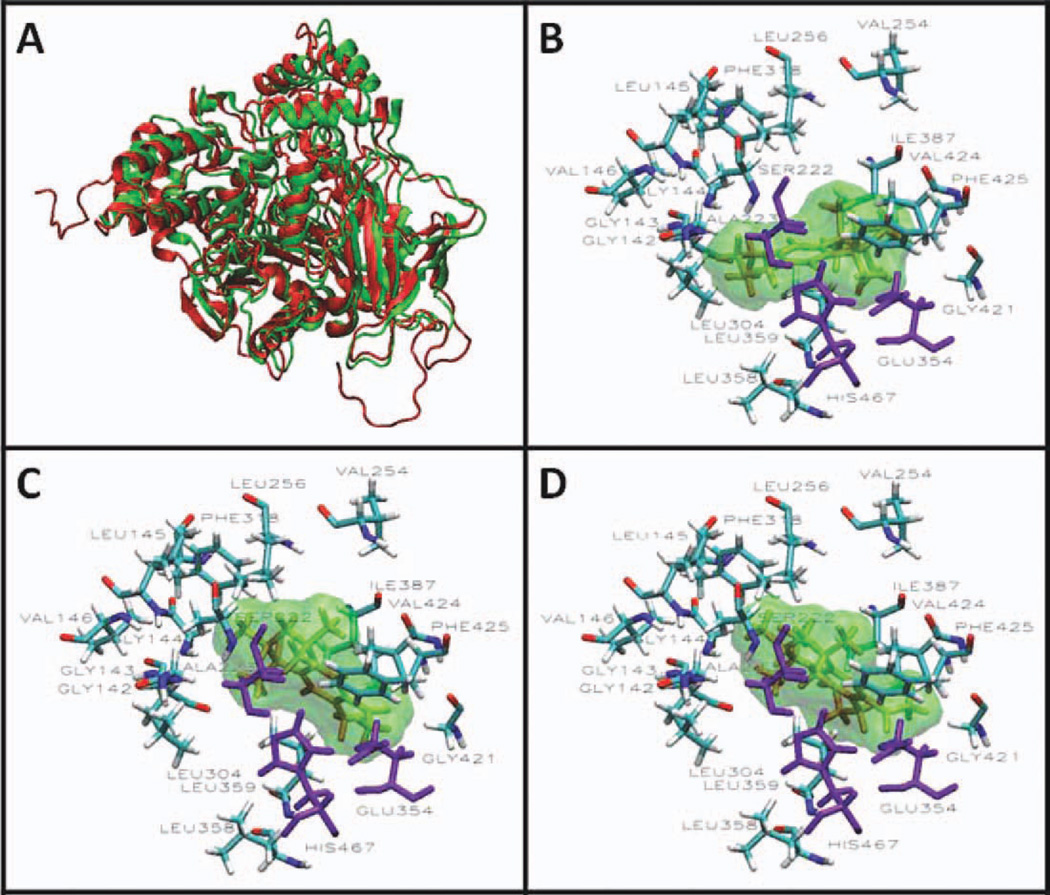
The AScore docking energies for the synthetic musk fragrances in 1YAH and PLE are shown in Table II. The values range from −10.67 to −13.15 kcal/mole for 1YAH and −12.44 to −13.33 kcal/mole for PLE. These reflect higher affinities than the −6.67 kcal/mole obtained for ethyl acetate in the X-ray crystal structure of hCE1 (1YAH). The low binding energies of fragrances indicate that these compounds engage in high affinity interactions with the enzyme. An examination of the binding energies suggests that the other synthetic musks will inhibit PMPMEase with similar potencies to Tonalide and Galaxolide. The results also suggest that a compound such as Versalide, which has shown evidence of neurotoxicity (Seabra et al., 1995, 2002), would constitute a potent inhibitor of PMPMEase given its PLE binding energy of −13.29 kcal/mol. Consistent with our expectations, the docking analysis reveals the close interactions between the hydrophobic fragrance molecules with the active site hydrophobic amino acids (Fig. 6). Similar in silico analyses of the substrates have revealed the close association of the hydrophobic amino acids with the polyisoprenyl moieties of the substrates (Duverna et al., 2010).
