Abstract
Ethnopharmacological relevance
Aqueous preparations of Vernonia guineensis Benth. (Asteraceae) are used in Cameroonian folk medicine as a general stimulant and to treat various illnesses and conditions including malaria, bacterial infections and helminthic infestations.
Materials and methods
10-g samples of the leaf and tuber powders of V. guineensis were extracted separately using dichloromethane, methanol and distilled water. The extracts were dried in vacuo and used in bioassays. These extracts and three compounds previously isolated from V. guineensis [vernopicrin (1), vernomelitensin (2) and pentaisovalerylsucrose (3)] were screened for antiplasmodial activity against chloroquine (CQ)-sensitive (Hb3) and CQ-resistant (Dd2) Plasmodium falciparum lines.
Results
Crude extracts and pure compounds from V. guineensis showed antiplasmodial activity against both Hb3 and Dd2. The IC50 values of extracts ranged from 1.64 – 27.2 μg/ml for Hb3 and 1.82 – 30.0 μg/ml for Dd2; those for compounds 1, 2 and 3 ranged from 0.47 – 1.62 μg/ml (1364 – 1774 nM) for Hb3 and 0.57 – 1.50 μg/ml (1644 – 2332 nM) for Dd2. None of the crude extracts or pure compounds was observed to exert toxic effects on the erythrocytes used to cultivate the P. falciparum lines.
Conclusion
In Cameroonian folk medicine, V. guineensis may be used to treat malaria in part due to the antiplasmodial activity of sesquiterpene lactones (1, 2), a sucrose ester (3) and perhaps other compounds present in crude plant extracts. Exploring the safety and antiplasmodial efficacy of these compounds in vivo requires further study.
Keywords: Vernonia guineensis, antiplasmodial activity, sesquiterpene lactone, sucrose ester, Cameroon
1.0 Introduction
Malaria remains endemic in most tropical countries, especially those in sub-Saharan Africa (Alonso et al., 2011; Snow et al., 2005). The emergence of Plasmodium falciparum parasites with reduced susceptibility to artemisinins and partner drugs (e.g., mefloquine, lumefantrine and piperaquine) that comprise artemisinin-based combination therapies (ACTs) are worrisome and challenge existing efforts to control, treat and eliminate P. falciparum malaria (Nyunt and Plowe, 2007, Fairhurst et al., 2012). The discovery and development of new antimalarial drugs thus remains a priority. Such discoveries may be made by identifying lead compounds derived from medicinal plants used by traditional healers to treat malaria or febrile illnesses in general.
The carrot-like tubers of Vernonia guineensis are commonly used in ethnomedicine as an adaptogen (to combat stress), a stimulant, an aphrodisiac, general poison antidote, a treatment for jaundice and prostate-related problems, as well as an antibacterial, anthelmintic and antimalarial agent (Iwu, 1993; Tchinda et al., 2002; Noumi 2010). Antitrypanosomal and anti-cancer compounds have been isolated from the root extract of this plant (Tchinda et al., 2003; Toyang et al., 2012a). Antibacterial and anthelmintic activities have been found in crude extracts and pure compounds isolated from V. guineensis (Donfack et al., 2012, Toyang et al., 2012b). To date, V. guineensis extracts and compounds have not been tested in vitro for potential antiplasmodial activities. In this context, we explored the in-vitro antiplasmodial activity of crude extracts and pure compounds from V. guineensis.
2.0 Materials and methods
2.1. Plant collection
The tubers and leaves of Vernonia guineensis Var. cameroonica C. D. Adams were collected in Baicham, Boyo Division, North West Region, Cameroon, in 2009. A voucher specimen was authenticated at the Limbe Botanic Garden, South West Region, Cameroon, and a voucher specimen (No. SCA 12431) deposited at the Limbe Botanic Garden Herbarium.
