Abstract
It is now widely accepted that the development of atherosclerotic lesions involves a chronic inflammatory response that includes both innate and adaptive immune mechanisms. However, it is still unclear precisely what induces the inflammatory response. Furthermore, inflammation within the blood vessel can be divided into direct mechanisms where the primary inflammatory events occur within the intima of the blood vessel and contribute to both the initiation and progression of the plaques and indirect mechanisms where inflammation at non-vascular sites can contribute to the progression of the lesions. The direct mechanisms include lipid deposition and modification, influx of lipoprotein associated factors and microparticles derived from many different cell types, and possibly bacterial and viral infection of vascular cells. Indirect mechanisms derive from inflammation related to autoimmune diseases, smoking, respiratory infection, and pollution exposure, and possibly periodontal disease and gastric infection. The mechanisms include secretion of cytokines and other inflammatory factors into the circulation with subsequent uptake into the plaques, egress and recruitment of activated inflammatory cells, formation of dysfunctional HDL and cross reactive autoantibodies.
Introduction
The connection between inflammation and atherosclerosis is not of recent origin. Microscopic observations beginning in the 19th century described the presence of inflammatory reddening and inflammatory cells within human plaques (1, 2). Today it is acknowledged that development of atherosclerosis is a unique type of chronic inflammatory response that involves both innate and adaptive immune mechanisms (3). The development of animal models with cholesterol feeding, blood vessel injury and systemic infection have further supported the role of inflammation in the initiation and progression of atherosclerosis. The development of immunocytochemical and flow cytometric approaches with inflammatory cell type specific antibodies has now clearly established that atherosclerotic plaques at all stages of development contain multiple types of inflammatory cells including macrophages, dendritic cells, CD4 and CD8 positive T lymphocytes, B lymphocytes, mast cells and occasionally neutrophils and other granulocytes (4, 5). There is also a rapidly emerging new paradigm that suggests that several types of inflammatory cells within plaques have multiple phenotypes and can even change phenotype after entry into the plaques depending on the exposure to specific micro-environmental cues (6). In this short review the focus will be on distinguishing between direct inflammatory mechanisms that occur within the wall of the blood vessel and are thought to be responsible for the initiation of the disease process and indirect mechanisms where inflammation at non-vascular sites contributes to the progression and destabilization of the plaques.
Direct Mechanisms: Pro-inflammatory factors that initiate the disease process
Because of page restrictions, I will not review the natural history of the development of atherosclerotic plaques but refer the reader to a number of excellent recently published reviews (5, 7, 8). However, what is still unknown is precisely what enters the normal human blood vessel that sets in motion the subsequent activation of the endothelium to express adhesion molecules and leads to the recruitment of inflammatory cells into the intima. Today, lipids are still the leading candidate and the lipid retention hypothesis has been supported by much experimental evidence (9). Although Anitschkow first demonstrated that cholesterol feeding to rabbits led to the formation of lipid loaded cells within the intima (10), more definitive evidence was provided by experiments demonstrating that LDL particles that were labeled with a non-degradable radioactive probe accumulated at lesion susceptible branch points within days of initiating cholesterol feeding in rabbits (11, 12). However, those experiments did not clarify whether the simple accumulation of LDL particles trapped by interactions with matrix molecules (9) is sufficient to activate the inflammatory response or whether the LDL particles must be modified by oxidation of the fatty acids, phospholipids, and cholesterol or by other modifications. There is experimental evidence showing that oxidized lipids induce expression of adhesion molecules by endothelial cells (13, 14) and that oxidation specific epitopes can be localized in the intima coincident with endothelial expression of adhesion molecules (15, 16). Furthermore, lipoproteins with characteristics of those oxidized in vitro can be isolated from human plaques (17). However, free cholesterol itself in a non-oxidized crystalline state, can also induce a similar degree of endothelial activation (18). Furthermore, there is now evidence for a role of free cholesterol in the induction of the NLRP3 inflammasome with consequent release of IL-1B (19). It is also probable that other factors that associate with the LDL particle or with HDL or remnant particles that enter the intima can induce an inflammatory response. These include endotoxin (20) complement factors (21) or enzymes such as myeloperoxidase (22, 23) that if catalytically active can oxidize proteins that then become pro-inflammatory. Another possibility are microparticles (also referred to as microvesicles) that have been reported to enter the artery wall (24, 25). These microparticles are derived from platelets, neutrophils, macrophages, lymphocytes, endothelial cells and other cell types. The microparticles bear the signature of the cell type of origin including membrane proteins and bioactive lipids (24, 25). Microparticles have been shown to activate endothelial expression of adhesion molecules and thus could play a role in the initiation of lesions (26).
Finally, there is evidence that a number of different infectious agents populate human atherosclerotic lesions (27) and that infection of mice and rabbits can accelerate lesion development (27). T cell clones have been isolated from human plaques that proliferate in response to C. pneumoniae antigens (28, 29). Whether infectious agents play a role in the initiation of lesions is not clear as we have reported that C. pneumoniae infection of non-hyperlipidemic mice does not initiate development of lesions but requires prior or simultaneous hyperlipidemia (30). It is also unclear whether these infectious agents directly infect cells within the blood vessel or whether the bacteria are carried by cells that have been infected at other locations. For example, there is evidence that monocytes or macrophages that are infected by C. pneumoniae in the lungs can migrate to the aorta (31). It is also unclear whether there is active infection within atherosclerotic plaques. In most cases, it has not been possible to re-isolate viable organisms from human or experimental plaques (27). However, active infection is not a requirement for eliciting an immune or inflammatory response as even heat killed organisms can activate toll-like receptors on the plasma membrane of endothelial cells, macrophages and dendritic cells (32). Inflammatory cells within human plaques express a number of different toll-like and NOD receptors and deficiency of TLR-2 or TLR-4 impairs the development of lesions in hyperlipidemic mice and reduces the accelerated development of lesions in response to C. pneumoniae infection (33–35). Engagement of toll-like receptors activates a number of different signal transduction pathways leading to the activation of NFkB and other transcription factors that regulate the expression of pro-inflammatory cytokines (36, 37).
