Abstract
Relay cells of dorsal lateral geniculate nucleus (LGN) receive a Class 1 glutamatergic input from the retina and a Class 2 input from cortical layer 6. Among the properties of Class 2 synapses is the ability to activate metabotropic glutamate receptors (mGluRs), and mGluR activation is known to affect thalamocortical transmission via regulating retinogeniculate and thalamocortical synapses. Using brain slices, we studied the effects Group I (DHPG) and Group II (DCG IV) mGluR agonists on retinogeniculate synapses. We showed that both agonists inhibit retinogeniculate EPSCs through presynaptic mechanisms, and their effects are additive and independent. We also found high frequency stimulation of the layer 6 corticothalamic input produced a similar suppression of retinogeniculate EPSCs, suggesting layer 6 projection to LGN as a plausible source of activating these presynaptic mGluRs.
Keywords: lateral geniculate nucleus, corticothalamic, thalamus
Introduction
Glutamatergic inputs in the thalamus and cortex have been classified into two types: Class 1 and Class 2. Class 1 inputs are thought to provide the main route for information transfer, whereas Class 2 inputs are thought to serve a generally modulatory function (reviewed in (Sherman and Guillery 2006; Sherman 2012). Among the modulatory properties of these Class 2 inputs is related to their ability to activate metabotropic glutamate receptors (mGluRs). Several studies of cortical circuitry indicate that Class 2 inputs in the thalamus and cortex can activate mGluRs that act to reduce the amplitude of synaptic transmission from Class 1 inputs (Lee and Sherman 2009; DePasquale and Sherman 2012; Lee and Sherman 2012). Since one of the first defined Class 2 pathways is the layer 6 corticothalamic input to thalamic relay cells (Reichova and Sherman 2004), and since there is recent evidence that activation of presynaptic mGluRs on retinal terminals can suppress retinogeniculate transmission (Govindaiah et al. 2012; Hauser et al. 2013), we sought to expand on this observation in brain slices from mice by further characterizing the role of mGluRs on retinogeniculate transmission and determining the role layer 6 input might have in this process. A preliminary report of these studies has been made (Lam and Sherman 2011b).
Methods
Preparation of brain slices
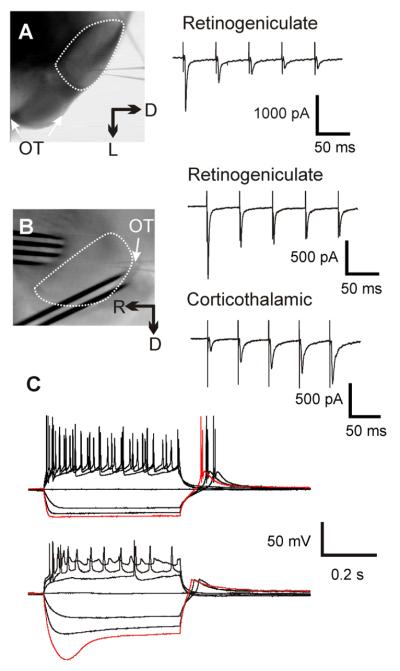
Our procedures followed the animal care guidelines of the University of Chicago and closely followed our previously published methodology (Lam and Sherman 2005; DePasquale and Sherman 2012; Lee and Sherman 2012). BALB/c mice (Harlan) of ages 12-21 days postnatal were deeply anaesthetized by inhalation of isoflurane, and their brains were quickly removed and chilled in ice-cold artificial cerebrospinal fluid (ACSF), which contained (in mM): 125 NaCl, 3 KCl, 1.25 NaH2PO4, 1 MgCl2, 2 CaCl2, 25 NaHCO3, 25 glucose. Their brains were sliced at 500 :m using a vibrating tissue slicer (Campden Instruments, Loughborough, UK). The slices were cut either coronally (Figure 1A) or parasagittally at an angle that preserved both corticothalamic and retinogeniculate inputs to the dorsal lateral geniculate nucleus (LGN, Figure 1B; (Turner and Salt 1998). These slices were then transferred to a holding chamber containing oxygenated ACSF and incubated at 30°C for at least 1 hour before each experiment.