2.2. Preparation of crude extracts, isolation of pure compounds, and thin layer chromatography (TLC) analysis
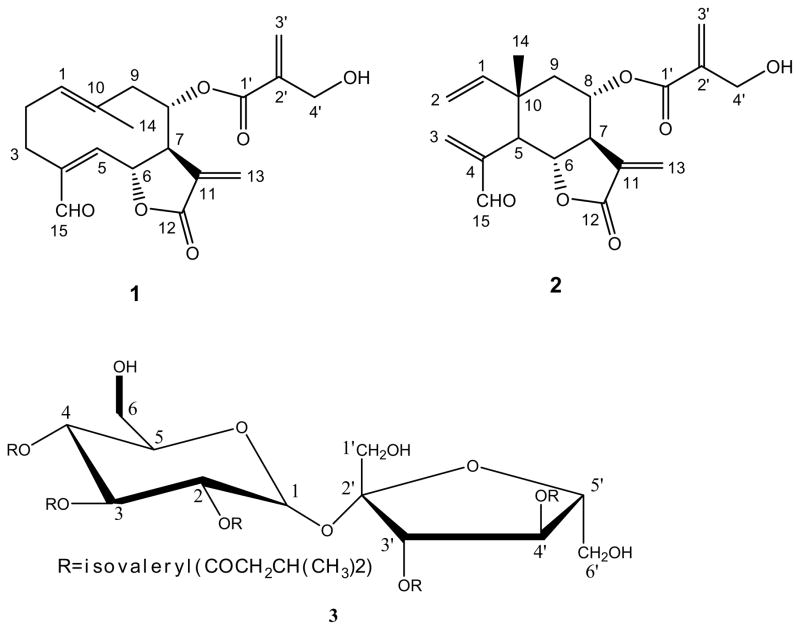
Samples of V. guineensis were extracted as described (Toyang et al., 2012b). Briefly, 10-g samples of dried V. guineensis leaf and tuber powders were separately extracted with 100 ml dichloromethane, methanol or distilled water twice at 37°C for 24 h. The samples were filtered (dichloromethane and methanol extracts) or centrifuged (water extracts) to separate the extract from the marc. The extracts were dried in vacuo to obtain solvent-free crude extracts for use in bioassays. Two sesquiterpene lactones (vernopicrin-1 and vernomelitensin-2) and one sucrose ester (pentaisovalerylsucrose-3) were available from recent isolations (Toyang et al., 2012a and c) and also used in this study. The sesquiterpene lactones and sucrose ester (Fig. 1) were isolated from the leaves and roots of V. guineensis, respectively. TLC analysis was carried out using normal phase silica plates coated with UV254 fluorescence indicator. The solvent system used was n-hexane/ethyl acetate (1:1). The developed plates were visualized under a UV station at 254 nm and 365 nm and any bands detected. The plates were further treated with H2SO4 spray and the Rf values of bands found in the crude extracts were compared with those of compounds 1, 2 and 3.
Figure 1.
Chemical structures of vernopicrin (1), vernomelitensin (2) and pentaisovalerylsucrose (3).
2.3. Bioassays
2.3.1. In-vitro antiplasmodial assay
The 50% inhibitory concentrations (IC50) of V. guineensis extracts and compounds were measured using a SYBR Green I-based DNA detection method (Smilkstein et al., 2004). Briefly, two laboratory-adapted P. falciparum lines, chloroquine (CQ)-sensitive Hb3 and CQ-resistant Dd2, were cultured as described (Moll et al., 2008). Stock solutions of crude extracts and pure compounds were diluted in cell culture water to 100 and 10 μg/ml, respectively. Two-fold serial dilutions of extracts (0.0977 – 100 μg/ml), compounds (0.00977 – 10 μg/ml), chloroquine (0.00078 – 0.8 μg/ml), and artesunate (0.0000469 – 0.0481 μg/ml) were added to 96-well plates. The plates were dried overnight in a dark sterile hood and stored for up to 1 week at 4°C. Suspensions of P. falciparum-infected erythrocytes (2% hematocrit, 1% parasitemia) were added to the drug-coated plates and incubated in an atmosphere of 5% CO2, 5% O2 and 90% N2 at 37°C for 72 h. The assay was terminated by freezing the plates at −20°C for 24 h. Parasite growth was evaluated using SYBR-Green I DNA dye fluorescence. Fluorescence was measured on a BMG Labtech FLUOstar Optima instrument (Ortenberg, Germany) at excitation and emission wavelengths of 485 nm and 530 nm, respectively. Analysis of data was performed with Graphpad Prism (Graphpad Software, La Jolla, CA). Normalized fluorescence was plotted against the logarithm of the drug concentration to yield the concentration of drug that produced 50% of the observed decline from the maximum fluorescence in the drug free wells (IC50).