As noted, there appear to be multiple phenotypes of inflammatory cells that populate the atherosclerotic plaque. In hyperlipidemic mice there are monocytes expressing both high and low levels of the Ly6C antigen coupled with the CCR2+ chemokine receptor and low levels of the CX3CR1 receptor that are recruited into the plaque. The monocytes expressing high levels of Ly6C are thought to be precursors of inflammatory macrophages that secrete pro-inflammatory cytokines (38–40). There are also subsets of monocytes and dendritic cells in humans such as monocytes expressing high levels of CD14 and low levels of CD16 that may be the source of pro-inflammatory macrophages and dendritic cells in human plaques (41). There are also both immature circulating blood DC antigen positive (BDCA 1+) myeloid versus (BDCA 2+) plasmacytoid dendritic cells that are not of monocyte origin (42). The sources of both the monocytes and dendritic cells is controversial as it is not clear that they are all bone marrow derived. Some cells may also be recruited from lymph nodes and the spleen following some degree of maturation (43–45). Mature dendritic cells and macrophages can also be recruited from the lesions back to lymph nodes and spleen during regression of plaques (46, 47) and are thought to present plaque derived autoantigens to naïve T and B cells (48). In fact, this may be the primary mechanism for how mature plaques regress as studies of plaque composition of regressed plaques where regression was induced by a variety of different approaches and in a number of different species show consistent reductions in the numbers of macrophages and macrophage-derived foam cells (46, 49–52).
Based primarily on in vitro observations, it is now thought that recruited monocytes can differentiate into both pro-inflammatory M1 and anti-inflammatory M2 phenotypes dependent on the combination of cytokines that they encounter (53) and cells expressing markers of both M1 and M2 macrophages are found in both human and mouse lesions (54, 55). Furthermore, these M1 and M2 macrophages can have a similar impact on the phenotype of T lymphocytes by stimulating formation of CD4+ T helper 1 versus T helper 2 cells or T regulatory cells from naïve T cells again dependent on antigen presentation and the types of cytokines they secrete (56). A similar paradigm is thought to exist for dendritic cells and may be mediated by infection with C. pneumoniae (57). However, this is an over-simplification of what is likely to occur in human plaques at different stages of lesion development as there are probably additional phenotypes of macrophages and dendritic cells than just pro- and anti-inflammatory (58, 59). Furthermore, it is still unclear precisely what induces macrophage and lymphocyte polarization within the plaques as factors other than cytokines can also have an impact on the phenotypes of these cells (58, 60–64). There is also emerging evidence that supports additional roles for other types of lymphocytes including CD8+ T cells, T regulatory cells and NK cells (5, 65, 66). A critical question to be addressed by future research is how these different phenotypes play roles in stimulating or preventing the progression of human plaques to clinically relevant stages of the disease.
Indirect Mechanisms: Inflammation at non-vascular sites that contributes to the progression of atherosclerotic lesions
It is well established that people with autoimmune diseases such as rheumatoid arthritis, systemic lupus erythematosus, psoriatic arthritis, and ankylosing spondylitis have an increased risk of mortality from cardiovascular disease (67). This gave the first hint that inflammatory disorders at sites other than the blood vessel could contribute in a number of ways to the chronic inflammation within the artery wall. Smoking is also a well established risk factor for CVD mortality as are respiratory infection, air pollution exposure and possibly gastric and periodontal infections (27, 68, 69). The outstanding question is what the mechanisms are by which all of these non-vascular inflammatory conditions contribute to the ongoing inflammation in atherosclerotic lesions.
Perhaps the most obvious answer is that cytokines and other factors that are generated at non-vascular sites can be released into the circulation and taken up into the wall of the blood vessel. There are increases in plasma cytokines associated with the above autoimmune diseases (67) and we have shown that respiratory infection of mice with C. pneumoniae stimulates an acute phase response with measureable increases in plasma cytokines (70). Exposure of mice to concentrated diesel exhaust or ambient particles derived from vehicular emissions and other sources of pollution also stimulates increases in cytokines (71–73). Air pollution exposure as well as smoking can lead to the oxidation of lung surfactant phospholipids (74) that may be picked up by circulating lipoproteins and delivered to the blood vessel. As noted, activated inflammatory cells such as C. pneumoniae infected monocytes and macrophages may also exit non-vascular sites of inflammation and subsequently be recruited to the atherosclerotic plaque (31). This may also be the case with inflamed peri-adventitial adipose tissue (75). There is emerging evidence that obese people with increased visceral fat have increased numbers of macrophages and lymphocytes in the stromal vascular compartment of the adipose tissue. These cells are also sources of cytokines such as TNF-alpha that are thought to contribute to insulin resistance in liver and muscle (76). It is quite conceivable that these activated inflammatory cells and/or secreted cytokines can enter the adventitia and vasa vasorum and be delivered to the plaque (75).
