Abstract
At therapeutic doses, acetaminophen (APAP) is a safe and effective analgesic. However, overdose of APAP is the principal cause of acute liver failure in the West. Binding of the reactive metabolite of APAP (NAPQI) to proteins is thought to be the initiating event in the mechanism of hepatotoxicity. Early work suggested that APAP-protein binding could not occur without glutathione (GSH) depletion, and likely only at toxic doses. Moreover, it was found that protein-derived APAP-cysteine could only be detected in serum after the onset of liver injury. On this basis, it was recently proposed that serum APAP-cysteine could be used as diagnostic marker of APAP overdose. However, comprehensive dose-response and time course studies have not yet been done. Furthermore, the effects of co-morbidities on this parameter have not been investigated. We treated groups of mice with APAP at multiple doses and measured liver GSH and both liver and plasma APAP-protein adducts at various timepoints. Our results show that protein binding can occur without much loss of GSH. Importantly, the data confirm earlier work that showed that protein-derived APAP-cysteine can appear in plasma without liver injury. Experiments performed in vitro suggest that this may involve multiple mechanisms, including secretion of adducted proteins and diffusion of NAPQI directly into plasma. Induction of liver necrosis through ischemia-reperfusion significantly increased the plasma concentration of protein-derived APAP-cysteine after a subtoxic dose of APAP. While our data generally support the measurement of serum APAP-protein adducts in the clinic, caution is suggested in the interpretation of this parameter.
Keywords: acetaminophen, hepatotoxicity, protein adducts, glutathione, cellular adduct release
INTRODUCTION
Acetaminophen (APAP) is one of the most commonly used drugs in the U.S. (Kaufman et al., 2002). At therapeutic doses it is a safe and effective analgesic, but high doses can cause severe liver injury. In fact, APAP overdose is the chief cause of acute liver failure throughout the West (Bernal, 2003; Gow et al., 2004; Larson et al., 2005; Canbay et al., 2009). Etiology may be a critical consideration in the treatment of patients with liver injury, particularly with regard to the need for liver transplant. APAP overdose patients are likely to recover without a new organ (Simpson et al., 2009), and it is advisable to avoid transplant whenever possible because of the lifelong immunosuppression and associated costs that follow. Moreover, diagnosis of intentional overdose can help clinicians to ensure that a patient receives proper psychiatric care, as non-fatal drug intoxication is a predictor of later suicide (Qin et al., 2009). Currently, diagnosis of APAP hepatotoxicity is made on the basis of patient-reported history and serum APAP concentration. The former can be unreliable, while the latter is limited by the relatively short serum half-life of the parent drug (1.5 – 3 h at therapeutic doses [Nelson and Moriaka, 1963; Cummings et al., 1967], about 6 h at hepatotoxic doses, and about 18 h at fatal doses [Schiødt et al., 2002]). Thus, a better diagnostic marker would be clinically useful.
Forty years ago a series of critical papers established that the mechanism of APAP-induced liver injury begins with the P450-catalyzed conversion of the drug to an electrophile that can react with glutathione (GSH) and bind to proteins (Mitchell et al., 1973a, 1973b; Jollow et al., 1973; Potter et al., 1973). This reactive metabolite is generally believed to be N-acetyl-p-benzoquinone imine (NAPQI) (Dahlin et al., 1984). It is now thought that binding to proteins, mitochondrial proteins in particular, causes oxidative stress and mitochondrial damage (Jaeschke et al., 2012) resulting in necrotic cell death (Gujral et al., 2002). Dose-response studies comparing liver APAP-protein adducts and GSH led to the idea that depletion of approximately 70% of total liver GSH is necessary for protein binding to begin (Mitchell et al., 1973b). It was assumed then that this could only occur at toxic doses. Later experiments using antibody-based assays to measure the cysteine adduct of APAP (APAP-CYS) on proteins showed that these adducts also appear in serum, but only after toxic doses or after the onset of injury in time course studies (Pumford et al., 1990; Roberts et al., 1991). This suggested that their presence in serum was the result of liver injury and cell contents release. New, more sensitive and specific techniques have been developed to measure APAP-CYS from proteins (Muldrew et al., 2002; McGill et al., 2011) and it was recently proposed that serum protein adducts could replace serum APAP as the primary diagnostic marker of APAP overdose (Davern et al., 2006). A major advantage is the much longer half-life of these adducts, in the range of 1–2 days (James et al., 2009). While this is a promising approach, comprehensive dose-response studies have yet to be done and there is evidence that APAP-CYS is present in serum even after therapeutic doses (Heard et al., 2011). Furthermore, the effects of polypharmacy and co-morbidities on this parameter are unknown, and the mechanism by which adducts appear in serum without cell death has not been investigated.
To address these issues, we performed detailed dose-response and time course studies to explore the relationships between liver GSH and protein binding, and serum APAP-protein adducts and toxicity. We hypothesized that extensive GSH depletion is not required for APAP-protein binding to occur in the liver and that APAP-protein adducts can form without toxicity. Although recent data in humans indicated the presence of APAP protein adducts in serum after therapeutic doses, i.e. without liver injury (Heard et al., 2011), we hypothesized that co-incidental liver injury can increase plasma APAP-protein adduct levels after subtoxic doses of APAP. To test this, we treated mice with APAP and induced necrosis through ischemia-reperfusion. Finally, primary mouse hepatocytes were used to study the appearance of adducts in extracellular fluid and to determine by what mechanism these adducts appear without liver injury. Overall, our data support the clinical use of this parameter, but urge consideration of potential confounding factors.
MATERIALS AND METHODS
Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Kansas Medical Center. Male C57Bl/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). The animals were kept in a temperature-controlled room with a 12 h light/dark cycle and ad libitum access to food and water. Food was removed 12–16 h prior to treatment with the indicated doses of acetaminophen or before hepatocyte isolation for cell culture. For in vivo studies, APAP was dissolved in warm saline and injected i.p. The concentration of each APAP solution was adjusted so that all mice received approximately the same volume. At the indicated time points, the mice were sacrificed by cervical dislocation. Blood was drawn from the caudal vena cava into heparinized syringes and plasma was obtained by centrifugation at 12,000 g for 3 min. A section was taken from the left lobe of each mouse liver and fixed in 10% phosphate-buffered formalin for histology. Remaining portions of the livers and whole kidneys were collected and flash frozen in liquid nitrogen for later biochemical analysis.
Ischemia-reperfusion surgery
Liver injury was induced by ischemia-reperfusion as described (Hasegawa et al., 2007). Briefly, each mouse was treated with 75 mg APAP / kg body weight. Under ketamine/xylazine/acepromazine anesthesia (i.m. injection), midline laparotomy was performed and the blood vessels supplying the left and median lobes were occluded with an atraumatic clamp at approximately 1 h post-APAP. After forty-five minutes, the clamp was removed to restore blood flow and the abdomen was closed using suture thread and wound clips. Body temperature was monitored with a rectal probe and maintained at 37.0 – 37.5 °C using a heat lamp. The animals were sacrificed while still under anesthesia after 1.5 h of reperfusion.
Primary hepatocyte culture
Primary mouse hepatocytes were isolated as described (Bajt et al., 2004). Briefly, the caudal vena cava was cannulated and the liver was perfused at 8 mL/min with warmed and oxygenated Hank’s buffer salt solution (HBSS) containing penicillin/streptomycin at pH 7.4 for 10 min. This was followed by perfusion with a solution of the same composition plus 1 mM Ca2+ and Mg2+ and 0.04% collagenase D (Roche Molecular Biochemicals, Mannheim, Germany). After perfusion, livers were minced and strained through a series of wire mesh filters. The cells were then pelleted by gentle centrifugation and washed three times before being re-suspended in cell culture medium and seeded on sterile collagen-coated dishes. The cells were maintained in Williams’ E medium supplemented with fetal bovine serum, insulin, and penicillin/streptomycin. Protein adducts in cells and supernatant were assessed after exposure to 5 mM APAP for 3 h. For some experiments, cultures were washed 4–5 times with 1× PBS and changed to serum- and insulin-free medium immediately before use. To monitor protein secretion in the absence of serum, the protein in the culture medium was concentrated using Amicon Ultra centrifugal filters with 3,000 Da MWCO (Millipore, Billerica, MA), separated by gel electrophoresis, and stained with Coomassie blue.
Biochemistry
Alanine aminotransferase (ALT) activity was measured using a kit from Pointe Scientific (Canton, MI). Glutamate dehydrogenase (GDH) was measured as described (McGill et al., 2012a). Liver total glutathione (GSH+GSSG) levels were measured using a modified Tietze assay as described (Jaeschke and Mitchell, 1990).
