Abstract
St. John's wort (Hypericum perforatum), a perennial herb native to Europe, is widely used and appears to be effective in treatment of mild to moderate depression. Hypericin, a singlet oxygen-generating photosensitizer that absorbs in both the visible and UVA range, is considered to be one of the bioactive ingredients, and commercial preparations are frequently calibrated to contain a standard concentration. Hypericin can accumulate in ocular tissues, including lenses, and can bind in vitro to α-crystallin, a major lens protein. Alpha-crystallin is required for lens transparency and also acts as a chaperone to ensure its own integrity and the integrity of all lens proteins. Because there is no crystallin turnover, damage to α-crystallin is cumulative over the lifetime of the lens, and can lead to cataracts, the principal cause of blindness worldwide. In this work we study hypericin photosensitization of α-crystallin and detect extensive polymerization of bovine α-crystallin exposed in vitro to hypericin and UVA. We use fluorescent confocal microscopy to visualize binding between hypericin and α-crystallin in a human lens epithelial (HLE) cell line. Further, we show that UVA irradiation of hypericin-treated HLE cells results in a dramatic decrease in α-crystallin detection concurrent with a dramatic accumulation of the tryptophan oxidation product N-formylkynurenine (NFK). Examination of actin in HLE cells indicates that this cytoskeleton protein accumulates NFK resulting from hypericin-mediated photosensitization. This work also shows that filtration of wavelengths <400 nm provides incomplete protection against α-crystallin modifications and NFK accumulation, suggesting that even by wearing UV blocking sunglasses, routine users of St. John's wort cannot adequately shield their lenses from hypericin-mediated photosensitized damage.
Introduction
There is a current trend to use herbs and other “natural” materials as a substitute for manufactured pharmaceuticals in the belief that natural products are inherently safer than synthetic ones. Regulation and safety testing for these natural products, however, ranges from far less stringent to non-existent, and unfortunately there appears to be little cognizance on the part of the general public that natural chemicals can have undesirable side effects. Because natural products are not regulated as drugs, their potential side effects and/or drug interactions are not always well understood.
St. John's wort (Hypericum perforatum) is a perennial herb native to Europe which has gained widespread use in the treatment of mild to moderate depression. One of the constituents of St. John's wort is hypericin (Fig. 1A), a photosensitizing agent that accumulates in specialized structures known as dark glands found in flowers and leaves. Because it is considered to be one of the active ingredients, hypericin is frequently used to standardize St. John's wort dosages, with most commercial preparations being standardized to 0.3%. Due to its high quantum yield of singlet oxygen (1O2) in both aqueous and lipophilic environments [1, 2], hypericin has also been investigated as a photodynamic therapy agent for cancer treatment [3]. Accordingly, St. John's wort is perhaps the most well studied of the currently prevalent herbal preparations and has been the subject of a number of reviews of both its efficacy in treating depression [4, 5] and its usefulness in anti-cancer therapy [3, 6, 7], as well as its potential side-effects and interactions with pharmaceuticals [8, 9]. There is also a sufficient awareness of St. John's wort/hypericin as a dermal photosensitizing agent that commercial preparations frequently carry a warning to that effect. More recently, however, evidence has been accumulating that hypericin is an effective photosensitizer in eye tissue as well [10-13].
Fig. 1.
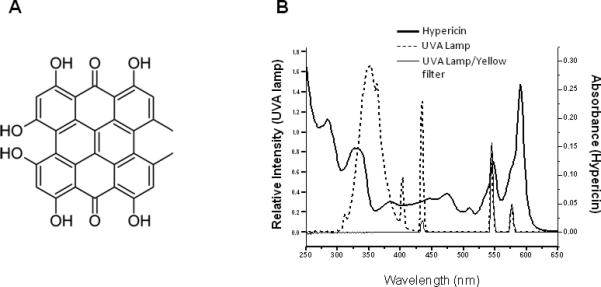
A) Structure of hypericin. B) Emission spectrum of UVA radiation source and emission spectrum of the same source filtered through the yellow-colored solution described in the materials and methods and used in the experiments shown in Figs. 2, 6 and 7 (left axis) plotted against the absorption spectrum of 5 μM hypericin in ethanol (right axis).
The human eye is composed of a succession of transparent tissues (cornea, lens, vitreous) that mediate transmission of light onto the retina. While the vitreous is an optically neutral tissue, both the cornea and the lens focus and filter incident light and UV radiation. The cornea, at the ocular surface, contributes two-thirds of the focusing power [14] and filters out all radiation below 295 nm. Wavelengths corresponding to both long UVB (295-315 nm) and UVA (315-400 nm) are absorbed by the adult human lens. Crystallins make up more than 90% of the total dry mass of the human lens with α-crystallin, composed of two homologous subunits (A and B), comprising approximately 40% of the total eye lens protein [15, 16]. Unlike most other proteins, the crystallins of the lens do not turn over, but accumulate throughout the lifetime of an individual, and any modifications or damage to these proteins persists. Cataracts, a clouding of the lens due to insolubilization of damaged lens proteins, are the leading cause of blindness in the world [17].
Over-the-counter preparations recommend a daily quantity of St. John's wort containing nearly 3 mg of hypericin, and at this dosage serum concentration can reach the order of 0.1 μM [18]. Hypericin is transported through the body by binding to endogenous macromolecules such as human serum albumin or low density lipoproteins [6], is capable of crossing the blood-ocular barrier [19] and accumulates in excised intact lenses [11, 12], and in cultured retinal and lens epithelial cell [10, 13, 20]. Because hypericin absorbs in both the UVA and visible range (Fig. 1B) it can photosensitize both lens and retinal tissue exposed to these wavelengths. Previous studies have shown that hypericin can photosensitize lens proteins in vitro [21], can cause cataracts in UV exposed excised bovine lenses [12] and can induce cell death in human lens epithelial culture [10]. Irradiated retinal cells containing hypericin show membrane damage and other forms of oxidative stress including decreases in mitochondrial activity [13]. Despite increasing evidence that hypericin can damage ocular tissues, there is little information available on the relationship between St. John's wort and cataracts. A single study using self-reported, questionnaire-derived data on both St. John's wort use and cataract occurrence reports an association between the two [22].