TABLE II.
Docking data for selected polycyclic musks
| Musk | Structure | 1YAH Ascore (kcal/mole) | PLE Ascore (kcal/mole) |
|---|---|---|---|
| Galaxolide (HHCB) | 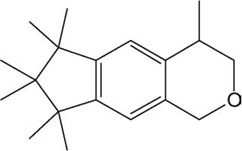 |
−12.32 | −12.82 |
| Tonalide (AHTN) |
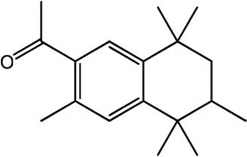
|
−12.41 | −12.79 |
| Versalide |
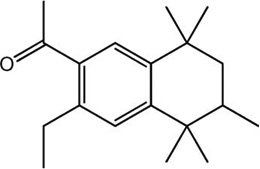
|
−12.76 | −13.29 |
| Phantolide |
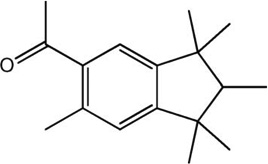
|
−12.13 | −12.66 |
| Traseolide | 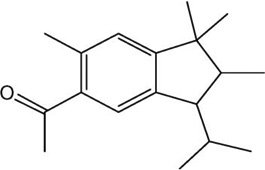 |
−13.15 | −13.33 |
| Crysolide | 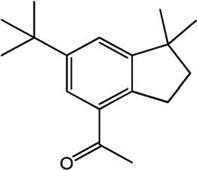 |
−12.91 | −13.25 |
| Cashmeran |  |
−10.67 | −12.44 |
Fig. 6.
Active site of PLE homology model showing the interactions between fragrance and the amino acids lining the active site. (A) The crystal structure of hCE1 (1YAH) was used as template to develop a homology model for the porcine liver enzyme (PLE) using the MODELLER program. The visualizations were created using Visual Molecular Dynamics (VMD). The overlapping cartoon ribbon structure of 1YAH (green) and the modeled PLE (red) is shown. Docking of Galaxolide (B), Tonalide (C), and Versalide (D) in the active site of PLE are displayed in the Licorice visualization. The active site amino acids are shown with the coloring method: Name (carbon atoms in blue, oxygen in red, nitrogen in dark blue, hydrogen in white). The fragrances are shown in orange Licorice and green Surf visualization. The catalytic triad is shown in purple. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
The structural and functional diversity of polyisoprenylated proteins, which account for an estimated 2% of eukaryotic proteins, suggest that significant deviations in their metabolism could spell adverse physiological consequences. Improper control of polyisoprenylated protein function results in diseases such as choroideremia (Tolmachova et al., 2006) or various cancers (Konstantinopoulos, 2007) at both ends of the cell viability spectrum. Substances that interfere with polyisoprenylated protein metabolism may alter their functional profiles resulting in disease. The active site of hCE1 (PMPMEase) which has been described as large, hydrophobic and flexible promotes the binding of a wide variety of mainly hydrophobic molecules (Bencharit et al., 2002; Redinbo et al., 2003). We therefore hypothesized that the hydrophobic nature of synthetic musks may lead them to strongly interact with the hydrophobic PMPMEase active site, reversibly inhibiting the enzymatic activity. The inhibition of PMPMEase activity by Galaxolide and Tonalide is consistent with the character of the compounds and the predicted tendency to interact with PMPMEase. Inhibition of the enzyme likely results in the accumulation of the methylated over the demethylated forms of polyisoprenylated proteins. It has been suggested that methylation/demethylation reactions of the terminal carboxylate, which is adjacent to the polyisoprenyl moiety may serve to regulate protein conformations and thus the protein–protein interactions and functional regulation of polyisoprenylated proteins (Zhang and Casey, 1996; Oboh and Lamango, 2008).
