Abstract
Despite conflicting evidence for the efficacy of hormone replacement therapy in cardioprotection of postmenopausal women, numerous studies have demonstrated reductions in ischemia/reperfusion (I/R) injury following chronic or acute exogenous estradiol (E2) administration in adult male and female, gonad-intact and gonadectomized animals. It has become clear that ovariectomized adult animals may not accurately represent the combined effects of age and E2 deficiency on reductions in ischemic tolerance seen in the postmenopausal females. E2 is known to regulate the transcription of several cardioprotective genes. Acute, non-genomic E2 signaling can also activate many of the same signaling pathways recruited in cardioprotection. Alterations in cardioprotective gene expression or cardioprotective signal transduction are therefore likely to result within the context of aging and E2 deficiency, and may help explain the reduced ischemic tolerance and loss of cardioprotection in the senescent female heart. Quantification of the mitochondrial proteome as it adapts to advancing age and E2 deficiency may also represent a key experimental approach to uncover proteins associated with disruptions in cardiac signaling contributing to age-associated declines in ischemic tolerance. These alterations have important ramifications for understanding the increased morbidity and mortality due to ischemic cardiovascular disease seen in postmenopausal females. Functional perturbations that occur in mitochondrial respiration and Ca2+ sensitivity with age-associated E2 deficiency may also allow for the identification of alternative therapeutic targets for reducing I/R injury and treatment of the leading cause of death in postmenopausal women.
Keywords: senescence, estrogen receptors, proteomics, cardioprotection
Introduction
Coronary heart disease (CHD) most commonly presents as an ischemic coronary event such as acute myocardial infarction (MI) or unstable angina (collectively termed acute coronary syndrome), and is the single largest killer of American men and women, accounting for one in every five U.S. deaths in 2004 [170]. The estimated annual incidence of myocardial infarction (heart attack) is 865,000 new and recurrent attacks, and the prevalence and mortality due to MI increases with age [170]. In women, longitudinal studies and clinical statistical reports indicate an important influence of the menopausal transition on the determination of cardiovascular risk with advancing age. The incidence of coronary heart disease (CHD) in postmenopausal women is 2–3 folds higher than in premenopausal women of the same age [105, 170]. Further, 23% of women age 40 and older who experience a first MI will die within one year, compared with 18% of men [170]. These reports implicate the loss of endogenous estradiol (E2) as an explanation, in part, for the reduced ischemic tolerance (IT) in postmenopausal women.
Despite statistical data suggesting a causative role for E2 deficiency in the age-associated increase in female cardiovascular risk, studies investigating the efficacy of exogenous hormone replacement therapy (HRT) on cardiovascular risk reduction have produced conflicting results. Observational and epidemiological reports, including the Nurses’ Health Study, demonstrated reduced risk for CVD and acute MI in women taking HRT for the management of menopausal symptoms [17, 59, 73, 189]. In contrast, mid-stage analysis of two randomized clinical trials, the Women’s Health Initiative (WHI) [131] and the Heart and Estrogen/Progestin Replacement Study (HERS) [87], showed evidence for increased MI and stroke risk in postmenopausal women treated with conjugated equine estrogens (CEE) alone or CEE plus medroxyprogesterone acetate. The WHI and HERS trials were terminated early as a result of the increased hazard to women receiving HRT.
One proposed explanation for the conflicting results among these studies is the age of the subjects and the timing of HRT administration in reference to the onset of menopause, in that HRT may be cardioprotective in younger women and those within the first several years of menopause, but ineffective or detrimental in older postmenopausal women [137]. A secondary analysis of the WHI data revealed a trend toward reduced CHD risk in women receiving HRT within 10 years of the onset of menopause and increased risk in women beyond 20 years of menopause, although statistical significance was not demonstrated [173]. The Kronos Early Estrogen Prevention Study (KEEPS), an ongoing clinical intervention trial, aims to identify the effects of early HRT administration in younger menopausal women [80]. Although the timing hypothesis may indeed prove to support short-term cardioprotection with HRT in younger postmenopausal women, the evidence for diminished efficacy and possible detrimental effects of HRT on CHD risk in older women, as well as concerns regarding increased breast and ovarian cancer risk with long-term HRT use [147, 172], demonstrate the need for alternative strategies in the treatment of ischemic heart disease in aging, postmenopausal women. A necessary first step in forging new therapeutic strategies to treat ischemic heart disease in aging women includes identification of the mechanisms which render the aged female heart vulnerable to ischemic insult. A major focus of the current review is to summarize what is known with regard to cardioprotective signaling in the aged, E2-deficient female heart, with particular focus on salient research challenges associated experimental models to recapitulate human menopause. Notably, the reader is referred to several recent complimentary reviews on mitochondrial aging and mechanisms of cell death [46, 57, 141].
Age, Estrogen Deficiency, and Ischemic Tolerance
Acute MI is caused by coronary occlusion, and current treatment options are focused on reducing the duration of ischemia by initiating reperfusion as quickly as possible. Mechanical (coronary angioplasty) or enzymatic (thrombolytic) interventions, however, are rarely performed soon enough to avert cell death during ischemia, and further are ineffective in preventing the extension of infarction at reperfusion [29, 52]. Extensive and ongoing research has thus focused on the identification of effective treatments for the reduction of ischemia/reperfusion (I/R) injury (termed cardioprotection), which may be implemented in a clinical setting of acute MI to limit infarct size and minimize loss of cardiac function. Premenopausal women have reduced risk for CHD relative to age-matched men [16], as well as a lower incidence of LV hypertrophy, coronary artery disease, and cardiac remodeling following MI [83]. The incidence of CHD increases in postmenopausal women, however, such that aged women have both reduced ischemic tolerance [10, 168] and increased mortality following MI [214] relative to age-matched men. In the paragraphs that follow, information regarding the influence of aging on cardioprotective signals is presented within the context of information gleaned from studies associated with the phenomenon of ischemic preconditioning (IPC). Particular emphasis is also placed on available experimental models of E2 deficiency.
Ischemia/Reperfusion Injury in Aging
Reduced IT and increased susceptibility of the heart to I/R injury is a hallmark adaptation of both aged human and animal hearts [2, 30, 93, 120, 128, 132, 158, 180, 192, 215]. The aged heart is also refractory to endogenous protection from interventions like IPC (described below), verifying inadequate protective cellular reserves [63, 64, 99]. The precise cellular mechanisms underlying this dysfunction, however, are incompletely understood. The problem is further exacerbated by the paucity of studies using females, limiting extrapolation of results. Reversal of cardioprotection with senescence is likely to involve aberrations in both intrinsic (i.e. excitation-contraction coupling) and extrinsic (adrenergic) inotropic regulatory mechanisms (for review see [99, 120]). However, alterations in cell signaling pathways related to metabolic and oxidative stress may also shift the balance from cell survival to cell death regulating pathways [39, 79, 102, 159, 174].
In distinction from aging, independent effects of E2 deficiency on cardiovascular risk have also been observed. As early as 1953, Wuest et al. noted the increased prevalence of coronary artery disease in autopsy studies of premenopausal women who had undergone oophorectomy [219], and numerous studies conducted throughout the ensuing five decades have demonstrated increased risk for CHD and myocardial infarction in both postmenopausal and oophorectomized premenopausal women [16, 42, 44, 60, 171]. Epidemiological data indicate the interaction of gender and aging, and the influence of menopause on the determination of cardiovascular risk in aging women. Animal and human studies have identified both functional and cellular alterations in ischemic tolerance and cardioprotection due to the independent and combined effects of aging and E2 deficiency. A notable limitation in identifying specific mechanistic underpinnings in the adult and aged female heart has been differences in experimental models used to recapitulate postmenopausal E2 deficiency.
Ovariectomy as a Model of E2 Deficiency in the Aged Rat
Given the discrepancies in observational and epidemiological data indicating the effects of menopause and HRT on cardiovascular risk in aging women, an animal model suitable for the experimental study of age- and E2-related cellular changes has presented significant challenges. Although the study of nonhuman primates has been purported as the model perhaps most applicable to the menopausal transition in humans [107, 217, 218], the feasibility of this approach is extremely limited, especially in the context of aging and in the physiological study of myocardial infarction. The feasibility of aging studies in other animal models commonly used to study I/R injury, such as the canine and porcine models, is also reduced by the relatively long lifespan of these animals and the limited availability of aged supply colonies. The relevance to the human heart of I/R studies performed in the rabbit, especially in aging, has also been questioned [4].
The clinical definition of menopause is the cessation of spontaneous menstrual cycling for at least one year, and occurs in women at an average age of 51 years [202]. In human and nonhuman primates, the cessation of menstruation is preceded by a gradual decline in the function of the hypothalamic-pituitary-gonadal (HPG) axis [218]. Plasma E2 concentrations in postmenopausal women have been reported to average about 30 pg/ml [26], compared with a cyclic variation from ~80 to 800 pg/ml in healthy, premenopausal women [190]. The menopausal transition in the rat is incompletely understood, and exhibits important differences from menopause in humans. Notably, the onset of senile anestrous is variable in rats [1, 33, 140, 179, 187], resulting in a state of persistent estrous followed by persistent diestrous, whereby sustained E2 levels are similar in magnitude to diestrous in adult animals. The age of ovarian decline and the timing of this progression may also vary between mice and rats, and also between different strains of the same species. Nevertheless, important similarities between menopause and ‘estropause’ (for recent review see [33, 140, 227]) include cessation of estrous cyclicity (~16 mo in F344 rats) and a progressive deterioration in HPG axis function thereafter [187] until senile anestrous. Interestingly, the menopausal transition in humans is also characterized by elevated E2 levels [74, 76, 213].
Given the complicated nature of the menopausal transition in rodents, surgical ovariectomy (OVX) has been used to create a model of menopause to more closely approximate the dramatic E2 deficiency observed in menopausal women. Ovariectomized adult rats represent, in fact, the most commonly used animal model of postmenopausal changes. However this model does not reflect the possible interactions between aging and E2 deficiency occurring in natural menopause [179]. Indeed, studies from our laboratory suggest a highly selective myocardial response to E2 deficiency in adult vs aged female rats with regard to alterations in mitochondrial protein targets [121]. Additional considerations when using the adult OVX model include the time course of changes in plasma E2 following OVX. Adult OVX animals reveal dramatic reductions in plasma E2 initially, which is followed by significant increases at 4, 5, and 6 months post-surgery (~30 pg/ml) [234]. The increase in plasma E2 post-OVX has been attributed to increased extragonadal aromatization of testosterone to E2. Other studies have demonstrated increased adiposity following OVX in rats [108]. The increases in extragonadal aromatization with time after OVX, as well as increased adiposity and potential metabolic alterations in the OVX rat, have important implications for the validity and applicability of the adult OVX model to age-associated E2 deficiency. An alternative approach for the study of menopause and cardioprotection includes use of age-appropriate rats in conjunction with OVX [90, 121, 122, 151, 198], which represents an often overlooked but critical design consideration of rodent studies to recapitulate postmenopausal E2 deficiency and reproductive senescence. At the very least, experimental design limitations should be acknowledged with regard to the interpretation of research findings and extent of the conclusions drawn. Given the cyclic nature of protein turnover and potential influence of circulating E2, some standardization of estrous cycle activity in rodents should also be considered. With regard to studies employing E2 replacement, assessment of circulating E2 levels should also be performed at routine intervals throughout the entire duration of replacement, to determine the physiological relevance and potential impact of dosages employed on observed responses.
Ischemic Preconditioning as a Model of Cardioprotection
IPC, in which brief intermittent periods of ischemia (I) and reperfusion (R) reduce myocardial damage during subsequent prolonged I/R injury [145], represents the most powerful and reproducible form of cardioprotection identified to date [144]. Two phases of cardioprotection have been characterized: an early or acute phase that lasts for two to three hours following the preconditioning stimulus, and a late phase that is effective beginning 24 hours following the stimulus and can last for three to four days [223]. Although still incompletely understood, much has been learned about the mechanisms by which acute IPC renders the heart resistant to I/R injury (for review, see [53, 144]), and by default, glean potential insight into aged-associated mechanisms of reductions in ischemic tolerance. While the direct therapeutic relevance of IPC is limited simply by the requirement that it must be invoked prior to the onset of an ischemic event, which is rarely foreseeable, the endogenous cellular pathways of IPC have come to be used as a model by which to study cardioprotective signaling and to identify targets for clinically-applicable interventions. These efforts have been encouraged by recent findings that I/R injury can be reduced by the activation of signaling pathways immediately prior to ischemia or at the beginning of reperfusion [236]. While initially thought to minimize infarction during ischemia, current thinking states that the protection afforded by IPC is realized primarily at reperfusion through a reduction in necrotic, apoptotic and potentially autophagic cell death, which are normally responsible for the extension of infarct size and are critically regulated by the mitochondria [58, 81, 237]. Substantial experimental evidence has promoted the mitochondria as the convergence point for the protective cellular signaling pathways of IPC, and has established the role of protein kinase C (PKC; and the PKCε isozyme in particular) as a critical mediator of this convergence [91]. In the paragraphs that follow, particular emphasis is placed on the potential role of PKCε modulation as a potential therapeutic strategy to improve ischemic tolerance with age-associated E2 deficiency.
Working Model of Cardioprotective Signaling
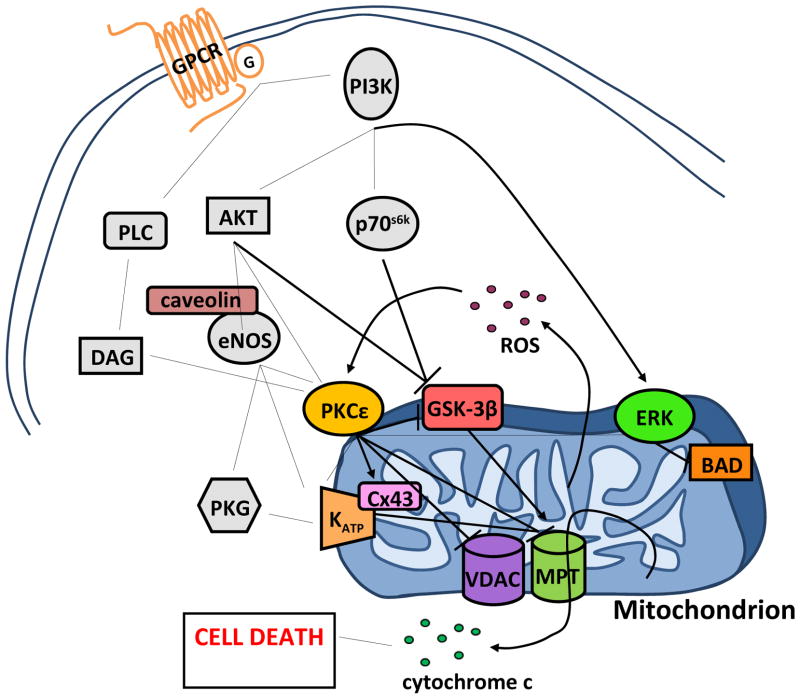
Although the molecular mechanisms of cardioprotection have yet to be fully elucidated, years of extensive study into IPC have characterized many cell signals associated with reductions in I/R injury in adult animals (Figure 1; adapted from [144]). Brief preconditioning cycles of I/R cause the release of agonists including adenosine [126], bradykinin [207], and opioids [182] from the ischemic myocardium, which act through G-protein coupled receptors to trigger multiple signaling cascades. The protection provided by each of these agonists can be blocked by inhibition of PKC [13, 71, 136, 175], illustrating the central importance of PKC as a common target in this signal transduction. Moreover, PKCε has been directly implicated in infarct sparing following global ischemia [92]. Low-level activation of PKCε has consistently been found to reduce hypoxic injury [167], and Mochly-Rosen and colleagues [37, 92] have provided direct evidence that isoform-specific activation of PKCε utilizing cell-permeating peptides prior to global ischemia is sufficient to reduce infarct size in adult male rats. Bradykinin and opioids stimulate PKCε by way of a complex phosphatidylinositol 3-kinase (PI3-K) pathway that involves activation of Akt, endothelial nitric oxide synthase (eNOS), guanylyl cyclase (GC), protein kinase G, and the opening of mitochondrial ATP-sensitive K+ channels (mitoKATP) [43, 153]. Subsequent K+ influx to the mitochondria leads to the generation of ROS, which act as a second messenger to activate PKCε [110]. Adenosine, in contrast to bradykinin and opioids, activates PKCε during IPC by a distinct pathway, since PI3-K inhibition does not block adenosine-stimulated cardioprotection. Although critically important to IPC, PKCε acts not as an effector of cardioprotection, but rather as a crucial intermediate in linking protective signaling to the mitochondria and initiating cellular protection at reperfusion. PKCε activates both the PI3-K/Akt and MEK1/2-ERK1/2 survival kinase cascades at reperfusion. Akt and ERK1/2 both phosphorylate and inactivate mitochondrial glycogen synthase kinase-3β (GSK-3β) [15, 199], which has been shown to result in strong inhibition of the mitochondrial permeability transition pore (MPTP) [95, 100], the hypothesized end-effector of IPC [82, 95].