This study was undertaken to determine if hypericin interacts with UVA radiation to alter the proteins of the human lens epithelial cell line HLE B-3 [23]. The singlet oxygen produced by UVA irradiation of photosensitizers, such as hypericin, can interact with the amino acid residues of proteins to chemically alter the structure of the side chains [24, 25]. Products of tryptophan reaction with 1O2 include hydroperoxides, hydroxytryptophans, kynurenine and N-formylkynurenine (NFK) [24, 25]. MALDI mass spectrometric analyses [21] performed on hypericin photosensitized calf lens α-crystallin detected oxidation of Met, His and Trp residues of both αA- and αB-crystallin subunits. Histidine residues were oxidized to dehydro-2-imidazolone while tryptophan residues were oxidized to hydroxytryptohan and NFK. Oxidation of Met residues was probably to methionine sulfoxide. The data from this study also suggested the possibility of cross-linking between His and Lys residues.
In this work we use confocal analysis in conjunction with anti-α-crystallin and anti-actin antibodies, and antiserum specific to NFK [26-29] to assess and directly visualize specific changes that occur in UVA-irradiated HLE cells. We show that UVA irradiation in the presence of hypericin results, concurrently, with the accumulation of NFK and decreased detection of α-crystallin. We also determine that hypericin photosensitization results in oxidation of actin tryptophan residues to NFK. Interposition of a filter that blocks wavelengths below 400 nm provides only incomplete protection against the observed hypericin-mediated photosensitized protein damage.
Materials and methods
Materials
Bovine α-crystallin, DMSO and hypericin were purchased from Sigma (St. Louis, MO). Stock solutions of hypericin were dissolved in DMSO and concentrations determined at 590 nm after dilution into 100% ethanol (ε590 = 46,000 M–1 cm–1). The previously described anti-NFK antiserum [26-28] was developed in our laboratory, and mouse monoclonal anti-αA and anti-αB crystallin and goat polyclonal anti-actin were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The (goat) anti-mouse IRdye 800 used in western analysis was from Li-Cor Biosciences (Lincoln, Nebraska) and the DAPI and all secondary antibodies used in the confocal experiments were purchased from Life Technologies (Rockville, MD).
UVA irradiation of bovine α-crystallin
Solutions (0.5 mL) of 1 mg mL–1 bovine α-crystallin in Chelex-treated, 100 mM phosphate buffer, pH 7.4, were placed in individual wells of a 24-well microtiter plate (Corning Incorporated, Corning, NY) with either hypericin in DMSO or DMSO alone. The samples were then either kept in the dark (D), exposed to UVA irradiation (U), or placed under the UVA lamps under a yellow-colored aqueous solution (Y) which filtered out all radiation of wavelengths below 400 nm. This filter consists of a solution of NaNO2 (50 g L-1), K2CrO4 (0.2 g L-1) and Na2CO3 91 g L-1) in a plastic flask with a vertical path length of exactly 1 cm. All treatments lasted for 40 min. The UVA radiation source (Fig. 1B) was composed of four parallel fluorescent UVA lamps (Houvalite F20T12BL-HO; National Biological Co. Twinsburg, OH) and the irradiance was measured with a YSI-Kettering Model 65A Radiometer (Yellow Springs Instrument Co., Yellow Springs, OH), and determined to be 10 J cm-2 for the non filtered UVA treatment (U).
For UVA irradiation in D2O, Chelex-treated 100 mM phosphate buffer, pH 7.4, was lyophilized and resuspended in an equal volume of D2O. Alpha-crystallin was then diluted 1:10 to a final protein concentration of 1 mg mL–1 and a final D2O concentration of 90%.
Western analyses
Gels containing 13 μg aliquots of α-crystallin and electrophoresed under reducing conditions were transferred to nitrocellulose. Western analyses were performed by incubating the filters in a mixture of mouse monoclonal anti-αA and anti-αB-crystallin (100 ng mL–1) followed by incubation with a 1:10,000 dilution of anti-mouse IRdye 800. Results were visualized using a Li-Cor Odyssey Infrared Imaging System (Lincoln, NE).
Cell culture
The extended lifespan human lens epithelial cell line HLE-B3 [23] purchased from ATCC (CRL-11421; Manassas, VA) was used in this study. Cells were grown in Eagle's MEM (Sigma) containing 2 mM L-glutamine, 50 μg mL-1 gentamicin and 20% FBS in an atmosphere of 5% CO2/95% air at 37 °C. Cells were fed thrice weekly and, after attaining confluence, were passaged using trypsin (0.125%)–EDTA (0.5 mM).
Cell treatments
HLE B-3 cells were seeded onto 35 mm plates containing 1.5 mm thick coverslips (MatTek, Ashland MA) and grown to approximately 80% confluence. For confocal experiments, cells were washed twice with PBS containing Ca2+ and Mg2+, and were then incubated for 1 h at 37 °C in the dark with either DMSO or hypericin in DMSO (final DMSO concentration for all treatments was 0.1%) in PBS containing Ca2+ and Mg2+. After washing three times, cells were then either kept in the dark for 40 min, exposed to 40 min of UVA irradiation or exposed to 40 min of UVA irradiation through a yellow filter which removed wavelengths <400 nm, as described above for the in vitro bovine α-crystallin treatments. All manipulations beginning with the addition of hypericin were carried out under dim red lighting.
