Abstract
Peripheral T-cell lymphomas (PTCL) are functionally and morphologically complex. EBV-positive B- cells have been reported in angioimmunoblastic T-cell lymphoma (AITL) and other PTCL and may mimic Hodgkin/Reed-Sternberg (HRS) cells, but EBV-negative HRS-like B-cells have not been described. We wished to assess the nature of the PTCL associated with HRS-like cells, and to determine whether EBV-negative HRS-like cells may be seen. We identified 57 PTCL cases reported as containing HRS-like cells. These included 32 AITL, 19 PTCL-NOS, 3 PTCL-NOS, follicular variant, 1 PTCL-NOS, T-zone variant and 2 adult T-cell leukemia/lymphoma (ATLL). All patients were adults, median age, 63, and presented with lymphadenopathy. The male: female ratio was 31:26 (1.2:1). Clonal TRG rearrangement was detected in 46/53 cases. 6/38 cases had a concomitant clonal immunoglobulin gene rearrangement. In 52/57 cases the HRS cells were positive for EBV. Five cases, three classified as AITL and two as PTCL-NOS, follicular variant, contained HRS-like cells negative for EBV. All PTCL with EBV-negative HRS cells had a TFH-immunophenotype. The neoplastic T-cells expressed CD3, CD4, and PD-1, and formed rosettes around the HRS-like cells. The HRS-like cells were positive for CD20 (variable intensity), PAX5, CD30 and CD15 (4/5). We conclude that both EBV positive and EBV negative HRS-like B-cells may occur in the background of PTCL; caution is needed to avoid misdiagnosis as CHL. The close interaction between the HRS-like cells and the rosetting PD-1-positive T-cells suggests a possible pathogenetic role in this phenomenon, and provides new insights into the abnormal B-cell proliferations that occur in the context of TFH malignancies.
Keywords: peripheral T-cell lymphoma, T-follicular helper cells, classical Hodgkin’s lymphoma, angioimmunoblastic T-cell lymphoma, Epstein Barr virus, PD-1, CD279
Introduction
Peripheral T-cell lymphomas are functionally and morphologically complex. In recent years much attention has focused on lymphomas derived from T-follicular helper cells (TFH). These include angioimmunoblastic T-cell lymphoma (AITL), but also the follicular variant of peripheral T-cell lymphoma, not otherwise specified (PTCL-NOS), and primary CD4-positive small medium cutaneous T-cell lymphoma. While all are accepted as clonal and neoplastic T-cell lymphoproliferations, there has been greater recognition in recent years of the abnormal B-cell expansions that can be a component of these tumors. This phenomenon has been described mainly in conjunction with AITL and more rarely with PTCL-NOS. Many of the B-cell lymphoproliferations are Epstein-Barr Virus (EBV) -positive, and it was postulated that the expansion of EBV-positive B-cells was related to defective immune surveillance secondary to underlying T-cell malignancy. 1–7
More recently EBV-negative B-cell expansions have been recognized, often with plasmacytic differentiation. 8,9 With the knowledge that most of the T-cell lymphomas were derived from TFH cells, it was hypothesized that the neoplastic T-cells functioned as helper cells, to promote B-cell proliferation.
In 1999, our group described Hodgkin-Reed-Sternberg (HRS)-like cells of B-cell derivation in the context of PTCL, with the majority of cases classified as AITL. 10 The HRS-like cells had the morphology and immunophenotype of classical Reed-Sternberg cells, and were EBV-positive. Other authors confirmed these observations. 4,11 Interestingly, the HRS-like cells appeared to be a transient phenomenon, perhaps due to defective immune surveillance, since the patients did not progress to clinically significant classical Hodgkin’s lymphoma (CHL). To date, instances of HRS-like cells negative for EBV are mentioned only in a report from a workshop on T-cell lymphomas, noting two such cases. 12
To better assess the nature of the T-cell lymphomas associated with HRS-like cells, and to determine if HRS-like cells negative for EBV may be seen, we reviewed all PTCL reported as containing HRS-like cells since our original report of 1999. We identified 57 mature T-cell lymphomas with HRS-like cells of B-cell lineage. Notably, in five cases, the HRS-like cells were negative for EBV (three AITL and two PTCL-NOS, follicular variant). Thus, this phenomenon cannot be attributed solely to defective surveillance for EBV, and suggests other mechanisms for the abnormal B-cell proliferation.
Material and Methods
Case selection
The pathology data base of the Hematopathology Section, Laboratory of Pathology, National Cancer Institute, was searched for mature T-cell lymphomas accrued since 1999 and reported as containing HRS-cells or a Hodgkin-like lesion. After initial review, fifty-seven T-cell lymphoma cases containing cells with the morphology and immunophenotype of HRS-cells, and the presence of one or more B-cell markers on the HRS-like cells, were chosen for this report. Histopathologic diagnosis of the T-cell malignancy was rendered by the authors according to the 2008 World Health Organization classification. 13 The study was approved by the NCI Institutional Review Board.
Immunohistochemistry studies
Immunohistochemistry studies were performed on available formalin-fixed paraffin-embedded tissue (FFPE) sections using the following antibodies: CD20, CD3, CD4, CD8, CD10, CD15, CD30, CD21, CD79a, PAX5, Oct-2, Bcl-6, MUM1, PD-1, IgD, kappa, lambda and LMP1 according to previously published techniques. 14,15 The panel of antibodies, clone, dilution and source are listed in table 1.
Table 1.
Antibodies used in the immunophenotypic analyses
| Antigen | Clone | Dilution | Source |
|---|---|---|---|
| CD3 | Polyclonal | 1:100 | Dako |
| CD4 | 1F6 | 1:40 | Novocastra |
| CD8 | C8/144B | 1:50 | Dako |
| CD10 | 56CF | 1:20 | Novocastra |
| CD15 | Leu-M1 | 1:20 | Becton Dickinson |
| CD20 | L26 | 1:200 | Dako |
| CD21 | 1F8 | 1:30 | Dako |
| CD30 | 1G12 | 1:80 | Novocastra |
| CD79a | JCB117 | 1:100 | Dako |
| PAX5 | 24 | 1:25 | BD Transl Labs |
| Oct-2 | CD-20 | 1:4000 | Santa Cruz |
| Bcl-6 | PG-B6p | 1:20 | Dako |
| MUM1 | MUM1p | 1:100 | Dako |
| PD-1 (CD279) | NAT | 1:50 | ABCAM |
| IgD | Polyclonal | 1:1000 | Dako |
| Kappa | Polyclonal | 1:25000 | Dako |
| Lambda | Polyclonal | 1:10000 | Dako |
| LMP1 | C-S 1–4 | 1:400 | Dako |
In situ hybridization for Epstein-Barr Virus (EBV) - encoded RNA (EBER)
In situ hybridization was performed on FFPE sections, using EBER1 DNP probe supplied by Ventana on an automated stainer (Ventana-Benchmark XT, Tucson, AZ). ISH iView blue plus system with alkaline phosphatase and nitroblue tetrozolium and 5-bromo-4-chloro-3-indolyl phosphate substrate, with Fast Red as contrast was used for visualization. To assess the appropriate staining, a positive control was run with the cases.