Histology
Formalin-fixed tissue samples were embedded in paraffin and 5 μm sections were cut. Sections were stained with hematoxylin and eosin (H&E) for evaluation of the areas of necrosis. Additional sections were stained for DNA fragmentation by using the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) in situ cell death assay (Roche) as previously described (Gujral et al., 2002).
APAP-protein adduct measurement
To remove low molecular weight compounds including APAP-GSH conjugates with the potential to interfere with detection and to isolate proteins, plasma samples, liver homogenates, cell lysates, and cell culture medium were filtered through Bio-Spin 6 columns (Bio-Rad, Hercules, CA) that were pre-washed with 10 mM sodium acetate buffer (pH 6.5). The filtered samples were digested overnight with proteases to free APAP-CYS. After digestion, remaining protein in the cell culture medium, cell lysate, and in some plasma samples was precipitated using cold isoproponal/methanol (McGill et al., 2011) and pelleted by centrifugation. The supernatants were evaporated at 55 °C and 16 psi and the protein-derived APAP-CYS-containing residues were re-suspended in small volumes of 10 mM sodium acetate. APAP-CYS in liver homogenates was prepared as described (Ni et al., 2012b). For time course and dose-response experiments, APAP-CYS was measured by LC-MS/MS (McGill et al., 2011). For ischemia-reperfusion and cell culture experiments, APAP-CYS was measured using HPLC with electrochemical detection (Muldrew et al., 2002; Ni et al., 2012b).
Statistics
The Shapiro-Wilk test was used to assess normality. For normally distributed data, one-way analysis of variance (ANOVA) was performed to test for significance, with Student-Newman-Keuls post-hoc comparison between groups. For non-normally distributed data, ANOVA was performed on ranks, followed by Dunn’s multiple comparisons. For all tests, P<0.05 was considered significant. Statistical analysis was done in SigmaPlot 11 (Systat Software Inc., San Jose, CA).
RESULTS
Dose-response of liver injury
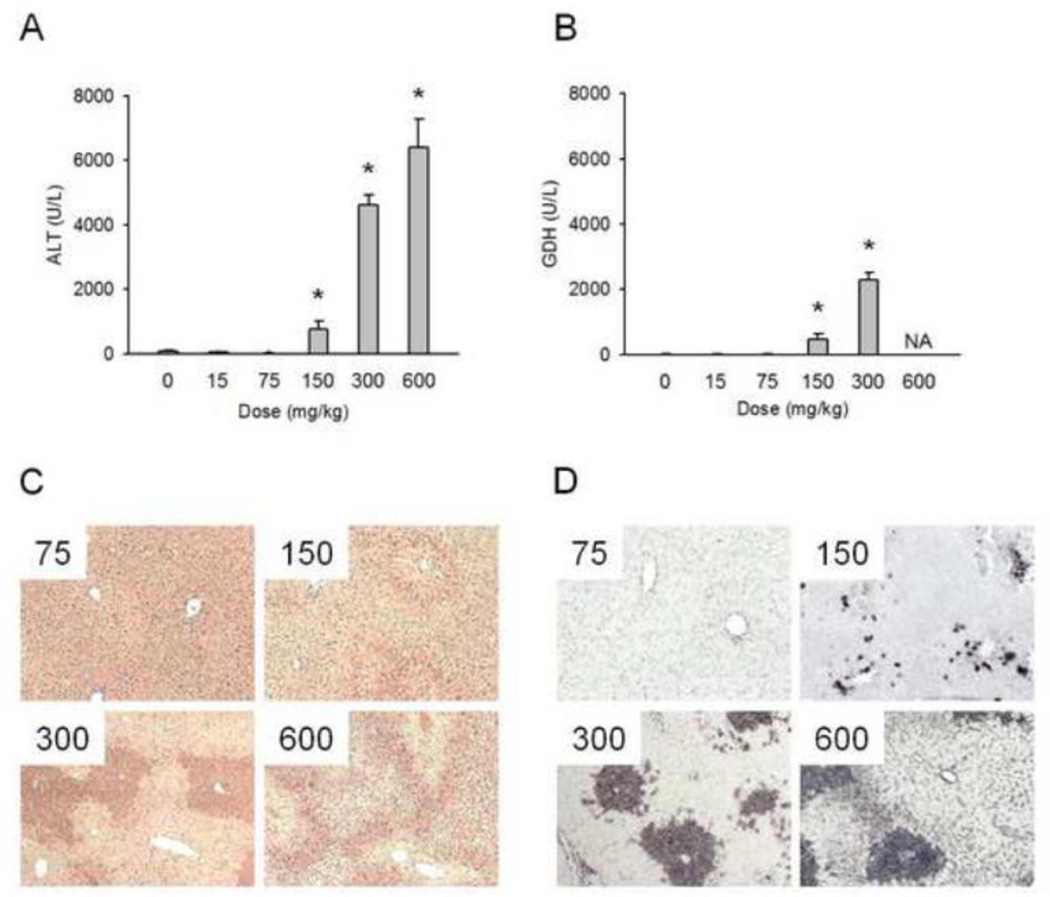
Mice were treated with various doses of APAP (15, 75, 150, 300, or 600 mg/kg) and sacrificed at the indicated times. As expected, APAP caused a dose-dependent increase in plasma levels of ALT and the mitochondrial enzyme GDH at 24 h (Fig. 1A,B). Our previous work suggested that GDH may be a biomarker of mitochondrial damage (McGill et al., 2012a). Neither enzyme was elevated after treatment with doses lower than 150 mg/kg, which produced injury in approximately half of the animals tested (Fig. 1A,B). The 300 and 600 mg/kg doses caused dramatic increases in plasma ALT activities (Fig. 1A). Unfortunately, hemorrhage limited the volume of plasma available for enzyme measurements after treatment with 600 mg/kg, so we could not collect a complete set of data for GDH at the highest dose. However, histology could be obtained and we observed increased necrosis and TUNEL staining with increasing doses (Fig. 1C,D), consistent with increasing plasma ALT and GDH activities.
Figure 1.
Dose-response of liver injury. Mice were treated with 0, 15, 75, 150, 300, or 600 mg APAP / kg bodyweight and sacrificed 24 h later. (A) Dose-response of plasma alanine aminotransferase (ALT) activities. (B) Dose-response of plasma glutamate dehydrogenase (GDH) activities. (C) Hematoxylin and eosin staining of liver sections. (D) TUNEL staining of liver sections. Data are expressed as mean ± SEM of n = 6 animals per time point. *P < 0.05 (compared to t=0); NA = Not Available.