Another indirect mechanism that has gained recent acceptance is the inflammation associated formation of dysfunctional HDL (77). For decades, HDL cholesterol has been thought of as the “good” cholesterol as epidemiologic data consistently demonstrated a strong association between plasma HDL cholesterol levels and a reduced risk of CVD (78). It is now becoming clear that HDL cholesterol may not be an adequate marker of reduced risk of CVD as recent clinical trials with agents that increase HDL cholesterol coupled with genome wide association studies have shown that there is no reduction in risk with elevation of HDL cholesterol (77). HDL is thought to play a protective role in CVD primarily by picking up cholesterol from the plaque and transporting it to the liver to be converted to bile and then excreted, a process referred to as reverse cholesterol transport. However, HDL also has anti-inflammatory and anti-oxidant properties that can be protective of atherosclerosis. These properties are most likely dependent on the proteins carried on the HDL particle such as the anti-oxidant enzyme paraoxonase (79, 80). Patients with rheumatoid arthritis and systemic lupus erythematosus have HDL with a reduced capacity to inhibit endothelial expression of adhesion molecules and the oxidation of LDL (61). In fact, patients with established coronary artery disease also appear to have dysfunctional HDL (81). Furthermore, mice infected with influenza A (82) or with C. pneumoniae (70) or exposed to concentrated ambient particles derived primarily from vehicular emissions also have dysfunctional HDL (83).
Finally, there are autoantibodies that recognize antigens within atherosclerotic plaques but that are thought to be generated in response to antigens generated elsewhere. For example, autoantibodies that recognize oxidized phospholipids within the plaques are known to be induced in response to oxidized phospholipids within bacterial cell walls or the membranes of apoptotic cells (84, 85). It is also conceivable that oxidized phospholipids generated from lung surfactant following exposure to air pollution may also contribute to the generation of these auotantibodies (70). There are also autoantibodies that recognize cholesterol and oxidized cholesterol (86, 87) and bacterial heat shock proteins and that cross react with human heat proteins (88). Thus, these autoantibodies could form antibody:antigen complexes within the developing plaque that could further contribute to the chronic inflammatory response of atherosclerosis.
Conclusions
It is now widely accepted that the development of atherosclerosis involves a chronic inflammatory response that includes both innate and adaptive immune mechanisms. However, there continue to be questions regarding the factors that directly stimulate these mechanisms within the intima of the blood vessel and set in motion the initiation and progression of the disease process. There are multiple possibilities that include lipid deposition and oxidation, accumulation of cell derived microparticles, and non-lipid lipoprotein associated factors. It is now clear that inflammation at non-vascular sites can contribute to the progression of atherosclerosis. These include multiple autoimmune diseases, smoking, respiratory infection and pollution exposure. There are still outstanding questions concerning the mechanisms by which non-vascular inflammation contributes to the chronic inflammation in the plaque. Possibilities include increases in plasma cytokines and other pro-inflammatory factors generated at non-vascular sites, migration and recruitment of already activated inflammatory cells and the formation of dysfunctional HDL. The mandate for the future is to verify that these direct and indirect mechanisms contribute to human atherosclerosis and to develop appropriate preventive and therapeutic approaches.
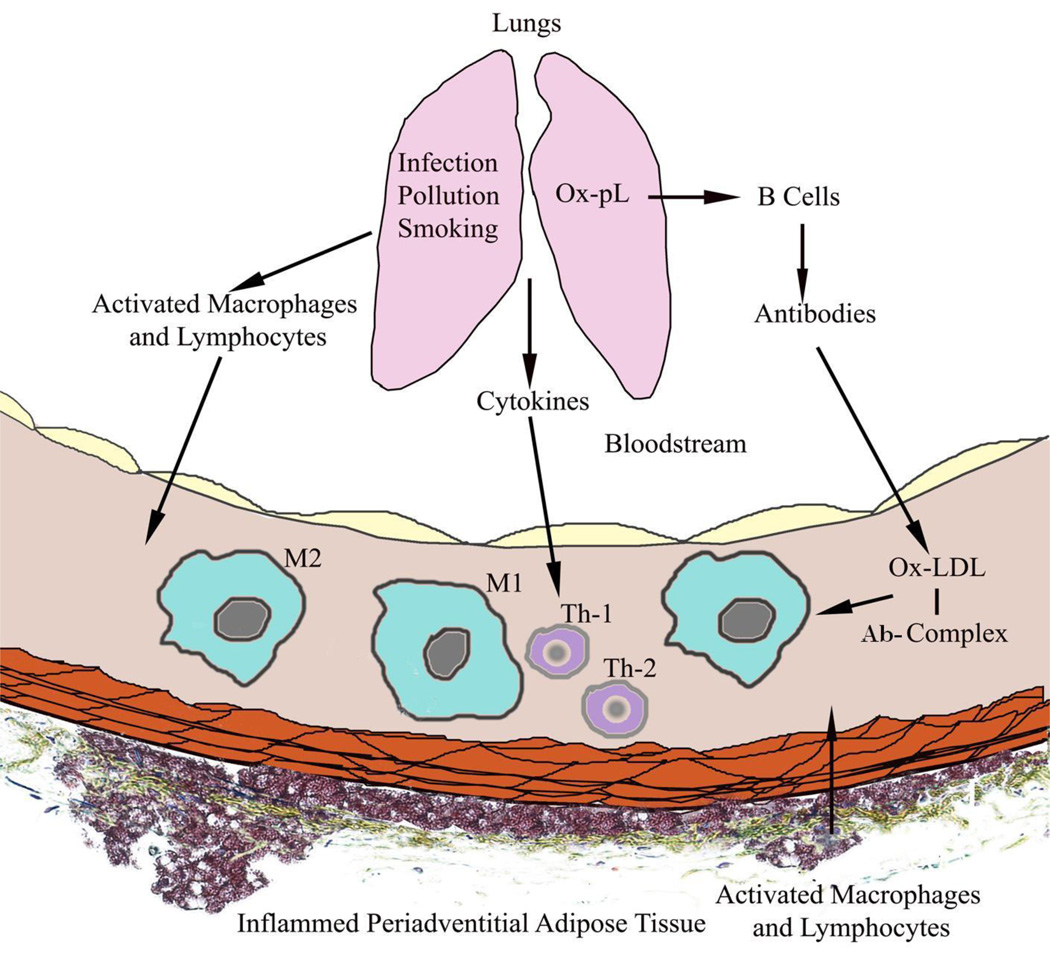
Figure 1. Potential Mechanisms of How Inflammation at Non-vascular Sites Contributes to Inflammation in Atherosclerotic Lesions.