Figure 1.
Simplified schematic of cardioprotective cellular signaling. Abbreviations: BAD, Bcl-2-associated death promoter; Cx43, connexin 43; diacylglycerol, DAG; eNOS, endothelial nitric oxide synthase; ERK, extracellular signal-regulated kinases; GSK-3β, glycogen synthase kinase-3β; MPTP, mitochondrial transition pore; PI3K, phosphoinositide 3-kinase; PLC, phospholipase C; PKCε, protein kinase Cε; PKG, protein kinase G; VDAC, voltage-dependent anion channel. Adapted from [144]).
The MPTP is a large conductance pore directly connecting the mitochondrial matrix to the cytosol. While its identity has not been firmly established [53], induction of the MPTP results in dissipation of the mitochondrial membrane potential which compromises the cell’s capacity for ATP production and hence volume regulation by Na+/K+ ATPase pumps, which leads to cellular swelling, lysis, and necrosis [75]. Mitochondrial swelling is also encouraged by MPTP formation, and lysis of the outer mitochondrial membrane results in cytochrome c release and the initiation of apoptosis [125].
PKCε prevents MPTP formation at reperfusion not only by activation of the survival kinases Akt and ERK1/2, but also by direct phosphorylation and inhibition of GSK-3β [100], activation of mitoKATP channels [70], and phosphorylation of possible MPTP regulatory proteins such as the voltage-dependent ion channel (VDAC) and the adenine nuclear transporter (ANT) [14]. Additionally, PKCε phosphorylates mitochondrial connexin43 (Cx43), which may cooperate with the mitoKATP channel in mitochondrial volume regulation and ROS production [183]. Further roles for PKCε in the regulation of cellular redox status through association with eNOS [164], and in the regulation of myocardial ATP synthesis by targeting of the mitochondrial F1ATPase [112] and cytochrome c oxidase subunit IV [154], have been demonstrated. The central role of PKCε in IPC suggests that cardioprotection is likely mediated by additional mitochondrial PKCε binding partners that have yet to be identified. How these signals may be influenced by age-associated E2 deficiency and reductions in ischemic tolerance is discussed in the next sections.
Aging and Protective Signaling
Animal models of I/R injury demonstrate impaired functional recovery and larger infarct size following I/R in the aged heart [3, 11, 193]. In addition, many studies demonstrate the reduced or abolished efficacy of IPC to reduce infarct size in the aged heart [6, 64, 130, 181, 186], although the majority of studies have been conducted in males. Nevertheless, clinical studies have also suggested a diminished capacity for cardioprotection following IPC or persistent angina in the aged human heart [5, 18, 123, 129]. At least in male animals and men, age-related declines in ischemic tolerance appear to correlate with alterations in cellular protein expression related to cardioprotective signal transduction. For instance, increased evidence of apoptosis and reduced induction of HSP70 was reported following ischemia in the aged rat heart [128, 150], and the loss of IPC-induced cardioprotection in the aged mouse heart was associated with reductions in gap junctional and mitochondrial Cx43 [21]. Tani et al. found in the middle-aged rat heart that the loss of IPC-induced cardioprotection was associated with altered PKC translocation, and that cardioprotection was achieved less effectively by PKC activation than by mitoKATP activation, suggesting that disruptions downstream of PKC signaling contributed to the loss of IPC with age [192]. Our laboratory previously demonstrated that impaired ischemic tolerance in aged male rats was associated with 1) increased basal PKCδ expression and could be improved by acute PKCδ inhibition (by Tat-δV1-1 administration) [114], and 2) reduced PKCε and increased GSK-3β at the mitochondria during ischemia and could be improved by acute PKCε activation (by Tat-ψεRACK administration) [112]. Impaired responsiveness to IPC in elderly patients undergoing coronary angioplasty has also been attributed to attenuated activation of KATP channels, since the KATP channel agonist nicorandil restored IPC-induced cardioprotection [123]. Chakravarti et al. described the altered expression of numerous proteins, primarily relating to cellular energetics at the mitochondria, in the aged male mouse heart through proteomic profiling experiments [34]. Examples of these include age-related reductions in mitochondrial aconitase 2, mitochondrial F1ATP synthase β, and NADH dehydrogenase subunit expression. Evidence also support a role for post-translational modifications or proteolysis, however the exact nature and quantity of these modifications was not further examined [35].
Senescence is also associated with enhanced cytochrome c release in male rats [113, 162] and expression of the proapoptotic proteins Bad, Bax and caspases [12, 32, 127] [233]. Age-related increases in reactive oxygen species (ROS) [72, 120, 146, 177] are also likely to contribute to increased apoptotic signaling. While ROS can be cytoprotective through activation of known survival signals such as PKCε [101] [138], increased ROS production in the face of declines in antioxidant defenses [222] is likely to contribute to increased I/R injury in the aged heart. Elevated nitric oxide (NO) production during I/R through NOS-dependent processes can also result in formation of more reactive oxidant species like superoxide (O−2) and peroxynitrite (ONOO−) [197, 212, 226] (i.e. NOS uncoupling) [239]. In females, links between E2 deficiency, aging, ROS and I/R injury are unclear, as studied in OVX younger animals may not accurately mimic the aged state [222]. In aged male rats, elevated O−2 production does occur during early reperfusion [155], however effects in aged females are unknown and are currently under investigation in a number of laboratories, including our own.
In female animals, reduced ischemic tolerance has been observed and is attributed to both the independent and combined effects of aging and E2 deficiency. Willems et al. demonstrated increased infarct size and impaired functional recovery following I/R in aged relative to adult female mice[216], and Hunter et al. showed that the increase in infarct size following I/R in aged relative to adult female rats was associated with decreased Akt and mitochondrial PKCε levels, as well as increased mitochondrial GSK-3β [89]. E2 deficiency alone reduces ischemic tolerance in the female heart, as Song et al. demonstrated increased infarct size following I/R and the loss of IPC-induced cardioprotection in adult OVX relative to adult ovary-intact female mice [188]. Kam et al. also showed, under hypercontractile conditions of elevated Ca2+, similar results using adult OVX rats[104]. Hunter et al. further found that aged OVX rats exhibited more severely impaired functional recovery and greater infarct size following I/R than was seen with aging or OVX alone, suggesting an additive detriment of aging and OVX in the female rat heart [89]. In support of a protective role for PKCε targeting in the aged, E2-deficient female rat heart, acute activation of PKCε prior to ischemia by local delivery of ψεRACK peptide has been associated with 1) improved functional recovery and reduced infarct size, 2) increased mitochondrial targeting of PKCε, and 3) candidate downstream signaling targets suggesting a role for activation of antioxidant enzymes as a mechanism of PKCε-mediated protection [122]. Specifically, mitochondrial Hsp10, GPX, and SOD2 (MnSOD) abundance are significantly increased with ψεRACK administration in aged OVX hearts (by ~10, 20, and 30%, respectively). Due to the brief time period of PKCε activation in these hearts (10 min), changes observed in this analysis are likely attributable to PKCε-stimulated mitochondrial translocation or import of identified proteins. Following ischemia, it is likely that improved levels of mitochondrial Hsp10, GPX and MnSOD2 observed in PKCε-treated aged OVX hearts are further influenced by protective effects limiting protein degradation. It is clear from the work of Zhang and colleagues [232] that I/R-induced alterations to the mitochondrial proteome of adult mice occur and are dependent upon severity of ischemia and specific protein abundance. How specific mitochondrial proteins are targeted for lysosomal and/or proteosomal degradation in the aged heart, and the dynamic regulation of these processes, is poorly understood and a necessary focus of future studies. Moreover, results are confounded by differing models of I/R injury including varying amounts of ischemic insult (i.e. duration of ischemia).
Nevertheless, it is likely that increases in GPX and SOD2 immediately following PKCε activation may serve to combat increased ROS production in the aged female heart [124]. In contrast, Hsp10 is a stress-response and chaperone protein shown to regulate mitochondrial pro-caspase-3 activation, and thus the initiation of apoptosis, through the formation of a complex with Hsp60 in the intermembrane space [176]. HSPs have recently been implicated in mitochondrial import of PKCε during I/R [28], and thus may contribute to observed increases in mitochondrial PKCε localization following acute PKCε activation in the aged female heart. Identification of candidate downstream PKCε signaling targets in mitochondria suggests a role for the regulation of oxidative stress as a mechanism of PKCε-mediated cardioprotection in the aged female heart. Studies are clearly needed to quantify the extent of interplay between ROS production and cell death in the aged female myocardium.
Estrogen Receptors (ER) and Cardioprotection
Effects of E2 in the heart are primarily mediated by two ER subtypes, ERα and ERβ, although the precise subcellular distribution of cardiac ER receptors remains to be elucidated. Recent evidence linking ERα and ERβ polymorphisms to adverse cardiac outcomes in women [160, 163, 206] suggest that ERα and ERβ may each play distinct roles in cardioprotection. Genomic actions mediated by nuclear ERα are well-described [50, 54] and involves ligand binding at E2 response elements. Non-genomic (rapid) effects of E2 are thought to be mediated by ERα and/or ERβ localized to the plasma membrane [8, 224, 230], and associated functions include Ca2+ homeostasis, anti-apoptosis and mitochondrial metabolism [224]. With regard to the latter, the recent demonstration that mitochondrial ERβs are present in human myocardium [224] have positioned ERβ as a potential regulator (or regulated target) of mitochondrial function and cell survival, perhaps through mitochondrial gene regulation [86]. Rapid ER signals are also known to regulate ER gene transcription in the myocardium [134]. In this regard, ERs are subject to post-translational modification (PTM) through phosphorylation, acetylation and sumoylation, which not only has the potential to influence ER activity, but may also influence ER stability and localization, particularly with aging (for review see [36, 62, 67, 231]).
While the importance of cardiac ER subtypes in I/R injury remains controversial, studies employing ERα and ERβ deficient mice have each demonstrated reductions in ischemic tolerance [69, 208]. However, it is important to note that ER deficiency in these models is not cardiac-specific, and some results are confounded by use of mice which encode a truncated ERα, as well as a metabolic phenotype which develops with age [27, 84]. Nevertheless, in mice completely null for ERα, greater I/R injury and impaired mitochondrial function [210, 229] is observed vs non-transgenics. Further, activation of ERα with the specific agonist, propyl pyrazole triol (PPT), protects the in vivo rabbit heart from I/R injury, while the specific ERβ activator, diarylpropiolnitrile (DPN) was without effect [24]. Recent studies also suggest a greater role for ERα vs ERβ in the modulation of endothelial progenitor cells and cardiac repair [48, 77]. Taken together, these data support a dominant role for ERα as the cardioprotective ER involved in I/R injury [24]. In contrast, Murphy and colleagues [69, 149] have provided equally compelling evidence that ERβ mediates gender differences in I/R injury using ERβ deficient mice under hypercontractile conditions or with DPN.
With regard to the potential cardioprotective role of non-genomic ER activation in reducing I/R injury in aged hearts, several recent findings implicate a possible role for selective ERα activation as follows [151]: 1) effectively reduced infarct size, 2) resulted in greater mitochondrial and particulate ERα localization coordinate with a protective pattern of PKCε activation, and 3) enhanced gene expression of the PKCε anchoring protein RACK2. Collectively, these results demonstrate a protective role for non-genomic ERα signaling in the aged female rat heart, the cellular basis of which may involve two distinct PKCε-dependent mechanisms. What is less clear are the mechanisms which underlie altered cardiac ER translocation. As noted above, post-translational modifications such as phosphorylation, acetylation and sumoylation (for review see [36, 38, 62, 67, 231]) are known to effect ER targeting, the effects of which are unstudied in aging. Since some non-genomic ER effects are specific to aged animals [151], it will be important that future studies incorporate true models of aging in conjunction with E2 deficiency to fully characterize the non-genomic ER response.
In contrast, acute ERβ activation does not appear to impact functional recovery following I/R injury in either adult or aged rats with varying degrees of E2-deficiency [198]. A logical interpretation of these results is that while classical genomic ERβ activation via chronic stimulation is possible, rapid, non-genomic signaling mechanisms downstream of ERβ may not be operative in the female rodent myocardium. However in this study, ERβ mRNA was not detected in either the adult or aged rat myocardium [198]. The lack of measureable ERβ in the F344 rat myocardium was surprising given results gleaned from past studies utilizing the ERβ knockout mouse model [68, 209, 211] mentioned heretofore. In this regard, ERβ expression in the rodent myocardium remains controversial [66, 94, 115, 178, 184, 221, 225], and the protein signal produced by ERβ antibodies in cardiac homogenates may be the result of cross-reactivity with ERα [198]. Combined with these previous findings, either ERβ signaling varies substantially between rat, rabbit and murine models or, cardioprotection observed in mouse models may be mediated indirectly through extra-cardiac ERβ signaling. For instance, DPN injection at the rostral ventrolateral medulla, an area associated with autonomic cardiovascular control, has been shown to reduce systemic arterial pressure in rats [185]. That ERβ activation can reduce systemic arterial pressure via autonomic influence indicates that additional autonomic cardioprotective mechanisms attributed to E2 may be mediated through ERβ. Indeed, E2-linked cardioprotection has been associated with reduced sympathetic input to the heart and vasculature during ischemia in female rats, resulting in reduced heart rate, mean arterial pressure, arrhythmia frequency, and overall improved ischemic tolerance vs males [55, 56]. Therefore it is plausible that hypertension and vascular dysfunction observed in whole body ERβ knockout mice as well as cardioprotection observed in chronic DPN treated mice may be explained by indirect ERβ effects on autonomic cardiac control and not direct effects on the myocardium [148, 157]. Future studies examining extra-cardiac effects of chronic ERβ stimulation, including vascular and neural mechanisms, may prove useful in elucidating possible therapeutic interventions with aging. Definitive studies on ER subtype distribution in adult and aged human myocardium are needed.
