Abstract
OBJECTIVE
This study summarizes the literature on the detection of cancer among indeterminate extracolonic findings on CT colonography in five targeted organs.
MATERIALS AND METHODS
We searched PubMed for English-language literature published between January 1, 1994 and December 31, 2010. We describe extracolonic findings in the kidney, lung, liver, pancreas, and ovary suspect for malignancy as they are associated with high mortality. For each organ, we calculated the median prevalence, positive predictive value (PPV), and false positive rate of malignancy, and a pooled false-positive rate across studies.
RESULTS
Of 91 publications initially identified, 24 were eligible for review. Indeterminate renal masses on CT colonography had 20.5% median PPV and low pooled false positive rate of 1.3% (95% CI 0.6–2.0). In contrast, indeterminate masses of the lung, liver, pancreas, and ovary had low PPV (medians ranged from 0–3.8%). Indeterminate masses of the ovary resulted in the highest pooled false-positive rate of 2.2%. Results were similar in studies of both screening and non-screening populations. We estimated the probability of false positive results through the detection of significant extracolonic findings as 46 per 1,000 for men and 68 per 1,000 for women.
CONCLUSIONS
Indeterminate renal masses newly detected on CT colonography have an estimated one in five chance of malignancy and therefore warrant further follow-up to provide a definitive diagnosis. Conversely, indeterminate masses of the lung, liver, pancreas, and ovary are associated with high false positive rates and merit more conservative clinical follow-up.
Keywords: CT colonography, extracolonic findings, neoplasm
Introduction
Computed tomographic (CT) colonography was first described as a method to assess colon neoplasia in 1994 [1]. CT colonography involves insufflation of the colon and rectum with gas, and the acquisition of thin-section CT images to visualize polyps and masses using both two-dimensional and three-dimensional interpretation [2]. Multiple studies have demonstrated that the accuracy of CT colonography is similar to traditional (optical) colonoscopy for detection of adenomas and colorectal cancer (i.e., sensitivity of 91.3% and specificity of 93.1% for lesions >5 mm) [3]. Both of these screening techniques require cathartic bowel preparation, which is a major deterrent to colorectal cancer screening for patients. However, because CT colonography does not require sedation, it has the potential to increase overall adherence to colorectal cancer screening [4] and is associated with fewer risks than optical colonoscopy.
Unlike traditional colonoscopy, CT colonography can identify extracolonic findings (i.e., outside the colon or rectal lumen) because the lung bases, abdomen, and pelvis are included in the examination. To guide management of extracolonic findings, the Working Group on Virtual Colonoscopy developed a rating of extracolonic findings using a scale of E1 to E4 [5]. Under this system, E3 and E4 findings are potentially significant to the patient’s health but incompletely characterized on CT colonography, and usually require further imaging and medical follow-up for definitive characterization. E3 findings (e.g., pulmonary nodules <1 cm or cystic renal or adnexal masses) are likely insignificant and might require non-urgent follow-up. E4 findings (e.g., solid renal masses or pulmonary nodules >1 cm) are likely significant and require urgent follow-up.
Whether the detection of potentially significant extracolonic findings results in a net benefit from the diagnosis and treatment of disease, or a net harm from the work-up of false positive findings is unclear [6, 7]. Understanding the outcomes (i.e., true positive and false positive rates) of detecting these findings could help clinicians in considering the necessity for follow-up. We performed a review of the literature of the CT colonography and extracolonic findings to determine the median prevalence and positive predictive value (PPV) of true disease, and false positive rate of extracolonic findings associated with high mortality, specifically indeterminate masses of the kidney, lung, liver, pancreas, and ovary that are suspected to be malignant. We also compared differences between populations receiving CT colonography for screening vs. non-screening presentation (i.e., with symptoms).
Materials and methods
Literature Review
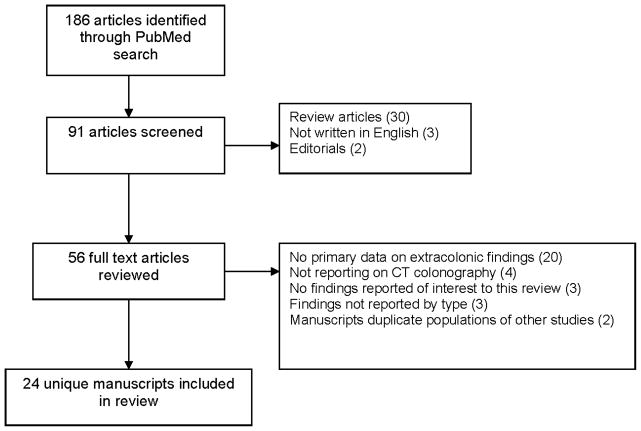
We performed a PubMed/MEDLINE search CT colonography literature under the subject headings CT colonography, extracolonic and virtual colonoscopy, limited to articles published from January 1, 1994 through December 31, 2010. We identified 91 unique manuscripts that were reviewed for inclusion (Figure 1).. Publications were excluded after review of the abstract if they were review articles (n=30), not written in English (n=3) or editorials (n=2). For the 56 remaining publications, full text articles were reviewed for further inclusion in the final review. We were specifically interested in extracolonic findings within the kidney, lung, liver, pancreas, and ovary that are suspected to be malignant. Studies were excluded if they did not contain any primary data on extracolonic findings on CT colonography (n=20), did not report on the use of CT colonography only (n=4); reported on extracolonic findings in organs other than our target organ sites (n=3), reported overall extracolonic findings combined but not by type (n=3) or included a study population that overlapped with another publication included in the review (n=2). The final study population included 24 unique publications. Two reviewers conducted separate assessments of the publications, and any discrepancies between the two reviews were adjudicated with another contributor.