2.3.2. Chemical injury to erythrocytes
To determine if the crude extracts and pure compounds caused chemical injury to erythrocytes, these cells (2% hematocrit) were incubated in the highest concentration of each drug under the same conditions of the drug response assay. Thin blood smears were stained with Giemsa and erythrocytes observed by light microscopy (100X) for any gross morphological changes.
2.4. Statistical analysis
The antiplasmodial assay experiments were run at least in duplicate with each experimental drug concentration duplicated during each run. The IC50 values of the different experiments were calculated and the data represented as mean ± SD. Comparison of activity against CQ-sensitive vs. CQ-resistant P. falciparum strains was carried out. P-values <0.05 were deemed significant.
3.0 Results
Compounds 1 and 2 were detected by TLC in the dichloromethane and methanol extracts, but not in the aqueous extract of the leaves of V. guineensis using (not shown). Compound 3 was detected by TLC in the dichloromethane extract but not in the methanol and aqueous extracts of the root biomass of V. guineensis (chromatogram not shown).
These pure compounds and crude extracts from V. guineensis inhibited the growth of Hb3 and Dd2 (Table 1). The differential drug susceptibility of Hb3 (CQ-sensitive) and Dd2 (CQ-resistant) to CQ was confirmed, with the former parasite line showing an IC50 = 28 nM and the latter an IC50 = 219 nM (Table 1). Both Hb3 and Dd2 were highly susceptible to artesunate (IC50 = 4–5 nM), as expected. The IC50 values of compounds 1, 2 and 3 were also similar for Hb3 and Dd2 and ranged from 1364 – 2130 nM for Hb3 and 1664 – 2332 nM for Dd2. The IC50 values of compounds 1, 2 and 3 were thus at least an order of magnitude higher than those for CQ and artesunate.
Table 1.
Antiplasmodial activity Vernonia guineensis crude extracts and isolated compounds
| Antiplasmodial activity IC50 (μg/ml) | |||
|---|---|---|---|
| Chloroquine resistant (Dd2) | Chloroquine sensitive (Hb3) | P-value (Dd2 vs. Hb3) | |
| Antimalarials | |||
| Chloroquine | 0.070 ± 0.017 (219 ± 52.2 nM) | 0.009 ± 0.0009 (28±2.75 nM) | 0.005 |
| Artesunate | 0.002 ± 0.0001 (5 ± 0.31 nM) | 0.0015 ± 0.0004 (4 ± 0.91 nM) | 0.112 |
| Pure compounds | |||
| Vernopicrin (1) | 0.807 ± 0.197 (2332 ± 569 nM) | 0.614 ± 0.110 (1774 ± 317 nM) | 0.138 |
| Vernomelitensin (2) | 0.569 ± 0.076 (1644 ± 219 nM) | 0.472 ± 0.0861 (1364 ± 248 nM) | 0.142 |
| Sucrose ester (3) | 1.495 ± 0.174 (1962 ± 228 nM) | 1.623 ± 0.167 (2130 ± 219 nM) | 0.329 |
| Crude extracts | |||
| VGRD | 3.162 ± 0.295 | 3.218 ± 0.310 | 0.802 |
| VGRM | 29.977 ± 1.212 | 27.084 ± 1.514 | 0.024 |
| VGRA | 26.115 ± 4.027 | 27.238 ± 3.316 | 0.682 |
| VGLD | 1.823 ± 0.503 | 1.635 ± 0.429 | 0.590 |
| VGLM | 3.980 ± 1.246 | 2.055 ± 0.894 | 0.046 |
| VGLA | 11.354 ± 1.148 | 9.546 ± 1.229 | 0.075 |
Root extracts: VGRD (dichloromethane); VGRM (methanol); VGRA (aqueous)
Leaf extracts: VGLD (dichloromethane); VGLM (methanol); VGLA (aqueous)
Results of four replicate experiments are presented as mean ± standard deviation.