Molecular studies
Studies of T-cell receptor γ chain (TRG) and immunoglobulin heavy and/or light chain rearrangement were available in 53 and 38 cases, respectively. Polymerase chain reaction (PCR) or genomic Southern Blot analysis (case 3) were carried out on whole tissue extracts from paraffin-embedded tissue. The PCR reactions were run according to the protocols previously detailed by Venkataraman et al. 16 and Eberle et al. 15 For the TRG locus, we used primers that interrogate all of the known Vγ family members and the J1/2, JP1/2, and JP joining segments. 17 For the IGH locus, we employed consensus primers directed against the joining region (JH) with forward primers for the VH framework (FR) III and VH FR-II; for the IgK analysis, the primer sets interrogated rearrangements involving the Vκ loci and Jκ (tube A), the Vκ locus and the κDE locus (tube B), and the κ-intron RSS locus and the κDE locus (tube B) (InvivoScribe Technologies, Inc, San Diego, CA). The products were analyzed by either acrylamide gel electrophoresis, or by capillary electrophoresis on an ABI 3130xl Genetic Analyzer (Applied Biosystems, Foster City, CA).
Results
Fifty-seven cases of T-cell lymphoma were identified based on the criteria stipulated. They were further subclassified as: AITL, 32 cases; PTCL-NOS, 19 cases; PTCL-NOS, follicular variant, 3 cases; PTCL-NOS, T-zone variant, 1 case. Twenty-six percent of PTCL-NOS (5/19) expressed TFH-cell markers (CD4 and CD10 with or without Bcl-6), but lacked classical features of AITL or the follicular variant. Two cases of adult T-cell leukemia/lymphoma (ATLL) were included, one of which was previously reported.16 A summary of the demographic, clinical and molecular data is presented in table 2. All patients were adults, with a median age of 63 years (n=57). The male:female ratio was 31:26 (1.2:1). All patients presented with lymphadenopathy, with rare involvement of extranodal sites: skin (1) and tongue (1).
Table 2.
Clinical parameters and molecular status of all PTCL with HRS-like cells
| Lymphoma type |
No cases |
Age Median (range) |
Gender M/F |
Location | TRG@* | IG@* |
|---|---|---|---|---|---|---|
| AITL | 32 | 65 (31–86) | 14/18 |
#LN: 8 axilla, 8 cervical, 11 inguinal, 3 supraclavicular, 2 NOS |
25 - c 2 - pc 2 - suspicious 1 – inconclusive |
4 - c 19 - pc |
| PTCL-NOS | 19 | 62 (35–78) | 12/7 |
&LN: 11 cervical, 2 inguinal, 1 periparotid, 1 supraclavicular, 1 axilla, 1 bone marrow; 1 NOS 1 tongue |
15 - c 1 - pc 1 - suspicious |
2 - c 7 - pc 1 - suspicious |
| PTCL-NOS, Follicular variant | 3 | 57 (52–67) | 2/1 | LN: 2 cervical, 1 inguinal, axilla and skin/soft tissue |
3 - c | 2- pc |
| PTCL-NOS, T-zone | 1 | 58 | 1/0 | LN: inguinal and axilla | 1 - c | 1 - pc |
| ATLL | 2 | 59.5 (53–66) | 2/0 | LN: 2 cervical | 2 - c | 2- pc |
| Total | 57 |
HRS – Hodgkin/Reed-Sternberg; AITL – angioimmunoblastic T-cell lymphoma; PTCL-NOS – peripheral T-cell lymphoma not otherwise specified; ATLL - adult T-cell leukemia/lymphoma; M – male; F – female; LN - lymph node; TRG@ - T-cell receptor γ chain rearrangement; IG@ - immunoglobulin gene rearrangement; c – clonal; pc – polyclonal; NOS- not otherwise specified
6 cases more than one location
4 cases more than one location
not all the cases were analyzed for TRG@ and IG@ by PCR
The neoplastic nature of the T-cell proliferation was further confirmed by a clonal TRG rearrangement, which was detected in 46/53 cases. In addition, in 6/38 cases, a clonal immunoglobulin gene rearrangement was found. In 52/57 cases, the HRS-like cells were positive for EBV (Fig. 1). In all EBV-positive cases (52) the HRS-like cells showed evidence of B-cell lineage. PAX5 was strongly positive in 37% and more weakly positive in 63%. CD20 was strongly expressed in 32%; variably expressed in 32% and negative in 36%. CD30 was positive in 100% of cases. The HRS-like cells were CD15-positive in 41/51 (80%) of cases, with 12/51 (24%) showing only focal staining. CD15 was negative in 10/51 (20%).
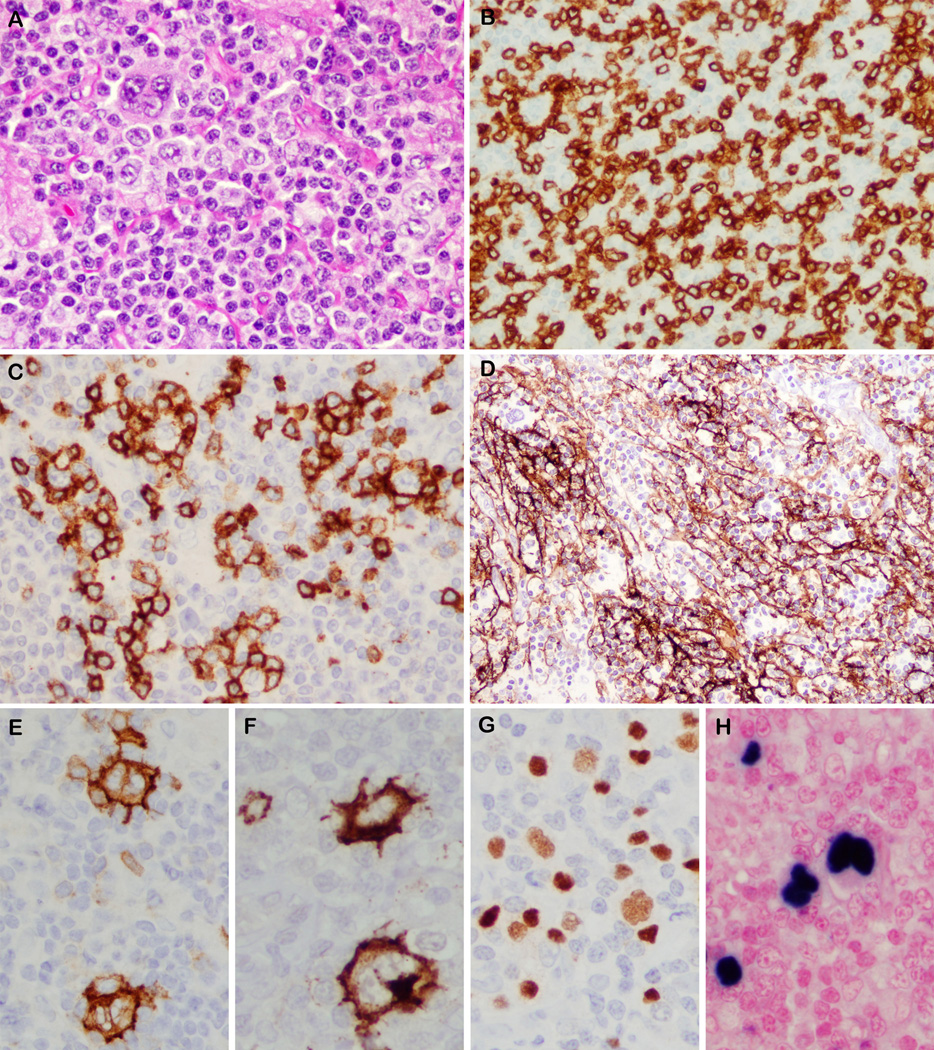
Figure 1.