FIGURE 1.
Selection of manuscripts included in the literature review
From each article, the reviewers abstracted the sample size, age and gender distribution, presence of patient symptoms, the number of incident extracolonic findings detected of interest, and the numbers of subjects with a diagnosed carcinoma. Many studies reported the number of extracolonic findings by type (e.g., renal mass), allowing multiple findings per person across types. For simplicity, we assumed that the number of reported findings by type represented the total number of individuals with that finding. Some studies did not report the follow-up or diagnosis of detected extracolonic findings. We included these studies in the review to demonstrate the range of reported results across studies, and their data are listed as not available when the data are missing.
Study populations were categorized as screening (i.e., patients presenting for CT colonography for screening and without symptoms of disease); non-screening (i.e., patients presenting for CT colonography due to symptoms of colorectal cancer); or mixed/unknown (i.e., the study population included both screening and non-screening populations or did not specify the patient population).
Targeted Extracolonic Findings
The extracolonic findings of interest in our analysis included incident indeterminate masses of the kidney, lung, liver, pancreas, and ovary suspect for primary neoplasm. These findings were targeted because they are associated with high-mortality diseases. We did not evaluate aortic abdominal aneurysms, which are also associated with high mortality, because they are definitively diagnosed on CT colonography. We also did not include extracolonic findings that were not associated with a primary tumor. For example, lymphadenopathy is a potentially significant finding, but it is associated with multiple potential diagnoses (e.g., primary lymphoma, metastatic disease) rather than a single disease, making it difficult to determine the false positive rate or PPV for this finding. Finally, we did not consider ovarian dermoids diagnosed on CT colonography as a true positive disease as these masses can be reliably diagnosed on unenhanced CT [8], and are associated with a low rate of malignant conversion (0.2–2%) [9, 10]. For ovarian findings, our calculations were restricted to the reported study sample of women within each publication.
Statistical Analysis
For each study, we used the available data to calculate the prevalence of true disease, the false positive rate, and the PPV associated with cancers of the kidney, lung, liver, pancreas, and ovary initially detected on CT colonography. Because disease status was verified only for individuals with a positive extracolonic finding on CT colonography, we only observed the total number of individuals with a negative finding. Therefore, to calculate prevalence and false positive rates, we assumed that CT colonography was 100% sensitive for detecting extracolonic disease present at the time of the examination, and all individuals without a reported extracolonic finding were true negatives. Our study-level outcomes were defined as:
Prevalence = the number of persons with the targeted disease per 1000
False positive rate (FPR) = the number of persons without the targeted disease who have extracolonic findings per 100
Positive predictive value (PPV) = the number of persons with disease-specific extracolonic findings who were diagnosed with the targeted disease per 100
Several studies only presented the number of extracolonic findings or the number of true positives. In these circumstances, we could not calculate all measures of interest. Using available data, we calculated the median prevalence and PPV of true disease, and the false positive rate as a summary across all studies. Results are presented stratified by population type. Further, we calculated study- and organ-specific binomial confidence intervals (CIs) for rate estimates for prevalence, PPV, and false positive rate. We used a beta-binomial model [11] to obtain maximum likelihood estimates of pooled false positive rates. Under the beta-binomial model, the number of events observed in the ith study is assumed to follow a binomial distribution with probability pi. Across studies, the event probabilities are assumed to follow a beta distribution with unspecified mean and variance. For example, to model false positive rates we assumed that for the ith study and jth outcome, the number of individuals with a false positive result out of Nij patients without disease follows a binomial (FPRij, Nij) distribution and that across studies, FPRij follows a beta distribution. Because studies focused on different populations, we did not calculate the pooled estimate of the true disease prevalence or PPV. All analyses were conducted using Stata v12 software [12, 13].
Results
Publications included in this review represented a range of sample sizes, participant ages and symptoms, and geographic populations from North America, Europe, Australia, and Asia. The findings across all organ sites are described in Table 1.
TABLE 1.