Figure 1.
Experimental setup and methods. A: Left, photomicrograph taken during a recording from a coronal slices. Right, an example of the EPSC response to optic tract stimulation. B: Left, photomicrograph taken during a recording from a parasagittal slice. Right, response to optic tract (upper) and corticothalamic axon (lower) stimulation. The dotted line in A and B encircles the LGN and arrows indicate the location of optic tract (OT). C: Response of a relay cell (upper) and an interneuron (lower) to current step injection. Interneuron can be distinguished by a distinctive “sag” in their response to hyperpolarizing current injection (red traces).
Physiological recording
A few threads of nylon filaments, attached to a platinum wire slice holder, were used to secure the slices in the bath during the experiment. The slice was carefully placed during the experiment so that the nylon threads did not interfere with electrophysiological recording and electrical stimulation.
The LGN was identified in the slice by its location and the presence of the optic tract at its lateral edge. Retinogeniculate synapses were stimulated using a bipolar electrode straddling the optic tract. For coronal slices, the electrode was placed at a location further ventral to the region shown in Figure 1A, and so it is not visible in the photomicrograph. The corticothalamic pathway was stimulated by placing a 4×1 electrode array across the incoming corticothalamic axons, near the LGN (Figure 1B) and the two electrodes with the lowest stimulation response threshold were used for bipolar stimulation. Electric current was generated using a stimulus isolator (A365, World Precision Instrument, Sarasota, FL, USA). Response threshold to optic tract thresholds were determined before each experiment, and the stimulation intensity used was 150% to 250% above threshold, which turned out to be between 40 μA and 200 μA.
Whole cell recordings were performed at room temperature (22 degree Celsius) using a visualized slice setup (Cox and Sherman 2000; Lam and Sherman 2005). Recording pipettes were pulled from borosilicate glass capillaries and had a tip resistance of 3-6 MΩ when filled with a pipette solution containing the following (in mM): 127 K-gluconate, 3 KCl, 1 MgCl2, 0.07 CaCl2, 10 HEPES, 2 Na2-ATP, 0.3 Na-GTP, 0.1 EGTA. The pH of the pipette solution was adjusted to 7.3 with KOH or gluconic acid, and the osmolality was 280-290 mOsm.
The experiments were performed in voltage-clamp mode at a holding potential of −60 mV, using an Axopatch 200B (Molecular Devices, Sunnyvale, CA, USA). The access resistance of each cell was constantly monitored throughout the recordings, and experiments were discontinued if the access resistance exceeded 30 MΩ. Gabazine (20 μM, SR95531) was included in the ACSF to prevent any disynaptic IPSCs from contaminating the results.
The LGN of mice contains both relay cells and interneurons (Arcelli et al. 1997), and so we identified interneurons by the presence of a distinctive “sag” in their response to hyperpolarization current injection (Figure 1C, (Pape and McCormick 1995; Zhu et al. 1999; Govindaiah and Cox 2006). We did not study these cells further, and thus all data reported here are from relay cells.
Photostimulation
Methods for photostimulation have been described by us previously (Lam and Sherman 2005; Lam and Sherman 2007; Lam and Sherman 2010; Lam and Sherman 2011a) and are briefly outlined here. Data acquisition and photostimulation were controlled by the program Tidalwave (Shepherd et al. 2003). Nitroindolinyl (NI)-caged glutamate (Canepari et al. 2001) was added to the recirculating ACSF to a concentration of 0.39 mM during recording. Focal photolysis of the caged glutamate was accomplished by a 2 msec pulsed UV laser (355 nm wavelength, frequency-tripled Nd:YVO4, 100 kHz pulse repetition rate, DPSS Laser, San Jose, CA, USA). The laser beam was directed into the side port of a double-port tube (U-DPTS) on top of an Olympus microscope (BX50WI) using UV-enhanced aluminum mirrors (Thorlabs, Newton, NJ, USA) and a pair of mirror-galvanometers (Cambridge Technology, Cambridge, MA, USA) and then focused onto the soma of the recording cells using a low-magnification objective (4×0.1 Plan, Olympus).