Time course and dose-response of liver GSH and APAP-protein adducts
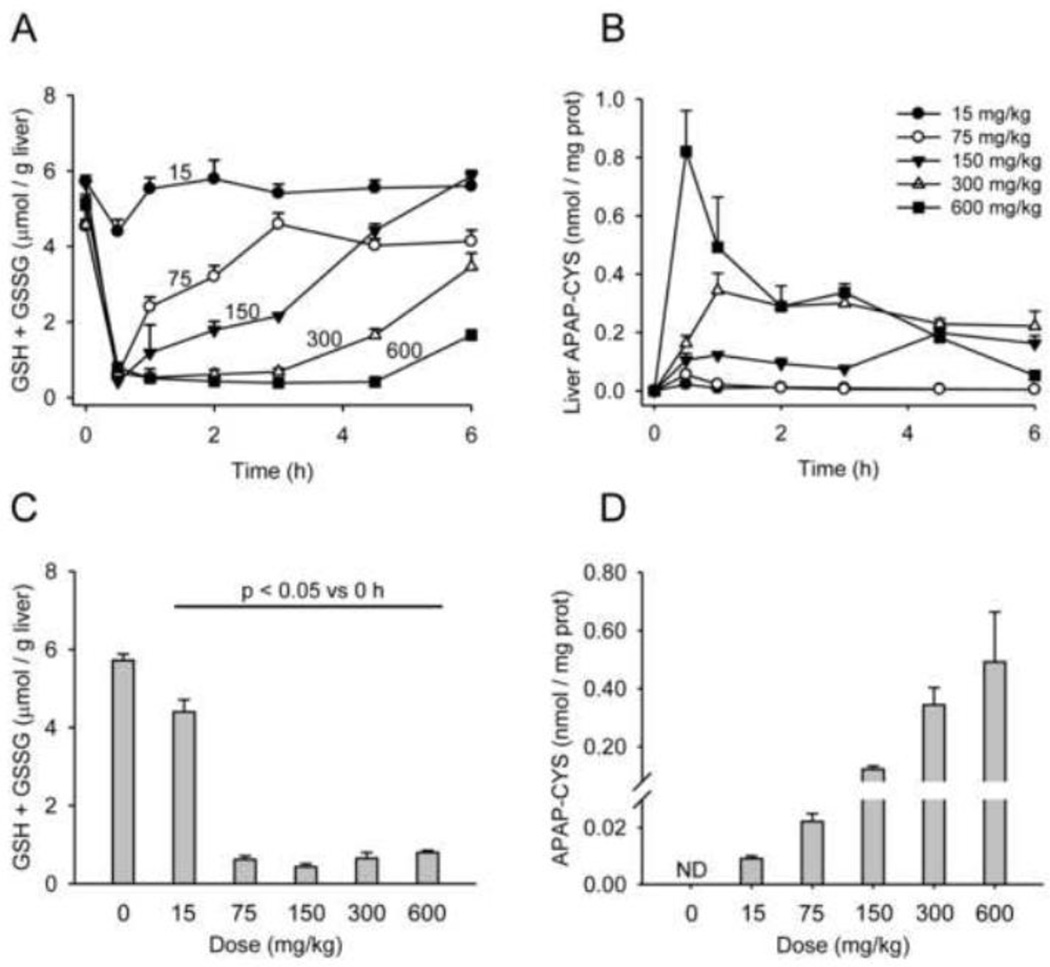
To explore the relationship between liver GSH and covalent binding of the reactive metabolite of APAP (NAPQI) to proteins, we measured GSH levels and APAP-protein adducts in the livers from these animals. It is well established that the cysteine adduct is the major protein adduct formed by NAPQI (Streeter et al., 1984). Upon reaction of NAPQI with the free sulfhydryl group of cysteine, the structure of NAPQI reverts to that of APAP and is often referred to as APAP-CYS (Muldrew et al., 2002). After proteolytic degradation of the protein, APAP-CYS is liberated, which is the molecule that is detected by the assay as previously described (Muldrew et al., 2002; McGill et al., 2011). Thus, our assay specifically measures APAP-CYS from proteins, not from APAP-GSH or free cysteine. Except for the lowest dose, GSH was depleted to the same extent within 0.5 h of each treatment (Fig. 2A,C). Significant differences in GSH levels were observed only during the time of GSH recovery (Fig. 2A). Importantly, protein-derived APAP-CYS could be detected in liver samples even after the 15 mg/kg dose, albeit at low levels (Fig. 2B,D). These data show that extensive GSH depletion is not necessary for protein binding to occur. Nevertheless, it is clear that GSH is an important scavenger of NAPQI.
Figure 2.
Dose-response and time course of total liver GSH (GSH + GSSG) and liver APAP-protein adducts. Mice were treated with 0, 15, 75, 150, 300, or 600 mg APAP / kg bodyweight and sacrificed at the indicated time points. (A) Total liver GSH over time. (B) Liver protein-derived APAP-CYS over time. (C) Total liver GSH 0.5 h after APAP treatment. (D) Liver proteinderived APAP-CYS concentration, 1 h after APAP treatment. Data are expressed as mean ± SEM of n = 6 animals per time point.
Time course and dose-response of liver injury and plasma APAP-protein adducts
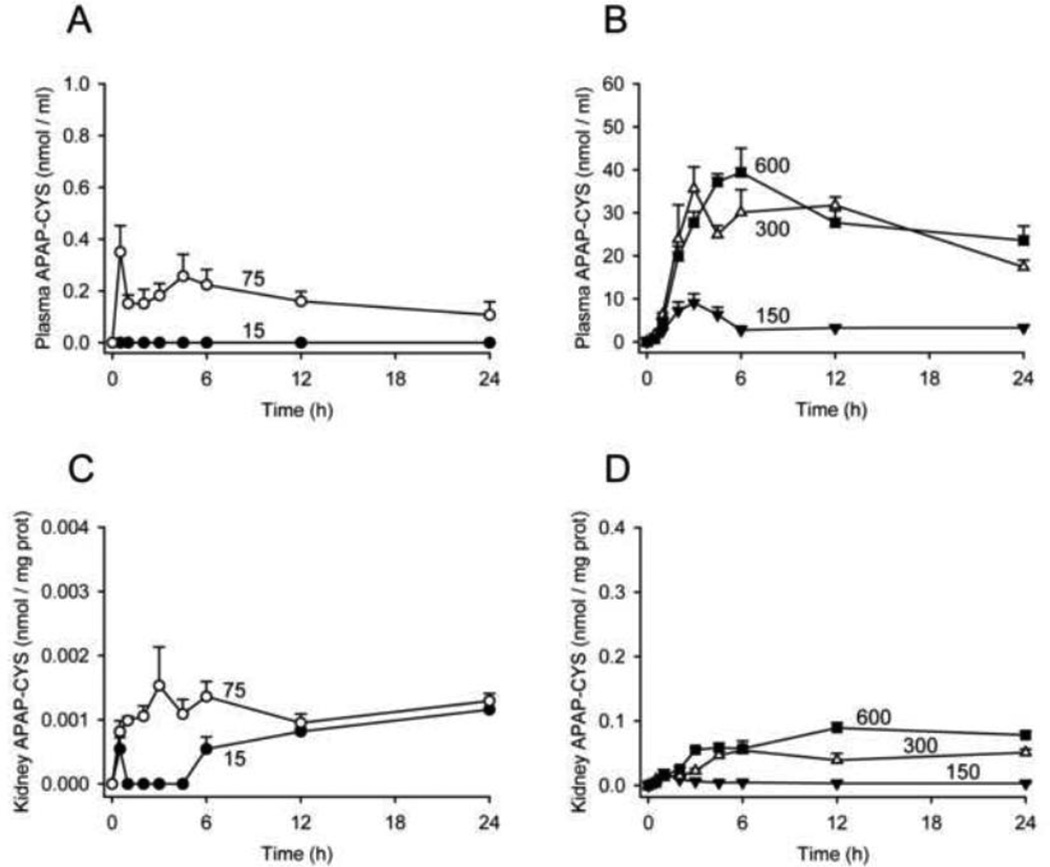
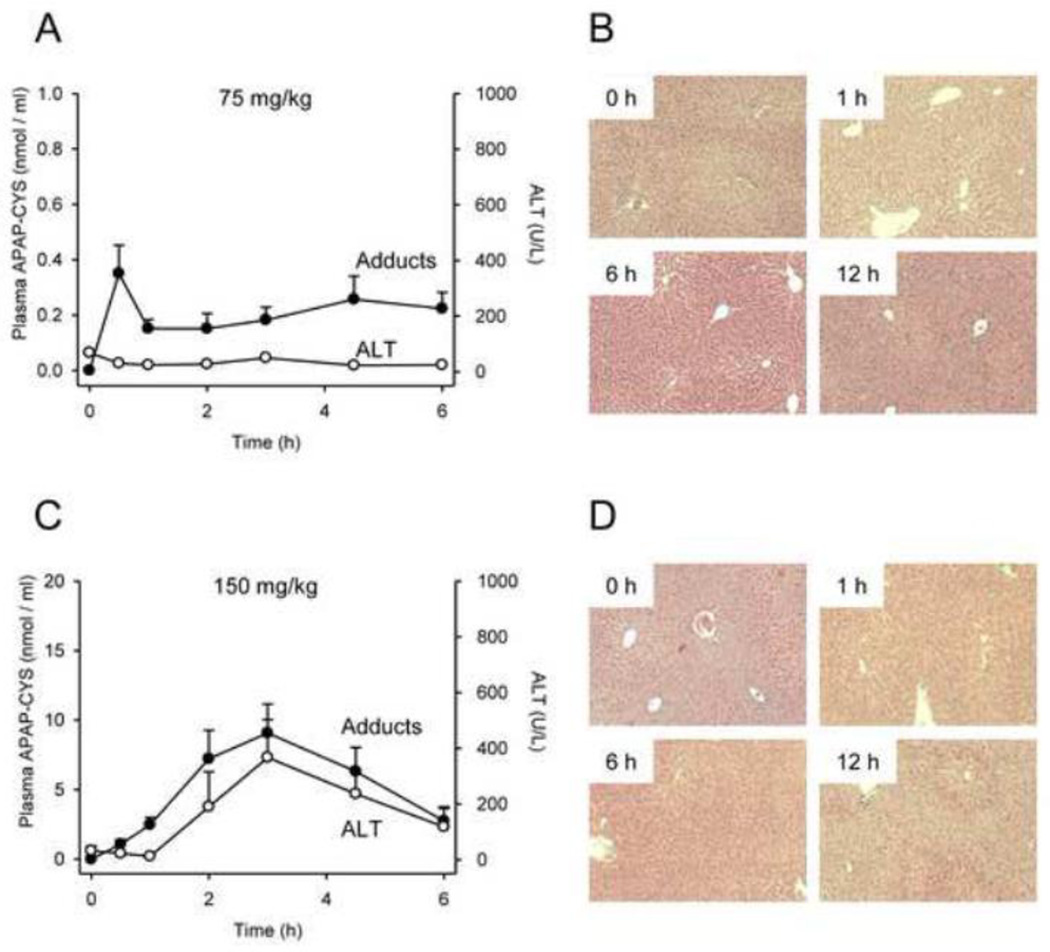
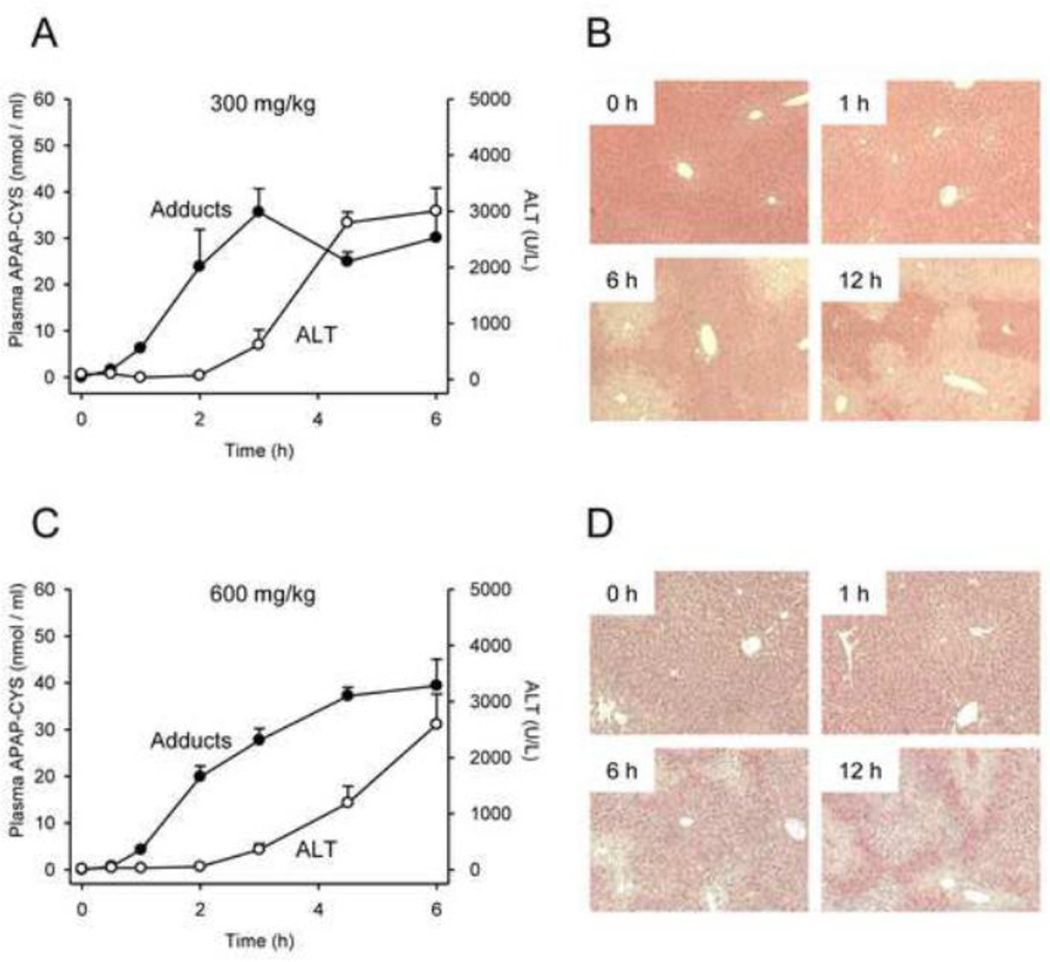
We next studied the dose-response of plasma APAP-protein adducts and compared these values with ALT. Protein-derived APAP-CYS concentration was below our lower limit of quantitation in all plasma samples from mice treated with the lowest dose (15 mg/kg). However, the adducts could be measured in plasma after treatment with the 75 mg/kg subtoxic dose, and increased