Synthetic musks are sold as components of various household consumer products. Widespread use of these compounds results in their discharge into sewage systems and consequently into the aquatic environment. Their mainly hydrocarbon molecular structures and their design to serve as fragrances implies that they have the tendency to be widely distributed, are persistent in the environment and have the tendency to bioaccumulate (Rimkus, 2004; Zhang et al., 2009). The degenerative effects of these compounds on human neuroblastoma and lung cancer cell lines are consistent with previously observed cytotoxicity on fish liver cells. The cytotoxicity profiles of the compounds is mirrored by their inhibition of purified porcine liver PMPMEase as well as PMPMEase in the cells as judged by the residual enzymatic activity following the treatment of cultured cells. Although other mechanisms for the observed toxicity cannot be precluded, the results appear to suggest a link between cell degeneration and PMPMEase inhibition.
A mechanism involving an interference with polyisoprenylated protein metabolism is understandable given that monomeric GTP-binding proteins are implicated in diseases at both ends of the cell viability spectrum either because of their hyperactivities (Konstantinopoulos, 2007) or due to loss-of-function mutations (Seabra et al., 1995, 2002). PMPMEase inhibition by Galaxolide and Tonalide would likely result in a buildup of methylated polyisoprenylated proteins coupled with the depletion of the unmethylated forms. Previous studies have shown a link between exposure to musks and the activation of estrogen receptors synthesis (Schreurs et al., 2002; Yamauchi et al., 2008). Some G-proteins are important polyisoprenylated signaling molecules whose ultimate functional expression involves gene expression (Parsa and Holland, 2004). The effects of some musks on the estrogen receptor expression could be an indirect consequence of the musks’ effects on proteins such as the G-proteins whose functional profiles may be impacted by the musk inhibition of PMPMEase. Besides employing nuclear receptors, estrogen signaling also occurs through the membrane-bound heterotrimeric G-protein-coupled receptor known as GPR30 (Vivacqua et al., 2006; Stormshak and Bishop, 2008). Given that the estrogen GPR30 is proliferative (Vivacqua et al., 2006) and its signaling is mediated by G-proteins whose c-subunit is polyisoprenylated, the musk effects on cell viability could also be a result of PMPMEase inhibition of the metabolism of the G protein γ-subunits. Despite these other possible mechanisms for the musks effects, recent observations of cell death in our laboratory caused by PMPMEase inhibition using compounds of such distinct chemically classes as the polyunsaturated fatty acids (Amissah et al., 2011) and specifically designed specific polyisoprenylated inhibitors (Aguilar et al., 2011) underscore the notion that PMPMEase inhibition is the likely reason for the musk effects on the cells.
Degenerative disorders such as Parkinson’s disease (PD) are believed to have genetic as well as environmental etiologic contributions (Barbeau et al., 1987; Veldman et al., 1998). The likelihood that PMPMEase inhibition may be involved in degenerative disorders is not only suggested by the cell degenerative effects in this study but by other studies in which knockouts (Bergo et al., 2001) or loss of function of key polyisoprenylation pathway proteins result in cell degeneration (Seabra et al., 1995). PMPMEase inhibition and treatment with the methyl donor, S-adenosyl-l-methionine (SAM) have the same endpoints of inducing polyisoprenylated protein methylation and the depletion of the unmethylated forms. The anatomical hallmark of PD is the degeneration of dopamine neurons in the brain (De Lau and Breteler, 2006; Shadrina et al., 2010). We previously showed that SAM injections into rat lateral ventricles cause transient PD-like effects that could be blocked by preceding administrations of polyisoprenylated cysteine analogs (Lamango and Charlton, 2000; Lamango et al., 2003). Exposure to compounds such as Galaxolide and Tonalide may play a role in some degenerative disorders by inhibiting PMPMEase in individuals who are predisposed to low levels of the enzyme activity. Bioaccumulation of the musks which have been detected at concentrations of up to 0.013 µg/g of wet human tissues, 2.1 µg/g of fish fillets and 0.440 µg/g of human milk may compound the PMPMEase inhibition by other common exposure chemicals such as organophosphorus pesticides (OPs) (Lamango, 2005; Oboh and Lamango, 2008). OPs inhibit PMPMEase as well as induce a degenerative syndrome known as organophosphorus pesticide-induced delayed neuropathy (OPIDN) through the inhibition of neuropathy target esterase (Glynn, 2000, 2006). On the other extreme, the effects of the musks on the cancer cell lines reveal a potential chemopreventive effect on individuals who may be at risk of developing neoplasms. This is not surprising as some hyperactive Ras and Rho are associated with about 30% of cancers.