This figure depicts how inflammation in the lungs induced by infection, smoking or air pollution can activate inflammatory cells that exit the lungs and home to the blood vessel (top) or how oxidation of lung surfactant phospholipids (Ox-pL) can induce autoantibodies that react with oxidized low density lipoproteins (Ox-LDL) forming pro-inflammatory antibody-antigen complexes (Ab-Complex). Activated inflammatory cells may also be recruited from inflamed peri-adventitial adipose tissues (bottom). M1 = pro-inflammatory macrophages, M2 = anti-inflammatory macrophages, Th-1 = CD4+ T helper 1 lymphocytes, Th-2 = CD4+ T helper 2 lymphocytes.
Highlights.
Inflammation in atherosclerosis can be divided into direct and indirect mechanisms
Direct mechanisms are inflammatory events occurring within the blood vessel
Direct mechanisms include lipid deposition, microparticles, and infectious agents
Indirect mechanisms derive from inflammatory events the occur at non-vascular sites
Non-vascular events include respiratory infection and air pollution exposure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel G. An historical perspective on atherosclerosis research in Germany. Atherosclerosis. 1999 May;144(1):1–6. doi: 10.1016/s0021-9150(99)00031-3. [DOI] [PubMed] [Google Scholar]
- 2.Nieto FJ. Infections and atherosclerosis: new clues from an old hypothesis? Am J Epidemiol. 1998 Nov 15;148(10):937–948. doi: 10.1093/oxfordjournals.aje.a009570. [DOI] [PubMed] [Google Scholar]
- 3.Hansson GK, Libby P, Schonbeck U, Yan ZQ. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res. 2002 Aug 23;91(4):281–291. doi: 10.1161/01.res.0000029784.15893.10. [DOI] [PubMed] [Google Scholar]
- 4.Soehnlein O, Weber C. Myeloid cells in atherosclerosis: initiators and decision shapers. Semin Immunopathol. 2009 Jun;31(1):35–47. doi: 10.1007/s00281-009-0141-z. [DOI] [PubMed] [Google Scholar]
- 5.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011 Mar;12(3):204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 6.Swirski FK, Weissleder R, Pittet MJ. Heterogeneous in vivo behavior of monocyte subsets in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009 Oct;29(10):1424–1432. doi: 10.1161/ATVBAHA.108.180521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011 Apr 29;145(3):341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011 May 19;473(7347):317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 9.Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007 Oct 16;116(16):1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 10.Finking G, Hanke H. Nikolaj Nikolajewitsch Anitschkow (1885–1964) established the cholesterol-fed rabbit as a model for atherosclerosis research. Atherosclerosis. 1997 Nov;135(1):1–7. doi: 10.1016/s0021-9150(97)00161-5. [DOI] [PubMed] [Google Scholar]
- 11.Schwenke DC, Carew TE. Initiation of atherosclerotic lesions in cholesterol-fed rabbits. II. Selective retention of LDL vs. selective increases in LDL permeability in susceptible sites of arteries. Arteriosclerosis. 1989 Nov-Dec;9(6):908–918. doi: 10.1161/01.atv.9.6.908. [DOI] [PubMed] [Google Scholar]
- 12.Schwenke DC, Carew TE. Initiation of atherosclerotic lesions in cholesterol-fed rabbits. I. Focal increases in arterial LDL concentration precede development of fatty streak lesions. Arteriosclerosis. 1989 Nov-Dec;9(6):895–907. doi: 10.1161/01.atv.9.6.895. [DOI] [PubMed] [Google Scholar]
- 13.Cominacini L, Garbin U, Pasini AF, Davoli A, Campagnola M, Contessi GB, et al. Antioxidants inhibit the expression of intercellular cell adhesion molecule-1 and vascular cell adhesion molecule-1 induced by oxidized LDL on human umbilical vein endothelial cells. Free Radic Biol Med. 1997;22(1–2):117–127. doi: 10.1016/s0891-5849(96)00271-7. [DOI] [PubMed] [Google Scholar]
- 14.Frostegard J, Haegerstrand A, Gidlund M, Nilsson J. Biologically modified LDL increases the adhesive properties of endothelial cells. Atherosclerosis. 1991 Oct;90(2–3):119–126. doi: 10.1016/0021-9150(91)90106-d. [DOI] [PubMed] [Google Scholar]
- 15.Rosenfeld ME, Palinski W, Yla-Herttuala S, Butler S, Witztum JL. Distribution of oxidation specific lipid-protein adducts and apolipoprotein B in atherosclerotic lesions of varying severity from WHHL rabbits. Arteriosclerosis. 1990 May-Jun;10(3):336–349. doi: 10.1161/01.atv.10.3.336. [DOI] [PubMed] [Google Scholar]
- 16.Cybulsky MI, Gimbrone MA., Jr Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991 Feb 15;251(4995):788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- 17.Yla-Herttuala S, Palinski W, Rosenfeld ME, Parthasarathy S, Carew TE, Butler S, et al. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989 Oct;84(4):1086–1095. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abela GS. Cholesterol crystals piercing the arterial plaque and intima trigger local and systemic inflammation. J Clin Lipidol. 2010 May-Jun;4(3):156–164. doi: 10.1016/j.jacl.2010.03.003. This is a short review that discusses how intracellular cholesterol crystals cause macrophage foam cell apoptosis which results in recruitment of more macrophages. Author proposes that this local inflammation eventually leads to systemic inflammation.