dose-dependently thereafter (Fig. 3A,B). Comparing adducts with ALT revealed that protein-derived APAP-CYS is detectable in plasma without increased ALT after a subtoxic dose (Fig. 4A) and appears in plasma as early as 0.5 – 1 h after treatment with a toxic dose, well before the onset of injury and ALT release (Figs. 4 and 5). These data contradict the earlier view that necrosis is required for release of adducted proteins (Davern et al., 2006), and are consistent with more recent work showing that adducts can be detected in serum from humans after therapeutic doses (Heard et al., 2011). Protein binding is also known to occur in the kidney after treatment with APAP (Mudge et al., 1978; McMurtry et al., 1978). However, our data show that the concentration of APAP-protein adducts in the kidney is very low, even after high doses of APAP (Fig. 3C,D). Thus, most of the plasma adducts in our study were probably liver-derived and the contribution of the kidneys is negligible. It may be important to note, however, that whole kidneys were used in these experiments. It is known that covalent binding and APAP-induced necrosis in the kidney occur primarily within the proximal tubules in the renal cortex (Emeigh Hart et al., 1991; Hart et al., 1994). The use of the entire organ may have resulted in under-estimation of the local production of adducts in areas of the kidney.
Figure 3.
Dose-response and time course of plasma and kidney APAP-protein adducts. Mice were treated with 0, 15, 75, 150, 300, or 600 mg APAP / kg bodyweight and sacrificed at the indicated time points. Protein-derived APAP-CYS was measured in plasma (A and B) and kidneys (C and D) from these animals. Data are expressed as mean ± SEM of n = 6 animals per time point.
Figure 4.
Time course of plasma APAP-protein adducts and liver injury after 75 mg/kg or 150 mg/kg dose. Mice were treated with 75 or 150 mg APAP / kg bodyweight and sacrificed at the indicated time points. (A,C) ALT and protein-derived APAP-CYS in plasma. (B,D) Liver histology time course. Data are expressed as mean ± SEM of n = 6 animals per time point.
Figure 5.
Time course of plasma APAP-protein adducts and liver injury after 300 mg/kg or 600 mg/kg dose. Mice were treated with 300 or 600 mg APAP / kg bodyweight and sacrificed at the indicated time points. (A,C) ALT and protein-derived APAP-CYS in plasma. (B,D) Liver histology time course. Data are expressed as mean ± SEM of n = 6 animals per time point.
