Abstract
Are there general rules to achieve efficient immunization against carbohydrate antigens? Thanks to technological advances in glycobiology and glycochemistry we entered in a new era in which the rational design of carbohydrate vaccines has become an achievable goal. Aim of this Tutorial Review is to present the most recent achievements in the field of semi and fully synthetic carbohydrate vaccines against viruses, bacteria and cancer. It is also pointed out that the understanding of the chemical and biochemical processes related to immunization allows the modern chemist to rationally design carbohydrate vaccines with improved efficiency.
1. Introduction: Sugar chemistry and immunology
Among the most abundant molecules found on the cell surfaces of bacteria, parasites, and viruses are carbohydrates.1 This explains why sugar-protein molecular recognition processes have pivotal roles in infections and in immune response to pathogens. Invading microorganisms use surface-exposed carbohydrate and protein molecules to adhere to target surfaces in order to withstand natural fluxes and perturbations. This initial adhesion step is considered essential for colonization and infection by pathogenic bacteria.2 On the other hand, host organism senses the presence of infectious agents through protein receptors that recognized specific pathogen associated molecular patterns (PAMP), often constituted by sugars. These Pattern Recognition Receptors (PRR), also named Toll-like Receptors (TLR), trigger the so-called innate immunity response that in turn activate adaptive immunity.3 In other words, sugar-protein interactions are used by both pathogens to adhere to tissues and infect host, and by the immunity apparatus of host organism to fight against infection.
The possibility to target such sugar-protein specific recognition events with small organic molecules containing carbohydrates suggests that sugar chemists can cooperate with immunologist to the development of increasingly potent and selective anti-infective drugs.
The design of high affinity sugar ligands for protein targets is not a simple task because protein-sugar interaction is weak when a single mono- or oligosaccharide (glycan) interacts with a protein, and dissociation constants in the range of mM are generally observed. Experimental observations and theoretical calculations indicate that the decrease in entropy that accompanies most sugar/protein interactions explains the low affinity. Significant negative entropy variation is due to loss of oligosaccharide conformational flexibility and reorganization of water molecules upon complex formation. The assembly of multiple glycans in the same molecule, thus obtaining a glycocluster or multivalent glycan, creates a high-avidity interaction with protein binding site(s) often reaching nM dissociation constant values. The so called “cluster glycoside effect”4 operates in protein-carbohydrate interactions in living cells, and can be reproduced in synthetic multivalent carbohydrate ligands which bind efficiently to protein targets. The possibility that multiple simultaneous interactions have unique collective properties that are qualitatively different from properties displayed by their constituents, that interact monovalently, suggested new strategies for the design of drugs and research reagents for biochemistry and biology. Synthetic clustered glycosides are biomimetics of natural glycoclusters because they imitate the complex glycan structures found on the surface of cells, and are therefore efficient protein ligands. Glycoclusters have been designed and synthesized to interfere in an array of biological processes, and their synthesis and properties have been exhaustively reviewed in this themed issue. .In this Tutorial Review the role of glycoclusters in vaccination is presented, with particular focus on fully synthetic carbohydrate vaccines.
Carbohydrate vaccines, in particular anti-tumour vaccines based on sugar epitopes, have been excellently reviewed with focus on the chemical synthesis5, 6 and on their immunological and pharmacological properties.7, 8
In synthetic vaccines carbohydrates can play a dual role: they act as antigens to elicit specific anti-carbohydrate immune response and as adjuvants to potentiate immune response. Sometimes these roles are quite distinct other times synergistically overlap. As other vaccines, carbohydrate vaccines have the final goal of inducing the production of specific, long-lived, antibody-mediated protection. This goal is achieved through a very complex cascade of biochemical events, some of them involving in turn sugar-protein interactions. As depicted schematically in Fig. 1, the intensity and efficiency of carbohydrate-specific antibody response depends from the synergic stimulation of innate and adaptive immunity. Immunity activation requires at a molecular levels an array of protein-protein and protein-sugar interactions, that can be modulated by synthetic carbohydrate clusters.
Fig. 1.
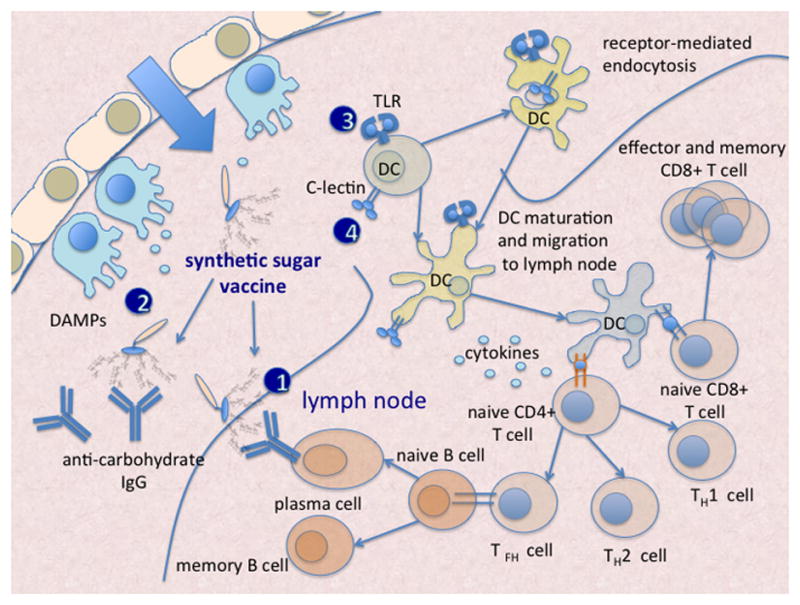
Clustered glycans have different roles in the immunization process: they can be 1) antigens 2) Danger Associated Molecular Patterns (DAMP) stimulating innate immunity response, 3) they can be ligands of TLRs and 4) of C-lectin receptors on the surface of dendritic cells, (DC) thus inducing DC activation and migration into lymph nodes.
2. Problems and solutions in carbohydrate vaccine development
The poor quality of antibody responses to carbohydrates is the major obstacle associated with developing carbohydrate-based vaccines.7 This is largely attributed to the T-cell independent immune responses, which are typically triggered by carbohydrate antigens.9 Oligo- and polysaccharides are able to activate B cells by binding to receptors of B-lymphocytes and inducing cross-linking of the Ig proteins, which leads to activation of the B-cell and production of low affinity antibodies. Such T-cell-independent responses are less robust, short-lived and primarily consist of immunoglobulin M (IgM) antibodies. To achieve a class switch to high-affinity IgG antibodies, the B-cells need to interact with helper T-cells. T-cells require, in turn, to be activated by binding to antigen-presenting cells (APCs), primarily dendritic cells, DCs (Fig. 1). These T cells-dependent responses, which are typically generated by protein and peptide epitopes, enable the generation of high affinity, class-switched antibodies and subsequently, long-lived antibody-mediated protection.
The classical method used to induce recruitment of CD4+ T cells for antibody responses is the conjugation of glycans to immunogenic carrier proteins, such as keyhole limpet hemocyanin (KLH) and ovalbumin (OVA), that are exogenous CD4+ T-cell epitopes.10 More recently, carrier proteins have been replaced by peptides or polysaccharides, as discussed later in this review.
Independently from the duration and intensity of the antibody response, another obstacle to efficient carbohydrate immunization is the inherent low affinity of antibodies to carbohydrates. Even the carbohydrate-specific IgG antibodies, produced upon T and B cell activation, typically bind to the corresponding sugar epitopes with dissociation constants in the μM range compared with protein-specific antibodies with nM dissociation constants.11 The affinity of this interaction can be increased by artificial assembly of several glycan units in multivalent glycoclusters that synergistically interact with different IgG molecules.
A third factor that reduces the efficacy of carbohydrate vaccines is the chemical heterogenicity of carbohydrate antigens extracted from natural sources and conjugated to proteins. This problem has been completely overcome by the advent of synthetic vaccines in which the carbohydrate part is prepared by multistep synthesis and not extracted by natural sources.
The few concepts of immunology given so far are however still not sufficient to fully understand how modern, fully synthetic vaccines work.
A quick journey into immunology: how vaccination works?
T-cell-B-cell cooperation is important for raising the production of efficient IgG antibodies against sugar epitopes, but is not the only element that can improve immune response. Another important factor that crucially determines the outcome of the adaptive immune response (i.e. the quality of antibodies produced) is the triggering of innate immune mechanisms.12, 13 The innate immunity system comprises cells and mechanisms that defend the host from infection by other organisms in a non-specific manner. Innate immunity cells (DCs and macrophages) are the first line of defense against foreign antigens and recognize minute amount of microbial molecular patterns (PAMPs) through TLRs with high sensitivity (pM) and selectivity.. Binding of PAMPs to TLRs activates innate immune responses, inflammatory signalling pathways and the production of cytokines that direct the subsequent adaptive immunity processes. DCs express the broadest repertoire of TLRs through which they can recognize a plethora of microbial compounds. After challenge with microbial or inflammatory stimuli, immature DCs undergo a complex process of maturation, resulting in their migration from tissues to secondary lymphoid organs and upregulation of major histocompatibility complex (MHC) and costimulatory molecules that are essential for T cell priming (Fig. 1).
