Abstract
Monoclonal gammopathy of undetermined significance of the immunoglobulin M class was diagnosed in 213 patients at the Mayo Clinic, 29 (14%) of whom developed lymphoma, Waldenström macroglobulinemia, or a related disorder over 1567 person-years of follow-up. The cumulative probability of progression was 10% at 5 years, 18% at 10 years, and 24% at 15 years, or approximately 1.5% per year. The concentration of serum monoclonal protein at diagnosis and the initial serum albumin value were the only independent predictors of progression with multivariate analysis.
By contrast, during 285 person-years of follow-up, 34 (71%) of 48 patients with smoldering Waldenström macroglobulinemia (SWM) progressed to Waldenström macroglobulinemia (WM), which required therapy, along with amyloid light chain (AL) amyloidosis (1) and lymphoma (1). The cumulative probability of progression was 6% at 1 year, 39% at 3 years, 59% at 5 years, and 65% at 10 years. The percentage of lymphoplasmacytic cells in the bone marrow, size of the serum monoclonal (M) spike, and hemoglobin value were significant independent risk factors for progression.
Introduction
Monoclonal gammopathy of undetermined significance (MGUS) is defined by the presence of a serum monoclonal (M) protein concentration <3 g/dL; fewer than 10% plasma cells in the bone marrow, and no end-organ damage, such as hypercalcemia, renal insufficiency, anemia, or lytic bone lesions related to the plasma cell proliferative process.1–3 The monoclonal gammopathy can consist of IgG, IgA, IgM or, infrequently, IgD. Only a few series of modest size, and with suboptimal durations of follow-up, focused on IgM monoclonal gammopathies.4–8 In this study, we compared the natural history of IgM MGUS with that of SWM, which is similar to IgM MGUS and has an indolent clinical course and must be differentiated from WM.9,10
Progression of IgM MGUS
MGUS of the IgM class was diagnosed in 213 patients at the Mayo Clinic who resided in the 11 counties (including Olmsted County) of southeastern Minnesota and were seen from 1960 to 1994.11 The 1980 population of Olmsted County was 92,006, with 312,559 people residing in the remaining 10 counties of the local region. Patients with SWM, lymphoma, or related lymphoproliferative disorders at the time of recognition of the IgM M protein were excluded. The primary endpoint of the study was progression to lymphoma, WM, or related disorders. Of the 213 patients with IgM MGUS, 58% (123) were men and 42% (90) were women. Their median age at diagnosis was 74 years (range, 24–94 years). Only 1% (3) were younger than 40 years of age, whereas almost two-thirds of the patients were older than 70 years.11
The serum M protein at diagnosis ranged from unmeasurable (visible as a small band on electrophoresis but not quantifiable by densitometry) to 2.6 g/dL (median, 1.2 g/dL). Only 53% (113) of patients had an M protein > 1.0 g/dL at diagnosis. The light chain was kappa in 70% (149) and lambda in 30% (64). The level of uninvolved (normal, polyclonal, or background) immunoglobulins was reduced in 35% of patients. Twenty-seven percent (58) of patients had a monoclonal light chain (kappa in 19% and lambda in 8%) in the urine, although the amount was small, with only 3 patients having more than 100 mg per 24 hours. Anemia, present in 17% (36), was due to conditions other than the monoclonal gammopathy.