The demonstration that rapid ERα activation reduces I/R injury in the aged female heart supports a key role for non-genomic ER signaling in the maintenance of cardioprotection. A better understanding of the non-genomic actions of E2 may lead to improved clinical therapeutic interventions for treating acute coronary syndrome in aged women, specifically selective modulation of cardiac ERs and non-genomic ER signaling in an attempt to harness the protection associated with E2 observed in adult women without increased cardiovascular risk observed from chronic HRT. In this regard, 17-β estradiol is the major physiological E2, but it has a similar affinity for both ERs. As noted, a number of selective ERα and ERβ agonists have been created and described; however, only a minority of these compounds has been evaluated extensively in vivo. The discovery of the GPCR30 has also reinforced the need for additional ER specific modulators. Selective estrogen receptor modulators (SERMs) may be of great utility and in understanding the role of ERs in ischemic tolerance with aging.
PI3K-Akt-GSK-3β Signaling and Estrogen
Interestingly, many of the protective actions mediated by rapid ER signaling involve downstream effectors known to be associated with IPC, such as PI3K-Akt, eNOS and PKCε (for review see [143]). Increased levels and/or activity of Akt have also been observed in female (vs male) animal and human myocardium [31, 235]. ERα-mediated nuclear transcription is also affected by Akt, and nuclear accumulation of Akt in human cardiocytes is increased 5.8-fold in adult women over men and reduced in postmenopausal women [31]. Collectively, these data suggest that the PI3K-Akt pathway is acutely activated by E2 and could be subject to modulation by aging. Urata and colleagues [204] recently demonstrated that E2 administration (18 hrs) in myocardiac H9c2 cells leads to a reduction in hydrogen peroxide (H2O2)-induced apoptosis through upregulation of glutaredoxin (GRX), which was abolished by the ER inhibitor ICI-182,780. Effects were presumed ERβ-mediated since these cells do not express ERα.
A target of Akt which has been proposed as a convergence point for many cardioprotective signals is inactivation of mitochondrial GSK-3β and associated apoptotic signaling. This model is supported by I/R- and OVX-dependent changes in mitochondrial pGSK-3β which mirror changes in pAkt in adult but interestingly not aged rats [88], suggesting dysregulated Akt-GSK-3β interactions in aged. Additional mechanisms by which rapid E2 signaling may influence GSK-3β and subsequent ischemic tolerance are worth noting. Recent studies suggest that GSK-3β can enhance ERα-mediated transcription [135], implicating the nuclear compartment as an potentially important site of regulation in the aged female heart. If this is so, several cardioprotective or apoptotic proteins that are modulated by E2 (such as heat shock proteins [78], ANT-1 [201], or Cx43 [228]) may show altered expression or activity, thus contributing to reduced ischemic tolerance in aged. Future studies are indicated to determine the role, if any, of altered gene expression in relation to cell survival with age-associated E2 deficiency.
Mitochondrial Mechanisms of Cell Death
Mitochondria are the main source of both ATP and reactive oxygen species (ROS) in the heart ideally positioning them as mediators of, and therapeutic targets for, ischemic CHD. Because of the pivotal role played by the mitochondria in the maintenance of cell survival and cardioprotection, it is logical that age-associated reductions in ischemic tolerance might arise from alterations in mitochondrial proteins. Given the estimate that 1000 to 2000 proteins are expressed in the mitochondria [133], it is likely that the adaptation of additional mitochondrial proteins in aging and/or E2 deficiency may contribute to the reductions in ischemic tolerance and increased I/R injury associated with advancing age and menopause. While correlational relationships between age-dependent declines in ischemic tolerance and altered expression and localization of cardioprotective signaling proteins have been noted in the female heart, the breadth and extent of protein changes have only recently been addressed.
Using a high throughput proteomics approach targeting the cardiac mitochondrial subproteome in adult and aged female rats, significant directional changes were observed in 67 proteins with aged and/or aged OVX, and 32 were unique to aged OVX [121]. Notably only 6 proteins were similarly altered in adult OVX, highlighting the specificity of the E2 deficiency response in adult vs aged female rats. Proteins affected by aging were primarily related to cellular metabolism, oxidative stress and cell death, with the largest change seen in monoamine oxidase-A (MAO-A), a potential source of oxidative stress. About 50% of the identified proteins altered in aged OVX were associated with mitochondrial ATP production [121]. Age-associated reductions in cardiac mitochondrial ATP production have been previously reported in male rodents, including declines in the rate of oxidative phosphorylation and the activity of electron transport chain (ETC) complexes III and IV [124]. A recent report on age-associated alterations in male rat cardiac mitochondrial gene transcripts also noted widespread downregulation of ETC complex RNA as well as decreased complex I and IV activity [165], while proteomic profiling in aged male mouse hearts demonstrated reduced expression of several mitochondrial ETC complex subunits [34]. In aged, E2-deficient female hearts, reduced quantity of protein subunits of ETC complex I (NADH dehydrogenase), II (succinate dehydrogenase), III (cytochrome bc1 complex), IV (cytochrome c oxidase), and V (F0F1 ATPase), and bidirectional changes in proteins involved in fatty acid substrate metabolism (acyl Co-A synthetase subunits) have been observed. In contrast, increases were primarily observed, in contrast, for proteins involved in carbohydrate and amino acid metabolism (pyruvate dehydrogenase subunits) and enzymes of the tricarboxylic acid cycle [121]. Increased levels of Hsp60 and mtHsp70 in aged OVX are consistent with previous studies in aged male hearts [45] and may be related to alterations in mitochondrial matrix protein import of nuclear-encoded enzymes, which may or may not be balanced by changes in proteolysis. Measurement of the activity and/or phosphorylation status [49] of these enzymes is indicated for a more comprehensive characterization of metabolic alterations and substrate utilization in the aged female heart.
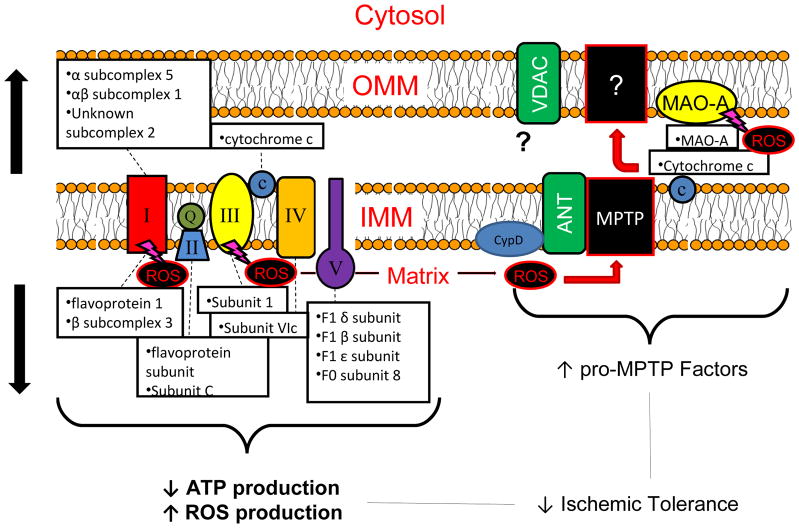
Nevertheless, dysregulated mitochondrial metabolism has been suggested as a contributory mechanism underlying impaired ischemic tolerance in the aged heart [99, 119, 165] and associated I/R injury (see Figure 2 for model summary). First, the reduced capacity for ATP production upon reperfusion leads to swelling, lysis, and initiation of necrotic and apoptotic cell death [53]. Observed reductions in the Na+/K+ ATPase and Ca2+ ATPase pumps in aged OVX hearts may further contribute to these detrimental events. Additionally, metabolic dysregulation is thought to contribute to cellular injury through increased mitochondrial ROS production in the aged heart [124]. Complex III, for example, has been identified as a major source of age-associated increases in mitochondrial superoxide radical (O2·) production both at baseline and in response to I/R [124]. High levels of ROS are generated during I/R from additional sources both within and outside the mitochondria, including ETC complex I [19, 203], the xanthine oxidase system [220], and vascular NADPH oxidase[7], and contribute to cellular injury through lipid peroxidation, protein oxidation, enzyme inactivation, and DNA damage [23]. Further, ROS can induce opening of the MPTP and therefore initiation of cell death by the facilitation of mitochondrial Ca2+ overload and/or the oxidation of thiol groups of ANT, a possible MPTP regulatory protein [106, 109, 238].
Figure 2.
Summary of proposed changes in mitochondrial electron transport complex and pro-apoptotic proteins which may contribute to reductions in ischemic tolerance with age-associated E2 deficiency. Abbreviations: ANT, adenine nucleotide translocator 1; ATP, adenosine triphosphate; CypD, cyclophilin D; IMM, inner mitochondrial membrane; MAO-A, monoamine oxidase-A; MPTP, mitochondrial permeability transition pore; OMM, outer mitochondrial membrane; ROS, reactive oxygen species; VDAC, voltage-dependent anion channel.
Indicative of possible increased ROS production in the aged, E2-deficient heart, altered expression of several mitochondrial proteins involved in the oxidative stress response have also been observed. A large increase (>90%) MAO-A, which is found in the outer mitochondrial membrane and represents a potent source of hydrogen peroxide (H2O2) during I/R [20, 200], has been noted in both aged and aged OVX hearts but not adult OVX [121]. Given recent evidence that MAO-A inhibition can reduce I/R injury in adult hearts (for review see [51, 103]), studies from our laboratory addressed the effects of acute MAO-A inhibition on mitochondrial respiration and subsequent I/R injury in the aged, E2-deficient rat heart [121]. While we observed a protective pattern of mitochondrial respiration in isolated mitochondria following MAO-A inhibition with clorgyline (predictive of mild mitochondrial uncoupling), acute MAO-A inhibition at varying doses and durations of exposure prior to I/R injury in vivo was unable to produce an infarct sparing effect in the aged female rat heart. We observed a similar lack of efficacy in isolated perfused hearts when clorgyline was delivered 15 min prior to I/R, suggesting that the aged female heart is refractory to protection by MAO-A inhibition. The mechanism of reduced cardioprotective efficacy of MAO-A inhibition in aged animals previously demonstrated in adult animals [20] is not immediately evident, but combined with the well-characterized refractoriness of the aged heart to ischemic intervention [99], likely includes an inability of age-associated changes in antioxidant machinery to combat overproduction of ROS associated with senescence.
In this regard, SOD2 (MnSOD), the mitochondrial SOD isoform that catalyzes the conversion of the strongly reactive O2· radical to less reactive H2O2 and molecular O2 [96], was increased by nearly 40% in aged OVX. It is likely that these increases represent compensatory adaptations to chronically increased ROS production in the aged female heart [124], and interestingly, our observation of increased SOD2 expression is in contrast to studies in male F344 rats demonstrating age-related increases in cardiac SOD2 activity [97, 162] but unaltered SOD2 expression [7, 205]. Increased levels in aged and aged OVX hearts of mitochondrial proteins involved in the initiation of cell death, including cytochrome c and possible MPTP regulatory proteins VDAC1 and ANT1 (Figure 2). The increased quantity of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a glycolytic enzyme that has been reported to play a pro-apoptotic role in the mitochondria through induction of the MPTP [195], was also noted. In this regard, direct measures of ROS are indicated in the aged female rat heart under conditions of I/R injury.
Although preserving ATP and limiting ROS production by inhibiting MPTP formation is a common strategy for cardioprotection, as noted above these strategies are often less effective in the aged [22, 64, 194]. While several groups have investigated changes in basal mitochondrial function with age, the results are variable, likely due to differences in isolation, mitochondrial subfractions, and measurement protocols. Respiration rates have been reported to increase [40, 47], decrease [41, 116, 161, 166, 196], or have variable effects depending on individual complexes or mitochondrial subpopulation [61, 85, 97, 117]. It is important to note that with the exception of Davies et al [47], all of these aging studies were performed on male heart mitochondria which may not necessarily extrapolate to aging in females. Given reported reductions of subunits in all five complexes of the ETC, subsequent studies [90] revealed that: 1) age significantly reduced the respiratory control index (RCI) at Complexes I and II, 2) Estrogen deficiency and age sensitized the mitochondria to Ca2+-overload, and 3) PPT increased mitochondrial RCI but did not improve Ca2+ sensitivity. No significant age-dependent changes in state 2 or state 3 respiration of Complexes I, II, IV (state 3 only) were observed, which are in agreement with oxygen consumption studies of similar mitochondrial populations in adult and 24 mo male rats [41, 61, 85]. However, in contrast to these studies, age-dependent decreases in the RCI’s for Complexes I and II were observed in females, which is consistent with increased mitochondrial uncoupling with aging. Although inhibition of ATP/ADP exchange and/or ATP synthase may also account for decreased RCI, ADP-induced respiration was not significantly decreased. Furthermore, it has been proposed that mitochondrial uncoupling may be a compensatory mechanism sacrificing ATP production efficiency to combat increased ROS production seen with aging in tightly-coupled mitochondria [25].
Cardiac calcium handling is perturbed with senescence and the aged myocardium is more sensitive to ROS- and Ca2+- induced MPTP opening [98, 118]. Similar to studies in male hearts, increases in Ca2+-sensitivity with aging occur in the female myocardium as evidenced by Ca2+-induced decreases in Complex I respiration and swelling [90]. Interestingly, the age-dependent reductions in Complex I respiration with Ca2+ are mirrored in adult mitochondria with OVX, suggesting that E2 may play a protective role with respect to Ca2+-sensitivity in adult animals. This hypothesis is supported by the observation that female mitochondria accumulate Ca2+ more slowly than do male [9], and that E2 supplementation reduces mitochondrial calcium accumulation [139]. That OVX does not worsen the age-dependent sensitization to Ca2+ suggests that age and OVX are sufficient to sensitize the mitochondria to Ca2+ to the same degree. Given discrepant results in respiration studies of mitochondria isolated from aged animals, more studies addressing measures in both subsarcolemmal and interfibrillar mitochondrial populations are indicated. Moreover, studies in true models of female aging are also sorely needed to reconcile the impact of associated changes in mitochondrial protein levels and functional outcomes.