Carbohydrates and sugar-protein interactions play pivotal roles in innate immunity. PAMPs of bacterial origin are constituted by polysaccharides that are specifically recognized by TLR4, and specific carbohydrate sequences and clusters interact with cell surface lectins in DCs and macrophages, thus inducing their activation.
Vaccines are thought to use mainly two types of immune triggers. First, they may contain PAMPs derived from the target pathogen. Second, vaccine components (called adjuvants) may induce the release of endogenous damage-associated molecular patterns (DAMPs), although this mechanism is less well studied. PAMPs and DAMPs can stimulate the innate immune system by activating Toll-like receptors (TLRs). TLR-derived signals are integrated directly or indirectly at the level of antigen-presenting cells (APCs) and in this way crucially condition the adaptive immune responses to the vaccine.14 PAMPs and DAMPs are detected directly by TLRs expressed by dendritic cells (DCs), leading to DC activation, maturation and migration to the lymph nodes (Fig. 1). Alternatively, PRR-mediated recognition of PAMPs and DAMPs by bystander cells may induce the release of tissue-derived factors, such as cytokines, that may cooperate in the activation and orientation of the DC response. The C-type lectin-dependent ligand internalization is also an important factor to elicit DC migration and T cell activation. In the lymph nodes, the activated DCs may present antigens to T cells, provide them with co-stimulatory signals and stimulate their differentiation by producing specific cytokines (Fig. 1). Depending on the cytokine milieu, CD4+ T cells may differentiate into various T helper (TH) cell subtypes thereby promoting the entry of these B cells into the plasma cell pathway or the germinal center pathway.
It is worth to emphasize the central function of cytokines in the immunization process. Cytokines are the chemical mediators that the immune system uses for cell-to-cell communication, and for activating macrophages, DCs, T-cells and B-cells. These small cell-signalling molecules are secreted by immunity cells and interact with specific cell surface receptors of other immunity cells, thus activating cascades of intracellular signalling. The stimulation of cytokines production has been used as an adjuvant strategy to potentiate the immune response to epitopes, especially in the field of cancer vaccines.
3. Rational design of synthetic carbohydrate vaccines
As outlined in Fig. 1, several points of the complex cascade of immunological events leading to antibodies production can be targeted in the rational design of vaccines. Modern, fully synthetic vaccines are multifunctional molecules containing different chemical units assembled by chemical bonds. Each of these moieties has a precise role in the cascade of events depicted in Fig. 1.
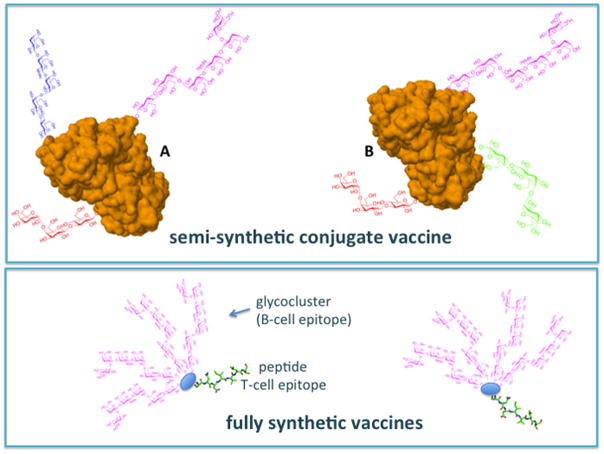
In Fig. 2 synthetic vaccines are presented as modular molecules composed by a combination of immunogenic units. A carbohydrate antigen, often a multivalent glycocluster, is conjugated (linked through a covalent bond) to one or more of the following elements: 1) a T-cell epitope (a carrier protein, a peptide or a polysaccharide), to trigger T-cell-B-cell cooperation; 2) a TLR ligand or a ligand of DC surface C-lectin, to stimulate DC maturation and cytokine production; 3) an adjuvant.
Fig. 2.
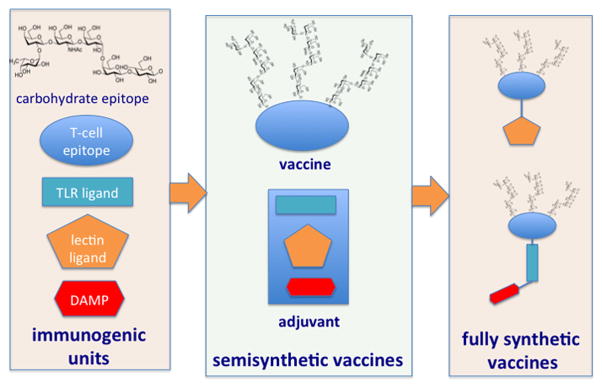
Semisythetic vaccines are composed by multivalent glycans conjugated to carrier proteins, the adjuvant is co-administered as a separate molecule. In fully synthetic vaccines the carbohydrate moiety recognized by B-cells and the peptide recognized by T-cells are combined with different types of adjuvants in the same molecule.
The majority of carbohydrate vaccines in use in clinic today, are composed by a glycan conjugated to a carrier protein, and belong to the category of semisynthetic vaccines (Fig. 2). In this case several copies of carbohydrate hapten are conjugated to a carrier protein, while the adjuvant is “external” i.e. is co-administered as part of vaccine formulation but is in fact a different molecule (or a mixture of molecules). The carbohydrate epitope is sometimes itself a TLR ligand, as in the case of “old”, very efficient vaccines composed by fragments of bacterial capsular polysaccharides, for example polysaccharides from Streptococcus pneumoniae, Neisseria meningitidis and Haemophilus influenza conjugated to a carrier parotein.15 Capsular polysacchardies are powerful TLR4 activators. One of the reasons of the high efficiency of these vaccines can be related to the antigen/adjuvant double role played by the carbohydrate.
A new generation of fully synthetic vaccines composed by a sugar epitope recognized by B-cells (B-cell epitope), a peptide recognized by T-cells (T-cell epitope) and, in some cases, an internal adjuvant is under clinical evaluation for a series of infectious diseases and for tumour immunotherapy. Synthetic vaccines totally lacking the T-cell epitope component and composed by the combination of clustered glycosides and adjuvants have also been developed. It has been observed that the co-localization of the above mentioned elements in the same molecule triggers a more potent antibody response than when the different elements are provided as separate molecules. When testing the activity of protein-conjugated sugar glycans as vaccines, a control immunization experiment is run by administering the carrier protein and the glycan as separated molecules, and a less efficient antibody production is generally observed.16 The potentiation of antibody response by monomolecular constructs comprising both B-cell and T-cell epitopes is therefore a matter of fact. However, the exact biochemical reason why immunogenic units should be joined in the same molecule rather than co-administered is still matter of debate.
From semisynthetic to fully synthetic vaccines
Several conjugate vaccines composed of naturally derived polysaccharides have excellent efficacy (usually approximately 90%) and safety profiles in the clinic.7 Three elements make these carbohydrate vaccines heterogeneous: i) the variability of chemical structures in naturally extracted glycans, ii) the variability of glycocluster/protein ratio in vaccines containing carrier proteins, iii) the use of adjuvants that are chemically non-homogeneous (Fig. 3).
Fig. 3.
Conjugate (semi-synthetic) vaccines are heterogeneous: the same vaccine preparation contains a mixture of molecules (such as molecules A and B) that differ by the number and type of glycans conjugated to the protein. Full synthetic vaccines are chemically homogeneous.
Naturally derived polysaccharide used for to producing conjugate vaccines that meet quality control and safety standards as required by European and US Agencies should be extracted and purified through generally costly process. The production of homogeneous glycans by chemical synthesis is therefore desirable for economical reasons. In the case of vaccines directed against tumour carbohydrates the natural abundance and availability of some of the key antigens is limited to minute nonusable quantities (often submilligram quantities) of pure product thus justifying synthetic efforts to have larger quantities. Another important reason to move forward synthetic vaccines is they present minimal batch-to-batch variability during manufacturing process and higher quality control standards are permitted, compared with the use of naturally derived agents.
An impressive synthetic effort has been done towards the production of homogeneous monovalent and multivalent glycans, the latter composed by several copies of the same glycan or by different glycan epitopes. The synthetic glycan has been then conjugated to protein carriers thus obtaining semisynthetic vaccines. Differently from other very influential scientists in this field17 that refer to synthetic glycans conjugated to proteins as fully synthetic vaccines, in this review the term semisynthetic will rather be adopted. A distinction is made here among protein-and polysaccharide-containing vaccines and fully synthetic vaccines in which the protein or polysaccharide has been replaced by a synthetic peptide or a multimeric peptide cluster (Fig. 3).