Summary of reviewed published literature of CT colonography by calculation of median test properties* across five target organ sites, stratified by screening and non-screening populations, through 2010.
| Target organ | No. of studies | False positive rate (per 100) | Prevalence (per 1000) | Positive Predictive Value (per 100) |
|---|---|---|---|---|
| Renal | ||||
| Screening | 6 | 1.0 | 0.6 | 1.5 |
| Non-screening | 13 | 1.0 | 4.2 | 25.0 |
| Mixed/Unknown | 3 | - | - | - |
| Overall | 22 | 1.1 | 2.2 | 20.5 |
| Lung | ||||
| Screening | 6 | 0.2 | 0.1 | 20.0 |
| Non-screening | 6 | 0.7 | 0.7 | 0 |
| Mixed/Unknown | 3 | - | - | - |
| Overall | 15 | 0.7 | 0.1 | 3.8 |
| Liver | ||||
| Screening | 4 | 0 | 1.2 | 0 |
| Non-screening | 8 | 1.8 | 0 | 0 |
| Mixed/Unknown | 2 | - | - | - |
| Overall | 14 | 1.3 | 0 | 0 |
| Pancreas | ||||
| Screening | 2 | 0.1 | 0 | 0 |
| Non-screening | 5 | 1.4 | 1.2 | 25.0 |
| Mixed/Unknown | 1 | - | - | - |
| Overall | 8 | 0.5 | 0.5 | 0 |
| Ovary | ||||
| Screening | 5 | 1.5 | 0 | 0 |
| Non-screening | 9 | 3.7 | 0 | 10.0 |
| Mixed/Unknown | 1 | - | - | - |
| Overall | 15 | 2.5 | 0 | 0 |
Summarized across studies with available data for the calculation.
- cannot be calculated due to missing data on the number of true positive results.
Renal findings were the most commonly cited extracolonic finding, reported in 22 publications (Tables 1 and 2). The overall median prevalence, PPV, and false positive rate was 3.2/1000 persons, 20.5%, and 1.1%, respectively (Table 1). The PPV was higher in non-screening populations (PPV = 25.0%) for detection of renal cancer compared to screening populations (PPV=1.5%).
TABLE 2.
Description of reviewed published manuscripts reporting extracolonic findings for renal cancer.
| Author | Year | Country | Sample size | Age range | Mean/Median Age | Men | Number of ECFa | Number of True Positives | False Positive Rate | Prevalence (per 1000) | PPV |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Screening populations | |||||||||||
| Ginnerup Pederson Bb [33] | 2003 | Denmark | 75 | 33–78 | 61 | 53.3 | 1 | 0 | 1.3 | 0 | 0 |
| Gluecker TM [21] | 2003 | US | 681 | 41–80 | 64 | 62.6 | 34 | 1 | 4.9 | 1.5 | 2.9 |
| Chin Mb [23] | 2005 | Australia | 432 | 50–69 | - | 53.2 | 6 | 1 | 1.2 | 2.3 | 16.7 |
| Kimberly JR [34] | 2008 | US | 143 | 44–83 | 57 | 48 | 1 | 0 | 0.7 | 0 | 0 |
| Pickhardt PJ [35] | 2010 | US | 10,286 | - | 59.8 | 52.3 | NA | 11 | - | 1.1 | - |
| Veerappan GR [18] | 2010 | US | 2277 | - | 59 | 56 | 16 | 3 | 0.6 | 0.1 | 18.8 |
| Non-screening populations | |||||||||||
| Edwards JT [19] | 2001 | Australia | 100 | 65 | 47 | 1 | NA | - | - | - | |
| Hellstrom M [36] | 2004 | Sweden | 111 | 19–86 | 66 | 59.0 | 4 | 1 | 2.7 | 9.0 | 25.0 |
| Pilch-Kowalczyk J [37] | 2004 | Poland | 71 | 20–85 | - | 40.8 | NA | 1 | - | 14.1 | - |
| Rajapaksa RC [38] | 2004 | US | 250 | - | 62.5 | 98 | 1 | 1 | 0 | 0.4 | 100 |
| Serracino-Inglott Fb [26] | 2004 | UK | 103 | 43–88 | 68 | 45.6 | 1 | 0 | 1.0 | 0 | - |
| Spreng A [22] | 2005 | Switzerland | 102 | 20–91 | 66 | 61.7 | NA | 1 | - | 9.8 | - |
| Yee J [39] | 2005 | US | 500 | 30–90 | 62.5 | 100 | 8 | 2 | 1.2 | 4.0 | 25.0 |
| Tolan D [28] | 2007 | UK | 400 | 70–96 | 79.6 | 37.5 | 4 | 2 | 0.5 | 5.0 | 50.0 |
| Khan KYb [24] | 2007 | UK | 225 | 62–81 | 74 | 40.4 | 3 | 0 | 1.3 | 0 | 0 |
| Flicker MS [20] | 2008 | US | 376 | 26–89 | 61 | 36.7 | 2 | 2 | 0 | 5.3 | 100.0 |
| Roberts-Thomson IC [25] | 2008 | Australia | 225 | 25–85 | 60 | 51.0 | 1 | 1 | 0 | 4.4 | 100.0 |
| Yucel C [27] | 2008 | US | 42 | 60–87 | 71 | 38.0 | 4 | NA | - | - | - |
| White TJ [40] | 2009 | US | 150 | 40–83 | 60.9 | 48.7 | NA | 3 | - | 2.0 | - |
| Mixed/Unknown | |||||||||||
| Hara AK [41] | 2000 | US | 264 | 33–88 | 64 | 55.3 | 9 | 2 | 2.7 | 7.6 | 22.2 |
| Iafrate F [42] | 2008 | Italy | 136 | 70–92 | 81 | NA | 1 | NA | - | - | - |
| Park SK [43] | 2009 | South Korea | 920 | 34–87 | 57.3 | 58.1 | 3 | NA | - | - | - |
ECF=extracolonic finding, PPV=positive predictive value, NA=not available, (-)=could not calculate; CI=confidence interval
Authors report extracolonic findings as “renal cysts” and report the number of cysts with follow-up. We counted the number of renal cysts with follow-up.