Conclusion
The increased prevalence of cardiovascular diseases in women following menopause coupled with the failure of HRT to demonstrate cardioprotection, has led many researchers to reexamine mechanisms of cardioprotection and subsequent loss of this cardioprotection in advancing age with E2 deficiency. It has become clear that ovariectomizing adult animals may not accurately represent the combined effects of age and E2 deficiency seen in postmenopausal females. E2 is known to regulate the transcription of several cardioprotective genes by action through ERα and ERβ, including eNOS and Akt, and females exhibit increased association of eNOS with the myocardial-specific caveolin-3 [152, 156, 191]. Acute, non-genomic signaling downstream of ER activation or E2 action at GPCRs, in addition, can activate many of the same signaling pathways recruited in cardioprotection, including PI3-K, Akt, and eNOS pathways [65, 142]. Alterations in cardioprotective gene expression or acute cardioprotective signal transduction are therefore likely to result in the context of aging and E2 deficiency, and may help explain the reduced ischemic tolerance and loss of cardioprotection in the senescent female heart. The assessment of cardioprotective signal transduction downstream of PKCε activation in aging and E2 deficiency may, further, allow for the identification of alternative therapeutic targets for reducing I/R injury in postmenopausal women. In this regard, recent findings [111, 113] demonstrating improved ischemic tolerance in aged male and female rats following acute PKC modulation extends the protective reach of PKC therapeutics to a model of senescence.
It is also clear that mitochondria play a central role in cardioprotection and research elucidating the mechanisms of this protection in the aged female heart is ongoing. Importantly, the vast majority of this research is being performed in adult models, rather than the population at risk for a cardiovascular event, i.e. the aged. Indeed, recent proteomic screens of mitochondria isolated from aged and E2-deficient rat hearts have revealed a highly selective response to E2 deficiency in aged vs adult, and perturbations of several ETC proteins may upset the stoichiometry of the ETC and contribute to increased ROS production. Importantly, quantification of ROS and characterization of the mitochondrial subproteome as it adapts to advancing age and E2 deficiency is indicated, which will allow for the identification of proteins and possible post-translational modifications associated with cardiac signaling disturbances contributing to age-associated declines in ischemic tolerance. Evidence-based medical treatments and therapies have helped to drastically reduce deaths due to CHD with ≈47% of the reduction in deaths in the US from 1980 to 2000 being attributed to their increased use [169]. This finding emphasizes the necessity of further research into the field of I/R injury to enable the continued development of these treatments and therapies particularly for aged, postmenopausal women.
Acknowledgments
This work was supported by the National Institutes of Health (NIH; HL091097, HL091097-01A2S1 AA019403 to DHK)
References
- 1.Aasum E, Cooper M, Severson DL, Larsen TS. Effect of BM 17.0744, a PPARa ligand, on the metabolism of perfused hearts from control and diabetic mice. Can J Physiol Pharmacol. 2005;83:183–190. doi: 10.1139/y04-139. [DOI] [PubMed] [Google Scholar]
- 2.Abete P, Calabrese C, Ferrara N, Cioppa A, Pisanelli P, Cacciatore F, Longobardi G, Napoli C, Rengo F. Exercise training restores ischemic preconditioning in the aging heart. J Am Coll Cardiol. 2000;36 (2):643–50. doi: 10.1016/s0735-1097(00)00722-1. [DOI] [PubMed] [Google Scholar]
- 3.Abete P, Cioppa A, Calabrese C, Pascucci I, Cacciatore F, Napoli C, Carnovale V, Ferrara N, Rengo F. Ischemic threshold and myocardial stunning in the aging heart. Exp Gerontol. 1999;34 (7):875–84. doi: 10.1016/s0531-5565(99)00060-1. [DOI] [PubMed] [Google Scholar]
- 4.Abete P, de Santis D, Condorelli M, Napoli C, Rengo F. A four-year-old rabbit cannot be considered the right model for investigating cardiac senescence. J Am Coll Cardiol. 2002;39(10):1701. doi: 10.1016/s0735-1097(02)01818-1. author reply 1701–2. [DOI] [PubMed] [Google Scholar]
- 5.Abete P, Ferrara N, Cacciatore F, Madrid A, Bianco S, Calabrese C, Napoli C, Scognamiglio P, Bollella O, Cioppa A, Longobardi G, Rengo F. Angina-induced protection against myocardial infarction in adult and elderly patients: a loss of preconditioning mechanism in the aging heart? J Am Coll Cardiol. 1997;30 (4):947–54. doi: 10.1016/s0735-1097(97)00256-8. [DOI] [PubMed] [Google Scholar]
- 6.Abete P, Ferrara N, Cioppa A, Ferrara P, Bianco S, Calabrese C, Cacciatore F, Longobardi G, Rengo F. Preconditioning does not prevent postischemic dysfunction in aging heart. J Am Coll Cardiol. 1996;27 (7):1777–86. doi: 10.1016/0735-1097(96)00070-8. [DOI] [PubMed] [Google Scholar]
- 7.Adler A, Messina E, Sherman B, Wang Z, Huang H, Linke A, Hintze TH. NAD(P)H oxidase-generated superoxide anion accounts for reduced control of myocardial O2 consumption by NO in old Fischer 344 rats. Am J Physiol Heart Circ Physiol. 2003;285 (3):H1015–22. doi: 10.1152/ajpheart.01047.2002. [DOI] [PubMed] [Google Scholar]
- 8.Alexaki VI, Charalampopoulos I, Kampa M, Artemissia-Phoebe N, Hatzoglou A, Gravanis A, Elias C. Activation of membrane estrogen receptors induce pro-survival kinases. J Steriod Biochem Mol Biol. 2006;98:97–110. doi: 10.1016/j.jsbmb.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Arieli Y, Gursahani H, Eaton MM, Hernandez LA, Schaefer S. Gender modulation of Ca(2+) uptake in cardiac mitochondria. J Mol Cell Cardiol. 2004;37 (2):507–13. doi: 10.1016/j.yjmcc.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 10.Arnstein PM, Buselli EF, Rankin SH. Women and heart attacks: prevention, diagnosis, and care. Nurse Pract. 1996;21(5):57–8. 61–4, 67–9. doi: 10.1097/00006205-199605000-00005. quiz 70-1. [DOI] [PubMed] [Google Scholar]
- 11.Azhar G, Gao W, Liu L, Wei JY. Ischemia-reperfusion in the adult mouse heart influence of age. Exp Gerontol. 1999;34 (5):699–714. doi: 10.1016/s0531-5565(99)00031-5. [DOI] [PubMed] [Google Scholar]
- 12.Azhar G, Liu L, Zhang X, Wei JY. Influence of age on hypoxiz/reoxygenation-induced DNA fragmentation and bcl-2, bcl-xl, ba and fas in the rat heart and brain. Mech Ageing Dev. 1999;112:5–25. doi: 10.1016/s0047-6374(99)00048-2. [DOI] [PubMed] [Google Scholar]
- 13.Baines CP, Goto M, Downey JM. Oxygen radicals released during ischemic preconditioning contribute to cardioprotection in the rabbit myocardium. J Mol Cell Cardiol. 1997;29 (1):207–16. doi: 10.1006/jmcc.1996.0265. [DOI] [PubMed] [Google Scholar]
- 14.Baines CP, Song CX, Zheng YT, Wang GW, Zhang J, Wang OL, Guo Y, Bolli R, Cardwell EM, Ping P. Protein kinase Cepsilon interacts with and inhibits the permeability transition pore in cardiac mitochondria. Circ Res. 2003;92 (8):873–80. doi: 10.1161/01.RES.0000069215.36389.8D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baines CP, Zhang J, Wang GW, Zheng YT, Xiu JX, Cardwell EM, Bolli R, Ping P. Mitochondrial PKCepsilon and MAPK form signaling modules in the murine heart: enhanced mitochondrial PKCepsilon-MAPK interactions and differential MAPK activation in PKCepsilon-induced cardioprotection. Circ Res. 2002;90 (4):390–7. doi: 10.1161/01.res.0000012702.90501.8d. [DOI] [PubMed] [Google Scholar]
- 16.Barrett-Connor E. Sex differences in coronary heart disease. Why are women so superior? The 1995 Ancel Keys Lecture. Circulation. 1997;95 (1):252–64. doi: 10.1161/01.cir.95.1.252. [DOI] [PubMed] [Google Scholar]
- 17.Barrett-Connor E, Bush TL. Estrogen and coronary heart disease in women. Jama. 1991;265 (14):1861–7. [PubMed] [Google Scholar]
- 18.Bartling B, Hilgefort C, Friedrich I, Silber RE, Simm A. Cardio-protective determinants are conserved in aged human myocardium after ischemic preconditioning. FEBS Lett. 2003;555 (3):539–44. doi: 10.1016/s0014-5793(03)01342-5. [DOI] [PubMed] [Google Scholar]
- 19.Batandier C, Leverve X, Fontaine E. Opening of the mitochondrial permeability transition pore induces reactive oxygen species production at the level of the respiratory chain complex I. J Biol Chem. 2004;279 (17):17197–204. doi: 10.1074/jbc.M310329200. [DOI] [PubMed] [Google Scholar]
- 20.Bianchi P, Kunduzova O, Masini E, Cambon C, Bani D, Raimondi L, Seguelas MH, Nistri S, Colucci W, Leducq N, Parini A. Oxidative stress by monoamine oxidase mediates receptor-independent cardiomyocyte apoptosis by serotonin and postischemic myocardial injury. Circulation. 2005;112 (21):3297–305. doi: 10.1161/CIRCULATIONAHA.104.528133. [DOI] [PubMed] [Google Scholar]
- 21.Boengler K, Konietzka I, Buechert A, Heinen Y, Garcia-Dorado D, Heusch G, Schulz R. Loss of ischemic preconditioning’s cardioprotection in aged mouse hearts is associated with reduced gap junctional and mitochondrial levels of connexin 43. Am J Physiol Heart Circ Physiol. 2007;292 (4):H1764–9. doi: 10.1152/ajpheart.01071.2006. [DOI] [PubMed] [Google Scholar]
- 22.Boengler K, Schulz R, Heusch G. Loss of cardioprotection with ageing. Cardiovasc Res. 2009;83(2):247–61. doi: 10.1093/cvr/cvp033. cvp033 [pii] [DOI] [PubMed] [Google Scholar]
- 23.Bognar Z, Kalai T, Palfi A, Hanto K, Bognar B, Mark L, Szabo Z, Tapodi A, Radnai B, Sarszegi Z, Szanto A, Gallyas F, Jr, Hideg K, Sumegi B, Varbiro G. A novel SOD-mimetic permeability transition inhibitor agent protects ischemic heart by inhibiting both apoptotic and necrotic cell death. Free Radic Biol Med. 2006;41 (5):835–48. doi: 10.1016/j.freeradbiomed.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Booth EA, Obeid NR, Lucchesi BR. Activation of estrogen receptor-a protects the in vivo rabbit heart from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2005;289:H2039–H2047. doi: 10.1152/ajpheart.00479.2005. [DOI] [PubMed] [Google Scholar]
- 25.Bratic I, Trifunovic A. Mitochondrial energy metabolism and ageing. Biochim Biophys Acta. 2010;1797(6–7):961–7. doi: 10.1016/j.bbabio.2010.01.004. S0005-2728(10)00005-8 [pii] [DOI] [PubMed] [Google Scholar]
- 26.Bremnes Y, Ursin G, Bjurstam N, Rinaldi S, Kaaks R, Gram IT. Endogenous sex hormones, prolactin and mammographic density in postmenopausal Norwegian women. Int J Cancer. 2007;121 (11):2506–11. doi: 10.1002/ijc.22971. [DOI] [PubMed] [Google Scholar]
- 27.Bryzgalova G, Gao H, Ahren B, Zierath JR, Galuska D, Steiler TL, Dahlman-Wright K, Nilsson S, Gustafsson JA, Efendic S, Khan A. Evidence that oestrogen receptor-alpha plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia. 2006;49:588–597. doi: 10.1007/s00125-005-0105-3. [DOI] [PubMed] [Google Scholar]
- 28.Budas GR, Churchill EN, Disatnik M, Sun L, Mochly-Rosen D. Mitochondrial import of PKCepsilong mediated by HSP90: a role in cardioprotection from ischaemia and reperfusion injury. Cardiovasc Res. 2010;88 (1):83–92. doi: 10.1093/cvr/cvq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Budas GR, Churchill EN, Mochly-Rosen D. Cardioprotective mechanisms of PKC isozyme-selective activators and inhibitors in the treatment of ischemia-reperfusion injury. Pharmacol Res. 2007;55 (6):523–36. doi: 10.1016/j.phrs.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Burns PG, Krukenkamp IB, Caldarone CC, Kirvaitis RJ, Gaudette GR, Levitsky S. Is the preconditioning response conserved in the senescent myocardium? Ann Thorac Surg. 1996;61:925–929. doi: 10.1016/0003-4975(95)01188-9. [DOI] [PubMed] [Google Scholar]
- 31.Camper-Kirby D, Welch S, Walker A, Shiraishi I, Setchell KD, Schaefer E, Kajstura J, Anversa P, Sussman MA. Myocardial Akt activation and gender: increased nuclear activity in females versus males. Circ Res. 2001;88 (10):1020–7. doi: 10.1161/hh1001.090858. [DOI] [PubMed] [Google Scholar]
- 32.Centurione L, Antonucci A, Miscia S, Grilli A, Rapino M, Grifone G, DiGiacomo V, DiGuiulio C, Falconi M, Cataldi A. Age-related death-survival balance in myocardium: an immunohistochemical and biochemical study. Mech Ageing Dev. 2002;123:341–350. doi: 10.1016/s0047-6374(01)00378-5. [DOI] [PubMed] [Google Scholar]
- 33.Chakraborty TR, Gore AC. Aging-Related Changes in Ovarian Hormones, Their Receptors, and Neuroendocrine Function. Experimental Biology and Medicine. 2004;229 (10):977–987. doi: 10.1177/153537020422901001. [DOI] [PubMed] [Google Scholar]
- 34.Chakravarti B, Oseguera M, Dalal N, Fathy P, Mallik B, Raval A, Chakravarti DN. Proteomic profiling of aging in the mouse heart: Altered expression of mitochondrial proteins. Arch Biochem Biophys. 2008 doi: 10.1016/j.abb.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Chakravarti B, Oseguera M, Dalal N, Fathy P, Mallik B, Raval A, Chakravarti DN. Proteomic profiling of aging in the mouse heart: Altered expression of mitochondrial proteins. Arch Biochem Biophys. 2008;474:22–31. doi: 10.1016/j.abb.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Chen D, Washbrook E, Sarwar N, Bates G, Pace P, Thirunuvakkarasu V, Taylor J, Epstein RJ, Fuller-Pace FV, Egly J, Coombes RC, Ali S. Phosphorylation of human estrogen receptor a at serine 118 by two distinct signal transduction pathways revealed by phosphorylation-specific antisera. Oncogene. 2002;21:4921–4931. doi: 10.1038/sj.onc.1205420. [DOI] [PubMed] [Google Scholar]