The fundamental difference between these two types of vaccines resides in the lower molecular homogeneity of protein- or polysaccharide-conjugated vaccines compared to vaccines containing chemically pure peptides.
Fully synthetic vaccines incorporate in the same molecule a synthetic glycan or glycocluster, a synthetic peptide as T-cell epitope and, sometimes, a third chemical moiety with the function of internal adjuvant. A total control on the chemical structure can be achieved in fully synthetic vaccines, while protein conjugation and external adjuvant are still sources of variability in semisynthetic vaccines.
Carbohydrate vaccines with different molecular architectures projected according to different strategies will be reviewed in next paragraphs. The common element of all these synthetic (or semisynthetic) vaccines is the presence of synthetic carbohydrate antigen in form of single glycans or glycoclusters.
4. Semisynthetic vaccines combining B-cell and T-cell epitopes
Sugar-protein conjugates
The first commercial vaccine containing a synthetic carbohydrate antigen was developed in Cuba against Haemophilus influenzae type b (Hib).18 In 1989, a team from Cuba embarked on a project to produce a new, more economical conjugate anti-Hib vaccine from a fully synthetic capsular polysaccharide antigen. The resulting synthetic pathway involves a one-step polycondensation reaction and produces oligomers with, on average, eight repeating units of polyribosylribitol with an 80% yield after purification by size-exclusion chromatography.18 The vaccine was evaluated in clinical trials in Cuba and showed long-term protective antibody titers that compared favourably to licensed products prepared with the Hib polysaccharide extracted from bacteria. This demonstrated for the first time the feasibility of the synthetic approach and also strongly indicated that chemical homogeneity of the saccharide part affords superior immunological properties. This successful example triggered the synthesis of increasingly complex carbohydrate structures to be conjugated to carrier proteins.
In the field of carbohydrate vaccines, anticancer vaccines have particular relevance. While antiviral and antibacterial vaccines are employed prophylactically to provide protection against infectious diseases, most experimental cancer vaccines are used therapeutically to evoke an immune response capable of eradicating an already existing disease.19
Anticancer therapy based on carbohydrate vaccination relies on the observation that some sugar units are exposed on the surface of tumour cells and are absent on normal healthy cells as result of aberrant glycosylation that characterize the majority of human cancers. Tumour cells may overexpress truncated versions of oligosaccharides, unusual terminal oligosaccharide sequences, and an increased sialylation (attachment of a sialic acid monosaccharide) of cell-surface glycolipids and glycoproteins. Several mechanisms have been proposed for the formation of Tumour-Associated Carbohydrate Antigens, TACAs, such as altered metabolism of tumour cells, changes in the tumour environment, and consequent changes in the expression of multiple genes of the glycosylation machinery.20
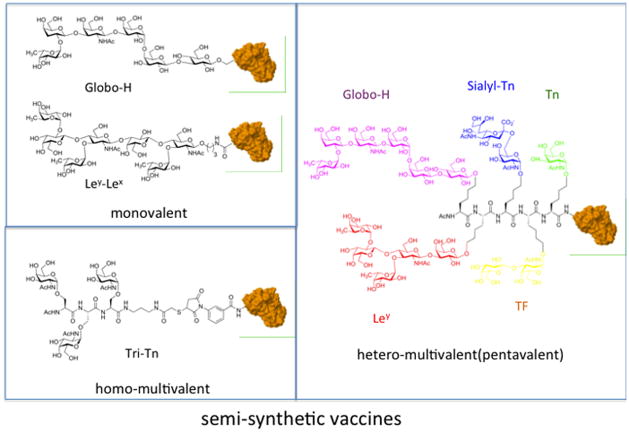
Worth of note, with the exception of few mono- and disaccharides (TF and Tn galactosides, Fig. 4), all TACAs are branched oligosaccharides and are therefore challenging targets for synthetic organic chemistry.5 This in part explains why the most important research groups in carbohydrate synthetic chemistry become involved in projects aimed at developing antitumour vaccines.
Fig. 4.
Synthetic glycans conjugated to a carrier protein: monovalent, homo-and hetero-multivalent vaccines.
The Livingston–Danishefsky team reported the challenging synthesis of Globo-H, Lewisy (Ley), Lewisx (Lex), Lewisb (Leb), KH-1, MUC-1, and the Tn, STn and TF-antigens.6 Boon’s group has developed a solid support and solution phase synthesis of the Ley, Lex, and the KH-1(Ley–Lex) antigens (Fig. 4). Other groups reported the synthesis of complex TACAs.21
The first-generation of semi-synthetic anticancer vaccines evaluated for their immunogenicity in human clinical settings were monovalent, i.e., containing a single carbohydrate antigen appropriately conjugated to a carrier protein. The carbohydrate antigens were synthesized with a reactive linker as glycosidic appendage (linked to the anomeric, C-1 carbon of the first reducing sugar) for subsequent covalent attachment to the immunogenic protein. Hexasaccharide Globo-H was first synthesized in 1996 and monovalent KLH-conjugate vaccine (Fig. 4) resulted safe, no significant toxicity was detected. In most patients, after immunization, both IgM and IgG antibodies were detected against synthetic Globo H. This was a further demonstration that a complex synthetic carbohydrate antigen can efficiently an immune response in humans. The vaccine is now in clinical Phase III for treatment of cancers of the breast, prostate, and ovary.22
Monovalent semisynthetic anticancer vaccines, composed by the synthetic tetrasaccharide Ley coupled to the protein carrier KLH using several different linkers were also proposed by Boons and coworkers.23 In this work was outlined how the chemical nature of the bifunctional linker connecting the glycan to the protein greatly influences the immune response towards the carbohydrate antigen. A surprisingly high IgG antibody titer was determined compared to previously reported immunizations with Ley-KLH conjugates. The maleimide linker used for conjugation turned out to be highly immunogenic and specific anti-linker antibodies were produced. When using the bromoacetyl electrophile for reaction with protein no antibodies were produced against the linker. Interestingly, the anti-linker response might be directed only against terminal hydrolyzed maleimides and not against maleimides connecting the saccharides to the protein, that is, internal maleimides. In this respect, it is important to realize that only 80% of the maleimides are functionalized by saccharides and the rest are hydrolyzed. This study called for an awareness of possible false positives stemming from the use of commercial kits in which the same linker is employed for conjugation of an antigen to different proteins. Moreover, as in this case, highly antigenic linkers can suppress antibody responses to weak antigens, such as carbohydrate antigens. This is another element in favour of replacement of carrier protein with a fully synthetic peptide construct, as will be discussed in the next paragraph.
A second generation of carbohydrate vaccines was constituted by smaller carbohydrate antigens (e.g., mono and disaccharides), those typically associated with mucins, assembled in glycoclusters and subsequently conjugated to proteins (Fig. 4). These can be defined as homo-multivalent (because several copies of the same carbohydrate are clustered) carbohydrate vaccines. It was determined that multiple repeats or clustering of the carbohydrates was required for a robust and efficient immune response to be generated.24 These vaccines were constituted by an antigen containing one or multiple copies of the human adenocarcinoma antigen sialyl-Tn, conjugated to the carrier protein.
A semisynthetic homo-multivalent clustered anti-HIV vaccine has been recently developed. In this molecule, cyclic peptide serves as multivalent scaffold for presenting several copies of a high-mannose glycan that is found on HIV protein gp120 and is efficiently recognized by human 2G12 anti-HIV antibodies.17 Three copies of a branched mannose nonasaccharide have been linked to one face of the cyclic peptide scaffold derived from the regioselectively addressable cyclic peptide (RAFT) developed by Mutter and Dumy.25 This homo-multivalent glycocluster was then covalently coupled to the purified outer membrane protein complex (OMPC) derived from Neisseria meningitidis. The conjugation was based on the reaction of maleimidated protein with the thiol group of a cysteine included in the cyclic peptide. Even within a particular cancer type, there is a considerable amount of variation in the level and nature of cell-surface carbohydrates expressed. A vaccine that targets several cancer-associated glycans should, in principle, lead to a stronger and more specific immune response than one that targets a single cancer glycan. With the aim of mirroring the diversity of glycan structures expressed on a single cancer cell, vaccine constructs were projected consisting in different tumour-associated glycans clustered in the same molecule and subsequently conjugated to a protein. These vaccines can be defined as hetero-multivalent because different glycans are assembled in the same molecule.
The synthesis of hetero-multivalent vaccines was based on the assembly of different glycans on a single molecule that would undergo a single conjugation step to a carrier. This approach has been preferred to that based on the conjugation of different carbohydrate antigens to the same carrier protein mainly because allows to obtain more homogeneous molecules.