The IC50 values of extracts were similar for Hb3 and Dd2, and ranged from 1.64 – 27.2 μg/ml for Hb3 and 1.82 – 30.0 μg/ml for Dd2. The IC50 values of root extracts ranged from 3.162 – 29.98 μg/ml, while those of leaf extracts ranged from 1.635 – 11.35 μg/ml and were generally higher (P<0.05) (Table 1). The dichloromethane root and leaf extracts were generally more potent than the methanol (P<0.05) and aqueous (P<0.05) extracts of the plant. The methanol and aqueous root extracts exhibited similar antiplasmodial activity at concentrations 8–10 times less than the potency of the dichloromethane root extract. The difference between the antiplasmodial activity of the dichloromethane and methanol leaf extracts, however, was not significant (P>0.05) compared to that between the dichloromethane and aqueous leaf extracts (P<0.05).
Microscopic observation of uninfected erythrocytes incubated for 72 h with the crude extracts of V. guineensis and compounds 1, 2 and 3 at the highest concentrations used in the drug response assay showed no morphological differences compared to positive control and CQ and artesunate negative controls.
4.0 Discussion
Plants remain the most frequently used source of antimalarial remedies in developing countries where access to relatively-expensive, modern chemotherapeutics may be limited. The possibility that plant-based remedies exert antiplasmodial effects has already been realized with Cinchona officinalis and Artemisia annua, source plants for quinine and artemisinin – two of the most effective antimalarial drugs ever developed. These plants have and continue to receive much attention as the demand for their associated compounds has often outweighed their supply (Meshnick and Dobson, 2001; de Ridder et al., 2008). Plants are thus likely to remain an important component of the world’s antimalarial arsenal. Here we have explored the putative antiplasmodial properties of V. guineensis.
The different solvent extracts of V. guineensis leaves and roots demonstrated variable activity against two P. falciparum lines, with the dichloromethane leaf and root extracts showing greater antiplasmodial activity than the other extracts. Vernopicrin (1) and vernomelitensin (2), two sesquiterpene lactones previously isolated from the acetone extract of V. guineensis (Toyang et al., 2012c), showed antiplasmodial activity with IC50 values of 0.614 and 0.807 μg/ml, and 0.472 and 0.569 μg/ml, respectively, against the CQ-sensitive and CQ-resistant P. falciparum lines. The Vernonia genus is known to be a rich source of sesquiterpene lactones and many species of this genus have yielded antiplasmodial sesquiterpenes with and without the lactone functionality (Kraft et al., 2003; Chea et al., 2006; Pillay et al., 2007; Chukwujekwu et al., 2009; Buskuhl et al., 2010). The sesquiterpenes identified from other Vernonia species have ranged in antiplasmodial activity with IC50 values of 0.2 – 4.8 μg/ml. Hirsutinolide, a sesquiterpene lactone isolated from V. scorpioides, exhibits the most potent antiplasmodial activity with an IC50 = 0.2 μg/ml (Buskuhl et al., 2010), whereas vernodalol isolated from V. colorata was the least active of the sesquiterpenes with an IC50 = 4.8 μg/ml (Kraft et al., 2003). Schmidt et al. (2009) reported the comparative antiprotozoal activity of 35 sesquiterpene lactones and found IC50 values ranging from 0.329 – 27.47 μg/ml against P. falciparum. With IC50 values <1 μg/ml against P. falciparum, compounds 1 and 2 appear to be among the most potent sesquiterpene lactones; by comparison, only 8.6% (3/35) of the sesquiterpene lactones assayed by Schmidt et al. (2009) showed IC50 values <1 μg/ml.
Pentaisovalerylsucrose (3), a sucrose ester isolated from the roots of V. guineensis, also demonstrated activity against the CQ-sensitive and CQ-resistant P. falciparum lines. To the best of our knowledge, this is the first report on the antiplasmodial activity of a sucrose ester. The activity of the dichloromethane extract of the roots and leaves of V. guineensis might be linked to the presence of compounds 1, 2 and 3 (Table 1) as evidenced by their detection in TLC analysis (Figure not shown), as well as their previous isolation from nonpolar fractions of the roots and leaves of V. guineensis (Toyang et al., 2012a, 2012c). The activity of the methanol extract of the plant’s leaves may also be linked to the presence of compounds 1 and 2 as these were detected by TLC in this extract (chromatogram not shown). The antiplasmodial activity of the aqueous extracts may be due to the presence of other metabolites yet to be identified as none of the three compounds tested were detected by TLC in the aqueous extracts. It is also unlikely that any of the three compounds were present to any significant degree in the aqueous extracts given that compounds 1, 2 and 3 in concentrations as low as 1 mg/ml were insoluble in aqueous solutions. Previous studies of V. guineensis roots also afforded two stigmastane derivatives, vernoguinosterol and vernoguinoside, with antitrypanosomal activity (Tchinda et al., 2002). It is unknown whether these compounds also possess antiplasmodial activities as they have not yet been investigated.