Confocal microscopy
At the end of treatment, cells were fixed and incubated with blocking solution (4% cold fish gelatin). Staining with primary antibodies was done simultaneously with rabbit polyclonal anti-NFK (1:250 dilution) and a mixture of mouse monoclonal anti-αA-crystallin and mouse monoclonal anti-αB-crystallin (1:500 dilutions) (Santa Cruz Biotechnology Inc., Santa Cruz, CA) or simultaneously with rabbit polyclonal anti-NFK (1:250 dilution) and goat polyclonal anti-actin (1:100 dilution) (Santa-Cruz Biotechnology Inc., Santa Cruz, CA) After washing, the cells were then stained simultaneously with all secondary antibodies at dilutions of 1:1000. For visualization of nuclei, cells were stained for 10 min in a solution of 1 μg mL-1 DAPI.
Cell viability
Cell viability was assayed using Cell Proliferation Kit I (MTT) from Roche (Mannheim, Germany) which measures the production of a formazan produced by only metabolically active cells. Cells were seeded onto the wells of microtiter plates and allowed to grow until 80–90% confluence. Cells were washed twice with PBS containing Ca2+ and Mg2+, and were then incubated for 1 h at 37 °C in the dark with either DMSO or hypericin in DMSO (final DMSO concentration for all treatments was 0.1%) in PBS containing Ca2+ and Mg2+. After washing three times, cells were then exposed to 40 min of UVA irradiation, and immediately after assayed for viability as described by the manufacturer. All manipulations beginning with the addition of hypericin were carried out under dim red lighting. The data presented represent the average for 3 wells per treatment and are expressed as a percentage of the positive (0 μM hypericin) control.
Results
Hypericin promotes polymerization of bovine α-crystallin exposed in vitro to UVA
An initial experiment was performed in vitro using purified bovine α-crystallin (Fig. 2A). The protein was mixed with hypericin in DMSO to a final concentration of 10 μM or with DMSO alone. The final concentration of DMSO (0.1%) was the same in all treatments. Samples were either kept in the dark (D), irradiated with UVA (U), irradiated with UVA filtered through a yellow filter to remove wavelengths <400 nm (Y), or irradiated with UVA in the presence of 10 mM sodium azide, which can act an efficient quencher of 1O2. Western blot analysis was performed using 1) anti-NFK, and 2) a mixture of anti-αA and anti-αB crystallin. While NFK was not detectable in any of the samples (data not shown), UVA irradiation of α-crystallin containing hypericin resulted in extensive polymerization of the protein monomers with a perceptible loss of αB-crystallin (Fig. 2, lower band) in the polymerized sample. This extensive polymerization was strongly attenuated, but not completely eliminated, by filtering out wavelengths <400 nm, and independently, in the presence of sodium azide. The hypericin-containing α-crystallin irradiated with unfiltered UVA accumulated polymers up to at least octomers, but the highest mw protein visible in the sample irradiated through the filtered UVA is a trimer.
Fig. 2.
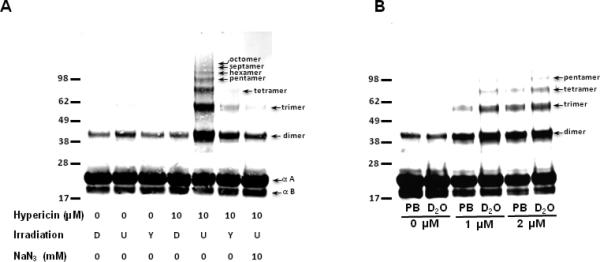
Western analysis of bovine α-crystallin photosensitized in the presence of hypericin. (A) α-crystallin (1 mg/ml) without and with 10 μM hypericin was either kept in the dark (D), exposed to 40 min of UVA irradiation (U), or exposed to 40 min of UVA irradiation filtered though a yellow solution to remove wavelengths < 400 nm (Y) as described in the materials and methods. An additional treatment contained 10 μM hypericin plus 10 mM NaN3 and was exposed to 40 min of unfiltered UVA irradiation. (B) α-crystallin (final concentration 1 mg/ml) without hypericin (0 μM) and with 1 or 2 μM hypericin as indicated was diluted into 100 mM Chelex-treated phosphate buffer pH 7.4 (PB) or into 100 mM chelex treated phosphate buffer pH 7.4 (PB) in D2O (final concentration D2O 90%). Protein samples were then UVA irradiated for 40 min. Protein (13 μg) from each treatment was electrophoresed under reducing conditions, transferred to nitrocellulose and subjected to western analysis using a mixture of anti-αA and anti-αB crystallin mouse monoclonal antibodies.
Experiments were also performed with higher concentrations of hypericin (50 and 100 μM) and the results subjected to western analysis. As with the experiments containing 10 μM hypericin NFK was not detected (data not shown). These higher concentrations of hypericin also failed to promote α-crystallin polymerization more extensive than that seen with 10 μM (data not shown).
To confirm the role of 1O2 in the protein polymerization observed in Fig. 2A UVA irradiation of α-crystallin was also performed in 90% D2O (Fig. 2B). Samples containing both 1 and 2 μM hypericin accumulated higher mw polymers in D2O than in phosphate buffer (PB). This indicates that hypericin-mediated polymerization of α-crystallin is produced by Type II (1O2) photochemistry.
Hypericin accumulates in HLE cells and colocalizes with α-crystallin
HLE cells were incubated with hypericin (1, 2 and 10 μM) or DMSO (0 μM). After washing the live unirradiated cells were visualized using the natural hypericin fluorescence resulting from excitation with a 561 nm laser (Fig. 3). Hypericin was detectable even at the lowest (1 μM) concentration used and was seen to accumulate in the membranes and interior of the cell.
Fig. 3.