Several unimolecular hetero-glycoclusters have been prepared by Danishefsky group and conjugated to carrier proteins thus obtaining semisynthetic vaccines that induced the production of antibodies against each of the component antigens.26
The most impressive example is the synthesis of pentavalent vaccine conjugate containing five different carbohydrate antigens known to be expressed in high levels on prostate cancer: Tn, TF, sTn, Lewis y, and Globo H (Fig. 4).16 The pentavalent epitope was conjugated to the carrier protein KLH or to the lipopeptide adjuvant Pam3Cys that is known to be a TLR2 ligand. Mice immunization experiments were performed by co-administering the adjuvant QS-21 to vaccines. The pentavalent hetero-glycocluster conjugated to KLH was quite immunogenic and the Pam3Cys conjugate was immunogenic too, but the antibodies titers were still somewhat lower than those observed for the KLH conjugate.
In the construction of monovalent, homo- and hetero-multivalent several types of linkers have been used to connect the carbohydrate part to the protein. These range from simple “classical” bifunctional linkers for protein conjugation, to more elaborate multivalent scaffolds constituted by linear16 or cyclic17 peptides.
Substituting carrier proteins with T-cell stimulating zwitterionic polysaccharides
The presence of the carrier proteins in vaccines can lead to some disadvantages. A strong immune response against the carrier protein can cause suppression of carbohydrate- specific antibody production (hapten-specific suppression).27 It has been reported by Kasper and coworkers that carrier proteins can be substituted in vaccines by zwitterionic polysaccharides (ZPSs) able to elicit an MHC II-mediated T-cell response.28 With the aim of developing an entirely carbohydrate vaccine, Andreana and coworkers conjugated the ZPS from Bacterioides fragilis to the tumour Tn epitope (the N-acetylgalactosamine, GalNAc) monosaccharide) thus obtaining a cancer vaccine.29 The polysaccharide was extracted and purified from the bacter B. fragilis and therefore presents the typical microhetergoeneity of natural polysaccharides. For this reason, similarly to protein-conjugate vaccines, this type of entirely carbohydrate vaccines should be considered as semisynthetic and is included in this paragraph.
The Tn epitope was covalently linked to the polysaccharide through a chemoselective technique based on the reaction of an hydroxylamine group inserted in the anomeric position of GalNAc and an aldehyde group obtained by sodium periodate oxidation of the ZPS part.29 The Tn-ZPS vaccine was tested on mice and carbohydrate-specific IgG3 antibodies were produced, that selectively bind to tumour expressing on the surface the Tn antigen.30
5. Fully synthetic vaccines combining T- and B-cell epitopes (and adjuvant)
The antiviral, antibacterial and anticancer vaccines above described are all semisynthtetic in the sense that a synthetic B-cell sugar epitope is conjugated to a nonhomogeneous T-cell epitope constituted by a protein or a polysaccharide. Totally synthetic vaccines have been developed in which the protein T-cell epitope has been substituted by a peptide.
Semisynthetic protein conjugate vaccines present the following disadvantages: 1) the conjugation chemistry is often difficult to control, which results in conjugates with ambiguities in composition 2) the production of carrier protein-specific antibodies 3) some linkers are B-cell epitopes, and hapten suppression can be elicited by unreacted linkers exposed on the surface of proteins.23
The design of fully synthetic vaccines is aimed to overcome these problems. Different chemical units with different functions (Fig. 2) are assembled in the same chemical entity that can be fully characterized by a chemical point view (Mass, IR and NMR spectra corresponding to a single molecular entity).
Synthetic vaccines are generally modular: several chemical units can be separately synthesized and subsequently assembled in a convergent way. Modular carbohydrate vaccines can be obtained by classical convergent synthesis using protecting groups. Alternatively, a large repertoire of orthogonal, chemoselective,31 and “click”32 reactions are available to organic chemist allowing the conjugation of large presynthesized molecular fragments with minimal or no use of protecting groups.
Three types of spatial architectures have been used for the construction of fully synthetic vaccines: 1) glycoclusters on dendrimeric structures, with a globular tertiary structure 2) glycoclusters assembled on organic templates (cyclic peptides) 3) glycoclusters or single glycans linked to other immunogenic units in a linear or branched arrangement.
Dendrimeric glycoclusters
Lo-Man and co-workers ideated a synthetic vaccine named Multiple Antigen Glycopeptide (MAG) with a dendrimeric architecture inspired to the Multiple Antigen Peptide (MAP) introduced by Tam in 1988.33 The pioneering work of Tam consisted on the use of a branched lysine peptide scaffold, usually constituted by seven lysines functionalized at the terminal amines with eight selected immunogenic peptide epitopes. The synthetic MAG is analogously composed of a dendrimeric lysine core structure with four arms. Each arm is linked to a CD4+ T cell epitope (PV peptide from the poliovirus type 1) with a monosaccharide tumour epitope (Tn, i.e. α-GalNAc) at the amino terminus34 (Fig. 5).
Fig. 5.
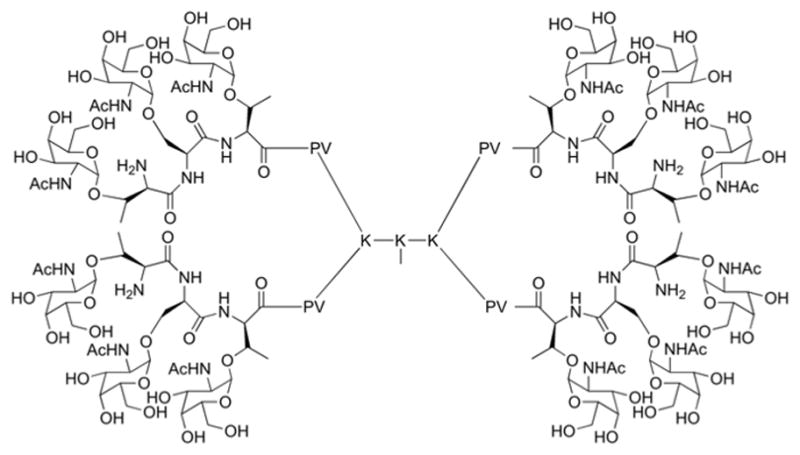
Fully synthetic dendrimeric (MAG) vaccines combining peptide T-cell epitope and sugar Tn (GalNAc) eptitopes
The tetrameric MAG-(PV-Tn)4 induced high titers of anti-Tn Antibodies (directed against the carbohydrate tumour epitope) and provided immunization against TA3/Ha adenocarcinoma cell line (expressing Tn epitope) in mice.35 With the aim of applying the MAG approach to human vaccination, “universal” CD4+ T-cell epitopes known to stimulate effective T-helper cell responses in humans were introduced.36 These trivalent dendrimeric MAG containing TT or PADRE peptides and Tn carbohydrate epitopes were tested in nonhuman primates (macaques and green monkeys) and found to induce strong anti-Tn IgG antibodies capable of specifically recognizing Tn-expressing human tumour cells. Moreover, these antibodies were able to mediate antibody-dependent cellular cytotoxicity against Tn-positive human tumour cell.
Glycoclusters assembled on cyclic peptides
Small cyclic peptides provide a great variety of tridimensional structures associated in nature to an array of biological functions. Mutter and coworkers pioneered the use of synthetic cyclic peptides as universal scaffolds in the construction of biomimetic molecules.25 The use of Mutter’s regioselectively addressable functionalized templates (RAFTs), as new scaffolds for the design of anticancer vaccines has been pioneered by Dumy and Renaudet.37 RAFTs are backbone-cyclized decapeptides containing two proline-glycine dipeptides as β-turns inducers that stabilize their conformation in solution. These cyclic templates offer the advantage of displaying two functional faces, one can be used for the attachment of several glycan units to form the glycocluster, while one or more copies of the T-cell epitope can be attached to the other face (Fig. 6).
Fig. 6.