- 19. Freigang S, Ampenberger F, Spohn G, Heer S, Shamshiev AT, Kisielow J, et al. Nrf2 is essential for cholesterol crystal-induced inflammasome activation and exacerbation of atherosclerosis. Eur J Immunol. 2011 Jul;41(7):2040–2051. doi: 10.1002/eji.201041316. Investigation of how treatment of cells with cholesterol crystals activates the transcription factor NF-E2-related 2 (Nrf2) and the Nod Like Receptor-related protein 3 (NLRP3) inflammasome and the role of NRF-2 in the induction of IL-1 and atherosclerosis. Supports the pro-inflammatory role of cholesterol crystals in atherogenesis.
- 20.Feingold KR, Funk JL, Moser AH, Shigenaga JK, Rapp JH, Grunfeld C. Role for circulating lipoproteins in protection from endotoxin toxicity. Infect Immun. 1995 May;63(5):2041–2046. doi: 10.1128/iai.63.5.2041-2046.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaisar T, Pennathur S, Green PS, Gharib SA, Hoofnagle AN, Cheung MC, et al. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest. 2007 Mar;117(3):746–756. doi: 10.1172/JCI26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaisar T, Shao B, Green PS, Oda MN, Oram JF, Heinecke JW. Myeloperoxidase and inflammatory proteins: pathways for generating dysfunctional high-density lipoprotein in humans. Curr Atheroscler Rep. 2007 Nov;9(5):417–424. doi: 10.1007/s11883-007-0054-z. [DOI] [PubMed] [Google Scholar]
- 23. Undurti A, Huang Y, Lupica JA, Smith JD, DiDonato JA, Hazen SL. Modification of high density lipoprotein by myeloperoxidase generates a pro-inflammatory particle. J Biol Chem. 2009 Nov 6;284(45):30825–30835. doi: 10.1074/jbc.M109.047605. Demonstration that MPO-catalyzed oxidation results in the loss the anti-apoptotic and anti-inflammatory properties of HDL mediated in part by loss of binding to SRB1. MPO oxidation of HDL also results in HDL activation of NF-kappaB and expression of vascular cell adhesion molecule by aortic endothelial cells. Supports the emerging concept that measuring the HDL function is more important than measuring HDL cholesterol.
- 24. Angelillo-Scherrer A. Leukocyte-derived microparticles in vascular homeostasis. Circ Res. 2012 Jan 20;110(2):356–369. doi: 10.1161/CIRCRESAHA.110.233403. Review article that discusses the role of leukocyte-derived microparticles in mediating both pro- and anti-inflammatory responses by altering endothelial functions. Further discussion of the role of these microparticles in angiogenesis.
- 25.Burnier L, Fontana P, Kwak BR, Angelillo-Scherrer A. Cell-derived microparticles in haemostasis and vascular medicine. Thromb Haemost. 2009 Mar;101(3):439–451. [PubMed] [Google Scholar]
- 26. Rautou PE, Leroyer AS, Ramkhelawon B, Devue C, Duflaut D, Vion AC, et al. Microparticles from human atherosclerotic plaques promote endothelial ICAM-1-dependent monocyte adhesion and transendothelial migration. Circ Res. 2011 Feb 4;108(3):335–343. doi: 10.1161/CIRCRESAHA.110.237420. Study showing that treatment of cultured endothelial cells with microparticles isolated from human carotid endarterectomy specimens led to the integration of microparticle associated ICAM-1 into the endothelial cell plasma membranes and that the transferred ICAM-1 is functional and leads to normal signal transduction and increases in monocyte adhesion. Provides a potential mechanism for how microparticles can be pro-inflammtory and pro-atherogenic.
- 27.Rosenfeld ME, Campbell LA. Pathogens and atherosclerosis: update on the potential contribution of multiple infectious organisms to the pathogenesis of atherosclerosis. Thromb Haemost. 2011 Nov;106(5):858–867. doi: 10.1160/TH11-06-0392. [DOI] [PubMed] [Google Scholar]
- 28.Curry AJ, Portig I, Goodall JC, Kirkpatrick PJ, Gaston JS. T lymphocyte lines isolated from atheromatous plaque contain cells capable of responding to Chlamydia antigens. Clin Exp Immunol. 2000 Aug;121(2):261–269. doi: 10.1046/j.1365-2249.2000.01294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mosorin M, Surcel HM, Laurila A, Lehtinen M, Karttunen R, Juvonen J, et al. Detection of Chlamydia pneumoniae-reactive T lymphocytes in human atherosclerotic plaques of carotid artery. Arterioscler Thromb Vasc Biol. 2000 Apr;20(4):1061–1067. doi: 10.1161/01.atv.20.4.1061. [DOI] [PubMed] [Google Scholar]
- 30.Blessing E, Campbell LA, Rosenfeld ME, Kuo CC. Chlamydia pneumoniae and hyperlipidemia are co-risk factors for atherosclerosis: infection prior to induction of hyperlipidemia does not accelerate development of atherosclerotic lesions in C57BL/6J mice. Infect Immun. 2002 Sep;70(9):5332–5334. doi: 10.1128/IAI.70.9.5332-5334.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moazed TC, Kuo CC, Grayston JT, Campbell LA. Evidence of systemic dissemination of Chlamydia pneumoniae via macrophages in the mouse. J Infect Dis. 1998 May;177(5):1322–1325. doi: 10.1086/515280. [DOI] [PubMed] [Google Scholar]
- 32.Blessing E, Kuo CC, Lin TM, Campbell LA, Bea F, Chesebro B, et al. Foam cell formation inhibits growth of Chlamydia pneumoniae but does not attenuate Chlamydia pneumoniae-induced secretion of proinflammatory cytokines. Circulation. 2002 Apr 23;105(16):1976–1982. doi: 10.1161/01.cir.0000015062.41860.5b. [DOI] [PubMed] [Google Scholar]
- 33.Naiki Y, Sorrentino R, Wong MH, Michelsen KS, Shimada K, Chen S, et al. TLR/MyD88 and liver X receptor alpha signaling pathways reciprocally control Chlamydia pneumoniae-induced acceleration of atherosclerosis. J Immunol. 2008 Nov 15;181(10):7176–7185. doi: 10.4049/jimmunol.181.10.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Curtiss LK, Tobias PS. The toll of Toll-like receptors, especially toll-like receptor 2, on murine atherosclerosis. Curr Drug Targets. 2007 Dec;8(12):1230–1238. doi: 10.2174/138945007783220605. [DOI] [PubMed] [Google Scholar]