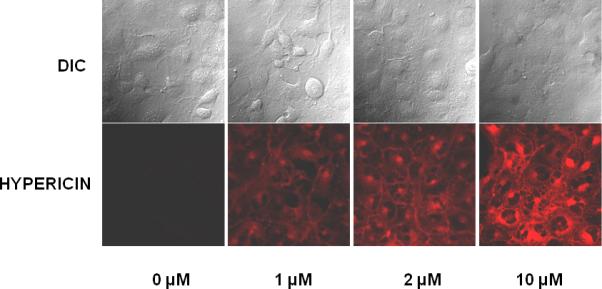
Visualization of hypericin in live HLE cells. Cells were incubated in the dark with hypericin in DMSO (1, 2 or 10 μM) or with DMSO alone (0 μM). After washing, natural hypericin fluorescence was used to visualize the cells (ex 561 nm).
Because α-crystallin and hypericin bind in vitro, as seen by the alteration of the hypericin absorption spectrum in the presence of α-crystallin [11, 30], we also assessed colocalization of hypericin with α-crystallin in unirradiated, fixed HLE cells. This experiment used the 561 nm laser to visualize the hypericin (green) and a mixture of anti-αA and anti-αB crystallin, followed by staining with anti-mouse 633 to visualize the α-crystallin (red) (Fig. 4). Areas of colocalization between hypericin and α-crystallin are seen as yellow areas in the overlay images of cells containing both 5 and 10 μM hypercin. This colocalization of hypericin with α-crystallin was quantified using Zen software, and the overlap coefficient for four images each from the 5 μM and 10 μM hypericin treated cells ranged from 54 to 65% and from 60 to 70%, respectively.
Fig. 4.
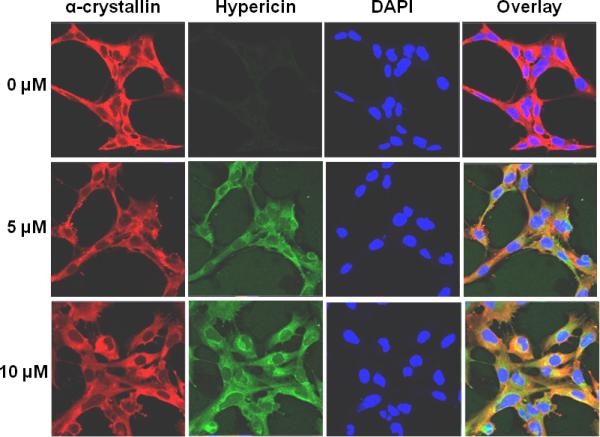
Colocalization of hypericin with α-crystallin in unirradiated HLE cells. HLE cells were incubated with either DMSO or hypericin in DMSO for 1 h at 37 °C in the dark. Following washing and fixation, cells were stained with a mixture of mouse monoclonal anti-αA and anti-αB crystallin (red) followed by staining with Alexa Fluor anti-mouse 633 and staining with DAPI to visualize cell nuclei (blue). Natural hypericin fluorescence (ex 561, shown in green) was used to image the photosensitizer presence in the cells. Alpha-crystallin (red), hypericin (green), DAPI (blue), and overlay of all three.
UVA irradiation of hypericin-containing HLE cells results in decreased detection of α-crystallin and accumulation of NFK
HLE cells incubated with DMSO alone (0 μM) or with 1, 5 or 10 μM hypericin in DMSO were irradiated with UVA. After fixation, the cells were stained with a mixture of anti-αA and anti-αB crystallin (red), with anti-NFK (green) and with DAPI (blue) to visualize cell nuclei. It is apparent in Figure 5 that α-crystallin visualization decreases as hypericin concentration increases. Concomitant with decreased α-crystallin detection, the samples display increased NFK accumulation. This decrease in α-crystallin visualization is clearly irradiation-dependent, as the comparable hypericin-containing, but non-UVA irradiated samples in Fig. 4 did not exhibit a comparable decline.
Fig. 5.
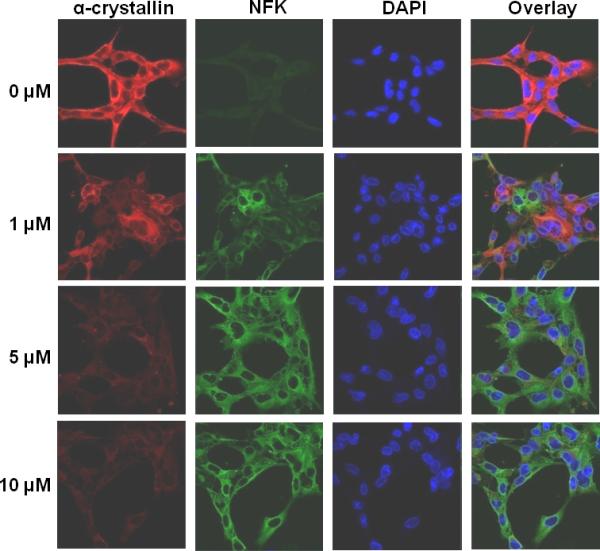
UVA irradiation of hypericin containing HLE cells results in decreased detection of α-crystallin and accumulation of NFK. HLE cells were incubated with either DMSO (0 μM) or hypericin in DMSO (1, 5 or 10 μM) for 1 h at 37 °C in the dark and, after washing with buffer, were exposed to 40 min of UVA irradiation. Fixed cells were stained simultaneously with a mixture of mouse monoclonal anti-αA and anti-αB crystallin and rabbit polyclonal anti-NFK followed by staining simultaneously with Alexa Fluor anti-mouse 633 and Alexa Fluor anti-rabbit 488. DAPI was used to stain cell nuclei. Alpha-crystallin (red), NFK (green), DAPI (blue) and overlay of all three.