Chemicals
Various agents were bath applied, including: the group I mGluR agonist, (R,S)-3,5-Dihydroxyphenylglycine (DHPG); the group II mGluR agonist (2S,2′R,3′R)-2-(2′,3′-Dicarboxycyclopropyl)glycine (DCG IV); the GABAA antagonist gabazine (SR 95531 hydrobromide) ; and the GABAB antagonist (3-minopropyl)(cyclohexylmethyl)phosphinic acid (CGP 46381). The G-protein antagonist GDP-β-S was included in the pipette solution for some cells to block postsynaptic mGluR responses. All the above reagents were purchased from Tocris (Minneapolis, MN, USA); caged glutamate and chemicals used for the ACSF and intracellular solution were purchased from Sigma (St. Louis, MO, USA).
Data Analysis
Data from these experiments were analyzed in Clampfit (Molecular Devices) and Excel (Microsoft). Statistical differences were tested using paired t-test using StatView (SAS Institute) or Origin (Microcal).
Results
Data were obtained from 50 geniculate relay cells, of which 37 were used to study retinogeniculate inputs, and 13, corticothalamic. We assumed that all postsynaptic responses evoked from stimulation of the optic tract were glutamatergic and monosynaptic, because: with GABA pathways blocked, there are no known multisynaptic inputs to geniculate relay cells that would be evoked, and the responses were smooth and monophasic. Furthermore, as expected, synapses were very reliable and at the stimulation intensity we used (150% - 250% of threshold), optic stimulation was always followed by large EPSC responses. These retinogeniculate EPSCs could be up to 2 nA and showed paired-pulse depression (Figure 1A & B, right), whereas corticothalamic EPSCs, evoked from stimulating the corticothalamic fibers, were much smaller (<500 pA) and showed paired-pulse facilitation (Figure 1B).
Additive effects for group I and II mGluR agonists on retinogeniculate EPSCs
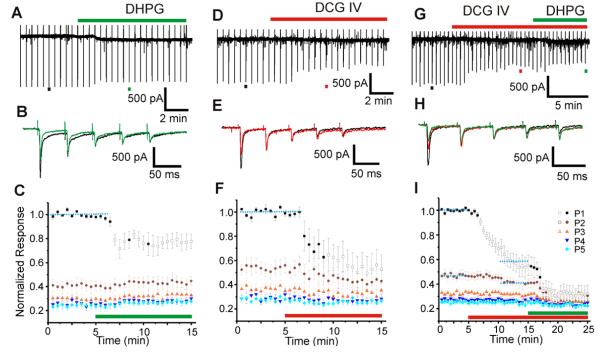
Retinogeniculate EPSCs were evoked in LGN relay cells once every 30 sec with optic tract stimulation that consisted of five 0.2 ms stimuli at 20 Hz, and these evoked EPSCs were used as a control against which to test effects of the mGluR agonists added to the bath. Figure 2A shows the recording trace of an experiment in which we tested the effects of the group I agonist DHPG (125 μM). Examples of evoked EPSCs (single trial, indicated by black and red bars underneath the trace in Figure 2A) to optic tract stimulation before (black) and after (green) DHPG application are shown in expanded time scale in Figure 2B. Application of DHPG strongly suppressed the first retinogeniculate EPSCs while having little effect on the subsequent responses to the test stimuli.
Figure 2.
Effects of mGluR agonists on retinogeniculate EPSCs. A-C: Effects of Group I agonist, DHPG. A: Voltage clamp recording during one experiment. The timing of DHPG application is indicated by the green bar above the trace, and the black and green bar beneath the trace indicate the EPSCs shown in B. B: Responses shown in an expanded time scale to the test stimulation of optic tract before (black) and after (green) DHPG application. C: Average normalized response of 5 experiments. EPSCs (P1-P5) in the response to a test pulse train are represented with different colors and symbols (see graph legend in I). Application of DHPG is indicated by the red bar. Responses significantly different from control (blue dotted line) are indicated with open symbols. D-F: Effects of Group II agonist, DCG IV. Conventions as in A-C. G-I: Independent and additive effects of Group I and Group II mGluR agonists. G: Recording of an experiment in which both DCG IV and DHPG were applied, with the red bar above the trace showing DCG IV application, and the green bar, DHPG. H: In an expanded time scale, selected EPSCs in control condition (black), in the presence of DCG IV (red) and both DCG IV and DHPG (green). I: Average normalized EPSCs of 10 experiments, displayed in the same format as C.