Angioimmunoblastic T-cell lymphoma with EBV-positive Hodgkin-Reed Sternberg-like cells. A. A large multinucleate cell is seen in a background of atypical lymphoid cells. B. CD3 highlights the cytologic atypia in the T-cells and presence of rosettes around Hodgkin-Reed Sternberg-like cell (upper left corner). C. T-cell rosettes are positive for CD10. D. Expanded CD21-positive follicular dendritic meshworks surround multinucleated cells (upper left corner). The Hodgkin-Reed Sternberg-like cells show strong membrane positivity for CD30 (E), CD15 (F), weak nuclear staining for PAX5 (G) and EBER positivity (H).
Five cases, three classified as AITL and two as PTCL-NOS, follicular variant, contained HRS-like cells negative for EBV. The features of these cases are discussed in greater detail below.
Clinical data of EBV-negative cases
The clinical features of the EBV-negative cases are summarized in table 3.
Table 3.
Clinical features of patients with T-cell lymphoma and HRS-like cells, EBV negative
| Case no |
Age (yr)/ Sex |
Clinical presentation and Treatment |
Diagnosis | Stage | Biopsy Site(s) | Outcome (months) |
|---|---|---|---|---|---|---|
| 1 | 65/M | Presented with skin rash, weight loss, generalized lymphadenopathy, splenomegaly Treated with 2xABVD, 4x DA- EPOCH, autologous bone marrow transplant |
AITL | IV | Inguinal LN | AWoD, 3 months after transplant |
| 2 | 75/F | Presented with respiratory insufficiency, mediastinal and supraclavicular lymphadenopathy |
AITL | Not known | Supraclavicular | Not known |
| 3 | 83/F | Presented with cervical and para- aortic lymphadenopathy; no other clinical data available |
AITL# | Not known | Cervical LN | Not known |
| 4 | 51/M | Presented with cervical lymphadenopathy, no constitutional symptoms, no hepatosplenomegaly. Patient has declined therapy. |
PTCL- NOS, follicular variant |
IV | Cervical LN | AWD (12 months) |
| 5 | 67/M | Presented with skin rash, inguinal and axillary lymphadenopathy, no hepatosplenomegaly, diagnosed and treated as CHL (8xABVD); LN recurrence at 2 years treated 2xCOPP and inguinal radiation; skin/soft tissue forearm and LN recurrence 7 years later recognized as PTCL. |
PTCL- NOS, follicular variant |
IV | Inguinal LNs and skin |
AWD |
Case 3 also showed some features of PTCL, NOS, follicular variant. Abbreviations: HRS – Hodgkin/Reed- Sternberg; EBV – Ebstein-Barr Virus; M - male; F – female; AITL – angioimmunoblastic T-cell lymphoma; PTCL-NOS – peripheral T-cell lymphoma not otherwise specified; CHL – classical Hodgkin’s lymphoma; LN – lymph node; AWoD, alive without disease; AWD, alive with disease; ABVD, doxorubicin, bleomycin, vinblastine and dacarbazine; DA-EPOCH, dose-adjusted etoposide, prednisone, oncovin, cyclophosphamide and hydroxydaunorubicin; COPP - cyclophosphamide, oncovin, procarbazine, prednisone
The patients ranged in age from 51 to 83 years (median 67 years) and included 3 men and 2 women. All presented with nodal involvement and three patients had stage IV disease. One patient (case 1) presented with skin rash, weight loss, splenomegaly and biochemically had high levels of lactate dehydrogenase, alkaline phosphatase and beta 2-microglobulin. Skin rash was also seen in case 5. Clinical follow-up was available in three of five patients. One patient is in complete remission and asymptomatic three months after systemic chemotherapy and autologous bone marrow transplant. One patient has persistent lymphadenopathy 12 months after diagnosis; the patient has refused therapy at this point in time. Case 5 was initially diagnosed and treated for CHL; recurrences developed in the inguinal lymph nodes at 2 years and in the skin/soft tissue of the right forearm and axillary lymph nodes at 7 years, at which time a diagnosis of T-cell lymphoma was established. He is currently undergoing therapy.
Pathologic findings
Cases 1, 2, 3: Angioimmunoblastic T-cell lymphoma
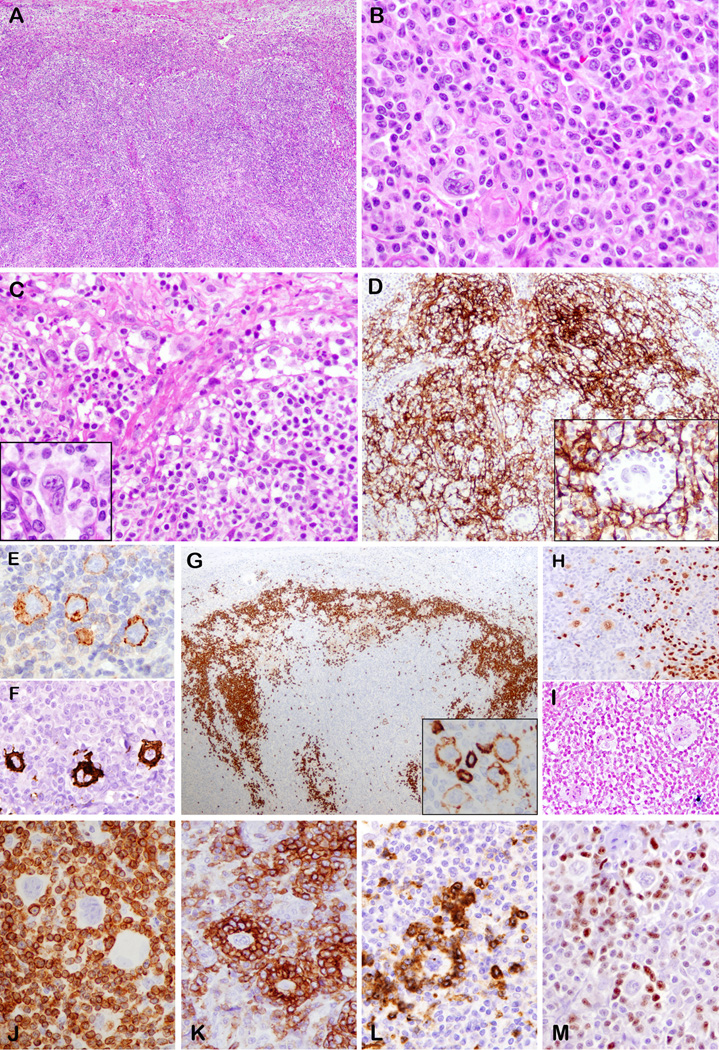
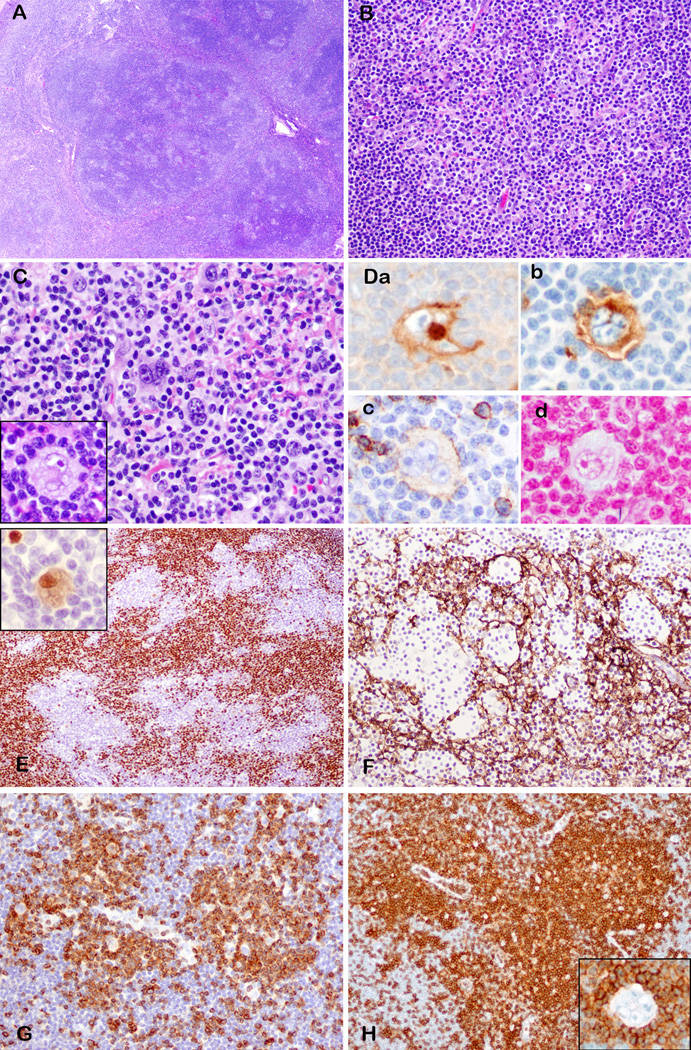
Lymph node architecture was partially effaced by a polymorphous cellular infiltrate, which focally breached the capsule involving the perinodal adipose tissue. The peripheral cortical sinus was preserved and dilated (Fig. 2A). The atypical cells were medium sized lymphocytes with round to angulated nuclei and abundant pale cytoplasm (Fig. 2B), and clustered around arborizing high endothelial venules. Hodgkin-like cells with abundant pale cytoplasm and uni-, bi or multilobated nuclei with prominent eosinophilic or basophilic nucleoli were distributed in the paracortex, amidst the paracortex. (Fig. 2B). In all three cases, the atypical HRS-like cells also had a focal intrasinusoidal distribution (Fig. 2C, detail in inset). The background contained small lymphocytes, histiocytes, plasma cells, eosinophils and rare polymorphonuclear leukocytes. Regressed and peripheralized follicles were observed (case 1 and 2), corresponding to AITL type III as per Attygalle et al. 18
Figure 2.