Blocking UVA irradiation decreases, but doesn't eliminate, hypericin-meditated photosensitization in HLE cells
To assess the potential protective effect of blocking wavelengths <400 nm, HLE cells were treated with either 10 μM hypericin in DMSO or with DMSO alone (0 μM). After washing, they were then either maintained in the dark, irradiated with UVA, or irradiated with UVA through a yellow filter (UVA/Y) that removes all radiation <400 nm. Figure 6 confirms that UVA irradiation of hypericin-containing cells results in parallel decreased α-crystallin detection and increased NFK accumulation. This experiment also shows that filtration of wavelengths <400 nm from the irradiation source by use of a yellow filter (UVA/Y) ameliorates, but does not completely eliminate, the effects of hypericin photosensitization.
Fig. 6.
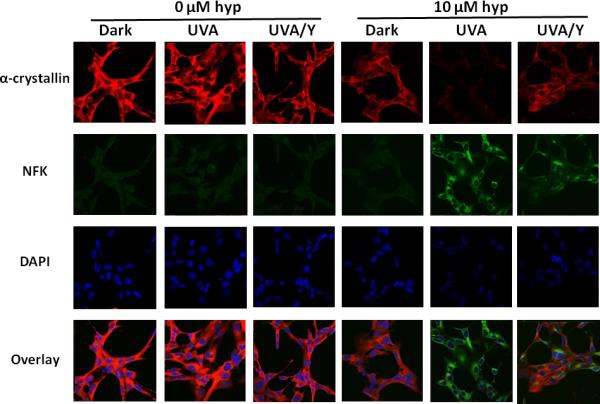
Partial protection of UVA-irradiated, hypericin-containing HLE cells by blocking of wavelengths <400 nm: The effect on NFK and α-crystallin detection. HLE cells were incubated with either DMSO (0 μM) or hypericin in DMSO (10 μM) for 1 h at 37 °C in the dark. After washing with buffer, the cells were either kept in the dark, exposed to 40 min of UVA irradiation, or exposed to 40 min of UVA irradiation through a yellow filter that removed wavelengths < 400 nm (UVA/Y). Fixed cells were stained simultaneously with a mixture of mouse monoclonal anti-αA and anti-αB-crystallin and rabbit polyclonal anti-NFK followed by staining simultaneously with Alexa Fluor anti-mouse 633 and Alexa Fluor anti-rabbit 488. DAPI was used to stain cell nuclei. Alpha-crystallin (red), NFK (green), DAPI (blue) and overlay of all three.
We also assessed the affect of hypericin photosensitization on actin in HLE cells (Fig. 7). In contrast to α-crystallin, the strength of the actin signal shows little, if any, diminution due to hypericin photosensitization. The actin, however, has clearly been altered by the hypericin-mediated photosensitization. Regions of NFK/actin colocalization are seen as yellow to orange colored areas in the overlay panel of the hypericin-containing, UVA exposed cells. Moreover, as also seen in Fig. 6, filtration of wavelengths <400 nm partially protects the cells from photosensitization. There is less NFK staining in the UVA/Y cells which, consequently, show less extensive NFK/actin colocalization.
Fig. 7.
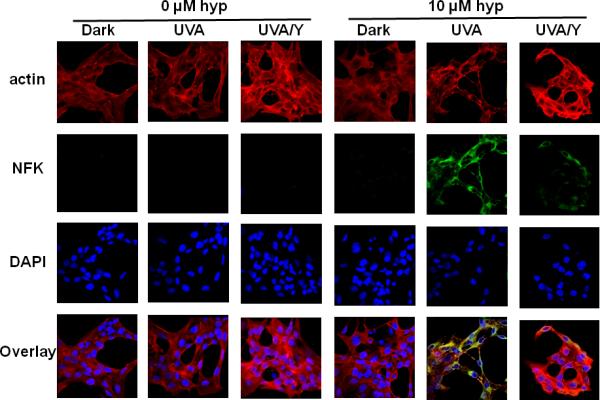
Partial protection of UVA-irradiated, hypericin-containing HLE cells by blocking of wavelengths <400 nm: The effect on NFK and actin detection. HLE cells were incubated with either DMSO (0 μM) or hypericin in DMSO (10 μM) for 1 h at 37 °C in the dark. After washing with buffer, the cells were either kept in the dark, exposed to 40 min of UVA irradiation, or exposed to 40 min of UVA irradiation through a yellow filter that removed wavelengths < 400 nm (UVA/Y). Fixed cells were stained simultaneously with a goat polyclonal anti-actin and rabbit polyclonal anti-NFK followed by staining simultaneously with Alexa Fluor anti-mouse 633 and Alexa Fluor anti-rabbit 488. DAPI was used to stain cell nuclei. Actin (red), NFK (green), DAPI (blue) and overlay of all three.
Cell viability decreases with increasing hypericin concentration
Cell viability assays were also performed to determine the effect of hypericin photosensitization on cell survival (Fig. 8). These assays indicated that hypericin was not toxic to the HLE cells in the absence of light (data not shown) and that toxicity increased with increasing hypericin concentration, with a 79.3% survival rate in cells treated with 2 μM hypericin and a 69% survival rate in cells treated with 10 μM.
Fig. 8.