Indeterminate lesions were described less frequently in other organs. Findings from the lung were the second most commonly cited extracolonic finding (Table 1 and 3). However, there was a low prevalence of malignancy detected and low overall PPV of 3.8% (Table 1). Similarly, findings indicating a mass in the liver and pancreas had a low median prevalence and PPV (Table 1). Extracolonic findings detected in the ovary were relatively common (Table 4), but the overall median PPV was zero. There were no striking differences between screening and non-screening populations for findings in the lung, liver, pancreas or ovary.
TABLE 3.
Description of reviewed published manuscripts reporting extracolonic findings for lung cancer.
| Author | Year | Country | Sample size | Age range | Mean/Median Age | Men | Number of ECFa | Number of True Positives | False Positive Rate | Prevalence (per 1000) | PPV |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Screening populations | |||||||||||
| Ginnerup Pederson B [33] | 2003 | Denmark | 75 | 33–78 | 61 | 53.3 | 1 | 1 | 0 | 1.3 | 100.0 |
| Gluecker TM [21] | 2003 | US | 681 | 41–80 | 64 | 62.6 | 26 | 1 | 3.7 | 0.1 | 3.8 |
| Chin M [23] | 2005 | Australia | 432 | 50–69 | - | 53.2 | 3 | 0 | 0.7 | 0 | 0 |
| Kimberly JR [34] | 2008 | US | 143 | 44–83 | 57 | 48 | 23 | NA | - | - | - |
| Pickhardt PJ [35] | 2010 | US | 10,286 | - | 59.8 | 52.3 | NA | 8 | - | 0.1 | - |
| Veerappan GRb [18] | 2010 | US | 2277 | - | 59 | 56 | 5 | 1 | 0.2 | 0.0 | 20.0 |
| Non-screening populations | |||||||||||
| Rajapaksa RC [38] | 2004 | US | 250 | - | 62.5 | 98 | 3 | NA | - | - | - |
| Spreng A [22] | 2005 | Switzerland | 102 | 20–91 | 66 | 61.7 | NA | 1 | - | 1.0 | - |
| Yee J [39] | 2005 | US | 500 | 30–90 | 62.5 | 100 | 7 | 0 | 1.4 | 0.0 | 0.0 |
| Tolan D [28] | 2007 | UK | 400 | 70–96 | 79.6 | 37.5 | 7 | 6 | 0.3 | 1.5 | 85.7 |
| Roberts-Thomson IC [25] | 2008 | Australia | 225 | 25–85 | 60 | 51.0 | 2 | NA | - | - | - |
| White TJ [40] | 2009 | US | 150 | 40–83 | 60.9 | 48.7 | NA | 1 | - | 0.7 | - |
| Unknown/Mixed | |||||||||||
| Hara AK [41] | 2000 | US | 264 | 33–88 | 64 | 55.3 | 8 | 0 | 3.0 | 0.0 | 0 |
| Iafrate F [42] | 2008 | Italy | 136 | 70–92 | 81 | NA | 1 | NA | - | - | - |
| Park SK [43] | 2009 | South Korea | 920 | 34–87 | 57.3 | 58.1 | 9 | NA | - | - | - |
ECF=extracolonic finding, PPV=positive predictive value, NA=not available, (-)=could not calculate
Only includes carcinoma detected from the E4 findings, even though additional cancer was detected among persons with E3 findings. The total number of E3 findings could not be determined.
TABLE 4.
Description of reviewed published manuscripts reporting extracolonic findings for ovarian cancer.