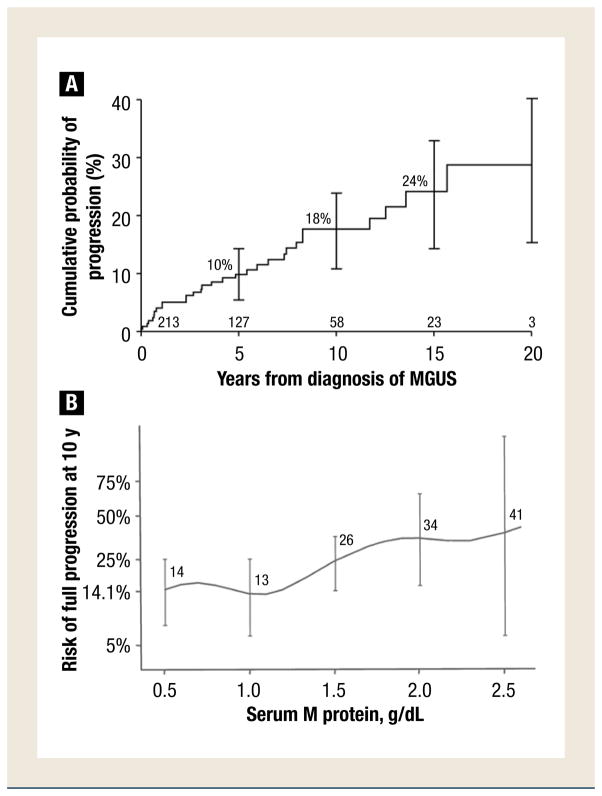
During follow-up for 1567 person-years (median, 6.3 person-years), during which time 71% (151) died, non-Hodgkin lymphoma, WM, AL amyloidosis, and chronic lymphocytic leukemia developed in 29 (14%) patients. Non-Hodgkin lymphoma was classified as lymphoplasmacytic (6 patients), diffuse large B-cell (5 patients), and mucosa-associated lymphoid tissue (2 patients), and 1 each of small lymphocytic, follicular, large cell, and unclassified B-cell lymphoma. WM developed in 6 patients, whereas AL amyloidosis was recognized in 3 patients, and chronic lymphocytic leukemia was recognized in 3 other patients. The cumulative probability of progression to one of these disorders was 10% at 5 years, 18% at 10 years, and 24% at 15 years (Figure 1A), for an overall average risk of progression of approximately 1.5% annually. Progression to a lymphoid neoplasm (29 patients) was 16 times that expected on the basis of incidence rates for those conditions in the general population.
Figure 1.
(A) Probability of Progression to Lymphoma, Waldenström Macroglobulinemia, AL Amyloidosis, or Chronic Lymphocytic Leukemia in 213 Residents of Southeastern Minnesota in Whom Monoclonal Gammopathy of Undetermined Significance (MGUS) of Immunoglobulin (Ig) M Class was Diagnosed From 1960 Through 1994. Error Bars Show 95% Confidence Intervals. The Numbers at the Bottom of the Horizontal Axis are the Numbers of Patients at Risk at Each Interval. Figure 1A Was Modified From Ref. 11. American Society of Hematology. (B) Relative Risk of Disease Progression 10 Years After 213 Residents of Southeastern Minnesota MGUS of IgM Class Were Diagnosed From 1960 Through 1994 by Monoclonal Protein Level at Diagnosis. Error Bars Show 95% Confidence Intervals
The risk factors evaluated for progression included sex, hemoglobin level, size of serum M protein, type of serum light chain, free light chain ratio, the difference between involved and uninvolved free light chains, reduction of uninvolved immunoglobulins, the presence of urinary monoclonal light chains, the serum albumin level, and the percentage of lymphoplasmacytic cells in the bone marrow. Of these, only the concentration of the M protein at diagnosis and the serum albumin value were independent predictors of progression on multivariate analysis. The risk for progression to lymphoma or a related malignancy 10 years after recognition of MGUS was 14%, for an initial M protein value of 0.5 g/dL or less, 26% for 1.5 g/dL, 34% for 2.0 g/dL, and 41% for 2.5 g/dL (Figure 1B). Reduction of uninvolved immunoglobulins and the presence of monoclonal light chain in the urine were not risk factors for progression.
The management of patients with IgM MGUS is not clearly defined. Patients should be evaluated 6 months after recognition of IgM MGUS. If the patient is stable at 6 months and if the serum M protein is ≥1.5 g/dL, then the patient should be followed up at annual intervals. If the M protein is <1.5 g/dL, then the patient may be followed up at 3 to 4 year intervals but should be told to seek medical advice if any unexplained symptoms develop.