Mechanisms of the appearance of APAP-protein adducts in plasma
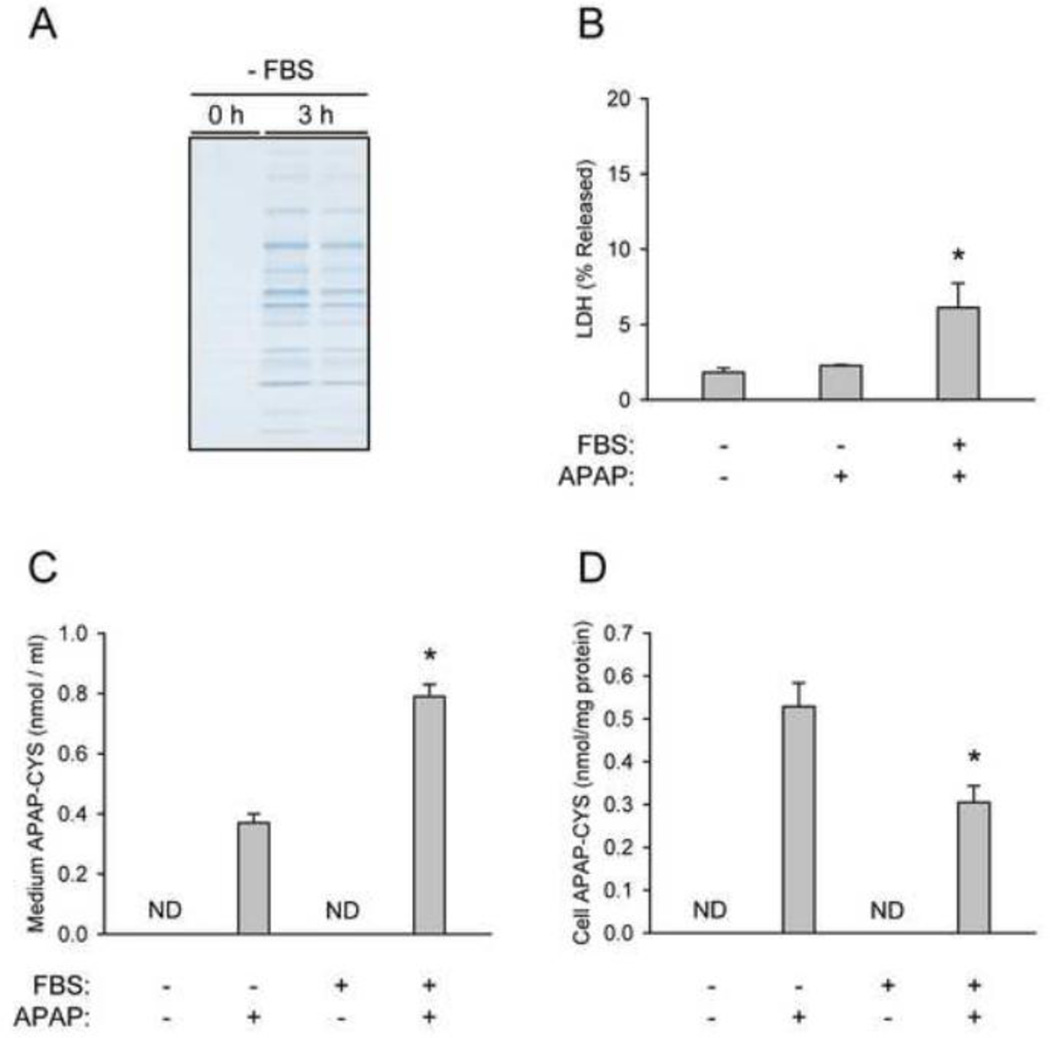
We hypothesized that the appearance of APAP-protein adducts in plasma occurs in one of two ways. Protein binding may take place exclusively within hepatocytes, followed by secretion or exocytosis of some of the adducted proteins into plasma. Alternatively, NAPQI could diffuse out of the hepatocyte and bind to plasma proteins in situ. We decided to take an in vitro approach to test these hypotheses. When cells were kept in serum-free medium (without APAP), we observed a clear increase in extracellular protein within 3 h (Fig. 6A), without an increase in cell death or enzyme release (Fig. 6B). These data suggest that primary mouse hepatocytes can actively secrete proteins into the culture medium. We then treated primary mouse hepatocytes with 5 mM APAP in the presence or absence of extracellular protein from FBS and measured protein-derived APAP-CYS in the culture medium. Importantly, we were able to measure APAP-protein adducts in medium from cultures without serum (Fig. 6C). This is consistent with the hypothesis that adducts are formed within hepatocytes and secreted. However, the concentration of adducts in the medium from this group was approximately half of that in medium from the group with serum. This could not be explained by a reduction in metabolism of APAP. In fact, protein binding within cells was increased in the absence of extracellular protein (Fig. 6D). One possible reason for the difference in medium adducts is that both adduct secretion and NAPQI diffusion are involved. Secretion of adducted proteins would explain the appearance of adducts in serum-free medium. Additional work is needed to fully understand the mechanism by which APAP-protein adducts appear in plasma.
Figure 6.
Mechanisms of the appearance of extracellular APAP-protein adducts. (A) Primary mouse hepatocytes were thoroughly washed and cultured in serum-free medium for 3 h after exposure to 5 mM APAP. Extracellular protein was measured using SDS-PAGE with Coomassie blue staining. (B) LDH release was measured in cultures of primary mouse hepatocytes treated with APAP in the presence or absence of fetal bovine serum (FBS) for 3 h. (C) Protein-derived APAP-CYS was measured in the culture medium of hepatocytes treated with APAP in the presence or absence of serum for 3 h. (D) Protein-derived APAP-CYS was measured in hepatocytes treated with APAP in the presence of absence of serum for 3 h. Data are expressed as mean ± SEM of 3–6 experiments. *P < 0.05 (compared to cultures with serum-free medium). ND = Not Detectable.
Effect of co-incidental liver injury on plasma APAP-protein adducts
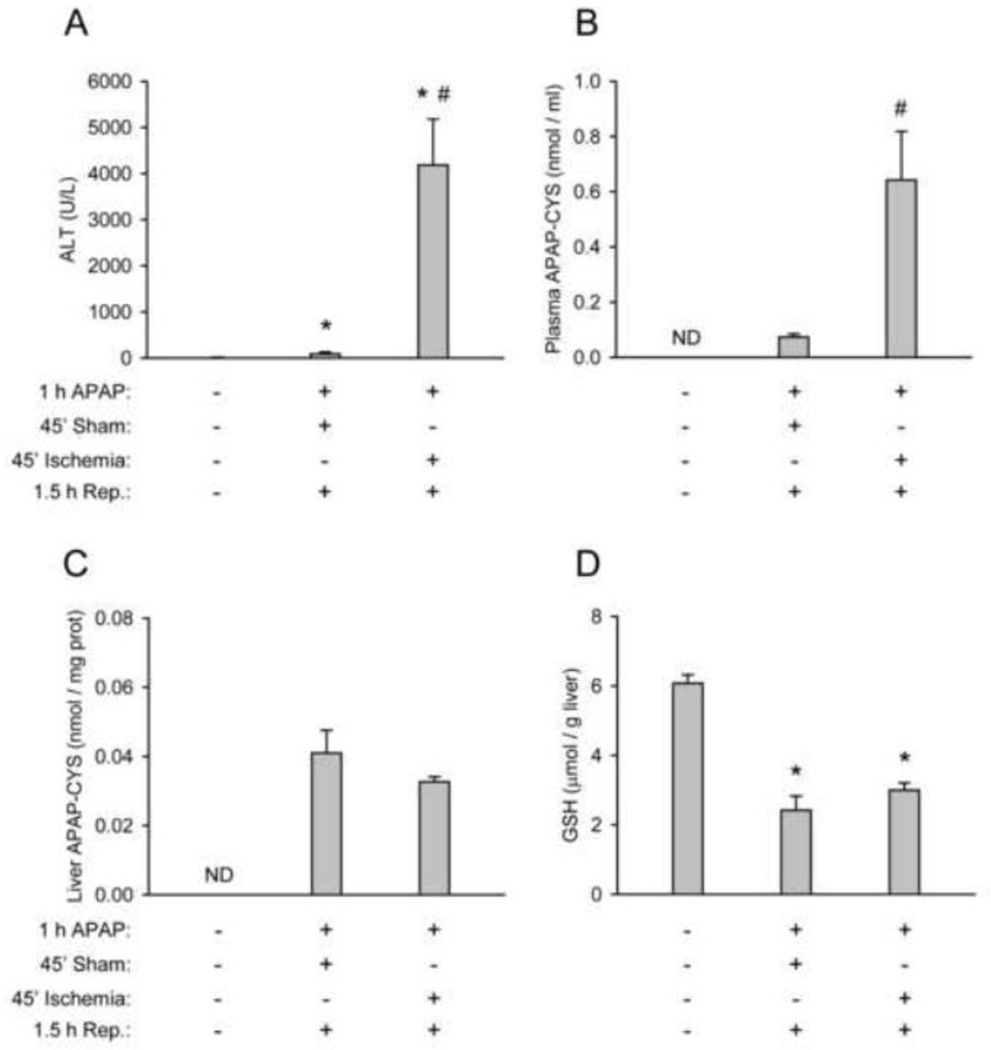
Although necrosis is not necessary for adduct release, it is possible that co-incidental liver injury could lead to artificially elevated plasma concentrations after a subtoxic dose. To test this, we treated mice with the subtoxic 75 mg/kg dose of APAP for 1 h and induced necrosis in the left lateral and median lobes with forty-five minutes of ischemia followed by 1.5 h of reperfusion. Sham controls were anesthetized in the same way and also underwent midline laparotomy, but were not subjected to ischemia. All animals were sacrificed at the same time point after APAP treatment. Ischemia-reperfusion resulted in significant liver injury and cell contents release, as indicated by plasma ALT (Fig. 7A). Only a very minor increase in ALT over control was also observed in the sham-treated animals. APAP-protein adducts could be detected in plasma from sham-operated mice, but was significantly increased in samples from the ischemia-reperfusion group (Fig. 7B). No difference in the metabolic activation of APAP could be discerned between the two groups, based on APAP adducts and GSH levels in the left lateral lobe (Fig. 7C,D). This is consistent with our time course data showing that NAPQI and liver adduct formation plateau by 1 h (Fig. 7B), before the mice were subjected to hepatic ischemia. These data show that plasma APAP-protein adduct concentrations can be increased by non-APAP-related liver necrosis after exposure to subtoxic doses.
Figure 7.
Ischemia-reperfusion liver injury increases plasma APAP-protein adduct levels. Mice were pretreated for 1 h with APAP at 75 mg/kg and subjected to ischemia-reperfusion of the liver. (A) Plasma ALT values. (B) Protein-derived APAP-CYS in plasma. (C) Protein-derived APAP-CYS in livers. (D) Total GSH (GSH + GSSG) in livers. Data are expressed as mean ± SEM of n = 4–6 animals per treatment group. *P < 0.05 (compared to untreated controls). #P < 0.05 (compared to sham). ND = Not Detectable.