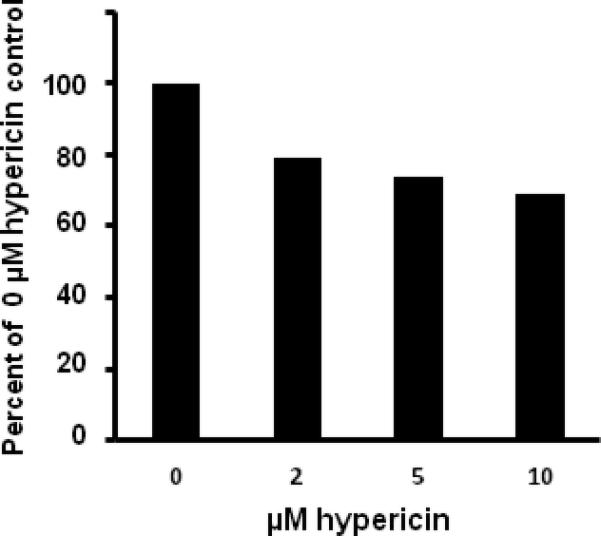
Cell viability after treatment with hypericin and UVA irradiation. Cells seeded into the wells of microtiter plates and allowed to grow until 80–90% confluence were incubated with either DMSO (0 μM) or hypericin in DMSO for 1 h at 37 °C in the dark. After washing with buffer the cells were exposed to 40 min of UVA irradiation and then assayed for viability. The data presented represent the average for 3 wells per treatment and are expressed as a percentage of the positive (0 μM hypericin) control.
Discussion
Clinical studies and meta-analyses suggest that St. John's wort can be used effectively for the treatment of mild to moderate depression [4, 5]. St. John's wort, however, can also produce adverse side effects such as altered metabolism of pharmaceuticals [8, 9, 31] and increased skin sensitivity to sunlight. Photosensitivity in domestic animals, termed hypericism, is a well-known veterinary disorder defined as a severe dermatitis of domestic herbivores following ingestion of St. John's wort, and commercial preparations of St. John's wort often carry warnings of skin photosensitization. To date, however, less emphasis has been placed on the potential for ocular-hypericin interactions.
Lens crystallin proteins do not turn over, and the lens fiber cells that differentiate into the lens itself accumulate throughout the lifetime of the eye. Photosensitizer damage to the lens is likewise cumulative and detectable as a cataract only after irreparable modifications have occurred. In this work we show that hypericin also binds efficiently to α-crystallin protein in HLE cells (Fig. 4) and can be detected in both living (Fig. 3) and fixed cells (Fig. 4). Photophysical characterization of hypericin in organic and biological media [1], and in the presence of α-crystallin [32], has provided substantial and convincing evidence for photo-induced 1O2 production but no detectable evidence for hypericin mediated radical production. In this work we corroborate these photochemical data with in vitro biochemical evidence that Type II, 1O2 photochemistry, is responsible for biologically significant modification of α-crystallin. In conjunction with D2O enhancement of bovine α-crystallin polymerization (Fig. 2B), we also saw azide inhibition of the same (Fig. 2A).
Because hypericin is hydrophobic it tends to aggregate in aqueous environments, leading to self quenching and a lower quantum yield of 1O2 [32]. The binding between hypericin and α-crystallin, however, decreases hypericin aggregation, extending its triplet lifetime [32] and increasing 1O2 yield. While previous studies have documented hypericin/α-crystallin interactions in vitro, in media and in liposomes [1, 32], our work provides strong evidence for colocalization of photosensitizer and protein in viable human lens epithelial cells (Fig. 4). This physical interaction, therefore, not only puts hypericin in maximal proximity to the protein but also increases its toxicity/phototoxicity.
In both live and fixed HLE cells, hypericin can be seen to accumulate in the cytoplasm (Figs. 3 and 4). While detection of α-crystallin is unaffected by the presence of hypericin (Fig. 4), UVA irradiation of HLE cells does cause a precipitous decrease in apparent protein amount (Figs. 5 & 6). Because the duration of the experiment is short and the cells are fixed immediately following irradiation, this decrease in detection can be attributed to loss of the α-crystallin epitopes recognized by the antibodies and not by an actual decline in protein content.
In these same cells (Fig. 5), NFK accumulation is inversely proportional to the decline in α-crystallin detection, and therefore, it is difficult to discuss the presence or absence of NFK amino acid residues in hypericin-photosensitized α-crystallin. A previous study on porphyrin photosensitization of HLE cells presented clear evidence that NFK colocalized with photosensitized α-crystallin [28], as none of the porphyrins used caused a decline in α-crystallin detection. Because the current work uses the same anti-α-crystallin antibodies, we can conclude that hypericin photosensitization produces α-crystallin alterations additional to or different from those produced in our porphyrin study. To determine if NFK colocalized with other proteins, we examined the cytoskeleton protein actin and saw that while there was little, if any, effect of hypericin photosensitization on actin detectability, there was clear evidence of NFK colocalization (Fig. 7). Because actin is essential for fiber cell elongation and differentiation, which is in turn essential for the transparency of the lens [33], damaging actin could also contribute to cataractogenesis. Finally, this experiment also corroborated the evidence in Fig. 6 showing that blocking wavelengths <400 nm provides only partial protection against hypericin-mediated protein modification.
Because proteins are the most abundant macromolecule in the cell, they are also a major target for oxidative modification. Exposure of proteins to 1O2 can cause fragmentation of the protein backbone, formation of cross-links and aggregates and alteration of the amino acid side chains of His, Met, Trp, Tyr and Cys [24, 25]. The photosensitization-mediated alterations in α-crystallin and other lens proteins such as actin are likely to create an environment conducive to accelerated protein damage. The tryptophan oxidation products NFK and kynurenine are both more efficient photosensitizers than tryptophan [24]. As lens proteins do not turn over [15, 16], the conversion of lens protein amino acid residues into more active photosensitizers will permanently increase the potential for light-mediated lens protein damage in the lens. This increased potential for photodamage will remain a permanent fixture of lens proteins, even in the eventuality that there is no further addition of exogenous photosensitizer. Viability determinations on hypericin-photosensitized HLE cells (Fig. 8) indicate that the conditions used in this work produced limited cell death. Because lens proteins do not turn over, the observation that hypericin photosensitization produces substantial post-translational modification of proteins in the absence of necrosis/apotosis has potentially greater impact on lens health than if the 1O2 stress outright killed the cells.