- 37.Chen L, Hahn H, Wu G, Chen CH, Liron T, Schechtman D, Cavallaro G, Banci L, Guo Y, Bolli R, Dorn GW, 2nd, Mochly-Rosen D. Opposing cardioprotective actions and parallel hypertrophic effects of delta PKC and epsilon PKC. Proc Natl Acad Sci U S A. 2001;98 (20):11114–9. doi: 10.1073/pnas.191369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheskis BJ, Greger JG, Nagpal S, Freedman LP. Signaling by estrogens. J Cell Physiol. 2007;213:610–617. doi: 10.1002/jcp.21253. [DOI] [PubMed] [Google Scholar]
- 39.Chimenti C, Kajstura J, Torella D, Urbanek K, Heleniak H, Colussi C, Di Meglio F, Nadal-Ginard B, Frustaci A, Leri A, Maseri A, Anversa P. Senescence and Death of Primitive Cells and Myocytes Leads to Premature Cardiac Aging and Heart Failure. Circ Res. 2003 doi: 10.1161/01.RES.0000093985.76901.AF. 01.RES.0000093985.76901.AF. [DOI] [PubMed] [Google Scholar]
- 40.Choksi KB, Papaconstantinou J. Age-related alterations in oxidatively damaged proteins of mouse heart mitochondrial electron transport chain complexes. Free Radic Biol Med. 2008;44(10):1795–805. doi: 10.1016/j.freeradbiomed.2008.01.032. S0891-5849(08)00072-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cocco T, Sgobbo P, Clemente M, Lopriore B, Grattagliano I, Di Paola M, Villani G. Tissue-specific changes of mitochondrial functions in aged rats: effect of a long-term dietary treatment with N-acetylcysteine. Free Radic Biol Med. 2005;38(6):796–805. doi: 10.1016/j.freeradbiomed.2004.11.034. S0891-5849(04)00964-5 [pii] [DOI] [PubMed] [Google Scholar]
- 42.Cochran R, Gwinup G. Coronary artery disease in young females. Possibility of oophorectomy as an etiologic agent. Arch Intern Med. 1962;110:162–5. doi: 10.1001/archinte.1962.03620200022005. [DOI] [PubMed] [Google Scholar]
- 43.Cohen MV, Baines CP, Downey JM. Ischemic preconditioning: from adenosine receptor to KATP channel. Annu Rev Physiol. 2000;62:79–109. doi: 10.1146/annurev.physiol.62.1.79. [DOI] [PubMed] [Google Scholar]
- 44.Colditz GA, Willett WC, Stampfer MJ, Rosner B, Speizer FE, Hennekens CH. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987;316 (18):1105–10. doi: 10.1056/NEJM198704303161801. [DOI] [PubMed] [Google Scholar]
- 45.Craig EE, Hood DA. Influence of aging on protein import into cardiac mitochondria. Am J Physiol Heart Circ Physiol. 1997;272 (41):H2983–H2988. doi: 10.1152/ajpheart.1997.272.6.H2983. [DOI] [PubMed] [Google Scholar]
- 46.Dai D-F, Rabinovitch PS, Ungvari Z. Mitochondria and Cardiovascular Aging. Circulation Research. 2012;110(8):1109–1124. doi: 10.1161/circresaha.111.246140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davies SM, Poljak A, Duncan MW, Smythe GA, Murphy MP. Measurements of protein carbonyls, ortho- and meta-tyrosine and oxidative phosphorylation complex activity in mitochondria from young and old rats. Free Radic Biol Med. 2001;31(2):181–90. doi: 10.1016/s0891-5849(01)00576-7. S0891584901005767 [pii] [DOI] [PubMed] [Google Scholar]
- 48.Dawn B, Bolli R. Increasing evidence that estrogen is an important modulator of bone marrow-mediated cardiac repair after acute infarction. Circulation. 2006;114 (21):2203–5. doi: 10.1161/CIRCULATIONAHA.106.658260. [DOI] [PubMed] [Google Scholar]
- 49.Deng N, Zhang J, Zong C, Wang Y, Lu H, Yang P, Wang W, Young GW, Wang Y, Korge P, Lotz C, Doran P, Liem DA, Apweiler R, Weiss JN, Duan H, Ping P. Phosphoproteome analysis reveals regulatory sites in major pathways of cardiac mitochondria. Molecular & Cellular Proteomics. doi: 10.1074/mcp.M110.000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deroo B, Korach K. Estrogen receptors and human disease. J Clin Invest. 2006;116:561–570. doi: 10.1172/JCI27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Di Lisa F, Canton M, Menabo R, Kaludercic N, Bernardi P. Mitochondria and cardioprotection. Heart Fail Rev. 2007;12:249–260. doi: 10.1007/s10741-007-9028-z. [DOI] [PubMed] [Google Scholar]
- 52.Downey JM, Cohen MV. Reducing infarct size in the setting of acute myocardial infarction. Prog Cardiovasc Dis. 2006;48 (5):363–71. doi: 10.1016/j.pcad.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 53.Downey JM, Davis AM, Cohen MV. Signaling pathways in ischemic preconditioning. Heart Fail Rev. 2007;12 (3–4):181–8. doi: 10.1007/s10741-007-9025-2. [DOI] [PubMed] [Google Scholar]
- 54.Driggers PH, Segars JH. Estrogen action and cytoplasmic signaling pathways. Part II: the role of growth factors and phosphorylation in estrogen signaling. Trends in Endocrinology and Metabolism. 2002;13:422–427. doi: 10.1016/s1043-2760(02)00634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Du X-J, Riemersma RA, Dart AM. Cardiovascular protection by oestrogen is partly mediated through modulation of autonomic nervous function. Cardiovascular Research. 1995;30(2):161–165. doi: 10.1016/s0008-6363(95)00030-5. [DOI] [PubMed] [Google Scholar]
- 56.Du XJ, Dart AM, Riemersma RA, Oliver MF. Sex difference in presynaptic adrenergic inhibition of norepinephrine release during normoxia and ischemia in the rat heart. Circ Res. 1991;68 (3):827–35. doi: 10.1161/01.res.68.3.827. [DOI] [PubMed] [Google Scholar]
- 57.Dutta D, Calvani R, Bernabei R, Leeuwenburgh C, Marzetti E. Contribution of Impaired Mitochondrial Autophagy to Cardiac Aging: Mechanisms and Therapeutic Opportunities. Circulation Research. 2012;110(8):1125–1138. doi: 10.1161/circresaha.111.246108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eefting F, Rensing B, Wigman J, Pannekoek WJ, Liu WM, Cramer MJ, Lips DJ, Doevendans PA. Role of apoptosis in reperfusion injury. Cardiovasc Res. 2004;61 (3):414–26. doi: 10.1016/j.cardiores.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 59.Falkeborn M, Persson I, Adami HO, Bergstrom R, Eaker E, Lithell H, Mohsen R, Naessen T. The risk of acute myocardial infarction after oestrogen and oestrogen-progestogen replacement. Br J Obstet Gynaecol. 1992;99 (10):821–8. doi: 10.1111/j.1471-0528.1992.tb14414.x. [DOI] [PubMed] [Google Scholar]
- 60.Falkeborn M, Schairer C, Naessen T, Persson I. Risk of myocardial infarction after oophorectomy and hysterectomy. J Clin Epidemiol. 2000;53 (8):832–7. doi: 10.1016/s0895-4356(00)00187-6. [DOI] [PubMed] [Google Scholar]
- 61.Fannin SW, Lesnefsky EJ, Slabe TJ, Hassan MO, Hoppel CL. Aging selectively decreases oxidative capacity in rat heart interfibrillar mitochondria. Arch Biochem Biophys. 1999;372(2):399–407. doi: 10.1006/abbi.1999.1508. S0003-9861(99)91508-4 [pii] [DOI] [PubMed] [Google Scholar]
- 62.Faus H, Haendler B. Post-transloational modifications of steriod receptors. Biomed Pharmacother. 2006;60:520–528. doi: 10.1016/j.biopha.2006.07.082. [DOI] [PubMed] [Google Scholar]
- 63.Fenton R, Dickson E, DJ Inhibition of phosphatase activity enhances preconditioning and limits cell death in the ischemic/reperfused aged rat heart. Life Sci. 2005;77:3375–3388. doi: 10.1016/j.lfs.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 64.Fenton RA, Dickson EW, Meyer TE, Dobson JG., Jr Aging reduces the cardioprotective effect of ischemic preconditioning in the rat heart. J Mol Cell Cardiol. 2000;32 (7):1371–5. doi: 10.1006/jmcc.2000.1189. [DOI] [PubMed] [Google Scholar]
- 65.Filardo EJ, Thomas P. GPR30: a seven-transmembrane-spanning estrogen receptor that triggers EGF release. Trends Endocrinol Metab. 2005;16 (8):362–7. doi: 10.1016/j.tem.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 66.Forster C, Kietz S, Hultenby K, Warner M, Gustafsson JA. Characterization of the ERbeta−/−mouse heart. Proc Natl Acad Sci U S A. 2004;101(39):14234–9. doi: 10.1073/pnas.0405571101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fu M, Wang C, Zhang X, Pestell RG. Acetylation of nuclear receptors in cellular growth and apoptosis. Biochem Pharmacol. 2004;68:1199–1208. doi: 10.1016/j.bcp.2004.05.037. [DOI] [PubMed] [Google Scholar]
- 68.Gabel SA, Walker VR, London RE, Steenbergen C, Korach KS, Murphy E. Estrogen receptor beta mediates gender differences in ischemia/reperfusion injury. J Mol Cell Cardiol. 2005;38 (2):289–97. doi: 10.1016/j.yjmcc.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 69.Gabel SA, Walker VR, London RE, Steenbergen C, Korach KS, Murphy E. Estrogen receptor beta mediates gender differences in ischemia/reperfusion injury. J Mol Cell Cardiol. 2005;38:289–297. doi: 10.1016/j.yjmcc.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 70.Garg V, Hu K. Protein kinase C isoform-dependent modulation of ATP-sensitive K+ channels in mitochondrial inner membrane. Am J Physiol Heart Circ Physiol. 2007;293 (1):H322–32. doi: 10.1152/ajpheart.01035.2006. [DOI] [PubMed] [Google Scholar]
- 71.Goto M, Liu Y, Yang XM, Ardell JL, Cohen MV, Downey JM. Role of bradykinin in protection of ischemic preconditioning in rabbit hearts. Circ Res. 1995;77 (3):611–21. doi: 10.1161/01.res.77.3.611. [DOI] [PubMed] [Google Scholar]
- 72.Gottlieb RA. Mitochondrial signaling in apoptosis: mitochondrial daggers to the breaking heart. Basic Res Cardiol. 2003;98:242–249. doi: 10.1007/s00395-003-0404-0. [DOI] [PubMed] [Google Scholar]
- 73.Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133 (12):933–41. doi: 10.7326/0003-4819-133-12-200012190-00008. [DOI] [PubMed] [Google Scholar]
- 74.Hale GE, Zhao X, Hughes CL, Burger HG, Robertson DM, Fraser IS. Endocrine Features of Menstrual Cycles in Middle and Late Reproductive Age and the Menopausal Transition Classified According to the Staging of Reproductive Aging Workshop (STRAW) Staging System. J Clin Endocrinol Metab. 2007;92(8):3060–3067. doi: 10.1210/jc.2007-0066. [DOI] [PubMed] [Google Scholar]
- 75.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion--a target for cardioprotection. Cardiovasc Res. 2004;61 (3):372–85. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 76.Hall JE. Neuroendocrine physiology of the early and late menopause. Endocrinol Metab Clin N Am. 2004;33:637–659. doi: 10.1016/j.ecl.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 77.Hamada H, Kim MK, Iwakura A, Ii M, Thorne T, Qin G, Asai J, Tsutsumi Y, Sekiguchi H, Silver M, Wecker A, Bord E, Zhu Y, Kishore R, Losordo DW. Estrogen receptors alpha and beta mediate contribution of bone marrow-derived endothelial progenitor cells to functional recovery after myocardial infarction. Circulation. 2006;114 (21):2261–70. doi: 10.1161/CIRCULATIONAHA.106.631465. [DOI] [PubMed] [Google Scholar]
- 78.Hamilton KL, Gupta S, Knowlton AA. Estrogen and regulation of heat shock protein expression in female cardiomyocytes: cross-talk with NF kappa B signaling. J Mol Cell Cardiol. 2004;36 (4):577–84. doi: 10.1016/j.yjmcc.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 79.Hansford RG. Lipid oxidation by heart mitochondria from young adult and senescent rats. Biochem J. 1978;170:285–295. doi: 10.1042/bj1700285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Harman SM, Brinton EA, Cedars M, Lobo R, Manson JE, Merriam GR, Miller VM, Naftolin F, Santoro N. KEEPS: The Kronos Early Estrogen Prevention Study. Climacteric. 2005;8 (1):3–12. doi: 10.1080/13697130500042417. [DOI] [PubMed] [Google Scholar]
- 81.Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol Heart Circ Physiol. 2005;288 (2):H971–6. doi: 10.1152/ajpheart.00374.2004. [DOI] [PubMed] [Google Scholar]
- 82.Hausenloy DJ, Yellon DM, Mani-Babu S, Duchen MR. Preconditioning protects by inhibiting the mitochondrial permeability transition. Am J Physiol Heart Circ Physiol. 2004;287 (2):H841–9. doi: 10.1152/ajpheart.00678.2003. [DOI] [PubMed] [Google Scholar]
- 83.Hayward CS, Kelly RP, Collins P. The roles of gender, the menopause and hormone replacement on cardiovascular function. Cardiovasc Res. 2000;46 (1):28–49. doi: 10.1016/s0008-6363(00)00005-5. [DOI] [PubMed] [Google Scholar]
- 84.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proceedings of the National Academy of Sciences. 2000;97(23):12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hofer T, Servais S, Seo AY, Marzetti E, Hiona A, Upadhyay SJ, Wohlgemuth SE, Leeuwenburgh C. Bioenergetics and permeability transition pore opening in heart subsarcolemmal and interfibrillar mitochondria: effects of aging and lifelong calorie restriction. Mech Ageing Dev. 2009;130(5):297–307. doi: 10.1016/j.mad.2009.01.004. S0047-6374(09)00009-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hsieh Y-C, Choudhry MA, Yu H-P, Shimizu T, Yang S, Suzuki T, Chen J, Bland KI, Chaudry IH. Inhibition of cardiac PGC-1{alpha} expression abolishes ER{beta} agonist-mediated cardioprotection following trauma-hemorrhage. FASEB J. 2006;20:1109–1117. doi: 10.1096/fj.05-5549com. [DOI] [PubMed] [Google Scholar]
- 87.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. Jama. 1998;280 (7):605–13. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 88.Hunter JC, Kostyak JC, Novotny JL, Simpson AM, Korzick DH. Estrogen Deficiency Decreases Ischemic Tolerance in Aged Rat Myocardium: Roles of PKC{delta},PKC{epsilon}, Akt, and GSK3{beta} Am J Physiol Regul Integr Comp Physiol. 2006 doi: 10.1152/ajpregu.00374.2006. 00374.2006. [DOI] [PubMed] [Google Scholar]