- 35.Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, et al. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci U S A. 2004 Jul 20;101(29):10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tobias P, Curtiss LK. Thematic review series: The immune system and atherogenesis. Paying the price for pathogen protection: toll receptors in atherogenesis. J Lipid Res. 2005 Mar;46(3):404–411. doi: 10.1194/jlr.R400015-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Huang Y, Li T, Sane DC, Li L. IRAK1 serves as a novel regulator essential for lipopolysaccharide-induced interleukin-10 gene expression. J Biol Chem. 2004 Dec 3;279(49):51697–51703. doi: 10.1074/jbc.M410369200. [DOI] [PubMed] [Google Scholar]
- 38.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003 Jul;19(1):71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 39.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007 Jan;117(1):195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007 Jan;117(1):185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Passlick B, Flieger D, Ziegler-Heitbrock HW. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood. 1989 Nov 15;74(7):2527–2534. [PubMed] [Google Scholar]
- 42.Van Vre EA, Van Brussel I, Bosmans JM, Vrints CJ, Bult H. Dendritic cells in human atherosclerosis: from circulation to atherosclerotic plaques. Mediators Inflamm. 2011;2011:941396. doi: 10.1155/2011/941396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009 Jul 31;325(5940):612–616. doi: 10.1126/science.1175202. First study to show that undifferentiated monocytes reside in the splenic red pulp and can be recurited to the heart following ischemic injury to participate in a wound healing response.
- 44.Swirski FK. The spatial and developmental relationships in the macrophage family. Arterioscler Thromb Vasc Biol. 2011 Jul;31(7):1517–1522. doi: 10.1161/ATVBAHA.110.221150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Robbins CS, Chudnovskiy A, Rauch PJ, Figueiredo JL, Iwamoto Y, Gorbatov R, et al. Extramedullary hematopoiesis generates Ly-6C(high) monocytes that infiltrate atherosclerotic lesions. Circulation. 2012 Jan 17;125(2):364–374. doi: 10.1161/CIRCULATIONAHA.111.061986. Using fate mapping techniques, the authors show the hematpoietic stem and progenitor cells from the bone marrow develop into Ly-6-C high monocytes in the splenic red pulp and are recruited from the spleen into atherosclerotic lesions in hyperlipidemic mice.
- 46.Llodra J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Natl Acad Sci U S A. 2004 Aug 10;101(32):11779–11784. doi: 10.1073/pnas.0403259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feig JE, Quick JS, Fisher EA. The role of a murine transplantation model of atherosclerosis regression in drug discovery. Curr Opin Investig Drugs. 2009 Mar;10(3):232–238. [PMC free article] [PubMed] [Google Scholar]
- 48.Packard RR, Maganto-Garcia E, Gotsman I, Tabas I, Libby P, Lichtman AH. CD11c(+) dendritic cells maintain antigen processing, presentation capabilities, and CD4(+) T-cell priming efficacy under hypercholesterolemic conditions associated with atherosclerosis. Circ Res. 2008 Oct 24;103(9):965–973. doi: 10.1161/CIRCRESAHA.108.185793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown BG, Zhao XQ, Sacco DE, Albers JJ. Lipid lowering and plaque regression. New insights into prevention of plaque disruption and clinical events in coronary disease. Circulation. 1993 Jun;87(6):1781–1791. doi: 10.1161/01.cir.87.6.1781. [DOI] [PubMed] [Google Scholar]
- 50.Daoud AS, Jarmolych J, Augustyn JM, Fritz KE. Sequential morphologic studies of regression of advanced atherosclerosis. Arch Pathol Lab Med. 1981 May;105(5):233–239. [PubMed] [Google Scholar]
- 51.Feig JE, Parathath S, Rong JX, Mick SL, Vengrenyuk Y, Grauer L, et al. Reversal of hyperlipidemia with a genetic switch favorably affects the content and inflammatory state of macrophages in atherosclerotic plaques. Circulation. 2011 Mar 8;123(9):989–998. doi: 10.1161/CIRCULATIONAHA.110.984146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feig JE, Rong JX, Shamir R, Sanson M, Vengrenyuk Y, Liu J, et al. HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proc Natl Acad Sci U S A. 2011 Apr 26;108(17):7166–7171. doi: 10.1073/pnas.1016086108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mantovani A, Garlanda C, Locati M. Macrophage diversity and polarization in atherosclerosis: a question of balance. Arterioscler Thromb Vasc Biol. 2009 Oct;29(10):1419–1423. doi: 10.1161/ATVBAHA.108.180497. Excellent review article that discusses the role of monocyte subpopulations that differentiate into both pro- and anti-atherogenic macrophages depending on what environmental signals they encounter.
- 54. Stoger JL, Gijbels MJ, van der Velden S, Manca M, van der Loos CM, Biessen EA, et al. Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis. 2012 Sep 26; doi: 10.1016/j.atherosclerosis.2012.09.013. One of the first studies to demonstrate via expression profiles and immunocytochemistry that both M1 and M2 polarized macrophages can be found in human atherosclerotic plaques and that M1 macrophages are more common in rupture prone shoulder regions of unstable plaques.