Angioimmunoblastic T-cell lymphoma with EBV-negative Hodgkin-Reed Sternberg-like cells (cases 1 and 2). A. Effaced lymph node with paracortical expansion and preserved and dilated peripheral cortical sinus. B. Polymorphous infiltrate composed of pleomorphic cells with uni or multilobated nuclei and prominent nucleoli, resembling Hodgkin-Reed Sternberg cells admixed with medium sized atypical lymphocytes. C. Dilated subcapsular sinus with atypical Hodgkin-Reed Sternberg-like cells (detail in inset) D. Hodgkin-Reed Sternberg-like cells (detail in inset) are embedded in expanded CD21-positive follicular dendritic cell meshworks. They are positive for CD30 (E), CD15 (F), variably positive for CD20 (G inset), weakly positive for PAX5 (H), but negative for EBER (I). G. CD20 stain shows also marginalized B-cell areas in the far cortex. The Hodgkin-Reed Sternberg-like cells are rosetted by neoplastic T-cells positive for CD3 (J), PD-1 (K), CD10 (L). M. HRS-cells are negative for Bcl-6, which stains some background T-cells. (A, B, C, F, G, H, K, L M – case 1; D, E I, J –case 2)
Cases 4 and 5: PTCL-NOS, follicular variant
The nodal architecture was altered by multiple irregular, disrupted follicles, reminiscent of progressive transformation of germinal centers (Fig. 3A). The nodules corresponded to expanded follicular mantles, highlighted by CD20 and IgD. Within the nodules, clusters and larger aggregates of small-medium sized T-lymphocytes with moderate pale cytoplasm and slight nuclear variability were seen (Fig. 3B, C). Amidst the T-cell nodules and in the focally expanded paracortex, there were single scattered large atypical lymphoid cells reminiscent of HRS cells (Fig. 3C, detail inset). The appearance resembled lymphocyte-rich CHL, imparting a moth-eaten appearance within the nodules. The background lymphocytes in the paracortex showed some atypia, but the atypical cytology was more readily appreciated in CD3-immunostained sections (Figure 4). A rich inflammatory background, characterized by varying numbers of eosinophils, neutrophils and plasma cells was also identified in case 5.
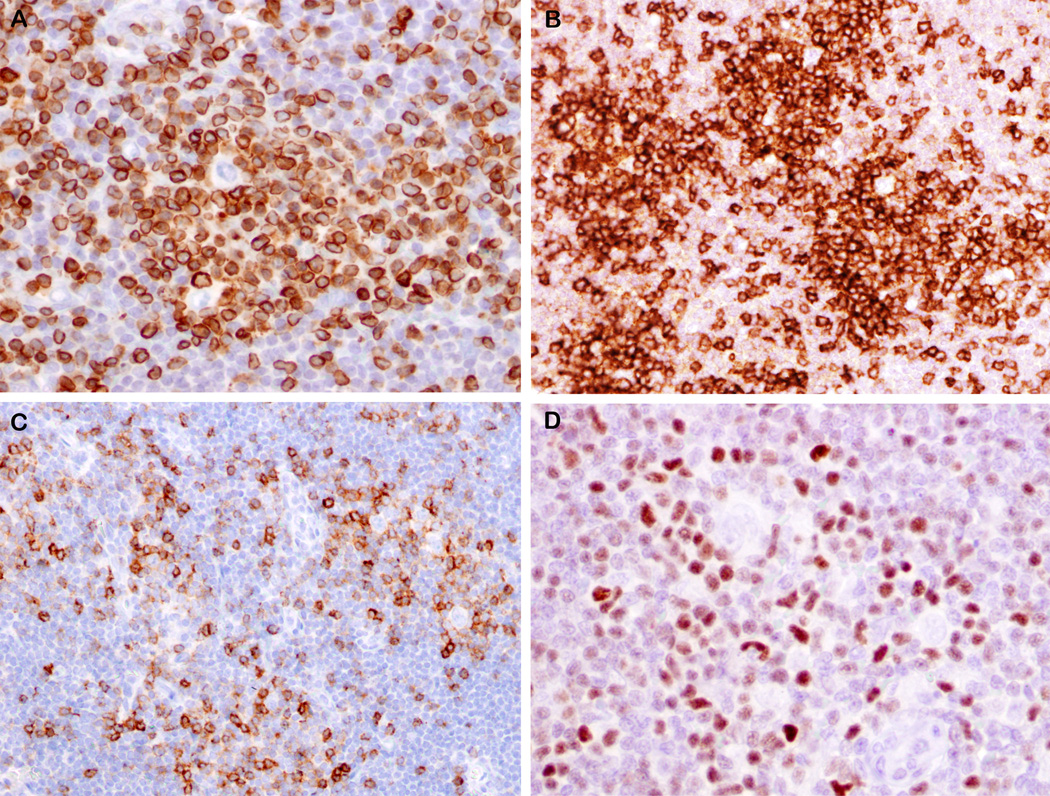
Figure 3.