- 89.Hunter JC, Kostyak JC, Novotny JL, Simpson AM, Korzick DH. Estrogen deficiency decreases ischemic tolerance in the aged rat heart: Roles of PKCdelta, PKCepsilon, Akt, and GSK3beta. Am J Physiol Regul Integr Comp Physiol. 2007;292 (2):R800–9. doi: 10.1152/ajpregu.00374.2006. [DOI] [PubMed] [Google Scholar]
- 90.Hunter JC, Machikas AM, Korzick DH. Age-Dependent Reductions in Mitochondrial Respiration are Exacerbated by Calcium in the Female Rat Heart. Gender Medicine. 2012;9(3):197–206. doi: 10.1016/j.genm.2012.04.001. http://dx.doi.org/10.1016/j.genm.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Inagaki K, Churchill E, Mochly-Rosen D. Epsilon protein kinase C as a potential therapeutic target for the ischemic heart. Cardiovasc Res. 2006;70 (2):222–30. doi: 10.1016/j.cardiores.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 92.Inagaki K, Hahn H, Dorn GW, 2nd, Mochly-Rosen D. Additive protection of the ischemic heart ex vivo by combined treatment with d-protein kinase c inhibitor and e-protein kinase c activator. Circulation. 2003;108:869–875. doi: 10.1161/01.CIR.0000081943.93653.73. [DOI] [PubMed] [Google Scholar]
- 93.Isoyama S, Nitta-Komatsubara Y. Acute and chronic adaptation to hemodynamic overload and ischemia in the aged heart. Heart Fail Rev. 2002;7 (1):63–9. doi: 10.1023/a:1013701923065. [DOI] [PubMed] [Google Scholar]
- 94.Jankowski M, Rachelska G, Donghao W, McCann SM, Gutkowska J. Estrogen receptors activate atrial natriuretic peptide in the rat heart. Proc Natl Acad Sci U S A. 2001;98 (20):11765–70. doi: 10.1073/pnas.201394198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Javadov SA, Clarke S, Das M, Griffiths EJ, Lim KH, Halestrap AP. Ischaemic preconditioning inhibits opening of mitochondrial permeability transition pores in the reperfused rat heart. J Physiol. 2003;549 (Pt 2):513–24. doi: 10.1113/jphysiol.2003.034231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jin ZQ, Zhou HZ, Cecchini G, Gray MO, Karliner JS. MnSOD in mouse heart: acute responses to ischemic preconditioning and ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2005;288 (6):H2986–94. doi: 10.1152/ajpheart.01144.2004. [DOI] [PubMed] [Google Scholar]
- 97.Judge S, Jang YM, Smith A, Hagen T, Leeuwenburgh C. Age-associated increases in oxidative stress and antioxidant enzyme activities in cardiac interfibrillar mitochondria: implications for the mitochondrial theory of aging. Faseb J. 2005;19 (3):419–21. doi: 10.1096/fj.04-2622fje. [DOI] [PubMed] [Google Scholar]
- 98.Juhaszova M, Rabuel C, Zorov DB, Lakatta EG, Sollott SJ. Protection in the aged heart: preventing the heart-break of old age? Cardiovasc Res. 2005;66 (2):233–44. doi: 10.1016/j.cardiores.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 99.Juhaszova M, Rabuel C, Zorov DB, Lakatta EG, Sollott SJ. Protection in the aged heart: preventing the heart-break of old age? Cardiovasc Res. 2005;66:233–244. doi: 10.1016/j.cardiores.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 100.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, Olson EN, Sollott SJ. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113 (11):1535–49. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kabir AM, Clark JE, Tanno M, Cao X, Hothersall JS, Dashnyam S, Gorog DA, Bellahcene M, Shattock MJ, Marber MS. Cardioprotection initiated by reactive oxygen species is dependent on activation of PKCepsilon. Am J Physiol Heart Circ Physiol. 2006;291 (4):H1893–9. doi: 10.1152/ajpheart.00798.2005. [DOI] [PubMed] [Google Scholar]
- 102.Kajstura J, Cheng W, Sarangarajan R, Li P, Li B, Nitahara JA, Chapnick S, Reiss K, Olivetti G, Anversa P. Necrotic and apoptotic myoctye cell death in the aging heart of Fischer 344 rats. Am J Physiol. 1996;271:H1215–H1228. doi: 10.1152/ajpheart.1996.271.3.H1215. [DOI] [PubMed] [Google Scholar]
- 103.Kaludercic N, Carpi A, Menabò R, Di Lisa F, Paolocci N. Monoamine oxidases (MAO) in the pathogenesis of heart failure and ischemia/reperfusion injury. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2011;1813 (7):1323–1332. doi: 10.1016/j.bbamcr.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kam KW, Qi JS, Chen M, Wong TM. Estrogen reduces cardiac injury and expression of beta1-adrenoceptor upon ischemic insult in the rat heart. J Pharmacol Exp Ther. 2004;309 (1):8–15. doi: 10.1124/jpet.103.058339. [DOI] [PubMed] [Google Scholar]
- 105.Kannel WB, Hjortland MC, McNamara PM, Gordon T. Menopause and risk of cardiovascular disease: the Framingham study. Ann Intern Med. 1976;85 (4):447–52. doi: 10.7326/0003-4819-85-4-447. [DOI] [PubMed] [Google Scholar]
- 106.Kanno T, Sato EE, Muranaka S, Fujita H, Fujiwara T, Utsumi T, Inoue M, Utsumi K. Oxidative stress underlies the mechanism for Ca(2+)-induced permeability transition of mitochondria. Free Radic Res. 2004;38 (1):27–35. doi: 10.1080/10715760310001626266. [DOI] [PubMed] [Google Scholar]
- 107.Keller ET, Zhang J, Yao Z, Qi Y. The impact of chronic estrogen deprivation on immunologic parameters in the ovariectomized rhesus monkey (Macaca mulatta) model of menopause. Journal of Reproductive Immunology. 2001;50 (1):41. doi: 10.1016/s0165-0378(00)00087-5. [DOI] [PubMed] [Google Scholar]
- 108.Kimura M, Irahara M, Yasui T, Saito S, Tezuka M, Yamano S, Kamada M, Aono T. The obesity in bilateral ovariectomized rats is related to a decrease in the expression of leptin receptors in the brain. Biochem Biophys Res Commun. 2002;290 (4):1349–53. doi: 10.1006/bbrc.2002.6355. [DOI] [PubMed] [Google Scholar]
- 109.Kinnally KW, Peixoto PM, Ryu SY, Dejean LM. Is mPTP the gatekeeper for necrosis, apoptosis, or both? Biochimica et Biophysica Acta. 2010;1813:616–622. doi: 10.1016/j.bbamcr.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Korichneva I, Hoyos B, Chua R, Levi E, Hammerling U. Zinc release from protein kinase C as the common event during activation by lipid second messenger or reactive oxygen. J Biol Chem. 2002;277 (46):44327–31. doi: 10.1074/jbc.M205634200. [DOI] [PubMed] [Google Scholar]
- 111.Korzick DH, Kostyak JC, Hunter JC, Saupe KW. Local delivery of PKC{varepsilon}-activating peptide mimics ischemic preconditioning in aged hearts through GSK-3beta but not F1-ATPase inactivation. Am J Physiol Heart Circ Physiol. 2007;293(4):H2056–2063. doi: 10.1152/ajpheart.00403.2007. [DOI] [PubMed] [Google Scholar]
- 112.Korzick DH, Kostyak JC, Hunter JC, Saupe KW. Local delivery of PKCepsilon-activating peptide mimics ischemic preconditioning in aged hearts through GSK-3beta but not F1-ATPase inactivation. Am J Physiol Heart Circ Physiol. 2007;293 (4):H2056–63. doi: 10.1152/ajpheart.00403.2007. [DOI] [PubMed] [Google Scholar]
- 113.Kostyak JC, Hunter JC, Korzick DH. Acute PKCd inhibition limits ischemia-reperfusion injury in the aged rat heart: role of GSK-3β. Cardiovasc Res. 2006;70:325–334. doi: 10.1016/j.cardiores.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 114.Kostyak JC, Hunter JC, Korzick DH. Acute PKCdelta inhibition limits ischaemia-reperfusion injury in the aged rat heart: role of GSK-3beta. Cardiovasc Res. 2006;70 (2):325–34. doi: 10.1016/j.cardiores.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 115.Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138 (3):863–70. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 116.Kumaran S, Subathra M, Balu M, Panneerselvam C. Age-associated decreased activities of mitochondrial electron transport chain complexes in heart and skeletal muscle: role of L-carnitine. Chem Biol Interact. 2004;148(1–2):11–8. doi: 10.1016/j.cbi.2003.10.010. S0009279704000286 [pii] [DOI] [PubMed] [Google Scholar]
- 117.Kwong LK, Sohal RS. Age-related changes in activities of mitochondrial electron transport complexes in various tissues of the mouse. Arch Biochem Biophys. 2000;373(1):16–22. doi: 10.1006/abbi.1999.1495. S0003-9861(99)91495-9 [pii] [DOI] [PubMed] [Google Scholar]
- 118.Lakatta EG, Sollott SJ. The “heartbreak” of older age. Mol Interv. 2002;2(7):431–46. doi: 10.1124/mi.2.7.4312/7/431. [pii] [DOI] [PubMed] [Google Scholar]
- 119.Lakatta EG, Sollott SJ. The “heartbreak” of older age. Mol Interv. 2002;2 (7):431–446. doi: 10.1124/mi.2.7.431. [DOI] [PubMed] [Google Scholar]
- 120.Lakatta EG, Sollott SJ. Perspectives on mammalian cardiovascular aging: humans to molecules. Comp Biochem Physiol A Mol Integr Physiol. 2002;132 (4):699–721. doi: 10.1016/s1095-6433(02)00124-1. [DOI] [PubMed] [Google Scholar]
- 121.Lancaster TS, Jefferson SJ, Hunter JC, Lopez V, Van Eyk JE, Lakatta EG, Korzick DH. Quantitative proteomic analysis reveals novel mitochondrial targets of estrogen deficiency in the aged female rat heart. Physiological Genomics. 2012;44(20):957–969. doi: 10.1152/physiolgenomics.00184.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lancaster TS, Jefferson SJ, Korzick DH. Local delivery of a PKCe-activating peptide limits ischemia reperfusion injury in the aged female rat heart. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2011;301(5):R1242–R1249. doi: 10.1152/ajpregu.00851.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lee TM, Su SF, Chou TF, Lee YT, Tsai CH. Loss of preconditioning by attenuated activation of myocardial ATP-sensitive potassium channels in elderly patients undergoing coronary angioplasty. Circulation. 2002;105 (3):334–40. doi: 10.1161/hc0302.102572. [DOI] [PubMed] [Google Scholar]
- 124.Lesnefsky EJ, Hoppel CL. Ischemia-reperfusion injury in the aged heart: role of mitochondria. Arch Biochem Biophys. 2003;420 (2):287–97. doi: 10.1016/j.abb.2003.09.046. [DOI] [PubMed] [Google Scholar]
- 125.Lim KH, Javadov SA, Das M, Clarke SJ, Suleiman MS, Halestrap AP. The effects of ischaemic preconditioning, diazoxide and 5-hydroxydecanoate on rat heart mitochondrial volume and respiration. J Physiol. 2002;545 (Pt 3):961–74. doi: 10.1113/jphysiol.2002.031484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu GS, Thornton J, Van Winkle DM, Stanley AW, Olsson RA, Downey JM. Protection against infarction afforded by preconditioning is mediated by A1 adenosine receptors in rabbit heart. Circulation. 1991;84 (1):350–6. doi: 10.1161/01.cir.84.1.350. [DOI] [PubMed] [Google Scholar]
- 127.Liu L, Azhar G, Gao W, Zhang X, Wei JY. Bcl-2 and Bax expression in adult rat hearts after coronary occlusion: age-associated differences. Am J Physiol. 1998;275:R315–R322. doi: 10.1152/ajpregu.1998.275.1.R315. [DOI] [PubMed] [Google Scholar]
- 128.Liu P, Xu B, Cavalieri TA, Hock CE. Age-related difference in myocardial function and inflammation in a rat model of myocardial ischemia-reperfusion. Cardiovasc Res. 2002;56 (3):443–53. doi: 10.1016/s0008-6363(02)00603-x. [DOI] [PubMed] [Google Scholar]
- 129.Longobardi G, Abete P, Ferrara N, Papa A, Rosiello R, Furgi G, Calabrese C, Cacciatore F, Rengo F. “Warm-up” phenomenon in adult and elderly patients with coronary artery disease: further evidence of the loss of “ischemic preconditioning” in the aging heart. J Gerontol A Biol Sci Med Sci. 2000;55 (3):M124–9. doi: 10.1093/gerona/55.3.m124. [DOI] [PubMed] [Google Scholar]
- 130.Lu R, Hu CP, Deng HW, Li YJ. Calcitonin gene-related peptide-mediated ischemic preconditioning in the rat heart: influence of age. Regul Pept. 2001;99 (2–3):183–9. doi: 10.1016/s0167-0115(01)00253-1. [DOI] [PubMed] [Google Scholar]
- 131.Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, Heckbert SR, Detrano R, Strickland OL, Wong ND, Crouse JR, Stein E, Cushman M. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349 (6):523–34. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 132.Mariani J, Ou R, Bailey M, Rowland M, Nagley P, Rosenfeldt F, Pepe S. Tolerance to ischemia and hypoxia is reduced in aged human myocardium. J Thorac Cardiovasc Surg. 2000;120 (4):660–7. doi: 10.1067/mtc.2000.106528. [DOI] [PubMed] [Google Scholar]
- 133.McDonald TG, Van Eyk JE. Mitochondrial proteiomics: undercover in the lipid bilayer. Basic Res Cardiol. 2003;98:219–227. doi: 10.1007/s00395-003-0417-8. [DOI] [PubMed] [Google Scholar]
- 134.Meldrum DR. Estrogen increases protective proteins following trauma and hemorrhage. Am J Physiol Regul Integr Comp Physiol. 2006;290 (3):R809–11. doi: 10.1152/ajpregu.00802.2005. [DOI] [PubMed] [Google Scholar]
- 135.Mendez P, Garcia-Segura LM. Phosphatidylinositol 3-kinase and glycogen synthase kinase 3 regulate estrogen receptor-mediated transcription in neuronal cells. Endocrinology. 2006;147:3027–3039. doi: 10.1210/en.2005-1224. [DOI] [PubMed] [Google Scholar]
- 136.Miki T, Cohen MV, Downey JM. Opioid receptor contributes to ischemic preconditioning through protein kinase C activation in rabbits. Mol Cell Biochem. 1998;186 (1–2):3–12. [PubMed] [Google Scholar]
- 137.Miller VM, Clarkson TB, Harman SM, Brinton EA, Cedars M, Lobo R, Manson JE, Merriam GR, Naftolin F, Santoro N. Women, hormones, and clinical trials: a beginning, not an end. J Appl Physiol. 2005;99 (2):381–3. doi: 10.1152/japplphysiol.00248.2005. [DOI] [PubMed] [Google Scholar]
- 138.Minners J, McLeod CJ, Sack MN. Mitochondrial plasticity in classical ischemic preconditioning-moving beyond the mitochondrial KATP channel. Cardiovasc Res. 2003;59 (1):1–6. doi: 10.1016/s0008-6363(03)00337-7. [DOI] [PubMed] [Google Scholar]