- 55.Khallou-Laschet J, Varthaman A, Fornasa G, Compain C, Gaston AT, Clement M, et al. Macrophage plasticity in experimental atherosclerosis. PLoS One. 2010;5(1):e8852. doi: 10.1371/journal.pone.0008852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Andersson J, Libby P, Hansson GK. Adaptive immunity and atherosclerosis. Clin Immunol. 2010 Jan;134(1):33–46. doi: 10.1016/j.clim.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 57.Ausiello CM, Fedele G, Palazzo R, Spensieri F, Ciervo A, Cassone A. 60-kDa heat shock protein of Chlamydia pneumoniae promotes a T helper type 1 immune response through IL-12/IL-23 production in monocyte-derived dendritic cells. Microbes Infect. 2006 Mar;8(3):714–720. doi: 10.1016/j.micinf.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 58.Adamson S, Leitinger N. Phenotypic modulation of macrophages in response to plaque lipids. Curr Opin Lipidol. 2011 Oct;22(5):335–342. doi: 10.1097/MOL.0b013e32834a97e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kadl A, Meher AK, Sharma PR, Lee MY, Doran AC, Johnstone SR, et al. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ Res. 2010 Sep 17;107(6):737–746. doi: 10.1161/CIRCRESAHA.109.215715. Demonstration by expression profiling that mouse macrophages treated with oxidized phospholipids develop a unique phenotype that express Nrf2 dependent redox-regulatory genes and have a different expression profile as compared to M1 or M2 polarized macrophages. Also show that macrophages with a similar expression profile are abundant in atherosclerotic plaques in LDLR−/− mice. Emphasizes the complexity of inflammatory cell phenotypic plasticity.
- 60. Hanna RN, Shaked I, Hubbeling HG, Punt JA, Wu R, Herrley E, et al. NR4A1 (Nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis. Circ Res. 2012 Feb 3;110(3):416–427. doi: 10.1161/CIRCRESAHA.111.253377. Study showing that hematopoietic cell deletion of nuclear receptor subfamily 4, group A, member 1 (NR4A1 or Nur77), a nuclear receptor shown to facilitate the development of Ly6C low monocytes, increases M1 macrophages and atherosclerosis in LDLR−/− mice. Further supports the potential anti-inflammatory role of M2 macrophages.
- 61. El Hadri K, Mahmood DF, Couchie D, Jguirim-Souissi I, Genze F, Diderot V, et al. Thioredoxin-1 promotes anti-inflammatory macrophages of the M2 phenotype and antagonizes atherosclerosis. Arterioscler Thromb Vasc Biol. 2012 Jun;32(6):1445–1452. doi: 10.1161/ATVBAHA.112.249334. Interesting study showing that treatment of macrophages with factors other than cytokines can impact macrophage polarization and atherosclerosis. Treatment of macrophages with thioredoxin-1 promoted cytokine mediated polarization of M2 macrophages and inhibited formation of M1 macrophages in vitro. Furthermore, administration of thioredoxin-1 to hyperlipidemic mice also stimulated formation of M2 macrophages and inhibited formation of M1 macrophages in liver and thymus and inhibited LPS stimulated M1 formation and atherosclerosis in the aorta.
- 62.Shiraishi D, Fujiwara Y, Komohara Y, Mizuta H, Takeya M. Glucagon-like peptide-1 (GLP-1) induces M2 polarization of human macrophages via STAT3 activation. Biochem Biophys Res Commun. 2012 Aug 24;425(2):304–308. doi: 10.1016/j.bbrc.2012.07.086. [DOI] [PubMed] [Google Scholar]
- 63.Cheng X, Folco EJ, Shimizu K, Libby P. Adiponectin Induces Pro-Inflammatory Programs in Human Macrophages and CD4+ T Cells. J Biol Chem. 2012 Sep 4; doi: 10.1074/jbc.M112.409516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Devaraj S, Jialal I. C-reactive protein polarizes human macrophages to an M1 phenotype and inhibits transformation to the M2 phenotype. Arterioscler Thromb Vasc Biol. 2011 Jun;31(6):1397–1402. doi: 10.1161/ATVBAHA.111.225508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Maganto-Garcia E, Tarrio ML, Grabie N, Bu DX, Lichtman AH. Dynamic changes in regulatory T cells are linked to levels of diet-induced hypercholesterolemia. Circulation. 2011 Jul 12;124(2):185–195. doi: 10.1161/CIRCULATIONAHA.110.006411. Important study showing that prolonged hypercholesterolemia and increasing atherosclerosis in mice reduces the number of T regulatory cells in both the circulation and atherosclerotic lesions of LDLR−/− mice but does not reduce the numbers of CD4+ T cells. Has implications for maintaining the protective role of T regulatory cells with lipid lowering therapy.
- 66.Gotsman I, Grabie N, Gupta R, Dacosta R, MacConmara M, Lederer J, et al. Impaired regulatory T-cell response and enhanced atherosclerosis in the absence of inducible costimulatory molecule. Circulation. 2006 Nov 7;114(19):2047–2055. doi: 10.1161/CIRCULATIONAHA.106.633263. [DOI] [PubMed] [Google Scholar]
- 67.Hahn BH, Grossman J, Chen W, McMahon M. The pathogenesis of atherosclerosis in autoimmune rheumatic diseases: roles of inflammation and dyslipidemia. J Autoimmun. 2007 Mar-May;28(2–3):69–75. doi: 10.1016/j.jaut.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 68.Mehta JL, Saldeen TG, Rand K. Interactive role of infection, inflammation and traditional risk factors in atherosclerosis and coronary artery disease. J Am Coll Cardiol. 1998 May;31(6):1217–1225. doi: 10.1016/s0735-1097(98)00093-x. [DOI] [PubMed] [Google Scholar]
- 69. Brook RD, Rajagopalan S, Pope CA, 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010 Jun 1;121(21):2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. Comprehensive review of the known and potential mechanisms by which exposure to air pollution may contribute to atherosclerosis and cardiovascular disease.