| Author | Year | Country | Sample size | Age range | Mean/Median Age | Number of ECFa | Number of True Positives | False Positive Rate | Prevalence (per 1000) | PPV |
|---|---|---|---|---|---|---|---|---|---|---|
| Screening populations | ||||||||||
| Ginnerup Pederson B [33] | 2003 | Denmark | 35 | 33–78 | 61 | 1 | 0 | 2.9 | 0 | 0 |
| Gluecker TM [21] | 2003 | US | 255 | 41–80 | 64 | 6 | 0 | 2.4 | 0 | 0 |
| Chin M [23] | 2005 | Australia | 202 | 50–69 | 3 | 0 | 1.5 | 0 | 0 | |
| Pickhardt PJ [17] | 2008 | US | 1199 | 40–90 | 58 | 13 | 0 | 1.1 | 0 | 0 |
| Veerappan GR [18] | 2010 | US | 1002 | 59 | 5 | 0 | 0.5 | 0 | 0 | |
| Non-screening populations | ||||||||||
| Morrin MM | 1999 | US | 28 | 22–96 | 1 | NA | - | - | - | |
| Edwards JT [19] | 2001 | Australia | 53 | 65 | 3 | 0 | 5.7 | 0 | 0 | |
| Serracino-Inglott F [26] | 2004 | UK | 56 | 43–88 | 68 | 5 | 1 | 7.3 | 17.9 | 20.0 |
| Spreng A [22] | 2005 | Switzerland | 39 | 20–91 | 66 | 3 | 1 | 5.3 | 25.6 | 33.3 |
| Khan KY [24] | 2007 | UK | 134 | 62–81 | 74 | 5 | 0 | 3.7 | 0 | 0 |
| Tolan D [28] | 2007 | UK | 250 | 70–96 | 79.6 | 5 | 0 | 2.0 | 0 | 0 |
| Flicker MS [20] | 2008 | US | 238 | 26–89 | 61 | 3 | 1 | 0.8 | 4.2 | 33.3 |
| Roberts-Thomson IC [25] | 2008 | Australia | 110 | 25–85 | 60 | 3 | 0 | 2.7 | 0 | 0 |
| Yucel C [27] | 2008 | US | 26 | 60–87 | 71 | 1 | NA | - | - | - |
| Unknown/Mixed | ||||||||||
| Park SK [43] | 2009 | South Korea | 385 | 34–87 | 57.3 | 1 | NA | - | - | - |
ECF=extracolonic finding, PPV=positive predictive value, NA=not available, (-)=could not calculate; CI=confidence interval
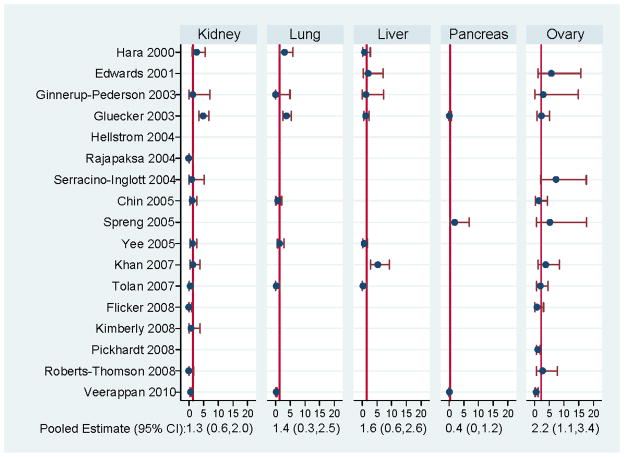
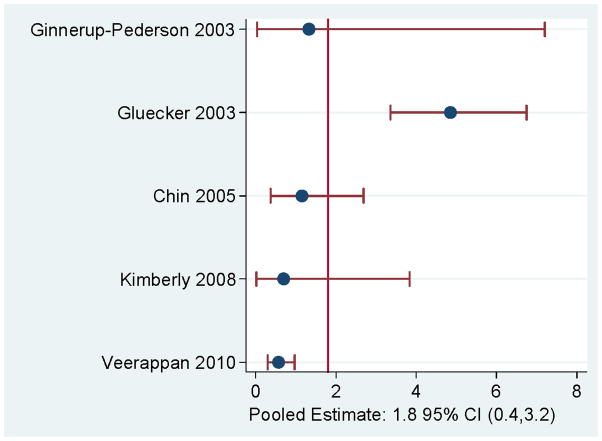
Pooled false positive rates were <2% for most findings (Figure 2). Ovarian findings had the highest pooled false positive rate (2.2%). For renal findings, the overall false positive rate was 1.3% overall (Figure 2) and increased to 1.8% among studies focused only on a screening population (Figure 3).
FIGURE 2.
False positive rates for malignancy per 100 disease-free individuals by target organ.
FIGURE 3.
False positive rates for malignancy per 100 disease-free individuals in screening populations for renal cancer.
If we assume independence of the extracolonic findings associated with the five cancer sites and use the pooled false positive rate for each extracolonic finding, then the estimated probability that CT colonography would detect a potentially significant false positive extracolonic finding defined from our analysis would be 46/1,000 in men and 68/1000 in women.
Discussion
Our literature review summarizes the reporting and outcomes of incident extracolonic findings detected on CT colonography. Indeterminate renal masses were associated with a high PPV and lowest false-positive rate, suggesting that CT colonography might provide useful clinical information about incident renal tumors, particularly in the non-screening population. In contrast, indeterminate masses of the liver, pancreas, and ovary were associated with a low PPVs suggesting that these lesions could be followed clinically with or without further imaging.
Screening for colorectal cancer is important given the burden of this disease in the US [14]. CT colonography has the potential to improve overall adherence rates to screening. However, both clinicians and patients should understand both the likelihood of newly detected extracolonic findings on CT colonography and the chances that these findings correspond to false positive results. Given that the incidence of the cancers included in this systematic review are rare in the US population, ranging from 6 to 60 new cases per 100,000 [14], it is not surprising that detection of these cancers on CT colonography is also rare. Our estimates of false positive findings associated with indeterminate masses for the five targeted organs were relatively low in the screening population. One example is the pooled low false positive rate for renal findings among a screening population. Our stratified analysis demonstrated that the lowest PPV was often found in screening populations, except for lung findings. We estimate that the work-up of extracolonic findings associated with these five cancer sites would result in 36 false positive findings for every 1000 disease-free men screened and 58 false positive findings for every 1000 disease-free women screened. The higher overall false positive rates among women are due to the much higher rate of false positive ovarian findings.