- 139.Morkuniene R, Arandarcikaite O, Ivanoviene L, Borutaite V. Estradiol-induced protection against ischemia-induced heart mitochondrial damage and caspase activation is mediated by protein kinase G. Biochim Biophys Acta. 2010;1797(6–7):1012–7. doi: 10.1016/j.bbabio.2010.03.027. S0005-2728(10)00133-7 [pii] [DOI] [PubMed] [Google Scholar]
- 140.Morrison JH, Brinton RD, Schmidt PJ, Gore AC. Estrogen, Menopause, and the Aging Brain: How Basic Neuroscience Can Inform Hormone Therapy in Women. J Neurosci. 2006;26(41):10332–10348. doi: 10.1523/jneurosci.3369-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Murphy E. Estrogen Signaling and Cardiovascular Disease. Circulation Research. 2011;109(6):687–696. doi: 10.1161/circresaha.110.236687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Murphy E, Steenbergen C. Gender-based differences in mechanisms of protection in myocardial ischemia-reperfusion injury. Cardiovasc Res. 2007;75 (3):478–86. doi: 10.1016/j.cardiores.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 143.Murphy E, Steenbergen C. Preconditioning: The Mitochondrial Connection. Annu Rev Physiol. 2006 doi: 10.1146/annurev.physiol.69.031905.163645. [DOI] [PubMed] [Google Scholar]
- 144.Murphy E, Steenbergen C. Preconditioning: the mitochondrial connection. Annu Rev Physiol. 2007;69:51–67. doi: 10.1146/annurev.physiol.69.031905.163645. [DOI] [PubMed] [Google Scholar]
- 145.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74 (5):1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 146.Nadal-Ginard B, Kajstura J, Anversa P, Leri A. A matter of life and death: cardiac myocyte apoptosis and regeneration. J Clin Invest. 2003;111 (10):1457–9. doi: 10.1172/JCI18611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nelson HD, Walker M, Zakher B, Mitchell J. Menopausal Hormone Therapy for the Primary Prevention of Chronic Conditions: A Systematic Review to Update the U.S. Preventive Services Task Force Recommendations. Annals of Internal Medicine. 2012:157. doi: 10.7326/0003-4819-157-2-201207170-00466. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 148.Nikolic I, Liu D, Bell JA, Collins J, Steenbergen C, Murphy E. Treatment with an estrogen receptor-beta-selective agonist is cardioprotective. J Mol Cell Cardiol. 2007;42(4):769–80. doi: 10.1016/j.yjmcc.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 149.Nikolic I, Liu D, Bell JA, Collins J, Steenbergen C, Murphy E. Treatment with an estrogen receptor-beta-selective agonist is cardioprotective. J Mol Cell Cardiol. 2007;42:769–780. doi: 10.1016/j.yjmcc.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 150.Nitta Y, Abe K, Aoki M, Ohno I, Isoyama S. Diminished heat shock protein 70 mRNA induction in aged rat hearts after ischemia. Am J Physiol. 1994;267 (5 Pt 2):H1795–803. doi: 10.1152/ajpheart.1994.267.5.H1795. [DOI] [PubMed] [Google Scholar]
- 151.Novotny JL, Simpson AM, Tomicek NA, Lancaster TL, Korzick DH. Rapid estrogen receptor-α activation improves ischemic tolerance in aged female rats through a novel PKCe-dependent mechanism. Endocrinology. 2009;150:889–896. doi: 10.1210/en.2008-0708. [DOI] [PubMed] [Google Scholar]
- 152.Nuedling S, Karas RH, Mendelsohn ME, Katzenellenbogen JA, Katzenellenbogen BS, Meyer R, Vetter H, Grohe C. Activation of estrogen receptor beta is a prerequisite for estrogen-dependent upregulation of nitric oxide synthases in neonatal rat cardiac myocytes. FEBS Lett. 2001;502 (3):103–8. doi: 10.1016/s0014-5793(01)02675-8. [DOI] [PubMed] [Google Scholar]
- 153.O’Rourke B. Evidence for mitochondrial K+ channels and their role in cardioprotection. Circ Res. 2004;94 (4):420–32. doi: 10.1161/01.RES.0000117583.66950.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ogbi M, Johnson JA. Protein kinase Cepsilon interacts with cytochrome c oxidase subunit IV and enhances cytochrome c oxidase activity in neonatal cardiac myocyte preconditioning. Biochem J. 2006;393 (Pt 1):191–9. doi: 10.1042/BJ20050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Oudot A, Martin C, Busseuil D, Vergely C, Demaison L, Rochette L. NADPH oxidases are in part responsible for increased cardiovascular superoxide production during aging. Free Radic Biol Med. 2006;40 (12):2214–22. doi: 10.1016/j.freeradbiomed.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 156.Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol. 2006;20 (9):1996–2009. doi: 10.1210/me.2005-0525. [DOI] [PubMed] [Google Scholar]
- 157.Pelzer T, Loza PA, Hu K, Bayer B, Dienesch C, Calvillo L, Couse JF, Korach KS, Neyses L, Ertl G. Increased mortality and aggravation of heart failure in estrogen receptor-beta knockout mice after myocardial infarction. Circulation. 2005;111(12):1492–8. doi: 10.1161/01.CIR.0000159262.18512.46. [DOI] [PubMed] [Google Scholar]
- 158.Pepe S. Dysfunctional ischemic preconditioning mechanisms in aging. Cardiovasc Res. 2001;49 (1):11–4. doi: 10.1016/s0008-6363(00)00283-2. [DOI] [PubMed] [Google Scholar]
- 159.Pepe S, Tsuchiya N, Lakatta EG, Hansford RG. PUFA and aging modulate cardiac mitochondrial membrane lipid composition and Ca2+ activation of PDH. Am J Physiol. 1999;276:H149–H158. doi: 10.1152/ajpheart.1999.276.1.H149. [DOI] [PubMed] [Google Scholar]
- 160.Peter I, Shearman AM, Vasan RS, Zucker DR, Schmid CH, Demissie S, Cupples A, Kuvin JT, Karas R, Mendelsohn M, Housman DE, Benjamin EJ. Association of estrogen receptor b gene polymorphisms with left ventricular mass and wall thickness in women. Am J Hypertens. 2005;18:1388–1395. doi: 10.1016/j.amjhyper.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 161.Petrosillo G, Matera M, Moro N, Ruggiero FM, Paradies G. Mitochondrial complex I dysfunction in rat heart with aging: critical role of reactive oxygen species and cardiolipin. Free Radic Biol Med. 2009;46(1):88–94. doi: 10.1016/j.freeradbiomed.2008.09.031. S0891-5849(08)00574-1 [pii] [DOI] [PubMed] [Google Scholar]
- 162.Phaneuf S, Leeuwenburgh C. Cytochrome c release from mitochondria in the aging heart: a possible mechanism for apoptosis with age. Am J Physiol Regul Integr Comp Physiol. 2002;282 (2):R423–30. doi: 10.1152/ajpregu.00296.2001. [DOI] [PubMed] [Google Scholar]
- 163.Phillips GB. Is atherosclerotic cardiovascular disease an endocrinological disorder? The estrogen-androgen paradox. J Clin Endocrinol Metab. 2005;90:2708–2711. doi: 10.1210/jc.2004-2011. [DOI] [PubMed] [Google Scholar]
- 164.Ping P, Takano H, Zhang J, Tang XL, Qiu Y, Li RC, Banerjee S, Dawn B, Balafonova Z, Bolli R. Isoform-selective activation of protein kinase C by nitric oxide in the heart of conscious rabbits: a signaling mechanism for both nitric oxide-induced and ischemia-induced preconditioning. Circ Res. 1999;84 (5):587–604. doi: 10.1161/01.res.84.5.587. [DOI] [PubMed] [Google Scholar]
- 165.Preston CC, Oberlin AS, Holmuhamedov EL, Gupta A, Sagar S, Syed RH, Siddiqui SA, Raghavakaimal S, Terzic A, Jahangir A. Aging-induced alterations in gene transcripts and functional activity of mitochondrial oxidative phosphorylation complexes in the heart. Mech Ageing Dev. 2008 doi: 10.1016/j.mad.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Preston CC, Oberlin AS, Holmuhamedov EL, Gupta A, Sagar S, Syed RH, Siddiqui SA, Raghavakaimal S, Terzic A, Jahangir A. Aging-induced alterations in gene transcripts and functional activity of mitochondrial oxidative phosphorylation complexes in the heart. Mech Ageing Dev. 2008;129(6):304–12. doi: 10.1016/j.mad.2008.02.010. S0047-6374(08)00041-9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Qiu Y, Ping P, Tang XL. Direct evidence that protein kinase C plays an essential role in the development of late preconditioning against myocardial stunning in conscious rabbits and that e is the isoform involved. Journal of Clinical Investigation. 1998;101:2182–2198. doi: 10.1172/JCI1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Robertson T, Kennard ED, Mehta S, Popma JJ, Carrozza JP, Jr, King SB, 3rd, Holmes DR, Cowley MJ, Hornung CA, Kent KM, Roubin GS, Litvack F, Moses JW, Safian R, Desvigne-Nickens P, Detre KM. Influence of gender on in-hospital clinical and angiographic outcomes and on one-year follow-up in the New Approaches to Coronary Intervention (NACI) registry. Am J Cardiol. 1997;80 (10A):26K–39K. doi: 10.1016/s0002-9149(97)00762-5. [DOI] [PubMed] [Google Scholar]
- 169.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart Disease and Stroke Statistics—2011 Update: A Report From the American Heart Association. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rosenberg L, Hennekens CH, Rosner B, Belanger C, Rothman KJ, Speizer FE. Early menopause and the risk of myocardial infarction. Am J Obstet Gynecol. 1981;139 (1):47–51. doi: 10.1016/0002-9378(81)90410-5. [DOI] [PubMed] [Google Scholar]
- 172.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. Jama. 2002;288 (3):321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 173.Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis KL, Stefanick ML. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. Jama. 2007;297 (13):1465–77. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 174.Saeedi R, Wambolt RB, Parsons H, Antler C, Leong HS, Keller A, Dunaway GA, Popov KM, Allard MF. Gender and post-ischemic recovery of hypertrophied rat hearts. BMC Cardiovasc Disord. 2006;8 doi: 10.1186/1471-2261-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sakamoto J, Miura T, Goto M, Iimura O. Limitation of myocardial infarct size by adenosine A1 receptor activation is abolished by protein kinase C inhibitors in the rabbit. Cardiovasc Res. 1995;29 (5):682–8. [PubMed] [Google Scholar]
- 176.Samali A, Cai J, Zhivotovsky B, Jones DP, Orrenius S. Presence of a pre-apoptotic complex of pro-caspase-3, Hsp60 and Hsp10 in the mitochondrial fraction of jurkat cells. Embo J. 1999;18 (8):2040–8. doi: 10.1093/emboj/18.8.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sanz A, Hiona A, Kujoth GC, Seo AY, Hofer T, Kouwenhoven E, Kalani R, Prolla TA, Barja G, Leeuwenburgh C. Evaluation of sex differences on mitochondrial bioenergetics and apoptosis in mice. Exp Gerontol. 2007;42 (3):173–82. doi: 10.1016/j.exger.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Saunders PT, Maguire SM, Gaughan J, Millar MR. Expression of oestrogen receptor beta (ER beta) in multiple rat tissues visualised by immunohistochemistry. J Endocrinol. 1997;154 (3):R13–6. doi: 10.1677/joe.0.154r013. [DOI] [PubMed] [Google Scholar]
- 179.Savonenko AV, Markowska AL. The cognitive effects of ovariectomy and estrogen replacement are modulated by aging. Neuroscience. 2003;119 (3):821–30. doi: 10.1016/s0306-4522(03)00213-6. [DOI] [PubMed] [Google Scholar]
- 180.Schulman D, Latchman DS, Yellon DM. Effect of aging on the ability of preconditioning to protect rat hearts from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2001;281 (4):H1630–6. doi: 10.1152/ajpheart.2001.281.4.H1630. [DOI] [PubMed] [Google Scholar]
- 181.Schulman D, Latchman DS, Yellon DM. Effect of aging on the ability of preconditioning to protect rat hearts from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2001;281 (4):H1630–6. doi: 10.1152/ajpheart.2001.281.4.H1630. [DOI] [PubMed] [Google Scholar]
- 182.Schultz JE, Rose E, Yao Z, Gross GJ. Evidence for involvement of opioid receptors in ischemic preconditioning in rat hearts. Am J Physiol. 1995;268 (5 Pt 2):H2157–61. doi: 10.1152/ajpheart.1995.268.5.H2157. [DOI] [PubMed] [Google Scholar]
- 183.Schwanke U, Konietzka I, Duschin A, Li X, Schulz R, Heusch G. No ischemic preconditioning in heterozygous connexin43-deficient mice. Am J Physiol Heart Circ Physiol. 2002;283 (4):H1740–2. doi: 10.1152/ajpheart.00442.2002. [DOI] [PubMed] [Google Scholar]
- 184.Schwend T, Gustafsson JA. False positives in MALDI-TOF detection of ERbeta in mitochondria. Biochem Biophys Res Commun. 2006;343(3):707–11. doi: 10.1016/j.bbrc.2006.02.164. S0006-291X(06)00482-7 [pii] [DOI] [PubMed] [Google Scholar]
- 185.Shih CD. Activation of estrogen receptor beta-dependent nitric oxide signaling mediates the hypotensive effects of estrogen in the rostral ventrolateral medulla of anesthetized rats. J Biomed Sci. 2009;16:60. doi: 10.1186/1423-0127-16-60. 1423-0127-16-60 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sniecinski R, Liu H. Reduced efficacy of volatile anesthetic preconditioning with advanced age in isolated rat myocardium. Anesthesiology. 2004;100 (3):589–97. doi: 10.1097/00000542-200403000-00019. [DOI] [PubMed] [Google Scholar]
- 187.Sone K, Yamamoto-Sawamura T, Kuwahara S, Nishijima K, Ohno T, Aoyama H, Tanaka S. Changes of estrous cycles with aging in female F344/n rats. Exp Anim. 2007;56 (2):139–48. doi: 10.1538/expanim.56.139. [DOI] [PubMed] [Google Scholar]
- 188.Song X, Li G, Vaage J, Valen G. Effects of sex, gonadectomy, and oestrogen substitution on ischaemic preconditioning and ischaemia-reperfusion injury in mice. Acta Physiol Scand. 2003;177 (4):459–66. doi: 10.1046/j.1365-201X.2003.01068.x. [DOI] [PubMed] [Google Scholar]