Peripheral T-cell lymphoma not otherwise specified, follicular variant, with Hodgkin-Reed Sternberg-like cells, EBV negative (case 4 and 5). A. The nodal architecture is altered by multiple irregular, disrupted follicles. B. Pale cluster of small-medium sized T-lymphocytes within the large nodule. C. Pleomorphic mono or binucleate Hodgkin-Reed Sternberg-like cells are seen amidst atypical T-cells (detail in inset). Hodgkin-Reed Sternberg-like cells strongly express CD30 (Da), CD15 (Db), weakly CD20 (Dc) and are negative for EBER (Dd). They are also weak positive for PAX5 (E inset). PAX5 (E) and CD21 (F) show the moth eaten appearance of nodules created by the atypical T-cell clusters positive for CD3 (G) and CD4 (H). Hodgkin-Reed Sternberg-like cells are rosetted by CD3 (G) and CD4 (H inset) positive T-cells. (A, B, C, E, F – case 4; D, G, H - case 5).
Figure 4.
Features of neoplastic T-cells in cases of PTCL, follicular variant, with EBV-negative HRS-like cells. A. CD3 immunostain highlights a large aggregate of atypical T-cells showing variation in nuclear size and shape. B. Several nodules of atypical T-cells are highlighted by PD-1 immunostain. Two nodules on right contain HRS-like cells. C. Many of the atypical T-cells are positive for CD10, with some rosetting HRS-like cells. D. Similarly, the atypical T-cells are positive for Bcl-6, while HRS-like cell is negative. The large size of the T-cell aggregates, and the atypical immunophenotype (CD10-posiitve, Bcl-6-positive, strong and uniform PD-1) are clues against the diagnosis of lymphocyte-rich CHL. (A, B, C – case 4; D – case 5).
Sections of the right forearm lesion (case 5) showed fibro-adipose tissue with a vaguely nodular atypical small lymphocytic infiltrate. In contrast to the prior lymph node biopsies, there were only rare HRS-like cells. Numerous eosinophils, histiocytes and focally prominent vessels were also present in the background.
Case 3, was notable for presence of overlapping features between AITL and the follicular variant of PTCL. It showed intrafollicular accumulations of atypical T-cells which surrounded the HRS-like cells. However, it was classified as AITL, based on dilated subcapsular sinuses, a similar population of cells in the paracortex mainly in association with a hyperplastic vascular network, and expanded FDC meshworks around the high endothelial venules.
Immunophenotypic findings
The immunophenotype of the malignant T-cells and HRS-like cells in all five cases is summarized in Table 4 and illustrated in Fig. 2, 3 and 4.
Table 4.
Immunophenotype of neoplastic T cell and HRS-like cells in the 5 EBV-negative T-cell lymphomas (cases 1, 2, 3 - AITL; cases 4, 5 - PTCL-NOS, follicular variant).
| Neoplastic T-cell phenotype | HRS-like cell phenotype | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | Case 2 | Case 3# | Case 4 | Case 5 | Case 1 | Case 2 | Case 3# | Case 4 | Case 5 | |
| CD20 | - | - | - | - | - | + v | + v | + v | + v | + v |
| PAX5 | - | - | - | - | - | + v | + v | + v | + w | + w |
| Oct-2 | - | - | - | + s | + f, w | + f | + f, w | |||
| CD79a | - | - | - | + f, w | + f, w | + f, w | ||||
| Kappa | - | - | - | - | - | - | ||||
| Lambda | - | - | - | - | - | - | ||||
| CD30 | + f | - | + f | - | - | + | + | + | + | + |
| CD15 | - | - | - | - | - | + | - | + f | + f | + |
| CD3 | + r | + cp, r | + w, r | + r | + r | - | - | - | - | - |
| CD4 | + r | + r | + r | + r | - | - | - | - | ||
| CD8 | - | - | - | - | - | - | ||||
| PD-1 | + r | + r | + r | + r | - | - | - | - | ||
| CD10 | + f, r | + f | + f | + f, r | - | - | - | - | - | - |
| Bcl-6 | + f, r | + f | +f | - | - | - | ||||
| MUM1 | - | - | - | + | + | + | ||||
| LMP1 | - | - | - | - | - | - | ||||
| CD21 | Expanded FDC |
Expanded FDC |
Expanded FDC |
Residual FDC |
Residual FDC |
- | - | - | - | - |
Abbreviations: HRS – Hodgkin/Reed-Sternberg; EBV – Ebstein-Barr Virus; AITL – angioimmunoblastic T-cell lymphoma; PTCL-NOS – peripheral T-cell lymphoma not otherwise specified; FDC –follicular dendritic cells; s- strong, w – weak, v – variable intensity, f – focal, r – rosettes, cp – cytoplasmic.
Case 3 also showed some features of PTCL, NOS, follicular variant.
Independent of the architectural pattern the atypical lymphoid cells in all cases had a TFH-immunophenotype. The cells were positive for CD3 (Fig. 2J, 3G, 4A), CD4 (Fig. 3H) and PD-1 (Fig. 2K, 4B) and negative for CD8. In case 3, CD3 was noticeably weaker in the neoplastic cells than in background lymphocytes. The atypical cells showed immunoreactivity for CD10 (case 1, 2, 3 and 4) (Fig. 2L, 4C) and Bcl-6 (case 1, 4 and 5) (Fig. 2M, 4D). Scattered clusters of T-cells were additionally positive for CD30 (case 1 and 3).
In all cases, HRS-like cells demonstrated strong membrane staining for CD30 (Fig. 2E, 3Da). All cases except one (case 2) showed at least focal positivity for CD15 (Fig. 2F, 3Db). CD20 stain showed a variable reactivity in all five cases (Fig. 2G inset, 3Dc). PAX5 was either of variable intensity (cases 1, 2 and 3) (Fig. 2H) or uniformly weak compared with the small B lymphocytes (cases 4 and 5) (Fig 3E, detail inset). HRS-like cells lacked expression of T-cell markers, but were rosetted by CD3 (Fig. 2J, 3G, 4A), CD4 (Fig. 3H, detail inset), PD-1 (Fig. 2K, 4B) and CD10 (Fig 2L, 4C) positive atypical T-cells (see table 4). They were negative for LMP1 in the cases studied (case 1, 3 and 4). In cases with available material, HRS-like cells also displayed positivity for CD79a (case 1, 2 and 5), Oct-2 (1, 2, 4 and 5), and MUM1/IRF4 (case 1, 4 and 5. HRS-cells did not show staining for Bcl-6 and immunoglobulin light chains.
CD20 and PAX5 revealed eitherregressed and peripheralized B-cell areas (case 1 and 2) (Fig. 2G) or expanded, disrupted, moth eaten ill-defined primary follicles, (3, 4 and 5) (Fig. 3E), which were also positive for IgD (case 4 and 5). CD21 demonstrated expansion of the FDC meshworks (case 1 and 2) (Fig. 2D). In the cases classified as PTCL, follicular variant, the FDC meshworks encircled clusters of atypical T-cells within the B nodules (case 4, 5) (Fig. 3F). In case 3, FDC meshworks were expanded around the high endothelial venules, but also defined clusters of atypical cells within the follicles.
In situ hybridization
HRS-like cells were negative for EBER ISH in all five cases (Fig. 2I, 3Dd). Occasional (cases 3, 4 and 5) or numerous (cases 1 and 2) bystander small lymphocytes were positive.