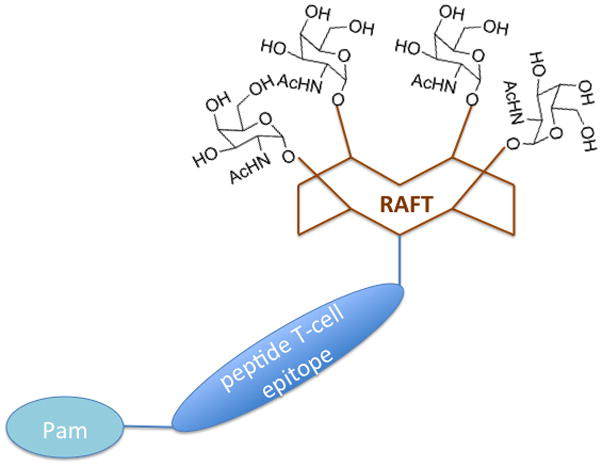
Fully synthetic RAFT carbohydrate vaccines
The first prototypical RAFT presented four copies of the Tn antigen on one side and one or two copies of the PV T-cell peptides (the same used in MAGs) on the other.37 The RAFT-PV-Tn4 was first used to immunize mice and to elicit Tn-specific IgG antibodies. Interestingly, only 0.1–1% of the antibodies recognized the peptidic part of the RAFT thus indicating that RAFT templates are non-immunogenic and can be used to elicit an immune response specifically directed against the native form of Tn displayed by cancer cells. Antibodies raised by RAFT-PV-Tn4 are able to recognize the native form of Tn, and RAFT binding to human Jurkat tumour cell line expressing the Tn antigen was observed by flow cytometry.37
As a development of this approach, RAFT platform was used to assemble in the same molecule four different immunogenic components: a clustered Tn sugar epitope, a CD8+ T cell epitope from ovalbumin (OVA257–264), a universal CD4+ T helper (Th) epitope (PADRE) and a palmitoyl tail as lipidic built-in adjuvant.38 Mice treated with this multicomponent synthetic vaccine were transplanted with melanoma cells (B16) expressing ovalbumin and only one mouse out of ten developed a detectable tumour, thus indicating that synthetic vaccine provided an effective protection from tumour development.
In a further study, the mechanism of action of RAFT-based synthetic vaccines was investigated.39 Two molecular architectures were synthesized, one with the adjuvant palmitoyl moiety attached to the N-terminal end of the T-cell peptide (linear RAFT), in the other the palmitic acid was attached between the two T-cell epitopes (HER and PADRE) thus forming a branched structure. The IgGs induced by both linear and branched constructs bind human breast tumour cell line MCF7 expressing Tn molecules. However, higher (350 fold) binding was observed for branched vaccine-induced IgGs than linear-induced IgGs. Interestingly, the position of the lipid moiety turned out to profoundly affect also the immunological mechanism of action of the molecules. DCs uptake of both linear and branched vaccines was studied as well as cross-presentation to T cells. It was observed that both linear HER-GLP-1 and branched HER-GLP-2 constructs are taken up easily by DCs, and both constructs induced DCs maturation through TLR2 stimulation. However, DC exposed to linear and branched vaccines were differently affected by inhibitors of the antigen internalization through endosome and specific presentation through MHC complexes, thus indicated that the epitopes exposed with different architectures undergo different biochemical pathways in DCs. The authors concluded that the position of the palmitoyl moiety within synthetic vaccine constructs greatly influence: (i) the magnitude of induced IgG and CD8+ T cell responses; (ii) the phenotypic and functional maturation of DCs; (iii) the cross-presentation pathway of the GLP constructs by DCs; and (iv) the level of therapeutic efficacy against established tumours.
Modular synthetic vaccines
Boons and coworkers first explored the possibility to evoke a carbohydrate-specific antibody response by using tri-component vaccine containing a carbohydrate B-cell epitope, a peptide T-cell epitope and a potent immunogenic moiety such as a TLR ligand (Fig. 7).
Fig. 7.
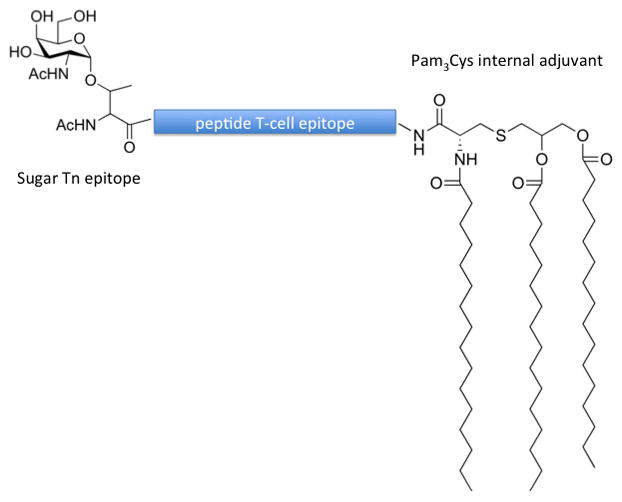
Molecular structure of three-component fully synthetic vaccine
A fully synthetic three-component anti-cancer vaccine composed of the Tn-antigen, a helper T-epitope derived from Neisseria meningitis, and the TLR ligand Pam3Cys was designed and synthesized, using a modular synthetic approach.40 In a subsequent study, two additional tri-component vaccine candidates were synthesized composed of the tumour-related MUC-1 glycopeptide, a well-documented helper T-cell epitope from polio virus, and either Pam2CysSK4 or Pam3CysSK4.41 Both Pam2CysSK4 and Pam3CysSK4 are TLR2 agonists (activators). The vaccine candidates were incorporated into liposomes and their antigenicity studied in murine hosts. Compound containing 3 palmitoyl units induced exceptionally high IgG antibody titers, while the molecule with 2 palmitoyl moieites induced lower IgG titer. It was observed that sera of mice immunized with both these molecules were able to recognize the native MUC1 antigen present on cancer cells. Authors also outlined that the activity of these vaccines containing palmitoyl-cysteine adjuvants is due to TLR2-dependent uptake by innate immunity cells.41 A fully synthetic three component vaccine has been recently developed containing a sugar tumour epitope Tn (B-cell antigen), a T-cell peptide antigen and the monosaccharide rhamnose.42 The function of L-rhamnose is that of an adjuvant. Human serum has been reported to contain large amounts of naturally occurring anti-rhamnose antibodies, thus, L-rhamnose-containing molecules potentiate immune response by the triggering anti-rhamnose antibody-mediated antigen uptake. The vaccine induced antibodies against the tumour Tn epitope and did not induce an unwanted dominant response to rhamnose itself, thus demonstrating the potential of use of the monosaccharide rhamnose as internal adjuvant.
6. Exploiting innate immunity: sugar vaccines containing TLR and lectin ligands
TLR ligands
TLR stimulation and innate immunity activation have a synergistic effect on adaptive immunity activation and antibody response. It has also been observed that the first contact with a TLR agonist opens a ‘temporal window’ for stimulation of another TLR that intensifies, complements and sustains the DC activation process.13 The integration of multiple stimuli over a defined ‘temporal window’ that is the incorporation in the same molecule of sugar epitopes and TLR agonists might allow a more effective response to carbohydrate epitopes.
According to the definition of modular vaccine adopted in that review (and schematically represented in Fig. 2) a synthetic carbohydrate vaccine can be formed by a sugar antigen conjugated to a TLR agonist. Toll-like receptor (TLR) agonists, such as the lipopeptide Pam3Cys, a TLR2 ligand, have been attached to TACAs. One of the first examples of synthetic vaccines not including in their design T-cell epitopes was reported by Toyokuni and coworkers, who covalently linked a dimeric Tn-antigen to Pam3Cys (Fig. 8).43
Fig. 8.
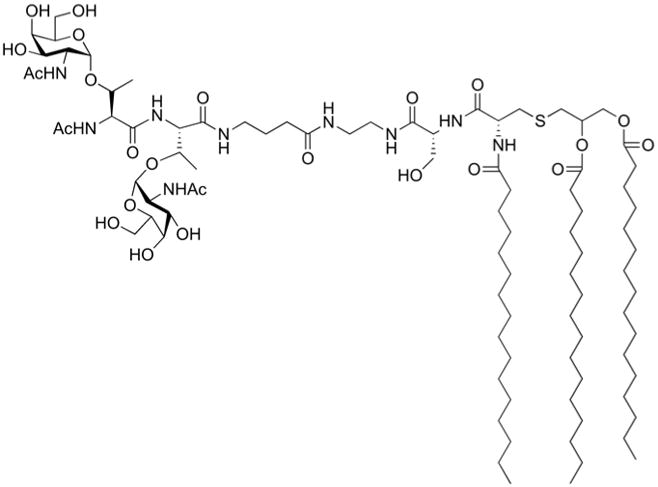
Fully synthetic vaccine composed by a synthetic glycan linked to TLR2 ligand Pam3Cys with the function of adjuvant
Although low titers of IgG antibodies were produced, this study showed that it is possible to elicit sugar-specific antibody production without any carrier protein or other T-cell epitope. Danishefsky and co-workers also explored this kind of synthetic vaccines, and several TACAs including monomeric Ley, a trimeric cluster of Ley, and a trimeric Tn-antigen cluster were attached to Pam3Cys. Mice immunized with the vaccine constructs elicited antibodies that recognized the natural epitope expressed by relevant cancer cell lines. However, mainly IgM antibodies were detected even upon administration of the saponin QS-21 as external adjuvant.