- 189.Stampfer MJ, Colditz GA, Willett WC, Manson JE, Rosner B, Speizer FE, Hennekens CH. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the nurses’ health study. N Engl J Med. 1991;325 (11):756–62. doi: 10.1056/NEJM199109123251102. [DOI] [PubMed] [Google Scholar]
- 190.Sullivan TR, Jr, Karas RH, Aronovitz M, Faller GT, Ziar JP, Smith JJ, O’Donnell TF, Jr, Mendelsohn ME. Estrogen inhibits the response-to-injury in a mouse carotid artery model. J Clin Invest. 1995;96 (5):2482–8. doi: 10.1172/JCI118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sun J, Picht E, Ginsburg KS, Bers DM, Steenbergen C, Murphy E. Hypercontractile female hearts exhibit increased S-nitrosylation of the L-type Ca2+ channel alpha1 subunit and reduced ischemia/reperfusion injury. Circ Res. 2006;98 (3):403–11. doi: 10.1161/01.RES.0000202707.79018.0a. [DOI] [PubMed] [Google Scholar]
- 192.Tani M, Honma Y, Hasegawa H, Tamaki K. Direct activation of mitochondrial K(ATP) channels mimics preconditioning but protein kinase C activation is less effective in middle-aged rat hearts. Cardiovasc Res. 2001;49 (1):56–68. doi: 10.1016/s0008-6363(00)00240-6. [DOI] [PubMed] [Google Scholar]
- 193.Tani M, Suganuma Y, Hasegawa H, Shinmura K, Ebihara Y, Hayashi Y, Guo X, Takayama M. Decrease in ischemic tolerance with aging in isolated perfused Fischer 344 rat hearts: relation to increases in intracellular Na+ after ischemia. J Mol Cell Cardiol. 1997;29 (11):3081–9. doi: 10.1006/jmcc.1997.0533. [DOI] [PubMed] [Google Scholar]
- 194.Tani M, Suganuma Y, Hasegawa H, Shinmura K, Hayashi Y, Guo X, Nakamura Y. Changes in ischemic tolerance and effects of ischemic preconditioning in middle-aged rat hearts. Circulation. 1997;95 (11):2559–66. doi: 10.1161/01.cir.95.11.2559. [DOI] [PubMed] [Google Scholar]
- 195.Tarze A, Deniaud A, Le Bras M, Maillier E, Molle D, Larochette N, Zamzami N, Jan G, Kroemer G, Brenner C. GAPDH, a novel regulator of the pro-apoptotic mitochondrial membrane permeabilization. Oncogene. 2007;26 (18):2606–20. doi: 10.1038/sj.onc.1210074. [DOI] [PubMed] [Google Scholar]
- 196.Tatarkova Z, Kuka S, Racay P, Lehotsky J, Dobrota D, Mistuna D, Kaplan P. Effects of aging on activities of mitochondrial electron transport chain complexes and oxidative damage in rat heart. Physiol Res. 2011;60(2):281–9. doi: 10.33549/physiolres.932019. 932019 [pii] [DOI] [PubMed] [Google Scholar]
- 197.Tiravanti E, Samouilov A, Zweier JL. Nitrosyl-heme complexes are formed in the ischemic heart: evidence of nitrite-derived nitric oxide formation, storage, and signaling in post-ischemic tissues. J Biol Chem. 2004;279 (12):11065–73. doi: 10.1074/jbc.M311908200. [DOI] [PubMed] [Google Scholar]
- 198.Tomicek NJ, Miller-Lee JL, Hunter JC, Korzick DH. Estrogen Receptor Beta Does Not Influence Ischemic Tolerance in the Aged Female Rat Heart. Cardiovascular Therapeutics. 2013;31(1):32–37. doi: 10.1111/j.1755-5922.2011.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tong H, Imahashi K, Steenbergen C, Murphy E. Phosphorylation of glycogen synthase kinase-3beta during preconditioning through a phosphatidylinositol-3-kinase--dependent pathway is cardioprotective. Circ Res. 2002;90 (4):377–9. doi: 10.1161/01.res.0000012567.95445.55. [DOI] [PubMed] [Google Scholar]
- 200.Toninello A, Salvi M, pIetrangeli P, Mondovi B. Biogenic amines and apoptosis: minireview article. Amino Acids. 2004;26:339–343. doi: 10.1007/s00726-004-0080-x. [DOI] [PubMed] [Google Scholar]
- 201.Too CK, Giles A, Wilkinson M. Estrogen stimulates expression of adenine nucleotide translocator ANT1 messenger RNA in female rat hearts. Mol Cell Endocrinol. 1999;150 (1–2):161–7. doi: 10.1016/s0303-7207(99)00002-7. [DOI] [PubMed] [Google Scholar]
- 202.Treloar AE. Menstrual cyclicity and the pre-menopause. Maturitas. 1981;3 (3–4):249–64. doi: 10.1016/0378-5122(81)90032-3. [DOI] [PubMed] [Google Scholar]
- 203.Turrens JF, Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem J. 1980;191 (2):421–7. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Urata Y, Ihara Y, Murata H, Goto S, Koji T, Yodoi J, Inoue S, Kondo T. 17B-estradiol protects against oxidative stress-induced cell death through the glutathione/glutaredoxin-dependent redox regulation of Akt in myocardiac H9c2 cells. J Biol Chem. 2006;281:13092–13102. doi: 10.1074/jbc.M601984200. [DOI] [PubMed] [Google Scholar]
- 205.van der Loo B, Bachschmid M, Labugger R, Schildknecht S, Kilo J, Hahn R, Palacios-Callender M, Luscher TF. Expression and activity patterns of nitric oxide synthases and antioxidant enzymes reveal a substantial heterogeneity between cardiac and vascular aging in the rat. Biogerontology. 2005;6 (5):325–34. doi: 10.1007/s10522-005-4807-1. [DOI] [PubMed] [Google Scholar]
- 206.Villablanca A, Lubahn D, Shelby L, Lloyd K, Barthold S. Susceptibility to early atherosclerosis in male mice is mediated by estrogen receptor a. Arterioscler Thromb Vasc Biol. 2004;24:1055–1061. doi: 10.1161/01.ATV.0000130467.65290.d4. [DOI] [PubMed] [Google Scholar]
- 207.Wall TM, Sheehy R, Hartman JC. Role of bradykinin in myocardial preconditioning. J Pharmacol Exp Ther. 1994;270 (2):681–9. [PubMed] [Google Scholar]
- 208.Wang M, Crisostomo P, Wairiuko GM, Meldrum DR. Estrogen receptor-{alpha} mediates acute myocardial protection in females. Am J Physiol Heart Circ Physiol. 2006;290:H2204–H2209. doi: 10.1152/ajpheart.01219.2005. [DOI] [PubMed] [Google Scholar]
- 209.Wang M, Crisostomo PR, Markel T, Wang Y, Lillemoe KD, Meldrum DR. Estrogen receptor beta mediates acute myocardial protection following ischemia. Surgery. 2008;144(2):233–8. doi: 10.1016/j.surg.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 210.Wang M, Tsai BM, Reiger KM, Brown JW, Meldrum DR. 17-b-Estradiol decreases p38 MAPK-mediated myocardial inflammation and dysfunction following acute ischemia. J Mol Cell Cardiol. 2006;40:205–212. doi: 10.1016/j.yjmcc.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 211.Wang M, Wang Y, Weil B, Abarbanell A, Herrmann J, Tan J, Kelly M, Meldrum DR. Estrogen receptor beta mediates increased activation of PI3K/Akt signaling and improved myocardial function in female hearts following acute ischemia. Am J Physiol Regul Integr Comp Physiol. 2009;296(4):R972–8. doi: 10.1152/ajpregu.00045.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wang P, Zweier JL. Measurment of Nitric Oxide and Peroxynitrite Generation in the Postischemic Heart. J Biol Chem. 1996;271 (46):29223–29230. doi: 10.1074/jbc.271.46.29223. [DOI] [PubMed] [Google Scholar]
- 213.Weiss G, Skurnick JH, Goldsmith LT, Santoro NF, Park SJ. Menopause and Hypothalamic-Pituitary Sensitivity to Estrogen. JAMA. 2004;292(24):2991–2996. doi: 10.1001/jama.292.24.2991. [DOI] [PubMed] [Google Scholar]
- 214.Wenger NK, Shaw LJ, Vaccarino V. Coronary heart disease in women: update 2008. Clin Pharmacol Ther. 2008;83 (1):37–51. doi: 10.1038/sj.clpt.6100447. [DOI] [PubMed] [Google Scholar]
- 215.Willems L, Zatta A, Holmgren K, Ashton KJ, Headrick JP. Age-related changes in ischemic tolerance in male and female mouse hearts. J Mol Cell Cardiol. 2005;38:245–256. doi: 10.1016/j.yjmcc.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 216.Willems L, Zatta A, Holmgren K, Ashton KJ, Headrick JP. Age-related changes in ischemic tolerance in male and female mouse hearts. J Mol Cell Cardiol. 2005;38 (2):245–56. doi: 10.1016/j.yjmcc.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 217.Wood CE, Sitruk-Ware RL, Tsong YY, Register TC, Lees CJ, Cline JM. Effects of estradiol with oral or intravaginal progesterone on risk markers for breast cancer in a postmenopausal monkey model. Menopause. 2007;14 (4):639–47. doi: 10.1097/01.gme.0000247017.41007.80. [DOI] [PubMed] [Google Scholar]
- 218.Wu JM, Zelinski MB, Ingram DK, Ottinger MA. Ovarian aging and menopause: current theories, hypotheses, and research models. Exp Biol Med (Maywood) 2005;230 (11):818–28. doi: 10.1177/153537020523001106. [DOI] [PubMed] [Google Scholar]
- 219.Wuest JH, Jr, Dry TJ, Edwards JE. The degree of coronary atherosclerosis in bilaterally oophorectomized women. Circulation. 1953;7 (6):801–9. doi: 10.1161/01.cir.7.6.801. [DOI] [PubMed] [Google Scholar]
- 220.Xia Y, Khatchikian G, Zweier JL. Adenosine deaminase inhibition prevents free radical-mediated injury in the postischemic heart. J Biol Chem. 1996;271 (17):10096–102. doi: 10.1074/jbc.271.17.10096. [DOI] [PubMed] [Google Scholar]
- 221.Xu Y, Arenas IA, Armstrong SJ, Davidge ST. Estrogen modulation of left ventricular remodeling in the aged heart. Cardiovasc Res. 2003;57(2):388–94. doi: 10.1016/s0008-6363(02)00705-8. S0008636302007058 [pii] [DOI] [PubMed] [Google Scholar]
- 222.Xu Y, Armstrong SJ, Arenas IA, Pehowich DJ, Davidge ST. Cardioprotection by chronic estrogen or superoxide dismutase mimetic treatment in the aged female rat. Am J Physiol Heart Circ Physiol. 2004;287 (1):H165–71. doi: 10.1152/ajpheart.00037.2004. [DOI] [PubMed] [Google Scholar]
- 223.Yamashita N, Hoshida S, Taniguchi N, Kuzuya T, Hori M. A “second window of protection” occurs 24 h after ischemic preconditioning in the rat heart. J Mol Cell Cardiol. 1998;30 (6):1181–9. doi: 10.1006/jmcc.1998.0682. [DOI] [PubMed] [Google Scholar]
- 224.Yang SH, Liu R, Perez EJ, Wen Y, Stevens SM, Jr, Valencia T, Brun-Zinkernagel AM, Prokai L, Will Y, Dykens J, Koulen P, Simpkins JW. Mitochondrial localization of estrogen receptor b. Proc Natl Acad Sci U S A. 2004;101 (12):4130–5. doi: 10.1073/pnas.0306948101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Yang SH, Liu R, Perez EJ, Wen Y, Stevens SM, Jr, Valencia T, Brun-Zinkernagel AM, Prokai L, Will Y, Dykens J, Koulen P, Simpkins JW. Mitochondrial localization of estrogen receptor beta. Proc Natl Acad Sci U S A. 2004;101(12):4130–5. doi: 10.1073/pnas.03069481010306948101. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Yasmin W, Strynadka KD, Schulz R. Generation of peroxynitrite contributes to ischemia-reperfusion injury in isolated rat hearts. Cardiovasc Res. 1997;33 (2):422–32. doi: 10.1016/s0008-6363(96)00254-4. [DOI] [PubMed] [Google Scholar]
- 227.Yin W, Gore AC. Neuroendocrine control of reproductive aging: roles of GnRH neurons. Reproduction. 2006;131(3):403–414. doi: 10.1530/rep.1.00617. [DOI] [PubMed] [Google Scholar]
- 228.Yu W, Dahl G, Werner R. The connexin43 gene is responsive to oestrogen. Proc Biol Sci. 1994;255 (1343):125–32. doi: 10.1098/rspb.1994.0018. [DOI] [PubMed] [Google Scholar]
- 229.Zhai P, Eurell TE, Cotthaus R, Jeffery EH, Bahr JM, Gross DR. Effect of estrogen on global myocardial ischemia-reperfusion injury in female rats. Am J Physiol Heart Circ Physiol. 2000;279:H2766–H2775. doi: 10.1152/ajpheart.2000.279.6.H2766. [DOI] [PubMed] [Google Scholar]
- 230.Zhai P, Eurell TE, Cotthaus RP, Jeffery EH, Bahr JM, Gross DR. Effects of dietary phytoestrogen on global myocardial ischemia-reperfusion injury in isolated female rat hearts. Am J Physiol Heart Circ Physiol. 2001;281 (3):H1223–32. doi: 10.1152/ajpheart.2001.281.3.H1223. [DOI] [PubMed] [Google Scholar]
- 231.Zhang D, Trudeau VL. Integration of membrane and nuclear estrogen receptor signaling. Comp Biochem Physiol A Mol Integr Physiol. 2006;144:306–315. doi: 10.1016/j.cbpa.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 232.Zhang J, Liem DA, Mueller M, Wang Y, Zong C, Deng N, Vondriska TM, Korge P, Drews O, MacLellan WR, Honda H, Weiss JN, Apweiler R, Ping P. Altered Proteome Biology of Cardiac Mitochondria Under Stress Conditions. Journal of Proteome Research. 2008;7(6):2204–2214. doi: 10.1021/pr070371f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zhang Y, Chong E, Herman B. Age-associated increases in the activity of multiple caspases in Fisher 344 rat organs. Exp Gerontol. 2002;37 (6):777–89. doi: 10.1016/s0531-5565(02)00013-x. [DOI] [PubMed] [Google Scholar]
- 234.Zhao L, O’Neill K, Brinton RD. Estrogenic agonist activity of ICI 182,780 (Faslodex) in hippocampal neurons: implications for basic science understanding of estrogen signaling and development of estrogen modulators with a dual therapeutic profile. J Pharmacol Exp Ther. 2006;319 (3):1124–32. doi: 10.1124/jpet.106.109504. [DOI] [PubMed] [Google Scholar]
- 235.Zhao X, Eghbali-Webb M. Gender-related differences in basal and hypoxia-induced activation of signal transduction pathways controlling cell cycle progression and apoptosis, in cardiac fibroblasts. Endocrine. 2002;18 (2):137–45. doi: 10.1385/ENDO:18:2:137. [DOI] [PubMed] [Google Scholar]
- 236.Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285 (2):H579–88. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 237.Zhao ZQ, Vinten-Johansen J. Myocardial apoptosis and ischemic preconditioning. Cardiovasc Res. 2002;55 (3):438–55. doi: 10.1016/s0008-6363(02)00442-x. [DOI] [PubMed] [Google Scholar]
- 238.Zorov DB, Juhaszova M, Yaniv Y, Nuss HB, Wang S, Sollott SJ. Regulation and pharmacology of the mitochondrial permeability transition pore. Cardiovasc Res. 2009;83 (2):213–225. doi: 10.1093/cvr/cvp151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zweier JL, Talukder MA. The role of oxidants and free radicals in reperfusion injury. Cardiovasc Res. 2006;70 (2):181–90. doi: 10.1016/j.cardiores.2006.02.025. [DOI] [PubMed] [Google Scholar]