- 70.Campbell LA, Yaraei K, Van Lenten B, Chait A, Blessing E, Kuo CC, et al. The acute phase reactant response to respiratory infection with Chlamydia pneumoniae: implications for the pathogenesis of atherosclerosis. Microbes Infect. 2010 Aug 12;(8–9):598–606. doi: 10.1016/j.micinf.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sakai M, Yamashita K, Takemoto N, Ohshima Y, Tsukimoto M, Shinkai Y, et al. Diesel exhaust (DE) aggravates pathology of delayed-type hypersensitivity (DTH) induced by methyl-bovine serum albumin (mBSA) in mice. J Toxicol Sci. 2009 Oct;34(5):483–492. doi: 10.2131/jts.34.483. [DOI] [PubMed] [Google Scholar]
- 72.Hiramatsu K, Azuma A, Kudoh S, Desaki M, Takizawa H, Sugawara I. Inhalation of diesel exhaust for three months affects major cytokine expression and induces bronchus-associated lymphoid tissue formation in murine lungs. Exp Lung Res. 2003 Dec;29(8):607–622. doi: 10.1080/01902140390240140. [DOI] [PubMed] [Google Scholar]
- 73.Takano H, Yoshikawa T, Ichinose T, Miyabara Y, Imaoka K, Sagai M. Diesel exhaust particles enhance antigen-induced airway inflammation and local cytokine expression in mice. Am J Respir Crit Care Med. 1997 Jul;156(1):36–42. doi: 10.1164/ajrccm.156.1.9610054. [DOI] [PubMed] [Google Scholar]
- 74.Deiuliis JA, Kampfrath T, Zhong J, Oghumu S, Maiseyeu A, Chen LC, et al. Pulmonary T cell activation in response to chronic particulate air pollution. Am J Physiol Lung Cell Mol Physiol. 2012 Feb 15;302(4):L399–L409. doi: 10.1152/ajplung.00261.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tavora F, Kutys R, Li L, Ripple M, Fowler D, Burke A. Adventitial lymphocytic inflammation in human coronary arteries with intimal atherosclerosis. Cardiovasc Pathol. 2010 May-Jun;(3):e61–e68. doi: 10.1016/j.carpath.2009.02.001. This is an important study that morphologically links adventitial and peri-adventital adipose inflammation with human coronary plaque characteristics such as hemorrhage, rupture, erosion, and thin fibrous caps. The study suggests that adventitial and peri-adventitial cells may contribute to human lesion development.
- 76.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011 Jun;121(6):2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fisher EA, Feig JE, Hewing B, Hazen SL, Smith JD. High-density lipoprotein function, dysfunction, and reverse cholesterol transport. Arterioscler Thromb Vasc Biol. 2012 Dec;32(12):2813–2820. doi: 10.1161/ATVBAHA.112.300133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977 May;62(5):707–714. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- 79.Heinecke JW. The protein cargo of HDL: implications for vascular wall biology and therapeutics. J Clin Lipidol. 2010 Sep-Oct;4(5):371–375. doi: 10.1016/j.jacl.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Davidson WS, Silva RA, Chantepie S, Lagor WR, Chapman MJ, Kontush A. Proteomic analysis of defined HDL subpopulations reveals particle-specific protein clusters: relevance to antioxidative function. Arterioscler Thromb Vasc Biol. 2009 Jun;29(6):870–876. doi: 10.1161/ATVBAHA.109.186031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Landmesser U. High Density Lipoprotein - Should we Raise it? Curr Vasc Pharmacol. 2012 Nov 1;10(6):718–719. doi: 10.2174/157016112803520710. [DOI] [PubMed] [Google Scholar]
- 82.Van Lenten BJ, Wagner AC, Nayak DP, Hama S, Navab M, Fogelman AM. High-density lipoprotein loses its anti-inflammatory properties during acute influenza a infection. Circulation. 2001 May 8;103(18):2283–2288. doi: 10.1161/01.cir.103.18.2283. [DOI] [PubMed] [Google Scholar]
- 83.Araujo JA, Barajas B, Kleinman M, Wang X, Bennett BJ, Gong KW, et al. Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress. Circ Res. 2008 Mar 14;102(5):589–596. doi: 10.1161/CIRCRESAHA.107.164970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Chou MY, Fogelstrand L, Hartvigsen K, Hansen LF, Woelkers D, Shaw PX, et al. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J Clin Invest. 2009 May;119(5):1335–1349. doi: 10.1172/JCI36800. Unique study where the authors generated reconstituted mice that only express natural IgM antibodies. They show that 30% of these antibodies recognize oxidation-specific epitopes and bind to atherosclerotic plaques and apoptotic cells. The study further supports the role of B cells and antibodies to oxidized lipids in atherosclerosis.
- 85.Hartvigsen K, Chou MY, Hansen LF, Shaw PX, Tsimikas S, Binder CJ, et al. The role of innate immunity in atherogenesis. J Lipid Res. 2009 Apr;50(Suppl):S388–S393. doi: 10.1194/jlr.R800100-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hwee Ming C, Kalyana S. Cholesterol oxides and natural autoantibodies. Atherosclerosis. 1998 Dec;141(2):347–348. doi: 10.1016/s0021-9150(98)00166-x. [DOI] [PubMed] [Google Scholar]
- 87.Horvath A, Biro A. Anti-cholesterol antibodies in human sera. Autoimmun Rev. 2003 Sep 2;(5):272–277. doi: 10.1016/s1568-9972(03)00034-x. [DOI] [PubMed] [Google Scholar]
- 88.Metzler B, Xu Q, Wick G. The role of (auto-) immunity in atherogenesis. Wien Klin Wochenschr. 1998 May 22;110(10):350–355. [PubMed] [Google Scholar]