These results highlight that a lack of a helper T-epitope, which is required to induce a class switch to IgG antibodies and affinity maturation, results mainly in the production of IgM antibodies. A unimolecular multi-antigenic construct comprising the Globo-H, Ley, STn, TF, and Tn-antigens all attached to the same peptide backbone was synthesized and conjugated to Pam3Cys.16 Mice were inoculated with the candidate vaccine in the presence of the adjuvant QS-21, and IgM antibodies against all antigens, were detected. However, when the same multi-antigenic construct was linked to the carrier protein KLH and co-administered with QS-21 in a murine host, both IgM and IgG antibodies were elicited and the antibodies recognized three different tumour cell-lines all expressing two or more of the five antigens on their respective cell surfaces. These observations suggested a superior efficiency of T-cell epitopes to induce IgG response compared to palmitoylated TLR2 ligands, and induced researchers to prefer vaccine constructs including carrier proteins or peptides and external adjuvants.
The TLR-activating moieties that have been included in the molecular structures of fully synthetic vaccines are palmitoyl chains or palmitoylated cysteines that are TLR2 agonists. However, the stimulation of other TLRs, in particular TLR4 and TLR7, can afford even more potent activation of innate immunity by triggering cytokine production. In particular, TLR4 stimulation is in principle the most potent way to activate innate immunity. Natural TLR4 agonists (bacterial endotoxin, LPS and lipid A) are however toxic molecules and cannot be used as vaccine adjuvants. Synthetic lipid A analogues with modified structures, as monophosphoryl lipid A (MPLA), are potent vaccine adjuvants. MPLA has been recently approved as adjuvant for Hepatitis B vaccination in Europe44 and has been used extensively in a variety of clinical vaccine testing. MPLA is a chemically modified derivative of lipopolysaccharide (LPS) with potent adjuvant activity, but is up to 10,000 fold less toxic than parent LPS molecule.
There is an increasing interest in the synthesis of TLR4 agonists. However, despite the very active research in this field, the chemical variety of newly discovered TLR4 agonists is still limited to lipid A analogues. Our research group is contributing to the discovery of new small-molecules active in modulating TLR4 activity with a structure different from lipid A.45, 46
Synthetic TLR agonists have been included in nanoparticle preparations47 and the combination of molecules activating different TLRs afforded very efficient immunostimulation.
However, the inclusion of TLR ligands other than palmitoylated peptides in fully synthetic molecules has still not been explored and is an interesting possibility for future vaccine development.
DC stimulating agents
Dendritic cells (DCs) are the primary antigen presenting cells of the immune system. Consequently, targeted delivery of vaccine components to DCs represents an attractive approach to enhance vaccine efficacy. It is known since 1997 that peptide and protein mannosylation facilitate the uptake of such peptides and proteins by innate immunity cells such as DC and macrophages that express mannose receptors on their surface.48
DC express surface C-lectins, namely to the Dendritic Cell-Specific Intercellular Adhesion Molecule-3-Grabbing Non-integrin (DC-SIGN) and to other mannose receptors (MR) that recognize mannosylated ligands, thus promoting their internalization and subsequent processing by the immune system. The first totally synthetic vaccine composed by a peptide epitope linked to a polymannose cluster as DC-stimulating agent was prepared thorough an elegant, convergent synthesis. Two orthogonal ligation steps allowed the condensation of unprotected peptide and glycan moieties through the formation of hydrazide and thioether bonds.49
Internalization studies of these lysine-based clusters functionalized with mannose or mannose bioisosters such as quinic and shikimic acid were done on cellular models presenting a mannose receptor.50 The same group designed a complementary strategy where the tetravalent system presenting quinic acids was covalently linked via a hydrazone ligation to a multilamellar lipidic vesicles vectors.51 These “onion” vesicles presented a mean size about 200 nm and contained surface aldehydes to be functionalized with the hydrazine presented at the tail of the multivalent system. The ligation reaction occurred with a high yield and several copies of the quinic acid functionalized dendrimer were attached to the surface of the lipidic vesicles. Rhodamine labeled vesicles were used to demonstrate the co-localization of the vesicles with the mannose receptor and the internalization of the vesicles into internal cellular compartments. These results demonstrated the potential application of these selective vectors which could incorporate lipophilic material to be selectively transported to target cells such as DCs.
A mannose-based antigen delivery system was adopted in the design and synthesis of a synthetic vaccine constituted by a glycocluster exposing 64 mannose units on a PAMAM dendrimer conjugated to the OVA protein.52 The Mannose Dendrimer-OVA conjugate (MDO) potently induced OVA-specific T cell response in vitro. It was found that the immunogenicity of MDO was due not exclusively to enhanced antigen presentation, but also to induction of DC maturation. Mice immunized with MDO generated strong OVA-specific CD4+/CD8+ T cell and antibody responses. MDO also targeted lymph node DC to cross-present OVA, leading to OTI CD8+ T cell proliferation. Moreover, upon challenge with B16-OVA tumour cells, tumours in mice pre-immunized with MDO either did not grow or displayed a much more delayed onset, and had slower kinetics of growth than those of OVA-immunized mice.
Bundle and co-worker developed efficient synthetic glycopeptide vaccines against candidiasis (Candida albicans infection) using a DC-based immunization approach.53 They discovered that antibodies specific for Candida albicans cell surface β-1,2-mannotriose β-(Man)3 protect mice. Several synthetic conjugates were synthesized composed by β-(Man)3 epitope corresponding to the Candida albicans cell wall β-mannan, and selected peptide carriers derived from cell wall proteins of the fungus that are known to be expressed during pathogenesis of human candidiasis. One of these fully synthetic carbohydrate vaccines, containing the N-terminal portion of the C. albicans cytosolic/cell surface protein fructose-bisphosphate aldolase (Fba), induced strong antibody responses and protective immunity against candidiasis in BALB/c mice.53 The trimannose glycan plays in these vaccines the double role of epitope and DC ligand.
Bundle’s group recently improved vaccine’s activity by coupling it to tetanus toxoid (TT).54 The addition of TT to the glycopeptide conjugate resulted in a self-adjuvanting vaccine that promotes robust antibody responses without the need for additional adjuvant, which is novel and represents a major step forward in vaccine design against disseminated candidiasis.
7. Glycosylated nanoparticles vaccines
Building on many years of basic and translational research, advances in the fields of nanotechnology and biomedical science are now converging to revolutionize the treatment of a range of diseases. To date, several types of particle-based therapeutics have been approved by the FDA for clinical use, including liposomes, albumin nanoparticles, and polymeric nanoparticles.55 In the field of immunology different nanocarriers have been used to display carbohydrate epitopes for vaccination. The opportunity of modifying the surface of nanoparticles to achieve simultaneous antigen-loading, adjuvant co-delivery, improved circulation times and targeting properties has increased the interest in nanoparticle-based vaccines.
Liposomes are regarded as attractive nanocarriers for the multivalent presentation of selected synthetic carbohydrate haptens, Th peptide epitopes and potent adjuvants. These versatile biocompatible (phospho)lipid vesicles are characterized by a low intrinsic immunogenicity and toxicity. They can carry multiple antigens either encapsulated, surface-bound, or associated with the bilayers, in addition to various amphiphilic adjuvants, such as different TLR ligands. As other nanoparticles, liposomal constructs can be efficiently captured and endocytosed by antigen presenting cells (APCs). Importantly, clusters of B-cell epitopes exposed on the surface of the vesicles efficiently target antigen specific B lymphocytes by multivalent interactions with the B-cell antigen receptors (BCR), increasing the potency of the humoral response.
We outlined that Boon’s three-component fully synthetic antitumour vaccines are an excellent example of efficient immunization by multiple presentation on liposomes.41
Another interesting liposome-based vaccine exposing carbohydrate epitopes has been developed against Shigella flexneri serotype 2a (SF2a), the major strain responsible of endemic shigellosis, or bacillary dysentery, in developing countries.56 Glycoliposomes carrying at their surface a T-cell epitope peptide and a synthetic pentasaccharide or a pentadecasaccharide corresponding, respectively, to one or three repeating units of O-specific polysaccharide moiety of Shigella flexneri, were synthesized. The T-cell epitope and the synthetic oligosaccharide were covalently conjugated to the surface of preformed liposomes via the maleimide functionalized lipopeptide anchor Pam3CAG-Mal, that also has the function of TLR2 ligand and adjuvant. The synthetic liposomal construct carrying the pentadecasaccharide (3 repeating units) induced a specific, long-lasting and protective immune response against SF2a, when administered by intramuscular injection to BALB/c mice.
The use of gold nanoparticles (GNP) as carriers for carbohydrate vaccines development has been recently investigated. Vaccine candidates against Streptococcus pneumonia were composed by 2-nm hybrid GNP displaying different ratios of the branched tetrasaccharide unit of S. pneumonia capsular polysaccharide (Pn14PS), and a T-helper peptide were prepared and the immunogenicity of the functionalized GNPs was studied in mice.57 GNP triggered specific anti-Pn14PS IgG antibodies. Cytokine levels confirmed that glyconanoparticles led to T-helper cell activation. The anti-saccharide antibodies promoted the phagocytosis of bacteria by human leukocytes, indicating the functionality of the antibodies.