Progression of SWM
SWM is a poorly described asymptomatic disorder with an increased risk of progressing to symptomatic WM, which requires therapy.12 It is characterized by the presence of a serum IgM value ≥3 g/dL and/or ≥10% bone marrow lymphoplasmacytic infiltration of the bone marrow but no evidence of end-organ damage, such as symptomatic anemia, constitutional symptoms, hyperviscosity syndrome, lymphadenopathy, or hepatosplenomegaly, which could be attributed to a lymphoplasmacytic proliferative disorder.13,14
Forty-eight patients diagnosed at the Mayo Clinic between 1974 and 1995 fulfilled the criteria for SWM. Their median age at diagnosis was 63 years. Seven (15%) were younger than 50 years old but only 1 (2%) was younger than 40 years of age at diagnosis. The liver was palpable in 12% (6), whereas the spleen was palpable in only 5% (2). Lymphadenopathy was noted in 10% (5). Hemoglobin <10 g/dL was found in 4 patients, but this was due to myelodysplastic syndrome, bronchopleural fistula with empyema, Barrett esophagus, and chronic renal failure, respectively. The serum M protein at diagnosis ranged from 1.5 to 5.2 g/dL (median, 3.3 g/dL). Only 25% (12) were <3 g/dL, whereas one-fifth had an M spike ≥4 g/dL. IgM kappa was present in 75% (36). Levels of uninvolved (normal, polyclonal) immunoglobulins were reduced in 47% (24). Proteinuria ranged from not measurable to 1.4 g per 24 hours (median, 0.05 g per 24 hours).
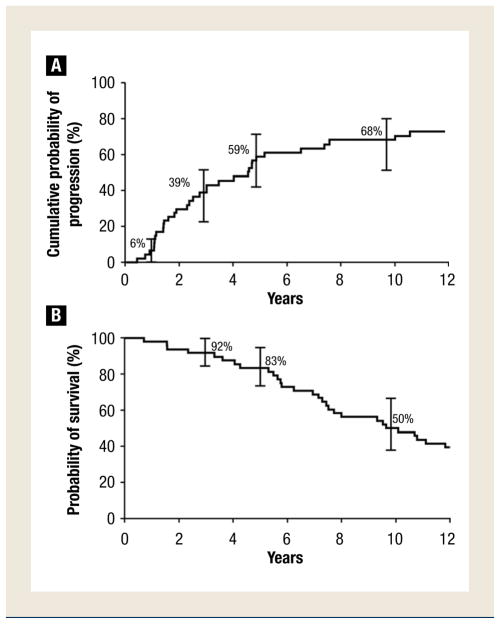
The patients were followed up for a median of 15.4 years (285 cumulative person-years). During this time, 34 (71%) progressed to WM that required chemotherapy, 1 patient progressed to AL amyloidosis and 1 patient progressed to lymphoma. The cumulative probability of progression to symptomatic WM that required therapy, amyloidosis, or lymphoma was 6% at 1 year, 39% at 3 years, 59% at 5 years, and 68% at 10 years (Figure 2A). The cumulative probability of progression was 12% per year for the first 5 years and then 2% per year for the next 5 years, which approaches that of MGUS. Thus, the longer a patient remains stable with SWM, the less likely is progression. The median time to progression was 4.6 years.
Figure 2.
(A) Cumulative Probability of Progression From Smoldering Waldenström Macroglobulinemia (SWM) to Symptomatic WM That Requires Therapy, Amyloidosis, or Lymphoma in the Cohort of 48 Patients With SWM. Error Bars Show 95% Confidence Intervals. (B) Probability of Survival in 48 Patients With SWM. Error Bars Show 95% Confidence Intervals
When considering the same potential risk factors mentioned above, the percentage of lymphoplasmacytic cells infiltrating the bone marrow was the most important predictor of progression on multivariate analysis. The size of the serum M spike and the hemoglobin value each contributed significantly to a model that already contained the percentage of lymphoplasmacytic cells as a significant risk factor for progression. During the first 5 years, the progression rate was 92% for the 13 patients who had 50% or more lymphoplasmacytic cells in the bone marrow at diagnosis compared with 46% for those with bone marrow infiltration <50% (P = .001). At 5 years, the risk of progression was 61% in those with an initial M protein ≥3 g/dL and ≥10% bone marrow lymphoplasmacytic involvement but only 49% among those having an M spike <3 g/dL and a bone marrow lymphoplasmacytic level ≥10% (P = .265). The baseline median level of lymphoplasmacytic infiltration in the bone marrow was 15% in the 12 patients who did not progress to active disease.
Seventy-five percent (36) of the 48 patients with SWM died during follow-up. Their overall survival was 83% at 5 years and 50% at 9.6 years (Figure 2B). The median survival after progression to symptomatic WM was 5.1 years. Only 5 patients with SWM were still alive and at risk for progression. These patients have now been followed-up for 14 to 24.6 years. Thus, the majority of patients with SWM will progress to symptomatic disease that requires therapy. The pertinent laboratory tests should be repeated 2 to 3 months after initial recognition of SWM to exclude early progression; if the results are stable, then the study should be repeated every 4 to 6 months for 1 to 2 years and then, if stable, rechecked at annual intervals. It is important to emphasize to patients that they seek medical care if they should develop any untoward symptoms.