DISCUSSION
Recent work in the field of APAP hepatotoxicity has focused on late events in the mechanism of injury. We now know that high doses of APAP can inhibit mitochondrial respiration (Meyers et al., 1988) and cause mitochondrial oxidative stress in both mice (Jaeschke, 1990; Cover et al., 2005; Fujimoto et al., 2009; Ramachandran et al., 2011b; Bajt et al., 2011) and human HepaRG cells (McGill et al., 2011). In mice, an initial oxidative stress leads to activation of the c-Jun N-terminal kinase (JNK) (Gunawan et al., 2006; Hanawa et al., 2008; Saito, et al., 2010) through apoptosis signal-regulating kinase 1 (ASK1) (Nakagawa et al., 2008) and mixed lineage kinase 3 (MLK3) (Sharma et al., 2012). The activated JNK translocates to mitochondria and exacerbates the oxidative stress and injury (Hanawa et al., 2008; Win et al., 2011; Ramachandran et al., 2011b; Jaeschke et al., 2012). The mitochondrial membrane permeability transition results in loss of mitochondrial membrane potential (Kon et al., 2004; Reid et al., 2005; Ramachandran et al., 2011a). Loss of mitochondrial membrane integrity and translocation of Bax into mitochondria cause release of endonucleases that can cleave nuclear DNA (Bajt et al., 2006, 2008, 2011). Importantly, these mechanisms are not limited to mice or human cell lines. There is evidence that mitochondrial damage and nuclear DNA fragmentation also occur in humans in vivo after APAP overdose (McGill et al., 2012a), leading to hepatocellular necrosis (McGill et al., 2012a; Antoine et al., 2012). While these mechanisms are critical in APAP-induced liver injury, it is important to remember that they occur downstream of the metabolic activation of APAP. Inhibiting NAPQI formation prevents the oxidative stress, JNK activation, and mitochondrial damage (Jaeschke et al., 2011). Unfortunately, the relationship between protein binding and liver injury is not fully understood. While a number of specific targets of NAPQI have been identified, the activities of these proteins are in general only modestly affected (Cohen et al., 1997; Qiu et al., 1998). Importantly, early work comparing APAP with the nonhepatotoxic isomer N-acetyl-m-aminophenol (AMAP) revealed that the reactive metabolite of APAP binds more to mitochondrial proteins (Tirmenstein and Nelson, 1989), suggesting that mitochondrial protein binding is particularly important. Consistent with this, we have shown that rats, which are less susceptible to APAP-induced liver injury, have lower APAP-protein adduct concentrations in mitochondria than mice (McGill et al., 2012b). However, these data are largely correlative. A recent in vitro study showed that AMAP can actually cause toxicity in liver slices from rats and humans and even mice at higher doses (Hadi et al., 2013). Unfortunately, no protein adducts were measured in these experiments (Hadi et al., 2013). Additional work is clearly needed to fully understand the connection between protein adducts and toxicity.
It has long been believed that extensive GSH depletion is required for protein binding to occur after APAP. Dose-response data supporting this were first published forty years ago (Mitchell et al, 1973b) and it has since become a paradigm in the study of electrophile-mediated hepatotoxicity. However, more recent work has challenged this idea. Protein adducts could be measured in human HepaRG cells as early as 1 h after treatment with APAP, well before any appreciable loss of GSH had occurred (McGill et al., 2011). Moreover, protein-derived APAP-CYS could be detected in serum from humans after only therapeutic doses (Heard et al., 2011). The discrepancy is likely due to the lack of multiple time points in the earlier dose-response data, which were collected 2 h post-APAP (Mitchell et al., 1973b). Our results show that the peak of protein adduct formation in the liver is reached by 0.5 – 1 h after administration of subtoxic doses and that adduct concentration decreases thereafter. Importantly, we were able to detect protein binding after treatment with 15 mg/kg APAP at these earlier time points, with only a minimal loss of liver GSH. Together, it is clear from these studies that some protein binding can occur without extensive GSH depletion and without toxicity.
Interestingly, liver GSH was similar at 0.5 h after APAP for all doses ≥ 75 mg/kg, while recovery of GSH showed a clear dose-response (Fig. 2A). It is worth noting that GSH recovered to near control levels by 12 h after all but the highest dose. Furthermore, our data show that APAP-protein adduct formation in the liver peaks within 1 – 2 h and that adduct concentration decreases beyond this time point, possibly as a result of autophagy of adducted proteins and mitochondria (Ni et al., 2012a). Thus, to make an accurate assessment of metabolic activation of APAP in mice it is necessary to measure GSH and/or protein adducts at an early time point, in the 0.5 – 2 h post-treatment range. Use of a single late time point, as done in most natural product testing experiments, does not give a reliable assessment of APAP metabolism and NAPQI formation (Jaeschke et al., 2011).
Etiology can be a consideration in determining whether or not an individual with liver injury is in need of a transplant. Patients with APAP hepatotoxicity are more likely to survive without a new organ than patients with liver injury due to other causes (Simpson et al., 2009). Moreover, when intentional overdose can be established, it is important to ensure that the patient receives proper care and counseling to avoid future incidents (Qin et al., 2009). Thus, accurate diagnosis of APAP overdose is critical. Presently, diagnosis is largely based on serum APAP concentrations. Unfortunately, APAP has a relatively short serum half-life in humans. The confident use of serum APAP requires a patient to present early after overdose, which is often not the case. Measurement of serum APAP-protein adducts, which have a half-life of 1–2 days (James et al., 2009), is a promising solution. Initially, this was based on the idea that adducts are released from dying hepatocytes (Davern et al., 2006) and are therefore a strong indication of APAP toxicity. However, data from this study and from other groups have shown that adducts can appear in plasma before ALT after a toxic dose and are detectable after subtoxic and even therapeutic doses (Heard et al., 2011). To deal with this, a threshold of ≥ 1.1 nmol/mL serum APAP-CYS combined with serum ALT ≥ 1,000 U/L has been proposed for diagnostic use (James et al., 2009). Our data are in general agreement with this. In our study, plasma levels of protein-derived APAP-CYS peaked at 0.35 ± 0.1 nmol/mL after treatment with the 75 mg/kg subtoxic dose, but reached 9.1 ± 2.1 nmol/mL after the lowest toxic dose tested (150 mg/kg). The results from our in vitro experiments support a role for secretion of adducted proteins into plasma as an important mechanism by which adducts appear there without liver injury. However, we cannot rule out the possibility that NAPQI formed in hepatocytes diffuses out of the cells and binds to plasma proteins in situ. The latter would be consistent with the earlier finding in mice of APAP-hemoglobin adducts in red blood cells, which do not express cytochrome P450 enzymes (Axworthy et al., 1988) and the identification of adducts on serum albumin (Switzar et al., 2013). It is likely that both mechanisms are involved.
While these data support the clinical use of plasma APAP-protein adducts, there may be circumstances that require special consideration. Though data from our study and others show that liver injury is not required for the appearance of APAP-protein adducts in plasma, it is possible that cell death and cell contents release can contribute to the plasma levels. APAP is a very popular drug and a person taking therapeutic doses of APAP could develop liver injury incidental to their APAP use, possibly resulting in higher concentrations of protein-derived APAP-CYS in plasma. Our data show that ischemia-reperfusion-induced liver injury can significantly increase APAP-protein adducts in plasma after a subtoxic dose of APAP (Fig. 7). Thus, care should be taken in the clinical interpretation of this parameter. Other possible causes of liver injury may need to be ruled out before a diagnosis of APAP overdose is made on the basis of plasma adducts.
CONCLUSIONS
APAP is a widely used drug and hepatotoxicity as a result of overdose is a major clinical issue. Protein binding is the critical initiating event in the mechanism of injury. However, contrary to early reports, APAP-protein binding can occur even after subtoxic doses, without extensive GSH depletion. It is likely that either a threshold of protein binding (particularly mitochondrial protein binding) needs to be achieved before the development of toxicity, or that specific binding targets are spared at low doses. Interestingly, while our data support the measurement of protein-derived APAP-CYS in plasma as a promising new diagnostic method, it is important to note that these adducts can appear in plasma without injury. Establishment of a sensitive but still specific threshold concentration is important. Our results show that co-incidental liver injury after a subtoxic dose of APAP can dramatically increase the plasma concentration of adducts. This may have important clinical implications. The effects of other diseases and polypharmacy on plasma APAP-protein adduct levels remain to be investigated.
HIGHLIGHTS.