Our group recently explored the possibility to mimic natural LPS aggregates with magnetic nanoparticle coated with bacterial LPS and we investigated the activity of these LPS-NP to stimulate TLR4 signaling in innate immunity cells.58 E. coli LPS was physically adsorbed on the surface of oleylamine-coated iron oxide nanoparticles (Fig. 9).
Fig. 9.
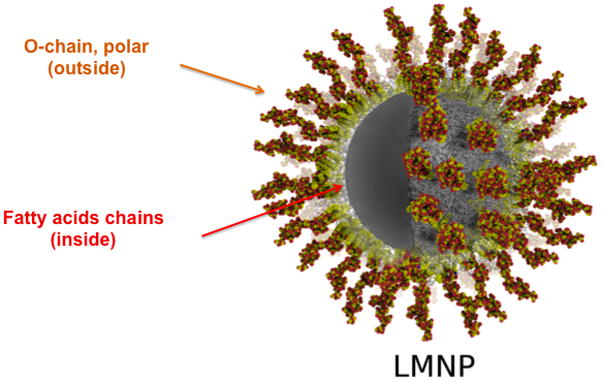
LPS-coated magnetic nanoparticle (LMNP): the lipophilic chains of lipid A interact with the hydrophobic chains of NP surface, while the hydrophilic oligosaccharide chain points outward, thus reproducing the conformation of a natural LPS aggregate.
The strong hydrophobic interaction between the lipid A moiety of LPS and the surface of nanoparticles made these LMNP particularly stable in aqueous solution. No LPS molecules were released from NP, however, NP-LPS activated TLR4-mediated cytokine production in macrophages. The use of immobilized LPS as strong vaccine adjuvant and the construction of nanoparticles exposing LPS together with carbohydrate antigens is an exciting perspective in nanomedicine.
8. Future perspectives
In this article an overview of the current state of carbohydrate vaccine research has been presented focusing on both chemical and biological aspects. The availability of efficient and convergent synthetic strategies combined with a growing knowledge of molecular mechanisms of immunization, allowed an impressive advancement in the field of synthetic carbohydrate vaccines. However, the rational design of synthetic vaccines only partially addressed the issues related to the intrinsic complexity of the vaccination process. From a medicinal chemistry point of view, to elicit a strong, specific and long-lived antibody response against sugars means to interfere with several complicated and mutually interplaying biochemical pathways. This task is orders of magnitude more complex than inhibiting an enzyme or blocking a receptor.
This probably explains why the advantage of the use of fully synthetic vaccines instead of carrier protein conjugates is controversial and has still not been definitively demonstrated. While some of Danishefsky’s semisynthetic vaccines based on KLH conjugates are in an advanced phase of clinical trials, the experimentation on humans of fully synthetic is still at a less advanced stage. However, the efficiency of MAG-Tn or RAFT-Tn or Tn-Pam3CS constructs in eliciting antibody response both in mice and non-human primates is a matter of fact documented by elegant and exhaustive studies.
The rational design of fully synthetic vaccines can be however improved by answering to the following, still open questions:
-
How the different immunogenic components should be mutually oriented to optimize vaccine activity? The rule leading the design of the majority of modular synthetic vaccines so far developed is that different components (sugar antigen, peptide antigen, adjuvant) have to be assembled in the same molecule. Few or no attention has so far been given to the tertiary structure of the vaccine.
In this context, studies on RAFT molecules have shown that the presentation of sugar and peptide epitopes on different sides of a rigid peptide scaffold could facilitate the interaction of both epitopes with relative receptors on B and T cells thus improving vaccine efficiency. In this sense, the design of dendrimeric MAG-Tn tested so far which present the peptide and the sugar directly linked could be improved by separating sugar and peptide epitopes in different spatial regions.
How linker’s chemical structure and size impact on vaccine efficiency? Several observation reported on this review suggest that some linkers can be more immunogenic that others, but extensive systematic studies comparing different linkers in the same biological context are still lacking.
-
New TLR agonists should be included in vaccine molecules. Several potent, small-molecule TLR agonists are currently available. As discussed in this review, very few TLR ligands have been included as adjuvants in vaccines, almost exclusively palmytoilated TLR2 ligands. TLR4, 7 and 9 potent agonists are available whose chemical structure can be opportunely modified to be conjugated to fully synthetic vaccines. Another interesting, still unexplored perspective, is the conjugation of these TLR-based adjuvants to the carrier proteins.
The complexity of vaccine chemistry and biology make the rational design of vaccines an intriguing and interdisciplinary domain, at the interface among chemistry, biology and medicine. Organic chemists can provide the biologists with unique, tailor-made chemical tools that can be used with the dual scope to develop drugs and to dissect immunological pathways, thus gaining information on the molecular mechanism of these processes.
Acknowledgments
Author acknowledges the US National Institutes of Health (NIH)/ National Institute of Allergy and Infectious Diseases (NIAID), project: “Regulation of MD-2 function and expression” (1R01AI059372), Finlombarda, Regione Lombardia (Italy), project: “Network Enabled Drug Design” (NEDD 14546), and the Italian Ministry of University and Research (MIUR), PRIN 2008, project: “Mono- and Multivalent Ligands for Human Galectins”.
Footnotes
Part of the multivalent scaffolds in glycosciences themed issue
Notes and references
- 1.Gagneux P, Varki A. Glycobiology. 1999;9:747–755. doi: 10.1093/glycob/9.8.747. [DOI] [PubMed] [Google Scholar]
- 2.Dwek RA. Chem Rev. 1996;96:683–720. doi: 10.1021/cr940283b. [DOI] [PubMed] [Google Scholar]
- 3.Iwasaki A, Medzhitov R. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 4.Lundquist JJ, Toone EJ. Chem Rev. 2002;102:555–578. doi: 10.1021/cr000418f. [DOI] [PubMed] [Google Scholar]
- 5.Buskas T, Thompson P, Boons GJ. Chem Commun (Camb) 2009:5335–5349. doi: 10.1039/b908664c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keding SJ, Danishefsky SJ. Proc Natl Acad Sci U S A. 2004;101:11937–11942. doi: 10.1073/pnas.0401894101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Astronomo RD, Burton DR. Nat Rev Drug Discov. 2010;9:308–324. doi: 10.1038/nrd3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heimburg-Molinaro J, Lum M, Vijay G, Jain M, Almogren A, Rittenhouse-Olson K. Vaccine. 2011;29:8802–8826. doi: 10.1016/j.vaccine.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snapper CM, Mond JJ. J Immunol. 1996;157:2229–2233. [PubMed] [Google Scholar]
- 10.Avery OT, Goebel WF. J Exp Med. 1931;54:437–447. doi: 10.1084/jem.54.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cygler M, Rose DR, Bundle DR. Science. 1991;253:442–445. doi: 10.1126/science.1713710. [DOI] [PubMed] [Google Scholar]
- 12.Pulendran B, Ahmed R. Nat Immunol. 2011;12:509–517. doi: 10.1038/ni.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Nat Immunol. 2005;6:769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwasaki A, Medzhitov R. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robbins JB, Schneerson R, Anderson P, Smith DH. JAMA. 1996;276:1181–1185. doi: 10.1001/jama.276.14.1181. [DOI] [PubMed] [Google Scholar]
- 16.Ragupathi G, Koide F, Livingston PO, Cho YS, Endo A, Wan Q, Spassova MK, Keding SJ, Allen J, Ouerfelli O, Wilson RM, Danishefsky SJ. J Am Chem Soc. 2006;128:2715–2725. doi: 10.1021/ja057244+. [DOI] [PubMed] [Google Scholar]