Results from 5 such experiments are combined and shown in Figure 2C. EPSCs were normalized to the size of the first response to the stimulation trains (P1), and the results were averaged across all 5 experiments. The average normalized amplitudes of all five EPSCs are then color-coded (see legends in Figure 2I) and plotted against time. The DHPG application reduced the first evoked EPSC, and evoked responses that are significantly different from the control (p<0.05) are indicated in the graph with open symbols. The DHPG, however, had no discernible effect on the other 4 evoked EPSCs in each train (p>0.05) (Figure 2C).
Effects of an mGluR II agonist, DCG IV (12 μM), were tested in five experiments and displayed in Figure 2D-F in a similar manner with the same conventions. Much like the effects of DHPG, DCG IV strongly suppressed the EPSC response to the first stimulus in the test pulses while leaving subsequent responses unaffected. As in Figure 2C, responses that are statistically significantly different (Students t-test, p<0.05) from control are indicated with open symbols in Figure 2F.
Figure 2G shows an example of a recording from an experiment in which we applied both DCG IV and DHPG, again with conventions as in Figure 2A-C. Similar experiments were repeated 10 times, 4 with the GABAB antagonist CGP 46381 (25 μM), included in the ACSF, and 6 without; we found no additional effect of adding this GABAB antagonist, and so we pooled the results of all 10 experiments in Figure 2I. Application of DCG IV suppressed retinogeniculate EPSCs, similar to that shown in Figure 2D-F. However, data here also show that subsequent additional DHPG application further suppressed retinogeniculate EPSCs even in the presence of DCG IV (Figure 2H&I), suggesting that the effects of these two mGluR agonists are independent and additive.
Presynaptic action for mGluR agonists
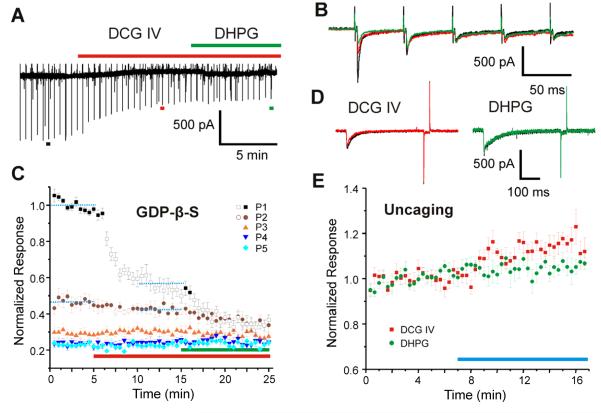
To determine whether DCG IV and DHPG act pre- or postsynaptically, 0.4 mM GDP-β-S was included in the pipette solution in 6 experiments during which we bath-applied these agonists (Figure 3A-C). Figure 3A shows an example of one such experiment, and Figure 3B shows the EPSC responses to selected test pulses before (black) and after (red & green) drug application in an expanded time scale, showing that inclusion of GDP-β-S, which interrupts the secondary messenger pathway of mGluR by inhibiting GTP-binding protein, did not discernibly affect the reduction of EPSC amplitude caused by application of the mGluR agonists. These data are shown in Figure 3C as the average normalized results for all 6 cells, following the format used in Figure 2C, F&I.
Figure 3.
Presynaptic action of mGluR agonists. A-C: GDP-β-S did not block the effects of mGluR agonists. A: Recording in which 0.4 mM GDP-β-S was included in the pipette solution. Application of agonists is shown by the bars (red, DCG IV; green, DHPG) above the trace. B: Selected EPSCs from A (indicated with colored bars in A) in control condition (black), in the presence of DCG IV (red) and in the presence of both DCG IV and DHPG (green). C: Average normalized responses of 6 experiments, displayed using the format shown in Figure 2C. D-E: Effects of mGluR agonists on response to photostimulation in a 0.2 mM Ca2+/3.8 mM Mg2+ ACSF solution containing 1 μM tetradotoxin. D: Example responses to photostimulation in control condition (black), after DCG IV (red, left) and DHPG (green, right) application. E: Effects of DCG IV (N=6, red squares) and DHPG application (N=5, green circles) on average normalized response to photostimulation with agonist application indicated by the blue bar.