Findings with a high PPV have potentially greater clinical value and therefore may warrant more complete evaluation. We found the highest PPV for indeterminate masses of the kidney (20%); consistent with the relatively high (and rising) incidence of renal cell carcinoma in the United States [15]. In contrast, PPVs were extremely low for indeterminate masses of the liver, pancreas, and ovary. Because PPV is closely related to disease prevalence when the sensitivity of a test is fixed [16], drawing conclusions about PPV across studies with different disease prevalence is difficult. However, our findings suggest potentially important differences across these extracolonic findings.
Indeterminate ovarian masses were not predictive of cancer, particularly in the screening population, suggesting that these masses should be followed conservatively. This conclusion is supported by prior work evaluating surgically resected adnexal lesions, which were found to be primarily benign tumors [17, 18]. In our review of ovarian findings, the majority of lesions that were benign on resection were identified as cystic ovarian lesions [17–22] corresponding to cysts (simple or complex), benign mucinous or serous cystadenomas, cystadenofibromas, mature teratomas or inflammatory lesions (abscesses) on pathology. We do not know if these findings demonstrated other concerning imaging features that would explain why these women underwent surgical intervention. Nonetheless, these results suggest that indeterminate cystic ovarian masses that are newly detected on CT colonography might benefit from less aggressive clinical follow-up, with or without imaging, than solid ovarian masses.
The need for clear guidelines relating to follow-up and reporting of extracolonic findings on CT colonography is underscored by the wide range of definitions of findings used by studies included in this review. Many studies describing indeterminate renal masses included only renal masses, while others included complex cystic masses, and some provided no finding definitions. Similar ambiguity was seen for other lesions, most often for ovarian findings, with several studies including cysts as indeterminate ovarian masses [18, 23–27] with either no lesion size, or a range of sizes included [19, 28]. Lesion size is relevant because most women receiving CT colonography are post-menopausal, and size is an important factor in ovarian lesion management for these women [29]. Although no size criteria exist for defining an indeterminate ovarian mass on CT, the literature suggests that lesions <1 cm can be ignored while those >3 cm should be evaluated with ultrasound [30]. Considering the high number of benign renal and ovarian cysts visualized on CT, high-priority follow-up should be assigned only to masses that meet strict criteria for indeterminate categorization. The establishment of such definitions could limit unnecessary follow-up of these findings, thereby reducing the range we observed for prevalence and false positive rates for the organs we investigated. However, whether establishing and adhering to guidelines will lead to improved patient outcomes is uncertain, particularly given the indolent growth of some cancers such as renal cancer [31].
While our study provides a useful overview of published work on extracolonic findings, it has some limitations. The studies we reviewed did not consistently report exam outcomes across the study sample. Many studies did not explicitly report the number of patients with extracolonic findings, instead reporting the number of extracolonic findings. Many studies did not describe either the receipt of additional work-up based on extracolonic findings or the outcomes of any additional work-up. Although some patients probably did not undergo additional work-up for the findings included in these studies because of underlying clinical conditions, prior knowledge of the finding, or patient choice was not usually specified. The result of reporting variability is that the number of patients with extracolonic findings or with true disease is not reported in several manuscripts. Other sources of variability included the wide range of patient ages, the inclusion of symptomatic patients at the time of CT colonography, and the use of different CT colonography protocols. Finally, we summarized targeted findings only from publications that reported specific findings. By excluding studies that did not report any findings, including null extracolonic findings for targeted diseases, we likely overestimated disease prevalence in the study population. Hence, our estimate of prevalence might overestimate the true cancer rate in the underlying population. Despite these limitations, we believe that the reported findings provide insight into extracolonic findings from CT colonography as used in current practice.
While the low overall rate of false positive findings for cancer from all indeterminate masses is reassuring, it points to the need for more judicious reporting and follow-up of newly detected indeterminate extracolonic findings, particularly when CT colonography is used as a colorectal cancer screening test used in the asymptomatic screening population. Even very low false positive rates can drive multiple subsequent tests that result in relatively few detected cancers. Patient distress and inconvenience [32] associated with extracolonic findings, the impact of extracolonic findings on the cost-effectiveness of CT colonography, and methods for prioritizing effective follow-up of extracolonic findings all deserve further study. As a first step, our results suggest that more priority should be given to follow-up of indeterminate renal masses detected on CT colonography, and that less emphasis could be given to cystic ovarian lesions. In addition, both research and clinical practice could be improved by creating clearer definitions of indeterminate extracolonic findings on CT colonography.