The adverse impact of synthetic musk exposure to developing organisms has been reported (Rimkus and Wolf, 1995; Chou and Dietrich, 1999; Carlsson et al., 2000). Knockouts of polyisoprenylation pathway enzymes result in embryonic lethality, cardiomyopathy (Bergo et al., 2001) and extensive degeneration (Perez-Sala, 2007; Tolmachova et al., 2006; Krishnan et al., 2008). Although the cancer cell lines used in this study do not adequately replicate endogenous cells which are normal, the current study linking the degenerative effects of synthetic musks to PMPMEase inhibition coupled with the ubiquity of polyisoprenylated proteins and their metabolizing enzymes is an indication that wide scale exposure to musks could have other detrimental health consequences.
Acknowledgments
Contract grant sponsor: NIH/NIGMS/SCORE.
Contract grant number: GM08111-35.
Contract grant sponsor: Pharmaceutical Research Center NIH/NCRR.
Contract grant number: G12 RR0 3020.
REFERENCES
- Aguilar B, Amissah F, Duverna R, Lamango NS. Polyisoprenylation potentiates the inhibition of polyisoprenylated methylated protein methyl esterase and the cell degenerative effects of sulfonyl fluorides. Curr Cancer Drug Targets. 2011;11:752–762. doi: 10.2174/156800911796191015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amissah F, Taylor S, Duverna R, Ayuk-Takem LT, Lamango NS. Regulation of polyisoprenylated methylated protein methyl esterase by polyunsaturated fatty acids and prostaglandins. Eur J Lipid Sci Technol. 2011;113:1321–1331. doi: 10.1002/ejlt.201100030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbeau A, Roy M, Bernier G, Campanella G, Paris S. Ecogenetics of Parkinson’s disease: Prevalence and environmental aspects in rural areas. Can J Neurol Sci. 1987;14:36–41. doi: 10.1017/s0317167100026147. [DOI] [PubMed] [Google Scholar]
- Bencharit S, Morton CL, Howard-Williams EL, Danks MK, Potter PM, Redinbo MR. Structural insights into CPT-11 activation by mammalian carboxylesterases. Nat Struct Biol. 2002;9:337–342. doi: 10.1038/nsb790. [DOI] [PubMed] [Google Scholar]
- Bencharit S, Morton CL, Hyatt JL, Kuhn P, Danks MK, Potter PM, Redinbo MR. Crystal structure of human carboxylesterase 1 complexed with the Alzheimer’s drug tacrine: From binding promiscuity to selective inhibition. Chem Biol. 2003;10:341–349. doi: 10.1016/s1074-5521(03)00071-1. [DOI] [PubMed] [Google Scholar]
- Bergo MO, Leung GK, Ambroziak P, Otto JC, Casey PJ, Gomes AQ, Seabra MC, Young SG. Isoprenylcysteine carboxyl methyltransferase deficiency in mice. J Biol Chem. 2001;276:5841–5845. doi: 10.1074/jbc.C000831200. [DOI] [PubMed] [Google Scholar]
- Carlsson G, Orn S, Andersson PL, Soderstrom H, Norrgren L. The impact of musk ketone on reproduction in zebrafish (Danio rerio) Mar Environ Res. 2000;50(1–5):237–241. doi: 10.1016/s0141-1136(00)00075-1. [DOI] [PubMed] [Google Scholar]
- Chou YJ, Dietrich DR. Toxicity of nitromusks in early lifestages of South African clawed frog (Xenopus laevis) and zebrafish (Danio rerio) Toxicol Lett. 1999;111(1–2):17–25. doi: 10.1016/s0378-4274(99)00167-8. [DOI] [PubMed] [Google Scholar]
- De Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5:525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- Duverna R, Ablordeppey SY, Lamango NS. Biochemical and docking analysis of substrate interactions with polyisoprenylated methylated protein methyl esterase. Curr Cancer Drug Targets. 2010;10:634–648. doi: 10.2174/156800910791859443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn P. Neural development and neurodegeneration: Two faces of neuropathy target esterase. Prog Neurobiol. 2000;61:61–74. doi: 10.1016/s0301-0082(99)00043-x. [DOI] [PubMed] [Google Scholar]