Extensive GSH depletion is not required for APAP-protein binding in the liver
APAP-protein adducts appear in plasma at subtoxic doses
Proteins are adducted in the cell and secreted out
Coincidental liver injury increases plasma APAP-protein adducts at subtoxic doses
Plasma APAP-protein adducts are diagnostically useful, but interpret with care
ACKNOWLEDGEMENTS
This investigation was supported in part by grants from McNeil Consumer Health, Inc. (to H.J. and D.R.), by the National Institutes of Health grants R01 DK070195 and R01 AA12916 to H.J., and by grants from the National Center for Research Resources (5P20RR021940-07) and the National Institute of General Medical Sciences (8 P20 GM103549-07) from the National Institutes of Health. Research reported in this publication was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM12345. CD Williams and MR McGill were supported by the “Training Program in Environmental Toxicology” (T32 ES007079-26A2) from the National Institute of Environmental Health Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Antoine DJ, Jenkins RE, Dear JW, Williams DP, McGill MR, Sharpe MR, Craig DG, Simpson KJ, Jaeschke H, Park BK. Molecular forms of HMGB1 and keratin-8 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity. J. patol. 2012;56:1070–1079. doi: 10.1016/j.jhep.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Axworthy DB, Hoffmann KJ, Streeter AJ, Calleman CJ, Pascoe GA, Baillie TA. Covalent binding of acetaminophen to mouse hemoglobin. Identification of major and minor adducts formed in vivo and implications for the nature of the arylating metabolites. Chem. Biol. Interact. 1988;68:99–116. doi: 10.1016/0009-2797(88)90009-9. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Cover C, Lemasters JJ, Jaeschke H. Nuclear translocation of endonuclease G and apoptosis-inducing factor during acetaminophen-induced liver cell injury. Toxicol. Sci. 2006;94:217–225. doi: 10.1093/toxsci/kfl077. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Farhood A, Lemasters JJ, Jaeschke H. Mitochondrial bax translocation accelerates DNA fragmentation and cell necrosis in a murine model of acetaminophen hepatotoxicity. J. Pharmacol. Exp. Ther. 2008;324:8–14. doi: 10.1124/jpet.107.129445. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Knight TR, Lemasters JJ, Jaeschke H. Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: protection by N-acetyl cysteine. Toxicol. Sci. 2004;80:343–349. doi: 10.1093/toxsci/kfh151. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Ramachandran A, Yan HM, Lebofsky M, Farhood A, Lemasters JJ, Jaeschke H. Apoptosis-induced factor modulates mitochondrial oxidant stress in acetaminophen hepatotoxicity. Toxicol. Sci. 2011;122:598–605. doi: 10.1093/toxsci/kfr116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal W. Changing patterns of causation and the use of transplantation in the United Kingdom. Semin. Liv. Dis. 2003;23:227–237. doi: 10.1055/s-2003-42640. [DOI] [PubMed] [Google Scholar]
- Canbay A, Jochum C, Bechmann LP, Festag S, Gieseler RK, Yüksel Z, Lütkes P, Saner FH, Paul A, Gerken G. Acute liver failure in a metropolitan area in Germany: a retrospective study (2002 – 2008) Z. Gastroenterol. 2009;47:807–813. doi: 10.1055/s-0028-1109058. [DOI] [PubMed] [Google Scholar]
- Cohen SD, Pumford NR, Khairallah EA, Boekelheide K, Pohl LR, Amouzadeh HR, Hinson JA. Selective protein covalent binding and target organ toxicity. Toxicol. Appl. Pharmacol. 1997;143:1–12. doi: 10.1006/taap.1996.8074. [DOI] [PubMed] [Google Scholar]
- Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, Jaeschke H. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J. Pharmacol. Exp. Ther. 2005;315:879–887. doi: 10.1124/jpet.105.088898. [DOI] [PubMed] [Google Scholar]
- Cummings AJ, King ML, Martin BK. A kinetic study of drug elimination: the excretion of paracetamol and its metabolites in man. Br. J. Pharmacol. Chemother. 1967;29:150–157. doi: 10.1111/j.1476-5381.1967.tb01948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlin DC, Miwa GT, Lu AY, Nelson SD. N-acetyl-p-benzoquinone imine: a cytochrome P-50-mediated oxidation product of acetaminophen. Proc. Natl. Acad. Sci. U.S.A. 1984;81:1327–1331. doi: 10.1073/pnas.81.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davern TJ, 2nd, James LP, Hinson JA, Polson J, Larson AM, Fontana RJ, Lalani E, Munoz S, Shakil AO, Lee WM Acute Liver Failure Study Group. Measurement of serum acetaminophen-protein adducts in patients with acute liver failure. Gastroenterology. 2006;130:687–694. doi: 10.1053/j.gastro.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Emeigh Hart SG, Beierschmitt WP, Bartolone JB, Wyand DS, Khairallah EA, Cohen SD. Evidence against deacetylation and for cytochrome P450-mediated activation in acetaminophen-induced nephrotoxicity in the CD-1 mouse. Toxicol. Appl. Pharmacol. 1991;107:1–15. doi: 10.1016/0041-008x(91)90325-9. [DOI] [PubMed] [Google Scholar]
- Hart SG, Beierschmitt WP, Wyand DS, Khairallah EA, Cohen SD. Acetaminophen nephrotoxicity in CD-1 mice. I. Evidence of a role for in situ activation in selective covalent binding and toxicity. Toxicol. Appl. Pharmacol. 1994;126:267–275. doi: 10.1006/taap.1994.1116. [DOI] [PubMed] [Google Scholar]
- Fujimoto K, Kumagai K, Ito K, Arakawa S, Ando Y, Oda S, Yamoto T, Manabe S. Sensitivity of liver injury in heterozygous Sod2 knockout mice treated with troglitazone or acetaminophen. Toxicol. Pathol. 2009;37:193–200. doi: 10.1177/0192623308329282. [DOI] [PubMed] [Google Scholar]
- Gow PJ, Jones RM, Dobson JL, Angus PW. Etiology and outcome of fulminant hepatic failure managed at an Australian liver transplant unit. J. Gastroenterol. Hepatol. 2004;19:154–159. doi: 10.1111/j.1440-1746.2004.03273.x. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H. Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol. Sci. 2002;67:322–328. doi: 10.1093/toxsci/67.2.322. [DOI] [PubMed] [Google Scholar]
- Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131:165–178. doi: 10.1053/j.gastro.2006.03.045. [DOI] [PubMed] [Google Scholar]
- Hadi M, Dragovic S, van Swelm R, Herpers B, van de Water B, Russel FG, Commandeur JN, Groothuis GM. AMAP, the alleged non-toxic isomer of acetaminophen, is toxic in rat and human liver. Arch. Toxicol. 2013;87:155–165. doi: 10.1007/s00204-012-0924-1. [DOI] [PubMed] [Google Scholar]
- Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J. Biol. Chem. 2008;283:13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa T, Ito Y, Wijeweera J, Liu J, Malle E, Farhood A, McCuskey RS, Jaeschke H. Reduced inflammatory response and increased microcirculatory disturbances during hepatic ischemia-reperfusion injury in steatotic livers of ob/ob mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292:G1385–G1395. doi: 10.1152/ajpgi.00246.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard KJ, Green JL, James LP, Judge BS, Zolot L, Rhyee S, Dart RC. Acetaminophen-cysteine adducts during therapeutic dosing and following overdose. BMC Gastroenterol. 2011;11:20. doi: 10.1186/1471-230X-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H. Glutathione disulfide formation and oxidant stress during acetaminophen-induced hepatotoxicity in mice in vivo: the protective effect of allopurinol. J. Pharmacol. Exp. Ther. 1990;255:935–941. [PubMed] [Google Scholar]
- Jaeschke H, McGill MR, Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug. Metab. Rev. 2012;44:88–106. doi: 10.3109/03602532.2011.602688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, McGill MR, Williams CD, Ramachandran A. Current issues with acetaminophen hepatotoxicity – a clinically relevant model to test the efficacy of natural products. Life Sci. 2011;88:737–745. doi: 10.1016/j.lfs.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Mitchell JR. Use of isolated perfused organs in hypoxia and ischemia/reperfusion oxidant stress. Methods Enzymol. 1990;186:752–759. doi: 10.1016/0076-6879(90)86175-u. [DOI] [PubMed] [Google Scholar]