Any effects of DCG IV and DHPG on postsynaptic glutamatergic responses were determined as follows. Synaptic responses were eliminated in these experiments with an ACSF containing 0.2 mM Ca2+/3.8 mM Mg2+ and 1 μM tetrodotoxin while we used photostimulation to determine any effects of mGluR agonists on direct actions of the uncaged glutamate. This experiment was repeated 6 times for DCG IV and 5 times for DHPG with the same laser power (24 mW, measured at the back-focal-plane), and Figure 3D shows that neither mGluR agonist affected the size of evoked responses to uncaged glutamate. Figure 3E shows the averaged normalized responses for these experiments, indicating that application of the mGluR agonists actually slightly increased the size of these responses.
Plausible contribution of corticothalamic inputs
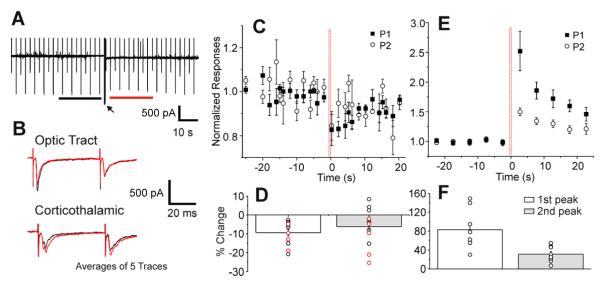
The above experiments indicate that activation of presynaptic mGluRs on retinogeniculate terminals reduces the amplitude of the first retinogeniculate EPSC evoked in a train. One possible source of glutamatergic input to relay cells that might produce such modulation is the input from layer 6 of visual cortex. We thus tested for this possibility by determining the effect of evoked corticothalamic input on retinogeniculate EPSCs in 13 cells using parasagittal slices. Unlike the experiments in Figures 2 and 3, test pulses of the optic tract consisted of only two 20 Hz stimuli delivered at intervals of 5 sec or 2 sec. Effects of the corticothalamic stimulation were tested by delivering high frequency stimulation (HFS; 125 Hz, 600 ms long) to the corticothalamic axons (arrow in Figure 4A), and the amplitudes of retinogeniculate EPSCs before and after the HFS were compared. HFS was used in an attempt to maximize the opportunity to evoke mGluR responses (e.g., (Beierlein et al. 2000; Grassi et al. 2002; Rush et al. 2002; Long et al. 2004).
Figure 4.
High frequency stimulation (HFS) of corticothalamic input suppresses retinogeniculate EPSCs. A: Recording from one experiment. The black and red bars beneath the trace indicate periods within which the averages in B are calculated. B: Reponses to optic tract and corticothalamic stimulation before (black) and after (red) HFS. Each is the average of 5 traces within the periods indicated in A. C: Average normalized responses to optic tract stimulation of 13 experiments. The vertical red lines indicate the timing of HFS. D: Percentage differences between the average responses to optic tract stimulation 20 sec before and after HFS (1st peak: t=-5.505, p<0.0001; 2nd peak: t=-2.305, p=0.0398, DF=12). Test pulses were delivered every 5 sec (black circles) or 2 sec (red circles). E: Average normalized response to stimulation of corticothalamic axons (N=8). F: Percentage differences between the average response to stimulation of corticothalamic input 20 sec before and after HFS (1st peak: t=5.444, p=0.0009; 2nd peak: t=5.250, p=0.0012, DF=7). Test pulses were delivered every 5 sec.