Acknowledgments
ACKNOWLEDGEMENT OF FINANCIAL SUPPORT:
This work was funded by the National Cancer Institute (U01CA097427; and KM1CA156715 to HZ) and the Agency for Healthcare Research and Quality (K12HS019482 to KJW). The authors thank Hannah Cohen-Cline for her diligence in retrieving and organizing the manuscripts included in this review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vining DJ, Gelfand DW, Bechthold RE, Scharling ES, Grishaw EK, Shifrin RY. Technical feasibility of colon imaging with helical CT and virtual reality. AJR Am J Roentgenol. 1994;162 (Suppl):104. [Google Scholar]
- 2.Parkins T. Computer lets doctor fly through the virtual colon. J Natl Cancer Inst. 1994;86:1046–1047. [Google Scholar]
- 3.Graser A, Stieber P, Nagel D, et al. Comparison of CT colonography, colonoscopy, sigmoidoscopy and faecal occult blood tests for the detection of advanced adenoma in an average risk population. Gut. 2009;58:241–248. doi: 10.1136/gut.2008.156448. [DOI] [PubMed] [Google Scholar]
- 4.Moawad FJ, Maydonovitch CL, Cullen PA, Barlow DS, Jenson DW, Cash BD. CT colonography may improve colorectal cancer screening compliance. AJR Am J Roentgenol. 2010;195:1118–1123. doi: 10.2214/AJR.10.4921. [DOI] [PubMed] [Google Scholar]
- 5.Zalis ME, Barish MA, Choi JR, et al. CT colonography reporting and data system: a consensus proposal. Radiology. 2005;236:3–9. doi: 10.1148/radiol.2361041926. [DOI] [PubMed] [Google Scholar]
- 6.Casarella WJ. A patient’s viewpoint on a current controversy. Radiology. 2002;224:927. doi: 10.1148/radiol.2243020024. [DOI] [PubMed] [Google Scholar]
- 7.Siddiki H, Fletcher JG, McFarland B, et al. Incidental findings in CT colonography: literature review and survey of current research practice. J Law Med Ethics. 2008;36:320–331. 213. doi: 10.1111/j.1748-720X.2008.00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Outwater EK, Siegelman ES, Hunt JL. Ovarian teratomas: tumor types and imaging characteristics. Radiographics. 2001;21:475–490. doi: 10.1148/radiographics.21.2.g01mr09475. [DOI] [PubMed] [Google Scholar]
- 9.Comerci JT, Jr, Licciardi F, Bergh PA, Gregori C, Breen JL. Mature cystic teratoma: a clinicopathologic evaluation of 517 cases and review of the literature. Obstet Gynecol. 1994;84:22–28. [PubMed] [Google Scholar]
- 10.Talerman A. Germ cell tumors of the ovary. In: Kurman RJ, editor. Blaustein’s pathology of the female genital tract. New York, NY: Springer-Verlag; 1994. pp. 849–914. [Google Scholar]
- 11.Young-Xu Y, Chan KA. Pooling overdispersed binomial data to estimate event rate. BMC Med Res Methodol. 2008;8:58. doi: 10.1186/1471-2288-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guimaraes P. A simple approach to fit the beta-binomial model. The Stata Journal. 2005;5:385–394. [Google Scholar]
- 13.StataCorp. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP; 2011. [Google Scholar]
- 14.Altekruse SF, Kosary FL, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2007. Bethesda, MD: National Cancer Institute; 2010. [Google Scholar]
- 15.Chow WH, Devesa SS, Warren JL, Fraumeni JF., Jr Rising incidence of renal cell cancer in the United States. JAMA. 1999;281:1628–1631. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 16.Gordis L. Epidemiology. 3. Philadelphia, PA: W. B. Saunders Company; 2004. [Google Scholar]
- 17.Pickhardt PJ, Hanson ME, Vanness DJ, et al. Unsuspected extracolonic findings at screening CT colonography: clinical and economic impact. Radiology. 2008;249:151–159. doi: 10.1148/radiol.2491072148. [DOI] [PubMed] [Google Scholar]
- 18.Veerappan GR, Ally MR, Choi JH, Pak JS, Maydonovitch C, Wong RK. Extracolonic findings on CT colonography increases yield of colorectal cancer screening. AJR Am J Roentgenol. 2010;195:677–686. doi: 10.2214/AJR.09.3779. [DOI] [PubMed] [Google Scholar]
- 19.Edwards JT, Wood CJ, Mendelson RM, Forbes GM. Extracolonic findings at virtual colonoscopy: implications for screening programs. Am J Gastroenterol. 2001;96:3009–3012. doi: 10.1111/j.1572-0241.2001.04679.x. [DOI] [PubMed] [Google Scholar]
- 20.Flicker MS, Tsoukas AT, Hazra A, Dachman AH. Economic impact of extracolonic findings at computed tomographic colonography. J Comput Assist Tomogr. 2008;32:497–503. doi: 10.1097/RCT.0b013e3181692091. [DOI] [PubMed] [Google Scholar]
- 21.Gluecker TM, Johnson CD, Wilson LA, et al. Extracolonic findings at CT colonography: evaluation of prevalence and cost in a screening population. Gastroenterology. 2003;124:911–916. doi: 10.1053/gast.2003.50158. [DOI] [PubMed] [Google Scholar]
- 22.Spreng A, Netzer P, Mattich J, Dinkel HP, Vock P, Hoppe H. Importance of extracolonic findings at IV contrast medium-enhanced CT colonography versus those at non-enhanced CT colonography. Eur Radiol. 2005;15:2088–2095. doi: 10.1007/s00330-005-2798-6. [DOI] [PubMed] [Google Scholar]