Molecular findings
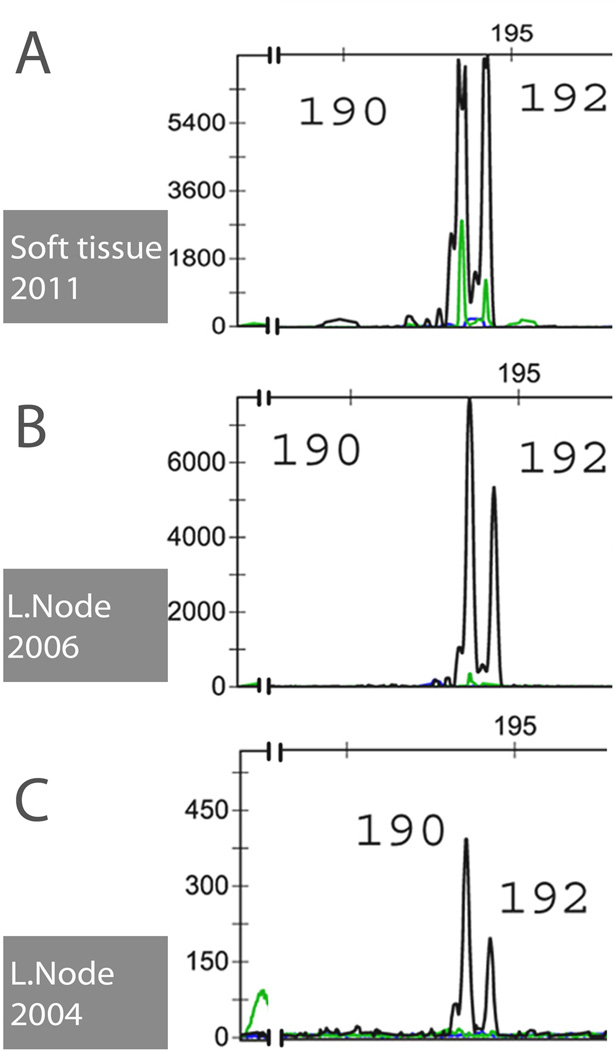
All five cases showed clonal TRG rearrangement. PCR identified bands of identical size in the lymph node and bone marrow biopsy in case 1, and identical peaks in the multiple biopsies of case 5 (Fig. 5), which indicated a common T-cell clone throughout the course. IG gene PCR was negative for clonality in all four cases examined.
Figure 5.
PCR studies of PTCL-NOS, follicular variant, containing HRS-like cells (Case 5). TRG PCR identifies two identical peaks, consistent with a clonal rearrangement in all three specimens tested: soft tissue 2011 (A), inguinal lymph nodes 2006 (B) and 2004 (C). This confirms that the same disease process is present in different sites and at different time points at molecular level. Color code of interrogated joining segments of TCR locus: black – J1/2; green – JP1/2 and blue – JP.
Discussion
EBV-positive B-cells are increased in many nodal T-cell lymphomas, but have been noted to be a characteristic feature of AITL for many years. 7 It was hypothesized that EBV-positive cells were expanded due to defects in immune surveillance, although EBV-positive cells can be abundant very early in the course, and may dominate the histological picture in some cases. 6,19 In 1999, Quintanilla-Martinez et al. noted that the EBV-positive cells could assume both the morphology and immunophenotype of HRS-cells. 10 This occurrence created diagnostic challenges because some of these cases were mistaken for CHL. Since our original report in 1999, we wished to assess the nature of the underlying T-cell lymphomas, and to examine more closely the relationship between the HRS-like cells and the neoplastic population. The current study confirms that HRS-like cells are mainly seen in AITL. 4,10,11. In the current series, 32/57 cases were classified as AITL, and another 3 were diagnosed as the follicular variant of PTCL-NOS, a tumor that like AITL is derived from TFH cells. In addition, 26% of PTCL-NOS expressed TFH-cell markers, but lacked classical features of AITL or the follicular variant.
It has generally been assumed that this process is passive, with the T-cell lymphoma allowing emergence of EBV-infected B-cells. This scenario also explains the development of EBV-positive HRS-like cells in ATLL, a lymphoma associated with marked immunosuppression. 16,20 However, in our review of 57 cases of T-cell lymphoma with HRS-like cells, we encountered 5 cases in which the HRS-like cells were negative for EBV. Thus, other pathogenetic mechanism must be sought. Notably, all five EBV-negative cases were T-cell lymphomas of TFH origin, three classified as AITL and two as PTCL-NOS, follicular variant.
Two main pathways can be hypothesized. First, the HRS-like cells could be driven by the microenvironment. In particular in the EBV-negative cases, but also in some of the EBV-positive cells, the neoplastic CD4-positive, PD-1-positive neoplastic T-cells intimately rosetted the HRS-like cells (case 1, 2, 4 and 5). TFH cells play a critical role in the generation of T-cell dependent B-cell responses, and promote the expansion of B-cells in the immune response.21 Thus, continued TFH help might aberrantly expand B-cells outside of normal physiological control. Another function of PD-1 is its interaction with its ligand, PDL-1, which helps to maintain an immunosuppressive environment. 22 Current tumor immunology trials are exploring ways to block PD-1, and promote anti-tumor activity by normal immune cells.22 Studies have shown that PDL-1 can be upregulated on the cells of Hodgkin’s lymphoma and EBV-positive post-transplant lymphoproliferative disease. 23 Thus, rosetting by PD-1 positive T-cells in these lymphomas might be protecting aberrant B-cell clones from immune surveillance, leading to emergence of the HRS-like cells we observed.
A second hypothesis, although somewhat less likely, is that EBV may be involved in a hit and run mechanism. 24 We saw frequent EBV-positive small lymphocytes in two of five cases, although the HRS-like cells were negative. Thus, the virus might be seen very early in the course of disease, 6 and might persist or disappear during disease progression. 25
Interestingly, as noted, all cases with EBV-negative HRS-like cells were T-cell lymphomas of TFH origin. Two were classified as AILT, and two as PTCL-NOS, follicular variant. A third case had overlapping features of both subtypes. While intrafollicular clusters of neoplastic T-cells were noted, the paracortical regions showed features of AITL with increased vascularity and expansion of FDC meshworks around high endothelial venules. Thus, our results confirm the close relationship of AITL and PTCL-follicular variant, and suggest that they may be different phases of the same condition. 5,26–29 The expansion of PD-1 positive lymphocytes may be playing a similar pathophysiological role in both lymphoma subtypes.
Regardless of EBV status, the HRS-like cells in T-cell lymphomas resembled their counterparts in classical Hodgkin lymphoma both morphologically and immunophenotypically. The HRS-like cells in our entire series were invariably positive for CD30 and the vast majority (45/56 studied) were positive for CD15, similar to earlier reports.4,10 However, one difference is that the B-cell program tends to be more completely preserved in the Hodgkin-like cells, in comparison with HRS cells of CHL. CD20 was expressed on the HRS-cells in 67% of cases, and in the majority of the positive cases (56%) nearly all of the HRS-like cells were positive to some degree, irrespective of EBV-status. As expected, PAX5 was uniformly positive, although it tended to be weakly expressed. Although CD79a was not uniformly investigated, interestingly it was positive in all three of the EBV-negative cases tested.
It can be challenging to distinguish CHL from T-cell lymphoma with HRS-like cells. The diagnosis of CHL was strongly considered in all five cases, and in one case (case 5), the patient was diagnosed and treated for CHL. It was only at the time of the second recurrence with subcutaneous involvement that the diagnosis of T-cell lymphoma was appreciated, when atypia of the T-cells was more apparent and interestingly, HRS-like cells were decreased.
The appearance most closely resembled the nodular variant of lymphocyte-rich CHL in cases 4 and 5, in which the HRS-like cells were observed within IgD-positive B-cell nodules resembling expanded mantle zones or primary follicles.30,31 A clue to the diagnosis was that the HRS-like cells were seen amidst sizable aggregates of T-cells (Fig. 4), rather than the single layer of rosetting T-cells characteristic of lymphocyte-rich CHL. Additionally, at least some of the T-cells in these aggregates expressed CD10 and Bcl-6, and all were strongly positive for PD-1. The diagnosis of AITL with HRS-like cells was easier to reach in cases 1 and 2, which contained atypical T-cells with clear cytoplasm, prominent vascularity, and regressed B-cell areas confined to the far cortex. 5 In 3 cases intrasinusoidal HRS-like cells also were observed, sometimes in clusters, a feature very rare in CHL. In all cases cytological atypia of the T-cells was easier to appreciate in immunostained sections.