Additionally, evidence also shows that partial perturbation of α-crystallin structure, such as may be caused by photooxidation, enhances the ability of hypericin to bind to α-crystallin [30]. Furthermore, α-crystallin functions as a chaperone in the lens for itself and the other crystallins and lens proteins, and altering this activity can promote cataractogenesis [34]. Thus, any α-crystallin modifications that impair chaperone function create an environment in which further protein damage can occur.
The ability to visualize and assess colocalization of proteins and potentially harmful oxidative agents provides a unique insight into cellular interactions. While processing of cellular components can produce oxidative artifacts that confound data analysis [35, 36], the type of analysis done in this study produces a snapshot of the conditions in living cells. In conjunction with other studies [10-12, 21, 30, 32], the data generated in this work demonstrate the potential for hypericin, and therefore St. John's wort, to cause irreversible, cataract-promoting changes in the protein structure of human lens α-crystallin and other proteins of the human lens. This work also shows (Fig. 6 and 7) that because both visible and UV wavelengths are effective at producing 1O2 from hypericin, only incomplete protection from hypericin photosensitization can be achieved by blocking UV irradiation (wavelength <400nm). This indicates that hypericin is likely to induce ocular toxicity even under visible light alone. We conclude that individuals using hypericin-containing St. John's wort should use extreme caution in protecting their eyes not only from UV radiation but also excessive visible light exposure.
Acknowledgements
The authors thank B. Jean Corbett and Mary Mason for their valuable help in the preparation of this manuscript. We are grateful to Jeff Tucker and Dr. Agnes Janoshazi for their expert help with confocal microscopy. This work was supported by the Intramural Research Program of the National Institutes of Health and the National Institute of Environmental Health Sciences.
References
- 1.Ehrenberg B, Anderson JL, Foote CS. Kinetics and yield of singlet oxygen photosensitized by hypericin in organic and biological media. Photochem. Photobiol. 1998;68:135–140. [PubMed] [Google Scholar]
- 2.Senthil V, Jones LR, Senthil K, Grossweiner LI. Hypericin photosensitization in aqueous model systems. Photochem. Photobiol. 1994;59:40–47. doi: 10.1111/j.1751-1097.1994.tb04999.x. [DOI] [PubMed] [Google Scholar]
- 3.Theodossiou TA, Hothersall JS, De Witte PA, Pantos A, Agostinis P. The multifaceted photocytotoxic profile of hypericin. Mol. Pharm. 2009;6:1775–1789. doi: 10.1021/mp900166q. [DOI] [PubMed] [Google Scholar]
- 4.Sarris J, Panossian A, Schweitzer I, Stough C, Scholey A. Herbal medicine for depression, anxiety and insomnia: A review of psychopharmacology and clinical evidence. Eur. Neuropsychopharm. 2011;21:841–860. doi: 10.1016/j.euroneuro.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Whiskey E, Werneke U, Taylor D. A systematic review and meta-analysis of Hypericum perforatum in depression: a comprehensive clinical review. Int. Clin. Psychopharmacol. 2001;16:239–252. doi: 10.1097/00004850-200109000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Krammer B, Verwanger T. Molecular response to hypericin-induced photodamage. Curr. Med. Chem. 2012;19:793–798. doi: 10.2174/092986712799034842. [DOI] [PubMed] [Google Scholar]
- 7.Karioti A, Bilia AR. Hypericins as potential leads for new therapeutics. Int. J. Mol. Sci. 2010;11:562–594. doi: 10.3390/ijms11020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Izzo AA. Interactions between herbs and conventional drugs: Overview of the clinical data. Med. Princip. Pract. 2012;21:404–428. doi: 10.1159/000334488. [DOI] [PubMed] [Google Scholar]
- 9.Rahimi R, Abdollahi M. An update on the ability of St. John's wort to affect the metabolism of other drugs. Expert Opin. Drug Metab. Toxicol. 2012;8:691–708. doi: 10.1517/17425255.2012.680886. [DOI] [PubMed] [Google Scholar]
- 10.He YY, Chignell CF, Miller DS, Andley UP, Roberts JE. Phototoxicity in human lens epithelial cells promoted by St. John's wort. Photochem. Photobiol. 2004;80:583–586. doi: 10.1562/0031-8655(2004)080<0583:PIHLEC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 11.Sgarbossa A, Angelini N, Gioffre D, Youssef T, Lenci F, Roberts JE. The uptake, location and fluorescence of hypericin in bovine intact lens. Curr. Eye Res. 2000;21:597–601. [PubMed] [Google Scholar]
- 12.Wahlman J, Hirst M, Roberts JE, Prickett CD, Trevithick JR. Focal length variability and protein leakage as tools for measuring photooxidative damage to the lens. Photochem. Photobiol. 2003;78:88–92. doi: 10.1562/0031-8655(2003)078<0088:flvapl>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Wielgus AR, Chignell CF, Miller DS, Van Houten B, Meyer J, Hu D-N, Roberts JE. Phototoxicity in human retinal pigment epithelial cells promoted by hypericin, a component of St. John's wort. Photochem. Photobiol. 2007;83:706–713. doi: 10.1562/2006-08-09-RA-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bassnett S, Shi Y, Vrensen GFJM. Biological Glass:structural determinants of eye lens transparancy. Phil. Trans. R. Soc. B. 2011;366:1250–1264. doi: 10.1098/rstb.2010.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andley UP. Crystallins in the eye: Function and pathology. Prog. Retin. Eye Res. 2007;26:78–98. doi: 10.1016/j.preteyeres.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Horwitz J. Alpha-crystallin. Exp. Eye Res. 2003;76:145–153. doi: 10.1016/s0014-4835(02)00278-6. [DOI] [PubMed] [Google Scholar]