- Glynn P. A mechanism for organophosphate-induced delayed neuropathy. Toxicol Lett Proc 42nd Cong Eur Soc Toxicol EUROTOX. 2006;162:94–97. doi: 10.1016/j.toxlet.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Gosser YQ, Nomanbhoy TK, Aghazadeh B, Manor D, Combs C, Cerione RA, Rosen MK. C-terminal binding domain of Rho GDP-dissociation inhibitor directs N-terminal inhibitory peptide to GTPases. Nature. 1997;387:814–819. doi: 10.1038/42961. [DOI] [PubMed] [Google Scholar]
- Kloog Y, Cox AD. Prenyl-binding domains: Potential targets for Ras inhibitors and anti-cancer drugs. Semin Cancer Biol. 2004;14:253–261. doi: 10.1016/j.semcancer.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Konstantinopoulos PA, Karamouzis MV, Papavassiliou AG. Post-translational modifications and regulation of the RAS superfamily of GTPases as anticancer targets. Nat Rev. 2007;6:541–555. doi: 10.1038/nrd2221. [DOI] [PubMed] [Google Scholar]
- Krishnan J, Lee G, Han SU, Choi S. Characterization of phototransduction gene knockouts revealed important signaling networks in the light-induced retinal degeneration. J Biomed Biotechnol. 2008;2008:327468. doi: 10.1155/2008/327468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamango NS. Liver prenylated methylated protein methyl esterase is an organophosphate-sensitive enzyme. J Biochem Mol Toxicol. 2005;19:347–357. doi: 10.1002/jbt.20100. [DOI] [PubMed] [Google Scholar]
- Lamango NS, Charlton CG. Farnesyl-cysteine analogs block SAM-induced Parkinson’s disease-like symptoms in rats. Pharmacol Biochem Behav. 2000;66:841–849. doi: 10.1016/s0091-3057(00)00274-4. [DOI] [PubMed] [Google Scholar]
- Lamango NS, Ayuk-Takem LT, Nesby R, Zhao W-Q, Charlton CG. Inhibition mechanism of S-adenosylmethionineinduced movement deficits by prenylcysteine analogs. Pharmacol Biochem Behav. 2003;76(3–4):433–442. doi: 10.1016/j.pbb.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Lamango NS, Duverna R, Ablordeppey SY, Zhang W. Porcine liver carboxylesterase requires polyisoprenylation for high affinity binding to cysteinyl substrates. Open Enzyme Inhibit J. 2009;2:12–27. doi: 10.2174/1874940200902010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebl B, Ehrenstorfer S. Nitro musks in human milk. Chemosphere. 1993;27:2253–2260. [Google Scholar]
- Müller S, Schmid P, Schlatter C. Occurrence of nitro and non-nitro benzenoid musk compounds in human adipose tissue. Chemosphere. 1996;33:17–28. doi: 10.1016/0045-6535(96)00160-9. [DOI] [PubMed] [Google Scholar]
- Mukherjee JJ, Jay FT, Choy PC. Purification, characterization and modulation of a microsomal carboxylesterase in rat liver for the hydrolysis of acyl-CoA. Biochem J. 1993;295(Part 1):81–86. doi: 10.1042/bj2950081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalivaeva NN, Turner AJ. Post-translational modifications of proteins: Acetylcholinesterase as a model system. Proteomics. 2001;1:735–747. doi: 10.1002/1615-9861(200106)1:6<735::AID-PROT735>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Oboh OT, Lamango NS. Liver prenylated methylated protein methyl esterase is the same enzyme as sus scrofa carboxylesterase. J Biochem Mol Toxicol. 2008;22:51–62. doi: 10.1002/jbt.20214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsa AT, Holland EC. Cooperative translational control of gene expression by Ras and Akt in cancer. Trends Mol Med. 2004;10:607–613. doi: 10.1016/j.molmed.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Perez-Sala D. Protein isoprenylation in biology and disease: General overview and perspectives from studies with genetically engineered animals. Front Biosci. 2007;12:4456–4472. doi: 10.2741/2401. [DOI] [PubMed] [Google Scholar]