- James LP, Letzig L, Simpson PM, Capparelli E, Roberts DW, Hinson JA, Davern TJ, Lee WM. Pharmacokinetics of acetaminophen-protein adducts in adults with acetaminophen overdose and acute liver failure. Drug Metab. Dispos. 2009;37:1779–1784. doi: 10.1124/dmd.108.026195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jollow DJ, Mitchell JR, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vitro. J. Pharmacol. Exp. Ther. 1973;187:195–202. [PubMed] [Google Scholar]
- Kaufman DW, Kelly JP, Rosenburg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA. 2002;287:337–344. doi: 10.1001/jama.287.3.337. [DOI] [PubMed] [Google Scholar]
- Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40:1170–1179. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiødt FV, Ostapowicz G, Shakil AO, Lee WM Acute Liver Failure Study Group. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J. Clin. Invest. 2012a;122:1574–1583. doi: 10.1172/JCI59755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Williams CD, Xie Y, Ramachandran A, Jaeschke H. Acetaminophen-induced liver injury in rats and mice: comparison of protein adducts, mitochondrial dysfunction, and oxidative stress in the mechanism of toxicity. Toxicol. Appl. Pharmacol. 2012b;264:387–394. doi: 10.1016/j.taap.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Yan HM, Ramachandran A, Murray GJ, Rollins DE, Jaeschke H. HepaRG cells: a human model to study mechanisms of acetaminophen hepatotoxicity. Hepatology. 2011;53:974–982. doi: 10.1002/hep.24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurtry RJ, Snodgrass WR, Mitchell JR. Renal necrosis, glutathione depletion, and covalent binding after acetaminophen. Toxicol. Appl. Pharmacol. 1978;46:87–100. doi: 10.1016/0041-008x(78)90139-4. [DOI] [PubMed] [Google Scholar]
- Meyers LL, Beierschmitt WP, Khairallah EA, Cohen SD. Acetaminophen-induced inhibition of hepatic mitochondrial respiration in mice. Toxicol. Appl. Pharmacol. 1988;93:378–387. doi: 10.1016/0041-008x(88)90040-3. [DOI] [PubMed] [Google Scholar]
- Mitchell JR, Jollow DJ, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J. Pharmacol. Exp. Ther. 1973a.;187:195–202. [PubMed] [Google Scholar]
- Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J. Pharmacol. Exp. Ther. 1973b;187:211–217. [PubMed] [Google Scholar]
- Mudge GH, Gemborys MW, Duggin GG. Covalent binding of metabolites of acetaminophen to kidney protein and depletion of renal glutathione. J. Pharmacol. Exp. Ther. 1978;206:218–226. [PubMed] [Google Scholar]
- Muldrew KL, James LP, Coop L, McCullough SS, Hendrickson HP, Hinson JA, Mayeux PR. Determination of acetaminophen-protein adducts in mouse liver and serum and human serum after hepatotoxic doses of acetaminophen using high-performance liquid chromatography with electrochemical detection. Drug Metab. Dispos. 2002;30:446–451. doi: 10.1124/dmd.30.4.446. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Maeda S, Hikiba Y, Ohmae T, Shibata W, Yanai A, Sakamoto K, Oqura K, Noguchi T, Karin M, Ichijo H, Omata M. Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibiting c-Jun N-terminal kinase activation. Gastroenterology. 2008;135:1311–1321. doi: 10.1053/j.gastro.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Nelson E, Morioka T. Kinetics of the metabolism of acetaminophen by humans. J. Pharm. Sci. 1963;52:864–868. doi: 10.1002/jps.2600520911. [DOI] [PubMed] [Google Scholar]
- Ni HM, Bockus A, Boggess N, Jaeschke H, Ding WX. Activation of autophagy protects against acetaminophen-induced hepatotoxicity. Hepatology. 2012a;55:222–232. doi: 10.1002/hep.24690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni HM, Boggess N, McGill MR, Lebofsky M, Borude P, Apte U, Jaeschke H, Ding WX. Liver-specific loss of Atg5 causes persistent activation of Nrf2 and protects against acetaminophen-induced liver injury. Toxicol. Sci. 2012b;127:438–450. doi: 10.1093/toxsci/kfs133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter WZ, Davis DC, Mitchell JR, Jollow DJ, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. 3. Cytochrome P-50-mediated covalent binding in vitro. J. Pharmacol. Exp. Ther. 1973;187:203–210. [PubMed] [Google Scholar]
- Pumford NR, Hinson JA, Benson RW, Roberts DW. Immunoblot analysis of protein containing 3-(cystein-S-yl)acetaminophen adducts in serum and subcellular liver fractions from acetaminophen-treated mice. Toxicol. Appl. Pharmacol. 1990;104:521–532. doi: 10.1016/0041-008x(90)90174-s. [DOI] [PubMed] [Google Scholar]
- Qin P, Jepsen P, Nørgård B, Agerbo E, Mortensen PB, Vilstrup H, Sørensen HT. Hospital admission for non-fatal poisoning with weak analgesics and risk for subsequent suicide: a population study. Psychol. Med. 2009;39:1867–1873. doi: 10.1017/S0033291709005741. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Benet LZ, Burlingame AL. Identification of the hepatic protein targets of reactive metabolites of acetaminophen in vivo in mice using two-dimensional gel electrophoresis and mass spectrometry. J. Biol. Chem. 1998;273:17940–17953. doi: 10.1074/jbc.273.28.17940. [DOI] [PubMed] [Google Scholar]
- Ramachandran A, Lebosfky M, Baines CP, Lemasters JJ, Jaeschke H. Cyclophilin D deficiency protects against acetaminophen-induced oxidant stress. Free Rad. Res. 2011a;45:156–164. doi: 10.3109/10715762.2010.520319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Lebofsky M, Weinman SA, Jaeschke H. The impact of partial manganese superoxide dismutase (SOD2)-deficiency on mitochondrial oxidant stress, DNA fragmentation and liver injury during acetaminophen hepatotoxicity. Toxicol. Appl. Pharmacol. 2011b;251:226–233. doi: 10.1016/j.taap.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid AB, Kurten RC, McCullough SS, Brock RW, Hinson JA. Mechanisms of acetaminophen-induced hepatotoxicity: role of oxidative stress and mitochondrial permeability transition in freshly isolated mouse hepatocytes. J. Pharmacol. Exp. Ther. 2005;312:509–516. doi: 10.1124/jpet.104.075945. [DOI] [PubMed] [Google Scholar]
- Roberts DW, Bucci TJ, Benson RW, Warbritton AR, McRae TA, Pumford NR, Hinson JA. Immunohistochemical localization and quantification of the 3-(cystein-S-yl)-acetaminophen protein adduct in acetaminophen hepatotoxicity. Am. J. Pathol. 1991;138:359–371. [PMC free article] [PubMed] [Google Scholar]
- Saito C, Lemasters JJ, Jaeschke H. c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol. Appl. Pharmacol. 2010;246:8–17. doi: 10.1016/j.taap.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiødt FV, Ott P, Christensen E, Bondesen S. The value of plasma acetaminophen half-life in antidote-treated acetaminophen overdosage. Clin. Pharmacol. Ther. 2002;71:221–225. doi: 10.1067/mcp.2002.121857. [DOI] [PubMed] [Google Scholar]
- Sharma M, Gadang V, Jaeschke A. Critical role for mixed-linease kinase 3 in acetaminophen-induced hepatotoxicity. Mol. Pharmacol. 2012;82:1001–1007. doi: 10.1124/mol.112.079863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson KJ, Bates CM, Henderson NC, Wigmore SJ, Garden OJ, Lee A, Pollok A, Masterton G, Hayes PC. The utilization of liver transplantation in the management of acute liver failure: comparison between acetaminophen and non-cetaminophen etiologies. Liver Transpl. 2009;15:600–609. doi: 10.1002/lt.21681. [DOI] [PubMed] [Google Scholar]
- Streeter AJ, Dahlin DC, Nelson SD, Baillie TA. The covalent binding of acetaminophen to protein. Evidence for cysteine residues as major sites of arylation in vitro. Chem. Biol. Interact. 1984;48:349–366. doi: 10.1016/0009-2797(84)90145-5. [DOI] [PubMed] [Google Scholar]
- Switzar L, Kwast LM, Lingeman H, Giera M, Pieters RH, Niessen WM. Identification and quantification of drug-albumin adducts in serum samples from a drug exposure study in mice. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2013;917–918C:53–61. doi: 10.1016/j.jchromb.2012.12.033. [DOI] [PubMed] [Google Scholar]
- Tirmenstein MA, Nelson SD. Subcellular binding and effects on calcium homeostasis produced by acetaminophen and a nonhepatotoxic regioisomer, 3’-hydroxyacetanilide, in mouse liver. J. Biol. Chem. 1989;264:9814–9819. [PubMed] [Google Scholar]
- Win S, Than TA, Han D, Petrovic LM, Kaplowitz N. c-Jun N-terminal kinase (JNK)-dependent acute liver injury from acetaminophen or tumor necrosis factor (TNF) requires mitochondrial Sab protein expression in mice. J. Biol. Chem. 2011;286:35071–35078. doi: 10.1074/jbc.M111.276089. [DOI] [PMC free article] [PubMed] [Google Scholar]