Figure 4A shows an example experiment, and Figure 4B (upper) shows in an expanded time scale the averaged responses to optical tract (upper) and cortical (lower) stimulation within the 20 sec before (black) and after (red) HFS was applied. In Figure 4C, the average normalized amplitudes of retinogeniculate responses to both test pulses are plotted against time. These data show that the retinogeniculate EPSC amplitudes in response to both test pulses were significantly decreased after the high frequency corticothalamic stimulation (Figure 4D, 1st peak: t=-5.505, p<0.0001; 2nd peak: t=-2.305, p=0.0398, DF=12). The reduction of EPSCs from cortical activation is less than that achieved by agonist application (e.g., compare Figure 2I with Figure 4D), but this is not unexpected, since the applied agonists presumably can activate all of the mGluRs available, whereas cortical activation is less likely to, because the released glutamate must travel an unspecified distance from the cortical terminals to retinal terminals, and also because in the slice one cannot assume that all relevant cortical axons are activated.
Furthermore, the amplitude of corticothalamic EPSCs was monitored at 5 sec intervals in 8 such experiments. Their average normalized amplitudes are shown in Figure 4E. In contrast to retinogeniculate responses, the amplitudes of corticothalamic EPSCs were significantly increased after the HFS (Figure 4F, 1st peak: t=5.444, p=0.0009; 2nd peak: t=5.250, p=0.0012, DF=7). Given the evidence in Figure 3D-E that effects on mGluR activation on glutamatergic EPSCs are presynaptic, it follows that these effects on corticothalamic EPSCs must also be presynaptic.
Discussion
We studied the effects Group I (DHPG) and Group II (DCG IV) mGluR agonists on retinogeniculate synapses. We showed that these agonists suppress retinogeniculate EPSCs, and their effects are additive and independent (Figure 2). Moreover, these agonists appear to act through presynaptic mechanisms as determined by the observations that EPSCs in response to the first pulse in a train of test stimulation being suppressed, while leaving the subsequent ones mostly unaffected (Figure 2C) and that blockage of postsynaptic mGluR effects via inclusion of GTP-β-S in the pipette solution did not ameliorate mGluR suppression of retinogeniculate transmission (Figure 3B&C). The possibility of a postsynaptic mechanism, such as a conductance change, cannot be entirely ruled out, but such a change is not supported by our results since application of mGluR agonists had no effect on postsynaptic responses evoked directly by photostimulation (Figure 3 D&E), and our main observations depended on evoked synaptic currents measured in voltage clamp recordings, which would be affected very little if at all by changes in baseline membrane conductance.
Many of our results thus are similar to those recently reported (Govindaiah et al. 2012; Hauser et al. 2013), except that we also show that both Group I and Group II mGluRs affect retinogeniculate transmission. The effects of Group I and Group II mGluR agonists are additive, suggesting separate and independent regulatory pathways for both receptor subtypes.
Govindaiah et al. (2012) also showed that high frequency stimulation of the optic tract mimicked mGluR suppression of retinogeniculate, which they interpreted as the activation of mGluR by glutamate spillover from retinogeniculate synapses. Our results show that the layer 6 corticothalamic projection to LGN is another plausible source of activating these mGluRs on retinal terminals (Figure 4). This is somewhat surprising, since it is commonly believed that retinogeniculate synapses are located near the soma, whereas the corticothalamic synapses are distributed further away in the dendritic tree of the relay cells (Wilson et al. 1984), suggesting that glutamated released from cortical terminals must travel quite a distance to affect retinal terminals. However, the evidence for a large separation of these different inputs onto dendrites of relay cells comes from other species, mostly cat (Wilson et al. 1984), and this feature may be different in mice, a point that needs to be determined. Nonetheless, this suggests a novel mechanism that layer 6 projection modulates information flow in the visual system.
We also found mGluR activation may slightly enhanced corticothalamic synapses (Figure 4 E&F). It is uncertain how significant this enhancement is since layer 6 neurons also indirectly inhibit thalamic relay cells through the thalamic reticular nucleus – the actual effect would not be known until the combined effect of mGluR activation on neurons of thalamic reticular nucleus and thalamus is determined. However, this observation suggests that firing of corticogeniculate axons sufficient to activate mGluRs (Viaene et al. 2013) could result in greater release of glutamate from these terminals, which in turn could lead to further reduction of retinogeniculate EPSCs. Such a process is consistent with recent evidence that activation of corticogeniculate axons reduces the gain of receptive fields measured in visual cortex (Olsen et al. 2012).