- Ramirez AJ, Brain RA, Usenko S, Mottaleb MA, O’Donnell JG, Stahl LL, Wathen JB, Snyder BD, Pitt JL, Perez-Hurtado P, Dobbins LL, Brooks BW, Chambliss CK. Occurrence of pharmaceuticals and personal care products in fish: Results of a national pilot study in the United States. Environ Toxicol Chem. 2009;28:2587–2597. doi: 10.1897/08-561.1. [DOI] [PubMed] [Google Scholar]
- Redinbo MR, Bencharit S, Potter PM. Human carboxylesterase 1: from drug metabolism to drug discovery. Biochem Soc Trans. 2003;31(Part 3):620–624. doi: 10.1042/bst0310620. [DOI] [PubMed] [Google Scholar]
- Reiner JL, Kannan K. A survey of polycyclic musks in selected household commodities from the United States. Chemosphere. 2006;62:867–873. doi: 10.1016/j.chemosphere.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Reiner JL, Wong CM, Arcaro KF, Kannan K. Synthetic musk fragrances in human milk from the United States. Environ Sci Technol. 2007;41:3815–3820. doi: 10.1021/es063088a. [DOI] [PubMed] [Google Scholar]
- Ribble D, Goldstein NB, Norris DA, Shellman YG. A simple technique for quantifying apoptosis in 96-well plates. BMC Biotechnol. 2005;5:12. doi: 10.1186/1472-6750-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimkus GG. Polycyclic musk fragrances in the aquatic environment. Toxicol Lett. 1999;111(1–2):37–56. doi: 10.1016/s0378-4274(99)00191-5. [DOI] [PubMed] [Google Scholar]
- Sommer C. In: The Role of Musk and Musk Compounds in the Fragrance Industry. Rimkus GG, editor; Springer; 2004. [Google Scholar]
- Rimkus GG, Wolf M. Nitro musk fragrances in biota from freshwater and marine environment. Chemosphere. 1995;30:641–651. [Google Scholar]
- Rimkus GG, Wolf M. Polycyclic musk fragrances in human adipose tissue and human milk. Chemosphere. 1996;33:2033–2043. doi: 10.1016/0045-6535(96)00321-9. [DOI] [PubMed] [Google Scholar]
- Salvito D. Synthetic musk compounds and effects on human health? Environ Health Perspect. 2005;113:A802–A803. doi: 10.1289/ehp.113-a802b. author reply A803–A804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavone A, Kannan K, Horii Y, Focardi S, Corsolini S. Polybrominated diphenyl ethers, polychlorinated naphthalenes and polycyclic musks in human fat from Italy: Comparison to polychlorinated biphenyls and organochlorine pesticides. Environ Pollut. 2010;158:599–606. doi: 10.1016/j.envpol.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Schnell S, Bols NC, Barata C, Porte C. Single and combined toxicity of pharmaceuticals and personal care products (PPCPs) on the rainbow trout liver cell line RTL-W1. Aquat Toxicol. 2009;93:244–252. doi: 10.1016/j.aquatox.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Schreurs RHMM, Quaedackers ME, Seinen W, van der Burg B. Transcriptional activation of estrogen receptor ERa and ERb by polycyclic musks is cell type dependent. Toxicol Appl Pharmacol. 2002;183:1–9. doi: 10.1006/taap.2002.9458. [DOI] [PubMed] [Google Scholar]