The diagnosis of composite lymphoma can also be questioned. However, a composite lymphoma involving both T and B-cell lineages is an unusual event, 32 and only exceptional reports describe occurrence of CHL EBV positive in the natural history of AITL. 5,6,33 The intimate association of HRS-like cells with the T-neoplastic proliferation and not with reactive-appearing T lymphocytes in an independent area makes the diagnosis of a composite lymphoma unlikely.34 The situation is somewhat analogous to the EBV-positive HRS-like cells observed in chronic lymphocytic leukemia (CLL) lymph nodes.35 If they are present in a background of CLL cells, and lack the inflammatory milieu of CHL, the diagnosis of composite lymphoma is not favored. 13,36
Neoplastic T-cells resembling Hodgkin’s cells can be observed in PTCL of diverse types, and some cases of PTCL may mimic CHL immunophenotypically, with expression of CD30 and CD15.14,15 Careful assessment of the immunophenotype will help in the recognition of such cases, as the Hodgkin-like cells will display T-cell associated antigens, and be negative for PAX5 or other B-lineage markers. Rare cases of CHL can aberrantly express T-cell antigens, but most are readily identified as CHL, 37,38 and will be negative for T-cell gene rearrangement by molecular studies.
The clinical significance of EBV-negative HRS-like cells in the context of T-cell lymphoma is uncertain. Similar to their EBV-positive counterparts, they could represent just an epiphenomenon, with eventual disappearance in the evolution of disease. 8,25 None of our cases showed histological progression to CHL or another B-cell lymphoma, although follow-up was limited to three cases. In our fifth case, the HRS-like cells decreased in number over the time, but did not disappear completely during the seven years of follow-up. It has been suggested that EBV-negative B-cell proliferations in AITL and other PTCL are more dependent on the T-cell component and subsequently more unstable and sensitive to therapy.8 Finally, they may represent a starting point for development of an EBV-positive or negative B-cell lymphoma. This possibility is supported by previous reports, which documented expansion of EBV-negative B-cell clones in AITL 3,5,6 as well as development of EBV-positive B-cell lymphomas in some cases in which the original AILT lacked detectable EBV-positive cells. 1,39
ACKNOWLEDGMENTS
This study was supported by the intramural research program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health. No other funding was received.
The authors would like to thank the following physicians who contributed clinical information or case materials utilized in this report: Dr. Norma E. Tartas, Alexander Fleming Institute, Buenos Aires, Argentina; Dr. Brian Berry, Royal Jubilee Hospital, Victoria, BC, Canada; Dr. Jeffrey Schrager, Integrated Oncology, New York, NY; Dr. Rachel Robbins, North Shore University Hospital, Glen Cove, NY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to disclose.
Bibliography
- 1.Abruzzo LV, Schmidt K, Weiss LM, et al. B-cell lymphoma after angioimmunoblastic lymphadenopathy: a case with oligoclonal gene rearrangements associated with Epstein-Barr virus. Blood. 1993;82:241–246. [PubMed] [Google Scholar]
- 2.Higgins JP, van de Rijn M, Jones CD, Zehnder JL, Warnke RA. Peripheral T-cell lymphoma complicated by a proliferation of large B cells. Am J Clin Pathol. 2000;114:236–247. doi: 10.1309/72CM-KAXF-66DE-4XVA. [DOI] [PubMed] [Google Scholar]
- 3.Brauninger A, Spieker T, Willenbrock K, et al. Survival and clonal expansion of mutating "forbidden" (immunoglobulin receptor-deficient) epstein-barr virus-infected b cells in angioimmunoblastic t cell lymphoma. J Exp Med. 2001;194:927–940. doi: 10.1084/jem.194.7.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zettl A, Lee SS, Rudiger T, et al. Epstein-Barr virus-associated B-cell lymphoproliferative disorders in angloimmunoblastic T-cell lymphoma and peripheral T-cell lymphoma, unspecified. Am J Clin Pathol. 2002;117:368–379. doi: 10.1309/6UTX-GVC0-12ND-JJEU. [DOI] [PubMed] [Google Scholar]
- 5.Attygalle AD, Kyriakou C, Dupuis J, et al. Histologic evolution of angioimmunoblastic T-cell lymphoma in consecutive biopsies: clinical correlation and insights into natural history and disease progression. Am J Surg Pathol. 2007;31:1077–1088. doi: 10.1097/PAS.0b013e31802d68e9. [DOI] [PubMed] [Google Scholar]
- 6.Willenbrock K, Brauninger A, Hansmann ML. Frequent occurrence of B-cell lymphomas in angioimmunoblastic T-cell lymphoma and proliferation of Epstein-Barr virus-infected cells in early cases. Br J Haematol. 2007;138:733–739. doi: 10.1111/j.1365-2141.2007.06725.x. [DOI] [PubMed] [Google Scholar]
- 7.Weiss LM, Jaffe ES, Liu XF, Chen YY, Shibata D, Medeiros LJ. Detection and localization of Epstein-Barr viral genomes in angioimmunoblastic lymphadenopathy and angioimmunoblastic lymphadenopathy-like lymphoma. Blood. 1992;79:1789–1795. [PubMed] [Google Scholar]
- 8.Balague O, Martinez A, Colomo L, et al. Epstein-Barr virus negative clonal plasma cell proliferations and lymphomas in peripheral T-cell lymphomas: a phenomenon with distinctive clinicopathologic features. Am J Surg Pathol. 2007;31:1310–1322. doi: 10.1097/PAS.0b013e3180339f18. [DOI] [PubMed] [Google Scholar]
- 9.Huppmann A, Roullet M, Raffeld M, Jaffe E. Angioimmunoblastic T-cell lymphoma partially obscured by an EBV-negative clonal plasma cell proliferation. Journal of Clinical Oncology. doi: 10.1200/JCO.2012.43.3797. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quintanilla-Martinez L, Fend F, Moguel LR, et al. Peripheral T-cell lymphoma with Reed-Sternberg-like cells of B-cell phenotype and genotype associated with Epstein-Barr virus infection. Am J Surg Pathol. 1999;23:1233–1240. doi: 10.1097/00000478-199910000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Xu Y, McKenna RW, Hoang MP, Collins RH, Kroft SH. Composite angioimmunoblastic T-cell lymphoma and diffuse large B-cell lymphoma: a case report and review of the literature. Am J Clin Pathol. 2002;118:848–854. doi: 10.1309/VD2D-98ME-MB3F-WH34. [DOI] [PubMed] [Google Scholar]
- 12.Warnke RA, Jones D, Hsi ED. Morphologic and immunophenotypic variants of nodal T-cell lymphomas and T-cell lymphoma mimics. Am J Clin Pathol. 2007;127:511–527. doi: 10.1309/QBLAMA321K9AD2XK. [DOI] [PubMed] [Google Scholar]
- 13.Swerdlow SHCE, Harris NL, editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: International Agency for Research on Cancer; 2008. [Google Scholar]