- 17.Krauss IJ, Joyce JG, Finnefrock AC, Song HC, Dudkin VY, Geng X, Warren JD, Chastain M, Shiver JW, Danishefsky SJ. J Am Chem Soc. 2007;129:11042–11044. doi: 10.1021/ja074804r. [DOI] [PubMed] [Google Scholar]
- 18.Verez-Bencomo V, Fernández-Santana V, Hardy E, Toledo ME, Rodríguez MC, Heynngnezz L, Rodriguez A, Baly A, Herrera L, Izquierdo M, Villar A, Valdés Y, Cosme K, Deler ML, Montane M, Garcia E, Ramos A, Aguilar A, Medina E, Toraño G, Sosa I, Hernandez I, Martínez R, Muzachio A, Carmenates A, Costa L, Cardoso F, Campa C, Diaz M, Roy R. Science. 2004;305:522–525. doi: 10.1126/science.1095209. [DOI] [PubMed] [Google Scholar]
- 19.Finn OJ. Nat Rev Immunol. 2003;3:630–641. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 20.Brooks S, Carter T, Royle L, Harvey D, Fry S, Kinch C, Dwek R, Rudd P. Anticancer Agents Med Chem. 2008;8:2–21. doi: 10.2174/187152008783330860. [DOI] [PubMed] [Google Scholar]
- 21.Cipolla L, Peri F, Airoldi C. Anticancer Agents Med Chem. 2008;8:92–121. doi: 10.2174/187152008783330815. [DOI] [PubMed] [Google Scholar]
- 22.Gilewski T, Ragupathi G, Bhuta S, Williams LJ, Musselli C, Zhang XF, Bornmann WG, Spassova M, Bencsath KP, Panageas KS, Chin J, Hudis CA, Norton L, Houghton AN, Livingston PO, Danishefsky SJ. Proc Natl Acad Sci U S A. 2001;98:3270–3275. doi: 10.1073/pnas.051626298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buskas T, Li Y, Boons GJ. Chemistry. 2004;10:3517–3524. doi: 10.1002/chem.200400074. [DOI] [PubMed] [Google Scholar]
- 24.Zhang S, Walberg LA, Ogata S, Itzkowitz SH, Koganty RR, Reddish M, Gandhi SS, Longenecker BM, Lloyd KO, Livingston PO. Cancer Res. 1995;55:3364–3368. [PubMed] [Google Scholar]
- 25.Dumy P, Eggleston IM, Esposito G, Nicula S, Mutter M. Biopolymers. 1996;39:297–308. doi: 10.1002/(sici)1097-0282(199609)39:3<297::aid-bip3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 26.Ragupathi G, Coltart DM, Williams LJ, Koide F, Kagan E, Allen J, Harris C, Glunz PW, Livingston PO, Danishefsky SJ. Proc Natl Acad Sci U S A. 2002;99:13699–13704. doi: 10.1073/pnas.202427599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herzenberg LA, Tokuhisa T. Nature. 1980;285:664–667. doi: 10.1038/285664a0. [DOI] [PubMed] [Google Scholar]
- 28.Tzianabos AO, Onderdonk AB, Rosner B, Cisneros RL, Kasper DL. Science. 1993;262:416–419. doi: 10.1126/science.8211161. [DOI] [PubMed] [Google Scholar]
- 29.De Silva RA, Wang Q, Chidley T, Appulage DK, Andreana PR. J Am Chem Soc. 2009;131:9622–9623. doi: 10.1021/ja902607a. [DOI] [PubMed] [Google Scholar]
- 30.De Silva RA, Appulage DK, Pietraszkiewicz H, Bobbitt KR, Media J, Shaw J, Valeriote FA, Andreana PR. Cancer Immunol Immunother. 2012;61:581–585. doi: 10.1007/s00262-012-1205-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peri F, Nicotra F. Chem Commun (Camb) 2004:623–627. doi: 10.1039/b308907j. [DOI] [PubMed] [Google Scholar]
- 32.Kolb HC, Finn MG, Sharpless KB. Angew Chem Int Ed Engl. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 33.Tam JP. Proc Natl Acad Sci U S A. 1988;85:5409–5413. doi: 10.1073/pnas.85.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bay S, Lo-Man R, Osinaga E, Nakada H, Leclerc C, Cantacuzène D. J Pept Res. 1997;49:620–625. doi: 10.1111/j.1399-3011.1997.tb01171.x. [DOI] [PubMed] [Google Scholar]
- 35.Lo-Man R, Bay S, Vichier-Guerre S, Dériaud E, Cantacuzène D, Leclerc C. Cancer Res. 1999;59:1520–1524. [PubMed] [Google Scholar]
- 36.Lo-Man R, Vichier-Guerre S, Perraut R, Dériaud E, Huteau V, BenMohamed L, Diop OM, Livingston PO, Bay S, Leclerc C. Cancer Res. 2004;64:4987–4994. doi: 10.1158/0008-5472.CAN-04-0252. [DOI] [PubMed] [Google Scholar]
- 37.Grigalevicius S, Chierici S, Renaudet O, Lo-Man R, Dériaud E, Leclerc C, Dumy P. Bioconjug Chem. 2005;16:1149–1159. doi: 10.1021/bc050010v. [DOI] [PubMed] [Google Scholar]
- 38.Bettahi I, Dasgupta G, Renaudet O, Chentoufi A, Zhang X, Carpenter D, Yoon S, Dumy P, BenMohamed L. Cancer Immunol Immunother. 2009;58:187–200. doi: 10.1007/s00262-008-0537-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Renaudet O, Dasgupta G, Bettahi I, Shi A, Nesburn AB, Dumy P, BenMohamed L. PLoS One. 2010;5:e11216. doi: 10.1371/journal.pone.0011216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buskas T, Ingale S, Boons GJ. Angew Chem Int Ed Engl. 2005;44:5985–5988. doi: 10.1002/anie.200501818. [DOI] [PubMed] [Google Scholar]
- 41.Ingale S, Wolfert MA, Gaekwad J, Buskas T, Boons GJ. Nat Chem Biol. 2007;3:663–667. doi: 10.1038/nchembio.2007.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarkar S, Lombardo SA, Herner DN, Talan RS, Wall KA, Sucheck SJ. J Am Chem Soc. 2010 doi: 10.1021/ja107029z. [DOI] [PubMed] [Google Scholar]
- 43.Toyokuni T, Hakomori S, Singhal AK. Bioorg Med Chem. 1994;2:1119–1132. doi: 10.1016/s0968-0896(00)82064-7. [DOI] [PubMed] [Google Scholar]
- 44.Kundi M. Expert Rev Vaccines. 2007;6:133–140. doi: 10.1586/14760584.6.2.133. [DOI] [PubMed] [Google Scholar]
- 45.Peri F, Piazza M. Biotechnol Adv. 2012;30:251–260. doi: 10.1016/j.biotechadv.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 46.Piazza M, Calabrese V, Damore G, Cighetti R, Gioannini T, Weiss J, Peri F. Chemmedchem. 2012;7:213–217. doi: 10.1002/cmdc.201100494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamdy S, Elamanchili P, Alshamsan A, Molavi O, Satou T, Samuel J. J Biomed Mater Res A. 2007;81:652–662. doi: 10.1002/jbm.a.31019. [DOI] [PubMed] [Google Scholar]
- 48.Engering AJ, Cella M, Fluitsma D, Brockhaus M, Hoefsmit EC, Lanzavecchia A, Pieters J. Eur J Immunol. 1997;27:2417–2425. doi: 10.1002/eji.1830270941. [DOI] [PubMed] [Google Scholar]
- 49.Grandjean C, Rommens C, Gras-Masse H, Melnyk O. Angew Chem Int Ed Engl. 2000;39:1068–1072. [PubMed] [Google Scholar]
- 50.Grandjean C, Angyalosi G, Loing E, Adriaenssens E, Melnyk O, Pancré V, Auriault C, Gras-Masse H. Chembiochem. 2001;2:747–757. doi: 10.1002/1439-7633(20011001)2:10<747::AID-CBIC747>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 51.Chenevier P, Grandjean C, Loing E, Malingue F, Angyalosi G, Gras-Masse H, Roux D, Melnyk O, Bourel-Bonnet L. Chem Commun (Camb) 2002:2446–2447. doi: 10.1039/b206980f. [DOI] [PubMed] [Google Scholar]
- 52.Sheng KC, Kalkanidis M, Pouniotis DS, Esparon S, Tang CK, Apostolopoulos V, Pietersz GA. Eur J Immunol. 2008;38:424–436. doi: 10.1002/eji.200737578. [DOI] [PubMed] [Google Scholar]
- 53.Xin H, Dziadek S, Bundle DR, Cutler JE. Proc Natl Acad Sci U S A. 2008;105:13526–13531. doi: 10.1073/pnas.0803195105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xin H, Cartmell J, Bailey JJ, Dziadek S, Bundle DR, Cutler JE. PLoS One. 2012;7:e35106. doi: 10.1371/journal.pone.0035106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Chem Soc Rev. 2012;41:2971–3010. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Said Hassane F, Phalipon A, Tanguy M, Guerreiro C, Bélot F, Frisch B, Mulard LA, Schuber F. Vaccine. 2009;27:5419–5426. doi: 10.1016/j.vaccine.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 57.Safari D, Marradi M, Chiodo F, Dekker HATh, Shan Y, Adamo R, Oscarson S, Rijkers GT, Lahmann M, Kamerling JP, Penadés S, Snippe H. Nanomedicine (Lond) 2012;7:651–662. doi: 10.2217/nnm.11.151. [DOI] [PubMed] [Google Scholar]
- 58.Piazza M, Colombo M, Zanoni I, Granucci F, Tortora P, Weiss J, Gioannini T, Prosperi D, Peri F. Angewandte Chemie-International Edition. 2011;50:622–626. doi: 10.1002/anie.201004655. [DOI] [PMC free article] [PubMed] [Google Scholar]