- Seabra MC, Ho YK, Anant JS. Deficient geranylgeranylation of Ram/Rab27 in choroideremia. J Biol Chem. 1995;270:24420–24427. doi: 10.1074/jbc.270.41.24420. [DOI] [PubMed] [Google Scholar]
- Seabra MC, Mules EH, Hume AN. Rab GTPases, intracellular traffic and disease. Trends Mol Med. 2002;8:23–30. doi: 10.1016/s1471-4914(01)02227-4. [DOI] [PubMed] [Google Scholar]
- Shadrina MI, Slominsky PA, Limborska SA. Molecular mechanisms of pathogenesis of Parkinson’s disease. Int Rev Cell Mol Biol. 2010;281:229–266. doi: 10.1016/S1937-6448(10)81006-8. [DOI] [PubMed] [Google Scholar]
- Shubert H-J. Skin diseases in workers at a perfume factory. Contact Dermatitis. 2006;55:81–83. doi: 10.1111/j.0105-1873.2006.00881.x. [DOI] [PubMed] [Google Scholar]
- Spencer PS, Sterman AB, Horoupian DS, Foulds MM. Neurotoxic fragrance produces ceroid and myelin disease. Science. 1979;204:633–635. doi: 10.1126/science.432669. [DOI] [PubMed] [Google Scholar]
- Spencer PS, Bischoff-Fenton MC, Moreno OM, Opdyke DL, Ford RA. Neurotoxic properties of musk ambrette. Toxicol Appl Pharmacol. 1984;75:571–575. doi: 10.1016/0041-008x(84)90194-7. [DOI] [PubMed] [Google Scholar]
- Stormshak F, Bishop CV. Board-invited review: Estrogen and progesterone signaling: Genomic and nongenomic actions in domestic ruminants. J Anim Sci. 2008;86:299–315. doi: 10.2527/jas.2007-0489. [DOI] [PubMed] [Google Scholar]
- Tolmachova T, Anders R, Abrink M, Bugeon L, Dallman MJ, Futter CE, Ramalho JS, Tonagel F, Tanimoto N, Seeliger MW, Huxley C, Seabra MC. Independent degeneration of photoreceptors and retinal pigment epithelium in conditional knockout mouse models of choroideremia. J Clin Invest. 2006;116:386–394. doi: 10.1172/JCI26617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujita T, Okuda H. Palmitoyl-coenzyme A hydrolyzing activity in rat kidney and its relationship to carboxylesterase. J Lipid Res. 1993;34:1773–1781. [PubMed] [Google Scholar]
- Veldman BAJ, Wijn AM, Knoers N, Praamstra P, Horstink MWIM. Genetic and environmental risk factors in Parkinson’s disease. Clin Neurol Neurosurg. 1998;100:15–26. doi: 10.1016/s0303-8467(98)00009-2. [DOI] [PubMed] [Google Scholar]
- Vivacqua A, Bonofiglio D, Recchia AG, Musti AM, Picard D, Ando S, Maggiolini M. The G protein-coupled receptor GPR30 mediates the proliferative effects induced by 17beta-estradiol and hydroxytamoxifen in endometrial cancer cells. Mol Endocrinol. 2006;20:631–646. doi: 10.1210/me.2005-0280. [DOI] [PubMed] [Google Scholar]
- Yamauchi R, Ishibashi H, Hirano M, Mori T, Kim JW, Arizono K. Effects of synthetic polycyclic musks on estrogen receptor, vitellogenin, pregnane X receptor, and cytochrome P450 3A gene expression in the livers of male medaka (Oryzias latipes) Aquat Toxicol. 2008;90:261–268. doi: 10.1016/j.aquatox.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Zhang FL, Casey PJ. Protein prenylation: Molecular mechanisms and functional consequences. Annu Rev Biochem. 1996;65:241–269. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]
- Zhang P, Song J, Yuan H. Persistent organic pollutant residues in the sediments and mollusks from the Bohai Sea coastal areas, North China: An overview. Environ Int. 2009;35:632–646. doi: 10.1016/j.envint.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Zhou H, Huang X, Gao M, Wang X, Wen X. Distribution and elimination of polycyclic musks in three sewage treatment plants of Beijing, China. J Environ Sci. 2009;21:561–567. doi: 10.1016/s1001-0742(08)62308-6. [DOI] [PubMed] [Google Scholar]