- 14.Barry TS, Jaffe ES, Sorbara L, Raffeld M, Pittaluga S. Peripheral T-cell lymphomas expressing CD30 and CD15. Am J Surg Pathol. 2003;27:1513–1522. doi: 10.1097/00000478-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Eberle FC, Song JY, Xi L, et al. Nodal involvement by cutaneous CD30-positive T-cell lymphoma mimicking classical Hodgkin lymphoma. Am J Surg Pathol. 2012;36:716–725. doi: 10.1097/PAS.0b013e3182487158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venkataraman G, Berkowitz J, Morris JC, Janik JE, Raffeld MA, Pittaluga S. Adult T-cell leukemia/lymphoma with Epstein-Barr virus-positive Hodgkin-like cells. Hum Pathol. 2011;42:1042–1046. doi: 10.1016/j.humpath.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawnicki LC, Rubocki RJ, Chan WC, Lytle DM, Greiner TC. The distribution of gene segments in T-cell receptor gamma gene rearrangements demonstrates the need for multiple primer sets. The Journal of molecular diagnostics : JMD. 2003;5:82–87. doi: 10.1016/s1525-1578(10)60456-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Attygalle A, Al-Jehani R, Diss TC, et al. Neoplastic T cells in angioimmunoblastic T-cell lymphoma express CD10. Blood. 2002;99:627–633. doi: 10.1182/blood.v99.2.627. [DOI] [PubMed] [Google Scholar]
- 19.Dunleavy K, Wilson WH, Jaffe ES. Angioimmunoblastic T cell lymphoma: pathobiological insights and clinical implications. Curr Opin Hematol. 2007;14:348–353. doi: 10.1097/MOH.0b013e328186ffbf. [DOI] [PubMed] [Google Scholar]
- 20.Ohshima K, Karube K, Hamasaki M, et al. Imbalances of chemokines, chemokine receptors and cytokines in Hodgkin lymphoma: classical Hodgkin lymphoma vs. Hodgkin-like ATLL. Int J Cancer. 2003;106:706–712. doi: 10.1002/ijc.11301. [DOI] [PubMed] [Google Scholar]
- 21.Ma CS, Deenick EK, Batten M, Tangye SG. The origins, function, and regulation of T follicular helper cells. J Exp Med. 2012;209:1241–1253. doi: 10.1084/jem.20120994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Current Opinion in Immunology. 2012;24:207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green MR, Rodig S, Juszczynski P, et al. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: implications for targeted therapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:1611–1618. doi: 10.1158/1078-0432.CCR-11-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Razzouk BI, Srinivas S, Sample CE, Singh V, Sixbey JW. Epstein-Barr Virus DNA recombination and loss in sporadic Burkitt's lymphoma. J Infect Dis. 1996;173:529–535. doi: 10.1093/infdis/173.3.529. [DOI] [PubMed] [Google Scholar]
- 25.Zaki MA, Wada N, Kohara M, et al. Presence of B-cell clones in T-cell lymphoma. Eur J Haematol. 2011;86:412–419. doi: 10.1111/j.1600-0609.2011.01597.x. [DOI] [PubMed] [Google Scholar]
- 26.Ikonomou IM, Tierens A, Troen G, et al. Peripheral T-cell lymphoma with involvement of the expanded mantle zone. Virchows Arch. 2006;449:78–87. doi: 10.1007/s00428-005-0123-z. [DOI] [PubMed] [Google Scholar]
- 27.Huang Y, Moreau A, Dupuis J, et al. Peripheral T-cell lymphomas with a follicular growth pattern are derived from follicular helper T cells (TFH) and may show overlapping features with angioimmunoblastic T-cell lymphomas. Am J Surg Pathol. 2009;33:682–690. doi: 10.1097/PAS.0b013e3181971591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhan HQ, Li XQ, Zhu XZ, Lu HF, Zhou XY, Chen Y. Expression of follicular helper T cell markers in nodal peripheral T cell lymphomas: a tissue microarray analysis of 162 cases. J Clin Pathol. 2011;64:319–324. doi: 10.1136/jcp.2010.084459. [DOI] [PubMed] [Google Scholar]
- 29.Agostinelli C, Hartmann S, Klapper W, et al. Peripheral T cell lymphomas with follicular T helper phenotype: a new basket or a distinct entity? Revising Karl Lennert's personal archive. Histopathology. 2011;59:679–691. doi: 10.1111/j.1365-2559.2011.03981.x. [DOI] [PubMed] [Google Scholar]
- 30.Ashton-Key M, Thorpe PA, Allen JP, Isaacson PG. Follicular Hodgkin's disease. Am J Surg Pathol. 1995;19:1294–1299. [PubMed] [Google Scholar]
- 31.Anagnostopoulos I, Hansmann ML, Franssila K, et al. European Task Force on Lymphoma project on lymphocyte predominance Hodgkin disease: histologic and immunohistologic analysis of submitted cases reveals 2 types of Hodgkin disease with a nodular growth pattern and abundant lymphocytes. Blood. 2000;96:1889–1899. [PubMed] [Google Scholar]
- 32.Abruzzo LV, Griffith LM, Nandedkar M, et al. Histologically discordant lymphomas with B-cell and T-cell components. Am J Clin Pathol. 1997;108:316–323. doi: 10.1093/ajcp/108.3.316. [DOI] [PubMed] [Google Scholar]
- 33.Tan LH, Tan SY, Tang T, et al. Angioimmunoblastic T-cell lymphoma with hyperplastic germinal centres (pattern 1) shows superior survival to patterns 2 and 3: a meta-analysis of 56 cases. Histopathology. 2012;60:570–585. doi: 10.1111/j.1365-2559.2011.04097.x. [DOI] [PubMed] [Google Scholar]
- 34.Gualco G, Chioato L, Van Den Berg A, Weiss LM, Bacchi CE. Composite lymphoma: EBV-positive classic Hodgkin lymphoma and peripheral T-cell lymphoma: a case report. Appl Immunohistochem Mol Morphol. 2009;17:72–76. doi: 10.1097/pai.0b013e31817c551f. [DOI] [PubMed] [Google Scholar]
- 35.Momose H, Jaffe ES, Shin SS, Chen YY, Weiss LM. Chronic lymphocytic leukemia/small lymphocytic lymphoma with Reed-Sternberg-like cells and possible transformation to Hodgkin's disease. Mediation by Epstein-Barr virus. Am J Surg Pathol. 1992;16:859–867. doi: 10.1097/00000478-199209000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Mao Z, Quintanilla-Martinez L, Raffeld M, et al. IgVH mutational status and clonality analysis of Richter's transformation: diffuse large B-cell lymphoma and Hodgkin lymphoma in association with B-cell chronic lymphocytic leukemia (B-CLL) represent 2 different pathways of disease evolution. Am J Surg Pathol. 2007;31:1605–1614. doi: 10.1097/PAS.0b013e31804bdaf8. [DOI] [PubMed] [Google Scholar]
- 37.Tzankov A, Bourgau C, Kaiser A, et al. Rare expression of T-cell markers in classical Hodgkin's lymphoma. Mod Pathol. 2005;18:1542–1549. doi: 10.1038/modpathol.3800473. [DOI] [PubMed] [Google Scholar]
- 38.Asano N, Oshiro A, Matsuo K, et al. Prognostic significance of T-cell or cytotoxic molecules phenotype in classical Hodgkin's lymphoma: a clinicopathologic study. J Clin Oncol. 2006;24:4626–4633. doi: 10.1200/JCO.2006.06.5342. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi T, Maruyama R, Mishima S, et al. Small bowel perforation caused by Epstein-Barr virus-associated B cell lymphoma in a patient with angioimmunoblastic T-cell lymphoma. J Clin Exp Hematop. 2010;50:59–63. doi: 10.3960/jslrt.50.59. [DOI] [PubMed] [Google Scholar]