- 17.Rao GN, Khanna R, Payal A. The global burden of cataract. Curr. Opin. Ophthamol. 2011;22:4–9. doi: 10.1097/ICU.0b013e3283414fc8. [DOI] [PubMed] [Google Scholar]
- 18.Schempp CM, Winghofer B, Langheinrich M, Schopf E, Simon JC. Hypericin levels in human serum and interstitial skin blister fluid after oral single-dose and steady-state administration of Hypericum perforatum extract (St. John's wort). Skin Pharmacol. Appl. Skin Physiol. 1999;12:299–304. doi: 10.1159/000066256. [DOI] [PubMed] [Google Scholar]
- 19.Higuchi A, Yamada H, Yamada E, Jo N, Matsumura M. Hypericin inhibits pathological retinal neovacularization in a mouse model of oxygen-induced retinopathy. Mol. Vis. 2008;14:249–254. [PMC free article] [PubMed] [Google Scholar]
- 20.Taroni P, Valentini G, Comelli D, D'Andrea C, Cubeddu R, Hu DN, Roberts JE. Time-resolved microspectrofluorimetry and fluorescence lifetime imaging of hypericin in human retinal pigment epithelial cells. Photochem. Photobiol. 2005;81:524–528. doi: 10.1562/2004-11-30-IR-385. [DOI] [PubMed] [Google Scholar]
- 21.Schey KL, Patat S, Chignell CF, Datillo M, Wang RH, Roberts JE. Photooxidation of lens α-crystallin by hypericin (active ingredient in St. John's wort). Photochem. Photobiol. 2000;72:200–203. doi: 10.1562/0031-8655(2000)072<0200:polcbh>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 22.Booth JN, McGwin G. The association between self-reported cataracts and St. John's wort. Curr. Eye Res. 2009;34:863–866. doi: 10.3109/02713680903144692. [DOI] [PubMed] [Google Scholar]
- 23.Andley UP, Rhim JS, Chylack LT, Jr., Fleming TP. Propagation and immortalization of human lens epithelial cells in culture. Invest. Ophthalmol. Vis. Sci. 1994;35:3094–3102. [PubMed] [Google Scholar]
- 24.Davies MJ. Singlet oxygen-mediated damage to proteins and its consequences. Biochem. Biophys. Res. Commun. 2003;305:761–770. doi: 10.1016/s0006-291x(03)00817-9. [DOI] [PubMed] [Google Scholar]
- 25.Davies MJ. The oxidative environment and protein damage. Biochim. Biophys. Acta. 2005;1703:93–109. doi: 10.1016/j.bbapap.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Ehrenshaft M, Bonini MG, Feng L, Chignell CF, Mason RP. Partial colocalization of oxidized, N-formylkynurenine-containing proteins in mitochondria and golgi of keratinocytes. Photochem. Photobiol. 2010;86:752–756. doi: 10.1111/j.1751-1097.2010.00718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ehrenshaft M, de Oliveira Silva S, Perdivara I, Bilski P, Sik RH, Chignell CF, Tomer KB, Mason RP. Immunological detection of N-formylkynurenine in oxidized proteins. Free Radic. Biol. Med. 2009;46:1260–1266. doi: 10.1016/j.freeradbiomed.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ehrenshaft M, Zhao B, Andley UP, Mason RP, Roberts JE. Immunological detection of N-formylkynurenine in porphyrin-mediated photooxided lens α-crystallin. Photochem. Photobiol. 2011;87:1321–1329. doi: 10.1111/j.1751-1097.2011.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Triquigneaux MM, Ehrenshaft M, Roth E, Silman I, Ashani Y, Mason RP, Weiner L, Deterding LJ. Targeted oxidation of Torpedo californica acetylcholinesterase by singlet oxygen: Identification of N-formylkynurenine tryptophan derivatives within the active-site gorge of its complex with the photosensitizer methylene blue. Biochem. J. 2012;448:83–91. doi: 10.1042/BJ20120992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Youssef T. Fluorescence study on the interaction between hypericin and lens proteins “α-crystallin”. Photochem. Photobiol. 2009;85:921–926. doi: 10.1111/j.1751-1097.2008.00511.x. [DOI] [PubMed] [Google Scholar]
- 31.Kober M, Pohl K, Efferth T. Molecular mechanisms underlying St. John's wort drug interactions. Curr. Drug Metab. 2008;9:1027–1037. doi: 10.2174/138920008786927767. [DOI] [PubMed] [Google Scholar]
- 32.Trevithick-Sutton CC, Chin KC, Contos SD, Foote CS. Lens α-crystallin and hypericin: A photophysical mechanism explains observed lens damage. Photochem. Photobiol. 2004;80:444–449. doi: 10.1562/0031-8655(2004)080<0444:LCAHAP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 33.Rao PV, Maddala R. The role of the lens actin cytoskeleton in fiber cell elongation and differentiation. Semin. Cell Develop. Bio. 2006;17:698–711. doi: 10.1016/j.semcdb.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng C, Xia C-H, Huang Q, Ding L, Horwitz J, Gong X. Altered chaperone-like activity of α-crystallins promotes cataractogenesis. J. Biol. Chem. 2010;285:41187–41193. doi: 10.1074/jbc.M110.154534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Brit. J. of Pharmocol. 2004;142:231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perdivara I, Deterding LJ, Przybylski M, Tomer KB. Mass spectrometric identification of oxidative modifications of tryptophan residues in proteins: Chemical artifact or post-translational modification? J. Am. Soc. Mass Spectrom. 2010;21:1114–1117. doi: 10.1016/j.jasms.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]