Conclusion
Patients with IgM monoclonal gammopathy of undetermined significance have a progression rate of approximately 1.5% per year and must be followed until death. Patients with smoldering Waldenström’s macroglobulinemia progress at a rate of 12% per year for the first 5 years and then decreases to almost the same as IgM MGUS.
Footnotes
Disclosure
The authors have stated that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kyle RA. Monoclonal gammopathy of undetermined significance. Natural history in 241 cases. Am J Med. 1978;64:814–26. doi: 10.1016/0002-9343(78)90522-3. [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA. ‘Benign’ monoclonal gammopathy — after 20 to 35 years of follow-up. Mayo Clin Proc. 1993;68:26–36. doi: 10.1016/s0025-6196(12)60015-9. [DOI] [PubMed] [Google Scholar]
- 3.Kyle RA, Therneau TM, Rajkumar SV, et al. Long-term follow-up of 241 patients with monoclonal gammopathy of undetermined significance: the original Mayo Clinic series 25 years later [see comment] Mayo Clin Proc. 2004;79:859–66. doi: 10.4065/79.7.859. [DOI] [PubMed] [Google Scholar]
- 4.Kyle RA, Garton JP. The spectrum of IgM monoclonal gammopathy in 430 cases. Mayo Clin Proc. 1987;62:719–31. doi: 10.1016/s0025-6196(12)65225-2. [DOI] [PubMed] [Google Scholar]
- 5.Hobbs JR, Carter PM, Cooke KB, et al. IgM paraproteins. J Clin Pathol. 1974;28:54–64. doi: 10.1136/jcp.s1-6.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stein RS, Ellman L, Bloch KJ. The clinical correlates of IgM M-components: an analysis of thirty-four patients. Am J Med Sci. 1975;269:209–16. doi: 10.1097/00000441-197503000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Tubbs RR, Hoffman GC, Deodhar SD, et al. IgM monoclonal gammopathy. Histopathologic and clinical spectrum. Cleve Clin Q. 1976;43:217–35. doi: 10.3949/ccjm.43.4.217. [DOI] [PubMed] [Google Scholar]
- 8.Waldenström JG. Benign monoclonal gammapathy. Acta Med Scand. 1984;216:435–47. doi: 10.1111/j.0954-6820.1984.tb05032.x. [DOI] [PubMed] [Google Scholar]
- 9.Baldini L, Goldaniga M, Guffanti A, et al. Immunoglobulin M monoclonal gammopathies of undetermined significance and indolent Waldenstrom’s macroglobulinemia recognize the same determinants of evolution into symptomatic lymphoid disorders: proposal for a common prognostic scoring system. J Clin Oncol. 2005;23:4662–8. doi: 10.1200/JCO.2005.06.147. [DOI] [PubMed] [Google Scholar]
- 10.Gobbi PG, Baldini L, Broglia C, et al. Prognostic validation of the international classification of immunoglobulin M gammopathies: a survival advantage for patients with immunoglobulin M monoclonal gammopathy of undetermined significance? Clin Cancer Res. 2005;11:1786–90. doi: 10.1158/1078-0432.CCR-04-1899. [DOI] [PubMed] [Google Scholar]
- 11.Kyle RA, Therneau TM, Rajkumar SV, et al. Long-term follow-up of IgM monoclonal gammopathy of undetermined significance. Blood. 2003;102:3759–64. doi: 10.1182/blood-2003-03-0801. [DOI] [PubMed] [Google Scholar]
- 12.Kyle RA, Benson JT, Larson DR, et al. Progression in smoldering Waldenstrom macroglobulinemia: long-term results. Blood. 2012;119:4462–6. doi: 10.1182/blood-2011-10-384768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23:3–9. doi: 10.1038/leu.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Owen RG, Treon SP, Al-Katib A, et al. Clinicopathological definition of Waldenstrom’s macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom’s Macroglobulinemia. Semin Oncol. 2003;30:110–5. doi: 10.1053/sonc.2003.50082. [DOI] [PubMed] [Google Scholar]