- 23.Chin M, Mendelson R, Edwards J, Foster N, Forbes G. Computed tomographic colonography: prevalence, nature, and clinical significance of extracolonic findings in a community screening program. Am J Gastroenterol. 2005;100:2771–2776. doi: 10.1111/j.1572-0241.2005.00337.x. [DOI] [PubMed] [Google Scholar]
- 24.Khan KY, Xiong T, McCafferty I, et al. Frequency and impact of extracolonic findings detected at computed tomographic colonography in a symptomatic population. Br J Surg. 2007;94:355–361. doi: 10.1002/bjs.5498. [DOI] [PubMed] [Google Scholar]
- 25.Roberts-Thomson IC, Tucker GR, Hewett PJ, et al. Single-center study comparing computed tomography colonography with conventional colonoscopy. World J Gastroenterol. 2008;14:469–473. doi: 10.3748/wjg.14.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serracino-Inglott F, Atkinson HD, Jha P, Parker I, Anderson DN. Early experiences with computed axial tomography colonography. Am J Surg. 2004;187:511–514. doi: 10.1016/j.amjsurg.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 27.Yucel C, Lev-Toaff AS, Moussa N, Durrani H. CT colonography for incomplete or contraindicated optical colonoscopy in older patients. AJR Am J Roentgenol. 2008;190:145–150. doi: 10.2214/AJR.07.2633. [DOI] [PubMed] [Google Scholar]
- 28.Tolan DJ, Armstrong EM, Chapman AH. Replacing barium enema with CT colonography in patients older than 70 years: the importance of detecting extracolonic abnormalities. AJR Am J Roentgenol. 2007;189:1104–1111. doi: 10.2214/AJR.07.2026. [DOI] [PubMed] [Google Scholar]
- 29.Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology. 2010;256:943–954. doi: 10.1148/radiol.10100213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson PT, Horton KM, Megibow AJ, Jeffrey RB, Fishman EK. Common incidental findings on MDCT: survey of radiologist recommendations for patient management. J Am Coll Radiol. 2011;8:762–767. doi: 10.1016/j.jacr.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 31.Volpe A, Panzarella T, Rendon RA, Haider MA, Kondylis FI, Jewett MA. The natural history of incidentally detected small renal masses. Cancer. 2004;100:738–745. doi: 10.1002/cncr.20025. [DOI] [PubMed] [Google Scholar]
- 32.Yee J, Rosen MP, Blake MA, et al. ACR Appropriateness Criteria on colorectal cancer screening. J Am Coll Radiol. 2010;7:670–678. doi: 10.1016/j.jacr.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Ginnerup Pedersen B, Rosenkilde M, Christiansen TE, Laurberg S. Extracolonic findings at computed tomography colonography are a challenge. Gut. 2003;52:1744–1747. doi: 10.1136/gut.52.12.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kimberly JR, Phillips KC, Santago P, et al. Extracolonic findings at virtual colonoscopy: an important consideration in asymptomatic colorectal cancer screening. J Gen Intern Med. 2009;24:69–73. doi: 10.1007/s11606-008-0835-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pickhardt PJ, Kim DH, Meiners RJ, et al. Colorectal and extracolonic cancers detected at screening CT colonography in 10,286 asymptomatic adults. Radiology. 2010;255:83–88. doi: 10.1148/radiol.09090939. [DOI] [PubMed] [Google Scholar]
- 36.Hellstrom M, Svensson MH, Lasson A. Extracolonic and incidental findings on CT colonography (virtual colonoscopy) AJR Am J Roentgenol. 2004;182:631–638. doi: 10.2214/ajr.182.3.1820631. [DOI] [PubMed] [Google Scholar]
- 37.Pilch-Kowalczyk J, Konopka M, Gibinska J, et al. Extracolonic findings at CT colonography - additional advantage of the method. Med Sci Monit. 2004;10 (Suppl 3):22–25. [PubMed] [Google Scholar]
- 38.Rajapaksa RC, Macari M, Bini EJ. Prevalence and impact of extracolonic findings in patients undergoing CT colonography. J Clin Gastroenterol. 2004;38:767–771. doi: 10.1097/01.mcg.0000139035.38568.18. [DOI] [PubMed] [Google Scholar]
- 39.Yee J, Kumar NN, Godara S, et al. Extracolonic abnormalities discovered incidentally at CT colonography in a male population. Radiology. 2005;236:519–526. doi: 10.1148/radiol.2362040166. [DOI] [PubMed] [Google Scholar]
- 40.White TJ, Avery GR, Kennan N, Syed AM, Hartley JE, Monson JR. Virtual colonoscopy vs conventional colonoscopy in patients at high risk of colorectal cancer--a prospective trial of 150 patients. Colorectal Dis. 2009;11:138–145. doi: 10.1111/j.1463-1318.2008.01554.x. [DOI] [PubMed] [Google Scholar]
- 41.Hara AK, Johnson CD, MacCarty RL, Welch TJ. Incidental extracolonic findings at CT colonography. Radiology. 2000;215:353–357. doi: 10.1148/radiology.215.2.r00ap33353. [DOI] [PubMed] [Google Scholar]
- 42.Iafrate F, Hassan C, Zullo A, et al. CT colonography with reduced bowel preparation after incomplete colonoscopy in the elderly. Eur Radiol. 2008;18:1385–1395. doi: 10.1007/s00330-008-0892-2. [DOI] [PubMed] [Google Scholar]
- 43.Park SK, Park DI, Lee SY, et al. Extracolonic findings of computed tomographic colonography in Koreans. World J Gastroenterol. 2009;15:1487–1492. doi: 10.3748/wjg.15.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]