5.0 Conclusion
The in-vitro antiplasmodial activity of crude extracts and purified compounds from V. guineensis may underlie the use of this plant in Cameroonian folk medicine to treat malaria. To the best of our knowledge, this is the first report on the antiplasmodial activity of extracts and compounds isolated from this plant. Further studies are needed to assess the safety and antiparasitic efficacy of vernopicrin, vernomelitensin and pentaisovalerylsucrose in in-vivo animal models. In addition, bioactivity-guided fractionation as well as metabolomic studies are recommended to identify other antiplasmodial compounds present in the roots and leaves of V. guineensis.
Acknowledgments
This study was supported in part by the Intramural Research Program of the NIAID, NIH. The authors are grateful to Alosyius N. Toyang, Valentine Lah and Therese Toyeng for assisting with the collection and processing of the plant material. We also acknowledge Harry Davis, Dr. Eugene Ateh and Dr. Valentina Turri for useful contributions to this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alonso PL, Brown G, Arevalo-Herrera M, Binka F, Chitnis C, Collins F, Doumbo OK, Greenwood B, Fenton Hall B, Levine MM, Mendis K, Newman RD, Plowe CV, Rodríguez MH, Sinden R, Slutsker L, Tanner M. A research agenda to underpin malaria eradication. Plos Medicine. 2011;8:1–8. doi: 10.1371/journal.pmed.1000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buskuhl H, Oliveira FL, Blind LZ, Freitas RA, Barison A, Campos FR, Corilo YE, Eberlin MN, Caramori GF, Biavatti MW. Sesquiterpene lactones from Vernonia scorpioides and their in vitro cytotoxicity. Phytochemistry. 2010;71:1539–1544. doi: 10.1016/j.phytochem.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Chea A, Hout S, Long C, Marcourt L, Faure R, Azas N, Elias R. Antimalarial Activity of Sesquiterpene Lactones from Vernonia cinerea. Chemical and Pharmaceutical Bulletin. 2006;54:1437–1439. doi: 10.1248/cpb.54.1437. [DOI] [PubMed] [Google Scholar]
- Chukwujekwu JC, Lategan CA, Smith PJ, Van Heerden FR, Van Staden J. Antiplasmodial and cytotoxic activity of isolated sesquiterpene lactones from the acetone leaf extract of Vernonia colorata. South African Journal of Botany. 2009;75:176–179. [Google Scholar]
- de Ridder S, van der Kooy F, Verpoorte R. Artemisia annua as a self-reliant treatment for malaria in developing countries. Journal of Ethnopharmacology. 2008;120:302– 314. doi: 10.1016/j.jep.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Donfack ARN, Toyang NJ, Wabo HK, Tane P, Awoufack MD, Kikuchi H, Tamokou JDD, Kuiate JR, Oshima Y. Stigmatane derivatives from the root extract of Vernonia guineensis and their antimicrobial activity. Phytochemistry Letters. 2012;5:596–599. [Google Scholar]
- Fairhurst RM, Nayyar GML, Breman JG, Hallett R, Vennerstrom JL, Duong S, Ringwald P, Wellems TE, Plowe CV, Dondorp AM. Artemisinin-Resistant Malaria: Research challenges, opportunities, and public health implications. American Journal of Tropical Medicine and Hygiene. 2012;87:231–241. doi: 10.4269/ajtmh.2012.12-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwu MM. Handbook of African Medicinal Plants. CRC Press; London: 1993. p. 415p. [Google Scholar]
- Kraft C, Jenett-Siems K, Siems K, Jakupovic J, Mavi S, Bienzle U, Eich E. Herbal remedies traditionally used against malaria. In vitro antiplasmodial evaluation of medicinal plants from Zimbabwe. Phytotherapy Research. 2003;17:123–128. doi: 10.1002/ptr.1066. [DOI] [PubMed] [Google Scholar]