Our observation extends the view of the layer 6 cells that make up the corticothalamic pathway, because these cells affect thalamocortical transmission via at least 4 different mechanisms both in the thalamus and in cortical layer 4: 1) the corticothalamic input activates both a direct EPSP and disynaptic IPSP (via the thalamic reticular nucleus) on relay cells, and in most cases, the IPSP is much stronger and regulated by neuromodulators (Lam and Sherman 2010); 2) as the present study indicates, it can activate presynaptic mGluRs on retinal terminals to reduce retinogeniculate transmission; 3) it activates postsynaptic mGluRs on thalamorecipient layer 4 cells in cortex, and in many cases, these are Group II mGluRs, which hyperpolarizes the target cells (Lee and Sherman 2009); and 4) it activates presynaptic mGluRs on thalamocortical terminals, which serves to reduce thalamocortical transmission (Lee and Sherman 2012). Thus these layer 6 corticothalamic cells have widespread effects on thalamocortical transmission, acting both at its source as well at its target, and overall these effects operate to suppress thalamocortical transmission.
Highlights.
Retinogeniculate terminals have metabotropic glutamate receptors (mGluRs)
These include both Groups I and II
When activated, they reduce retinogeniculate ESPCs
corticogeniculate input activates these mGluRs and reduces retinogeniculate ESPCs
Acknowledgement
This work was supported by NIH Grants from the NIDCD (DC008794) and NEI (EY022338).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Arcelli P, Frassoni C, Regondi MC, De Biasi S, Spreafico R. GABAergic neurons in mammalian thalamus: A marker of thalamic complexity? Brain Res Bull. 1997;42:27–37. doi: 10.1016/s0361-9230(96)00107-4. [DOI] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. A network of electrically coupled interneurons drives synchronized inhibition in neocortex. Nat Neurosci. 2000;3:904–910. doi: 10.1038/78809. [DOI] [PubMed] [Google Scholar]
- Canepari M, Nelson L, Papageorgiou G, Corrie JE, Ogden D. Photochemical and pharmacological evaluation of 7-nitroindolinyl-and 4-methoxy-7-nitroindolinyl-amino acids as novel, fast caged neurotransmitters. J Neurosci Methods. 2001;112:29–42. doi: 10.1016/s0165-0270(01)00451-4. [DOI] [PubMed] [Google Scholar]
- Cox CL, Sherman SM. Control of dendritic outputs of inhibitory interneurons in the lateral geniculate nucleus. Neuron. 2000;27:597–610. doi: 10.1016/s0896-6273(00)00069-6. [DOI] [PubMed] [Google Scholar]
- DePasquale R, Sherman SM. Modulatory effects of metabotropic glutamate receptors on local cortical circuits. J Neurosci. 2012;32:7364–7372. doi: 10.1523/JNEUROSCI.0090-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindaiah G, Cox CL. Excitatory actions of synaptically released catecholamines in the rat lateral geniculate nucleus. Neurosci. 2006;137:671–683. doi: 10.1016/j.neuroscience.2005.09.021. [DOI] [PubMed] [Google Scholar]
- Govindaiah G, Wang T, Gillette MU, Cox CL. Activity-dependent regulation of retinogeniculate signaling by metabotropic glutamate receptors. The Journal of Neuroscience. 2012;32:12820–12831. doi: 10.1523/JNEUROSCI.0687-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi S, Frondaroli A, Pettorossi VE. Different metabotropic glutamate receptors play opposite roles in synaptic plasticity of the rat medial vestibular nuclei. J Physiol. 2002;543:795–806. doi: 10.1113/jphysiol.2002.023424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser JL, Edson EB, Hooks BM, Chen C. Metabotropic glutamate receptors and glutamate transporters shape transmission at the developing retinogeniculate synapse. J Neurophysiol. 2013;109:113–123. doi: 10.1152/jn.00897.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam YW, Sherman SM. Mapping by laser photostimulation of connections between the thalamic reticular and ventral posterior lateral nuclei in the rat. J Neurophysiol. 2005;94:2472–2483. doi: 10.1152/jn.00206.2005. [DOI] [PubMed] [Google Scholar]