- Meshnick SR, Dobson M. The history of antimalarial drugs. In: Rosenthal P, editor. Antimalarial Chemotherapy. Mechanisms of Action, Resistance, and New Directions in Drug Discovery. Humana, Totowa; New Jersey: 2001. pp. 15–16. [Google Scholar]
- Moll K, Ljungström I, Perlmann H, Scherf A, Wahlgren M. MR4/ATCC. Manassas, Virginia. Paris, France: BioMalPar; 2008. Methods in malaria research. [Google Scholar]
- Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrobial Agents and Chemotherapy. 2004;48:1803–1806. doi: 10.1128/AAC.48.5.1803-1806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noumi E. Ethno medicines used for treatment of prostatic disease in Foumban, Cameroon. African Journal of Pharmacy and Pharmacology. 2010;4:793–805. [Google Scholar]
- Nyunt MM, Plowe CV. Pharmacological advances in the global control and treatment of malaria: combination therapy and resistance. Clinical Pharmacology and Therapeutics. 2007;82:601–605. doi: 10.1038/sj.clpt.6100361. [DOI] [PubMed] [Google Scholar]
- Pillay P, Vleggaar R, Maharaj VJ, Smith PJ, Lategan CA, Chouteau F, Chibale K. Antiplasmodial hirsutinolides from Vernonia staehelinoides and their utilization towards a simplified pharmacophore. Phytochemistry. 2007;68:1200–1205. doi: 10.1016/j.phytochem.2007.02.019. [DOI] [PubMed] [Google Scholar]
- Schmidt TJ, Nour AMM, Khalid SA, Kaiser M, Brun R. Quantitative structure – Antiprotozoal activity relationships of sesquiterpenes lactones. Molecules. 2009;14:2062–2076. doi: 10.3390/molecules14062062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrobial Agents Chemotherapy. 2004;48:1803–1806. doi: 10.1128/AAC.48.5.1803-1806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchinda AT, Tsopmo A, Tane P, Ayafor JF, Connolly JD, Sterner O. Vernoguinosterol and vernoguinoside, trypanocidal stigmastane derivatives from Vernonia guineensis (Asteraceae) Phytochemistry. 2002;59:371–374. doi: 10.1016/s0031-9422(01)00448-4. [DOI] [PubMed] [Google Scholar]
- Tchinda AT, Tsopmo A, Tane P, Ayafor JF, Connolly JD. Stigmatane derivatives and isovaleryl sucrose esters from Vernonia guineensis (Asteraceace) Phytochemistry. 2003;63:841–846. doi: 10.1016/s0031-9422(03)00326-1. [DOI] [PubMed] [Google Scholar]
- Toyang NJ, Wabo HK, Ateh EN, Davis H, Tane P, Kimbu SF, Sondengam LB, Bryant J. In-vitro anti-prostate cancer and ex vivo antiangiogenic activity of Vernonia guineensis Benth. (Asteraceae) tuber extracts. Journal of Ethnopharmacology. 2012a;141:866–871. doi: 10.1016/j.jep.2012.03.021. [DOI] [PubMed] [Google Scholar]
- Toyang NJ, Ateh EN, Keiser J, Vargas M, Bach H, Tane P, Sondengam LB, Davis H, Bryant J, Verpoorte R. Toxicity, antimicrobial and anthelmintic activities of Vernonia guineensis Benth. (Asteraceae) crude extracts. Journal of Ethnopharmacology. 2012b doi: 10.1016/j.jep.2012.10.016. In Press. [DOI] [PubMed] [Google Scholar]
- Toyang NJ, Wabo HK, Ateh EN, Davis H, Tane P, Sondengam LB, Bryant J, Verpoorte R. Cytotoxic and antiangiogenic sesquiterpene lactones from the leaves of Vernonia guineensis Benth. (Asteraceae) Journal of Ethnopharmacology. 2012c doi: 10.1016/j.jep.2013.01.022. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waako PJ, Katuura E, Smith P, Folb P. East African medicinal plants as a source of lead compounds for the development of new antimalarial drugs. African Journal of Ecology. 2007;45:102–106. [Google Scholar]