- Lam YW, Sherman SM. Different topography of the reticulothalmic inputs to first- and higher-order somatosensory thalamic relays revealed using photostimulation. J Neurophysiol. 2007;98:2903–2909. doi: 10.1152/jn.00782.2007. [DOI] [PubMed] [Google Scholar]
- Lam YW, Sherman SM. Functional organization of the somatosensory cortical layer 6 feedback to the thalamus. Cereb Cortex. 2010;20:13–24. doi: 10.1093/cercor/bhp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam YW, Sherman SM. Functional organization of the thalamic input to the thalamic reticular nucleus. J Neurosci. 2011a;31:6791–6799. doi: 10.1523/JNEUROSCI.3073-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam YW, Sherman SM. Metabotropic glutamate receptor agonists modulate the input-output relationship of retinogeniclulate synapses. Soc Neurosci Abstr. 2011b [Google Scholar]
- Lee CC, Sherman SM. Glutamatergic inhibition in sensory neocortex. Cereb Cortex. 2009;19:2281–2289. doi: 10.1093/cercor/bhn246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Sherman SM. Intrinsic modulators of auditory thalamocortical transmission. Hear Res. 2012;287:43–50. doi: 10.1016/j.heares.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long MA, Landisman CE, Connors BW. Small clusters of electrically coupled neurons generate synchronous rhythms in the thalamic reticular nucleus. J Neurosci. 2004;24:341–349. doi: 10.1523/JNEUROSCI.3358-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen SR, Bortone DS, Adesnik H, Scanziani M. Gain control by layer six in cortical circuits of vision. Nature. 2012;483:47–52. doi: 10.1038/nature10835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, McCormick DA. Electrophysiological and pharmacological properties of interneurons in the cat dorsal lateral geniculate nucleus. Neurosci. 1995;68:1105–1125. doi: 10.1016/0306-4522(95)00205-w. [DOI] [PubMed] [Google Scholar]
- Reichova I, Sherman SM. Somatosensory corticothalamic projections: Distinguishing drivers from modulators. J Neurophysiol. 2004;92:2185–2197. doi: 10.1152/jn.00322.2004. [DOI] [PubMed] [Google Scholar]
- Rush AM, Wu J, Rowan MJ, Anwyl R. Group I metabotropic glutamate receptor (mGluR)-dependent long-term depression mediated via p38 mitogen-activated protein kinase is inhibited by previous high-frequency stimulation and activation of mGluRs and protein kinase C in the rat dentate gyrus in vitro. J Neurosci. 2002;22:6121–6128. doi: 10.1523/JNEUROSCI.22-14-06121.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM, Pologruto TA, Svoboda K. Circuit analysis of experience-dependent plasticity in the developing rat barrel cortex. Neuron. 2003;38:277–289. doi: 10.1016/s0896-6273(03)00152-1. [DOI] [PubMed] [Google Scholar]
- Sherman SM. Thalamocortical interactions. Curr Opin Neurobiol. 2012;17:417–422. doi: 10.1016/j.conb.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Exploring the Thalamus and its Role in Cortical Function. MIT Press; Cambridge, MA: 2006. [Google Scholar]
- Turner JP, Salt TE. Characterization of sensory and corticothalamic excitatory inputs to rat thalamocortical neurones in vitro. Journal of Physiology. 1998;510:829–843. doi: 10.1111/j.1469-7793.1998.829bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaene AN, Petrof I, Sherman SM. Activation requirements for metabotropic glutamate receptors. Neurosci Lett. 2013 doi: 10.1016/j.neulet.2013.02.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JR, Friedlander MJ, Sherman SM. Fine structural morphology of identified X- and Y-cells in the cat’s lateral geniculate nucleus. Proc Roy Soc Lond B. 1984;221:411–436. doi: 10.1098/rspb.1984.0042. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Uhlrich DJ, Lytton WW. Properties of a hyperpolarization-activated cation current in interneurons in the rat lateral geniculate nucleus. Neurosci. 1999;92:445–457. doi: 10.1016/s0306-4522(98)00759-3. [DOI] [PubMed] [Google Scholar]